The authors claim Canadian physicians use numerous methods to obtain unfunded oral chemotherapies, including falsifying claims on access forms and submitting special requests to government agencies.
Abstract
Purpose:
Previous studies have shown hematologists and medical oncologists may not accept the financial limits set by governing agencies on patient access to oral chemotherapy. The purpose of this study was to capture the methods physicians used to overcome barriers to accessing chemotherapeutic regimens for their patients.
Methods:
A total of 640 medical oncologists and hematologists across Canada were surveyed using a 13-item Web-based survey tool. The survey was delivered by e-mail with three follow-up reminders. After a response period of 3 months, results were collated and analyzed with descriptive statistics.
Results:
Of the 640 invitations, 568 were successfully delivered, and 183 responses were received (response rate, 32.0%). Among respondents, 101 treated solid malignancies (55.2%), 49 treated nonsolid malignancies (26.8%), and 33 treated both (18.0%). To overcome funding barriers, participating oncologists enrolled patients onto clinical trials (90.5%), used compassionate access programs (96.1%), and made special requests to government (91.8%). Other methods included writing false claims on forms to fit funding criteria for drugs (31.1%) and using leftover drug supplies (31.0%). Physicians felt their inability to obtain unfunded medications had a negative impact on their patients' clinical outcomes (56.0%) and psychosocial quality of life (73.0%). Only 28.5% of physicians contacted their governing body with concerns about oral chemotherapy funding.
Conclusion:
Canadian physicians use numerous methods to obtain unfunded oral chemotherapies, including falsifying claims on access forms and submitting special requests to government agencies. Further study is warranted to explore the disconnection between policymakers and physicians with regard to funding of oral chemotherapies.
Introduction
The foundation of Canada's universal health care system is established on equality of access to treatment. Access to cancer drugs has been defined as “the ability to obtain recommended cancer drug treatments in a timely manner and without financial hardship.”1(pi) With advances in cancer care, there has been a shift toward orally administered drug regimens.2–8 Novel antineoplastic agents can place a heavy financial burden on patients, institutions, and agencies. Provincial cancer agencies and hospitals pay for drugs used in hospital such as intravenously administered chemotherapy, whereas oral chemotherapy taken at home are not covered. Publically funded drug programs across Canada are managed independently by each province with their own formulary and reimbursement rules.9 Cancer drug approval in Canada typically occurs under the jurisdiction of provincial cancer agencies, with reliance on drug advisory committees and government review boards. Both the efficacy as per the literature and the cost-benefit ratio of medications are taken into account. Oral chemotherapy, unlike intravenous, is not covered, resulting in patients with cancer in different provinces with differing access to care.9 Inconsistent access to anticancer drugs across Canada exists, despite the foundation that access to care is based on need and evidence rather than ability to pay or place of residence.9
In a previous study, it was shown that medical oncologists do not allow priority-setting decisions to affect access to cancer drugs from which they feel their patients would benefit.2 However, the methods physicians use to overcome limitations imposed by funding bodies has not been studied extensively. In addition, the amount of time a medical oncologist or hematologist spends on administrative work for funding can have a significant toll on his or her practice, taking away from direct care and other activities. In a previous qualitative study in which 46 medical oncologists from Ontario were interviewed, the following types of measures used were identified: lying on forms, using local hospitals to cover the drugs, and appealing to other government funding programs.2 The frequency of these measures and overall impact on a large scale have not been assessed. Such approaches can be viewed as unethical because they breach the standard of available care in a jurisdiction. Perceived harms of such practices include patient exposure to medications that lack similar efficacy (ie, using out-of-country medications) and increased cost of medical care if toxicity ensues. Many institutions across Canada have employed specialists for the role of drug access coordinator (DAC) to assist with the significant burden of barriers to cancer drugs. A DAC supports patients in finding ways to cover the cost of their medications or accessing drug programs when needed. Health professionals such as social workers, nurses, and other allied professionals perform the role of a DAC in addition to their required duties.
This study was a nationwide study across Canada, surveying all practicing oncologists and hematologists, to assess the methods used to obtain unfunded oral chemotherapy and quantify the proportion of physicians using each of these alternative means. Furthermore, the impact on time and resources was assessed.
Methods
Participants
Licensed medical oncologists and hematologists across Canada were identified through the use of publically available medical directories. A total of 640 physicians were surveyed using a confidential Web-based survey tool.
Survey Development
The validity of the survey questions has not been evaluated, but the questions are similar to those used by Berry et al2 in their qualitative study of Ontario oncologists and priority-setting decisions. The survey was developed in collaboration with and piloted by medical oncologists, pharmacists, and hematologists; further consultation with the survey development unit was completed.
Demographics
To account for differences in the amount of direct patient care, demographic characteristics, including total years spent in hematology/oncology practice as well as number of half-day clinics per week, were collected. In addition, the province in which each physician practiced was recorded to determine if interprovincial differences existed as a result of independent funding programs.
Resources
To determine the amount of time physicians spent on obtaining unfunded oral drugs for patients, we asked physicians how many hours on average were spent per working week in the past year on obtaining funding and whether their institutions had a DAC.
Approaches
To quantify the use of a variety of methods to obtain access to unfunded oral chemotherapy, a Likert scale question was developed (weekly, monthly, every 6 months, annually, never, do not know) to allow respondents to select the frequency of using given methods to access drugs. The top seven methods identified by data from the Berry et al2 study and physician experience at St Michael's Hospital (Toronto, Ontario, Canada) were: one, shipping drugs from other countries; two, referring patients to other countries for treatment; three, enrolling patients onto clinical trials; four, filing false claims on forms or dictations to fit funding criteria for drugs; five, using leftover drug supplies from other patients; six, completing special request forms to government agencies; and seven, using compassionate access program. Compassionate access programs refer to programs that provide access to new and unapproved drugs when no other treatments are available. These are provided through single-patient access and for patients not enrolled onto a clinical trial.
Impact
We determined the impact of physicians' inability to obtain provincial reimbursements for expensive, unfunded medications on direct patient care by asking a subset of questions with the possible responses of positive impact, no impact, or negative impact. Specifically, we asked whether physicians perceived an impact on their physician-patient relationships, patients' clinical outcomes, or patients' psychosocial quality of life. Similarly, questions pertained to what type of impact (improved access, no impact, or impaired access) the following had on patients' drug access: patients' socioeconomic status, patients having private drug insurance, and media coverage of new cancer drugs.
Solutions
A Likert scale (ranging from very likely to very unlikely) was used to assess whether respondents felt the following would improve patients' access to oral chemotherapy medicine: direct engagement of treating physicians in funding decisions about new medicines, increased patient awareness of all options for therapy (funded and unfunded), increased media attention to heighten public awareness of gaps in medication funding, and increased funding for public agency drug access programs (eg, Trillium program). Ontario's Trillium program ensures no one pays more than 5% of his or her household income for oral chemotherapy. These approaches to improve drug access were selected based on institutional consensus during survey development. Finally, we asked whether the responding physician had contacted his or her provincial governing body with concerns about funding for oral chemotherapy.
Survey Procedure
The survey was administered in December 2010 through an e-mail message, with a link to the survey contained the questions described. Three additional e-mail reminders were then subsequently sent. Responses were collected from December 2010 to February 2011.
Statistical Analysis
Descriptive statistics were performed to analyze the data and were presented as proportions, means, or medians. Preplanned subgroup analysis included differences based on practice location. This study was approved by the St Michael's Hospital Research Ethics Board.
Results
Demographics
A majority of respondents were based in an academic setting and practiced in either Ontario (n = 90; 49.7%), British Columbia (n = 24; 13.3%), Quebec (n = 18; 9.9%), or Alberta (n = 19; 10.5%; Table 1). Of all responding physicians, 101 treated solid malignancies (55.2%), 49 treated nonsolid malignancies (26.8%), and 33 treated both (18.0%).
Table 1.
Demographics of Medical Oncologists and Hematologists (n = 183)
| Demographic | No. | % |
|---|---|---|
| Sex | ||
| Female | 101 | 60.5 |
| Male | 66 | 39.5 |
| Practice setting | ||
| University based | 134 | 80.2 |
| Comprehensive cancer center (not university affiliated) | 17 | 10.2 |
| Community based | 18 | 10.8 |
| Province of primary practice | ||
| Alberta | 19 | 10.5 |
| British Columbia | 24 | 13.3 |
| Manitoba | 9 | 5.0 |
| New Brunswick | 4 | 2.2 |
| Newfoundland and Labrador | 2 | 1.1 |
| Nova Scotia | 10 | 5.5 |
| Ontario | 90 | 49.7 |
| Prince Edward Island | 2 | 1.1 |
| Quebec | 18 | 9.9 |
| Saskatchewan | 3 | 1.7 |
| Treats | ||
| Solid malignancies | 101 | 55.2 |
| Nonsolid/hematologic malignancies | 49 | 26.8 |
| Both | 33 | 18.0 |
| Years spent in practice | ||
| 0-4 | 31 | 18.6 |
| 5-9 | 36 | 21.6 |
| 10-25 | 71 | 42.5 |
| > 25 | 29 | 17.4 |
| No. of half-day clinics per week* | ||
| < 3 | 28 | 16.8 |
| 3-6 | 108 | 64.7 |
| > 6 | 31 | 18.6 |
| Hours spent per working week on obtaining drugs for patients* | ||
| 0 | 5 | 3.3 |
| 1-4 | 97 | 64.2 |
| 5-9 | 22 | 14.6 |
| > 10 | 27 | 17.9 |
| Practices at institution with drug access coordinator | ||
| Yes | 93 | 61.6 |
| No | 58 | 38.4 |
| Has contacted provincial body with chemotherapy funding concerns | ||
| Yes | 52 | 28.5 |
| No | 115 | 62.8 |
In past year on average.
Resources
Sixty-four percent of physicians spent an average of 1 to 4 hours obtaining funding for oral drugs for patients per week, and 61.6% indicated their institution had a DAC. Approximately 15% of physicians spent an average of 5 to 9 hours per week on obtaining funding for oral drugs, and an additional 17.9% of physicians spent more than 10 hours per week on obtaining funding for oral drugs.
Approaches
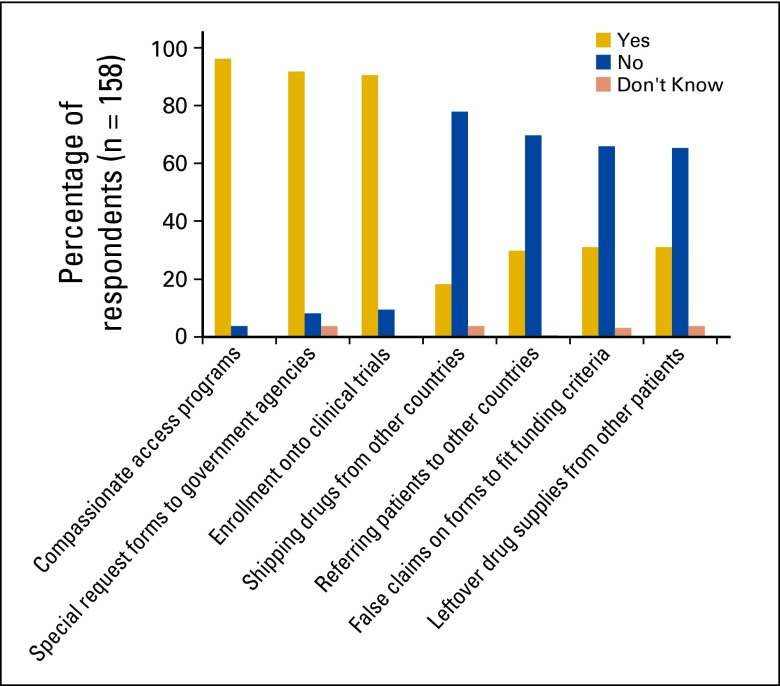
Methods to obtain access to unfunded oral chemotherapies ranged from enrolling patients onto clinical trials to referring patients to other countries for treatment (Fig 1). The frequency and use of each method in the past year was noted (Appendix Table A1, online only). Respondents in the past year used compassionate access programs (96.1%) and special request forms to government agencies (91.8%) to obtain access to unfunded chemotherapy regimens. Of all physicians who responded, 31.0% admitted to false claims on forms or dictations to fit funding criteria for drugs. The frequencies of reported use of false claims on forms or dictations to fit funding criteria for drugs in the past year were weekly (1.9%), monthly (7.0%), every 6 months (19.6%), and annually (3.2%).
Figure 1.
Responses to question: “In the past year, have you used the following to gain access to unfunded oral chemotherapy treatment?”
Impact
The perceived psychosocial and clinical implications of not obtaining oral chemotherapies for patients are summarized in Table 2. This included an inability to prescribe the treatments physicians preferred, which negatively affected patient outcomes and psychosocial quality of life. However, physicians felt this did not affect their physician-patient relationships. Factors perceived as increasing patients' access to publically unfunded medications were private insurance and media coverage, whereas low socioeconomic status was prohibitive (Table 2).
Table 2.
Factors and Impact of Inability to Obtain Unfunded Medications for Patients (n = 158)
| Factor | Positive Impact |
No Impact |
Negative Impact |
Do Not Know |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Inability to obtain expensive unfunded medications for patients has impacted: | ||||||||
| Your physician-patient relationships, trust, credibility | 10 | 6.3 | 85 | 53.8 | 49 | 31.0 | 14 | 8.9 |
| Your patients' clinical outcomes, overall survival, toxicity, and adverse effects | 7 | 4.4 | 33 | 20.9 | 88 | 55.7 | 30 | 19.0 |
| Your patients' perceived psychosocial quality of life, anxiety, depression | 5 | 3.2 | 23 | 14.6 | 115 | 72.8 | 15 | 9.5 |
| Factors that affect patients' drug access | ||||||||
| Patients' low socioeconomic status | 12 | 7.6 | 26 | 16.5 | 116 | 73.4 | 4 | 2.5 |
| Patients having private drug insurance | 138 | 87.3 | 12 | 7.6 | 6 | 3.8 | 2 | 1.3 |
| Media coverage of new cancer drugs | 60 | 38.0 | 75 | 47.5 | 8 | 5.1 | 15 | 9.5 |
Solutions
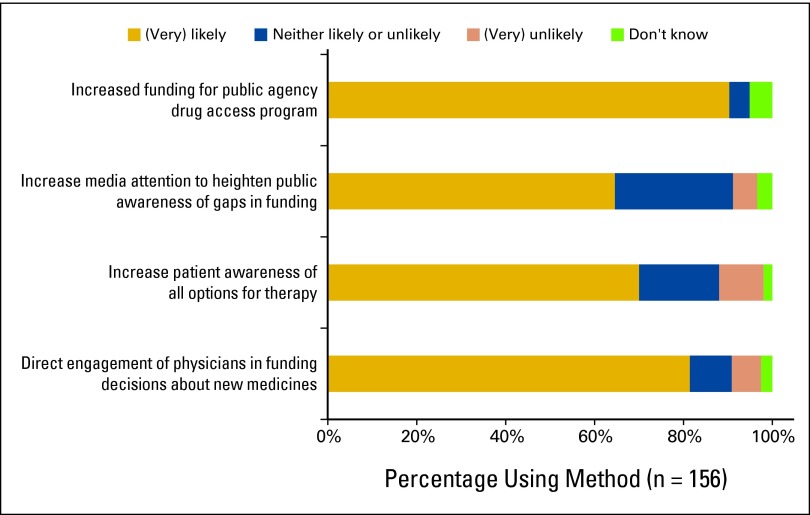
There was general agreement that improvements to drug funding would occur if physicians were directly involved in government priority-setting decisions (81.5% responded this method would very likely improve access; Appendix Fig A1, online only). However, only 28.5% of physicians had contacted their provincial governing body with concerns about funding for oral chemotherapy within the past year (Table 1). Additional solutions included increased funding for public agency drug access programs (90.4%) and increasing patient awareness of available drug options (70.1%). Forty-eight percent of physicians felt that increasing media coverage of funding gaps would improve access.
Regional Differences
Regional differences across Canada were compared according to the six-region model of British Columbia, the Prairies (Alberta, Saskatchewan, and Manitoba), Ontario, Quebec, Atlantic Canada (New Brunswick, Prince Edward Island, Nova Scotia, and Newfoundland and Labrador), and Northern Canada (Yukon, Northwest Territories, and Nunavut; Appendix Table A2, online only). Compassionate access programs, special request forms to government agencies, and enrolling patients onto clinical trials were highly used across all regions of Canada. Eleven percent of physicians in Quebec reported shipping drugs from other countries compared with 20.8% of physicians in British Columbia. The highest rate of referring patients to other countries was in Ontario (38.0%), followed by Atlantic Canada (10.0%), the Prairies (16.1%), British Columbia (12.5%), and Quebec (11.1%). Of all physicians situated in Ontario, 40.2% had made false claims on forms or dictations to fit funding criteria. This method was used to a lesser degree in Atlantic Canada (30.0%), the Prairies (19.3%), Quebec (16.7%), and British Columbia (4.2%).
Interpretation
This study illustrates that accessing unfunded chemotherapy is a concern significantly affecting the daily clinical practice of prescribing physicians. Our findings suggest medical oncologists and hematologists heavily rely on compassionate access programs (96.1%) and special request forms to government agencies (91.8%) to obtain access to unfunded chemotherapy regimens. Of all physicians surveyed, 31.1% admitted to false claims on forms or dictations to fit funding criteria for drugs. It is speculated that this is a conservative estimate because of self-report.
Because medical oncologists and hematologists wish to provide the optimal care for patients with the most effective agents possible, it is not surprising many alternatives are exhausted to overcome barriers to unfunded drugs physicians feel are life saving, prolong disease progression, or improve quality of life. More than half of physicians surveyed felt that their inability to obtain expensive unfunded medications for their patients negatively affected their patients' clinical outcomes, and 72.8% felt that this had an impact on patients' psychosocial quality of life. However, most physicians had not expressed their concerns to governing bodies.
This study expands on the methods that physicians use to obtain drugs, such as using leftover drug supplies or gaming the system.2 Our findings assert that clinicians do not accept limits to drugs from which they feel their patients would benefit. This may lead to nonstandardized chemotherapy regimens for patients despite comparable cancers and can be dependent on physicians' ability and efforts to overcome barriers. A previous study suggested that external factors such as media coverage can influence priority-setting decisions.10 Our findings show that almost half of physicians believed it had no impact, whereas 38.0% believed it improved access. This difference may be the result of differing effectiveness of lobbying bodies based on different disease types, with the great success of breast cancer lobbying bodies as an example in the funding of intravenous trastuzumab.10
The impact of physicians' inability to obtain unfunded medications for their patients' psychosocial quality of life and overall care has been noted in previous studies.11–14 Patients with low incomes who do not meet eligibility requirements for public insurance may face significant financial distress.11 Studies have shown physicians may avoid disclosure of expensive antineoplastic therapies to patients with lower socioeconomic status who are not covered through private insurance.12–14 Physician parameters such as practicing in an academic or community setting as well as links to pharmaceutical companies and funding agencies play a role in drug access.
With 19 public and a myriad of private drug plans across Canada, interprovincial inequalities are bound to exist in access to chemotherapy.1 A lack of pan-Canadian standards results in no standard uniformity for coverage or cost of cancer drugs in each province.9 Changing oncologic practices in drug regimens add to provincial differences in cancer approaches. Descriptively, differences were found in the proportion of medical oncologists and hematologists in each province who used methods to overcome funding barriers. For example, in comparison with British Columbia, two provinces cover fewer than half of the 21 new cancer drugs.15
In terms of coverage, the three westernmost provinces (British Columbia, Alberta, and Saskatchewan) publically cover all cancer drugs taken at home, whereas provinces such as Ontario cover drugs depending on patient eligibility criteria.1 If a drug is listed on the formulary, patients are fully covered without deductibles. However, in the seven other provinces, coverage ranges significantly.15 Some, but not all, provinces have implemented catastrophic drug insurance schemes that cover out-of-hospital drug treatment costs exceeding a certain threshold.16 Disparities exist because of the limitations on accessing listed drugs rather than the selection of drugs on a formulary, such as eligibility restrictions, higher out-of-pocket costs, and drug use restrictions. In addition, provinces such as Alberta are better able to afford health care investments compared with Ontario and Nova Scotia.16 There was a trend in regional differences, with unconventional methods to obtain access to unfunded oral chemotherapy used less frequently in western compared with eastern provinces.
These issues reflect broader issues in the Canadian health care systems managed at the provincial level and are a result of the failure to include pharmaceuticals in health insurance plans.16 Potential reasons for these differences are fiscal transfers, large income disparities among provinces, and complicated politics.16 Other Organisation for Economic Co-operation and Development countries provide universal health care programs that cover pharmaceuticals.16 The Canadian health care system can be generalizable to other nations relying on public taxes for funding or those facing rising cancer drug costs.
The health care system in Great Britain, the National Health Service, is entirely public, funded from the general budget and delivered by government institutions. European countries that rely exclusively on private health insurance are able to achieve universal coverage for their citizens.16 The United States has a complex system of taxpayer-financed insurance schemes that coexist with a private insurance sector. Some Americans are left without insurance coverage because they have pre-existing conditions, do not qualify for government programs, cannot afford to buy insurance, or have employers do not provide health benefits.16
Our findings may be limited by the response rate of 32.0%; however, this is typical of most physician surveys, especially Web- and e-mail–based surveys.17,18 Another significant limitation is response bias; physicians may overestimate their frequency or use of various means to gain access to unfunded oral chemotherapies. It is quite high that 17.9% of physicians reported spending more than 10 hours—more than a full workday—completing paperwork to obtain funding for oral chemotherapy for patients. As with any self-administered questionnaire, responses are self-reported and can only capture physicians' interpretations, which may not reflect the true nature of physicians' practice. Although this is important to note, it is crucial to take into consideration the views of physicians. Barriers to unfunded oral chemotherapy can take a toll on practice workloads and be morally distressing, which ultimately affects physicians' role as care providers. Caution was taken to ensure anonymity for the respondents and encourage candor in responses. However, nonresponse biases may exist; nonresponders may have chosen not to respond because they may not have problems obtaining drugs, they may have been deterred by some of the confrontational questions, or they may have had other time-consuming demands. The distribution of respondents, however, was nationwide.
A majority of respondents were located in an academic setting, which may not be reflective of the entire medical oncologist and hematologist population in Canada. This is most likely attributed to the lack of protected time for community-based practices for nonclinical use. A bias exists because physicians who practice in large, academic institutions are more likely to participate in clinical trials that use expensive agents and thus are more likely to recommend them to patients. Nevertheless, it has been suggested physicians based in an academic setting may be more able to predict future trends of drug-regimen care.19 Despite these limitations, this study shows that the nationwide issue of drug access is significant. This adds to the evidence of physicians who have voiced profound concerns.2,5
Discussion
Our findings demonstrate that physicians do not accept restrictions set by provincial agencies and spend a significant proportion of their time obtaining access to unfunded oral chemotherapies. The study has captured methods currently used to access unfunded expensive anticancer drugs nationally. Physicians feel their inability to obtain drug access has negatively affected patient outcomes and quality of life. Funding for certain drugs may lessen how much clinicians work around governing agencies' funding systems. Greater awareness of differing access to oral chemotherapy may provide an avenue for discussion and promotion of a uniform nationwide drug formulary. Potential solutions to address this issue include direct engagement of physicians in decisions and public awareness of gaps in funding. As the cost of more-effective cancer treatments continues to outstrip the funds available, knowledge exchange among all stakeholders and collaborative action seem indicated.
Acknowledgment
Presented in part at the 2011 American Society of Clinical Oncology Breast Cancer Symposium, September 8-10, 2011, San Francisco, CA.
Appendix
Table A1.
Question: In the Past Year, on Average, How Often Have You Used the Following to Gain Access to Unfunded Oral Chemotherapy Treatment? (n = 158)
| Method | Frequency |
Yes |
Never |
Do Not Know |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weekly |
Monthly |
Every 6 Months |
Annually |
|||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Shipping drugs from other countries | 2 | 1.7 | 4 | 2.5 | 7 | 4.4 | 16 | 10.1 | 29 | 18.7 | 124 | 78.5 | 5 | 3.2 |
| Referring patients to other countries for treatment | 0 | 0.0 | 3 | 1.9 | 16 | 10.1 | 28 | 17.7 | 47 | 29.7 | 111 | 70.3 | 0 | 0.0 |
| Enrolling patients onto clinical trials | 35 | 22.2 | 56 | 35.4 | 45 | 28.5 | 8 | 5.1 | 144 | 91.2 | 15 | 9.5 | 0 | 0.0 |
| False claims on forms or dictations to fit funding criteria for drugs | 3 | 1.9 | 11 | 7.0 | 31 | 19.6 | 5 | 3.2 | 50 | 31.7 | 104 | 65.8 | 4 | 2.5 |
| Using leftover drug supplies from other patients | 3 | 1.9 | 13 | 8.2 | 25 | 15.8 | 9 | 5.7 | 50 | 31.6 | 103 | 65.2 | 5 | 3.2 |
| Special request forms to government agencies | 59 | 37.3 | 46 | 29.1 | 33 | 20.9 | 8 | 5.1 | 146 | 92.4 | 12 | 7.6 | 0 | 0.0 |
| Compassionate access programs | 41 | 25.9 | 68 | 43.0 | 40 | 25.3 | 3 | 1.9 | 152 | 96.1 | 6 | 3.8 | 0 | 0.0 |
Table A2.
Provincial Differences in Methods Used to Obtain Access to Oral Chemotherapies*
| Method | British Columba (n = 24) |
Prairies (n = 31)† |
Ontario (n = 92) |
Quebec (n = 18) |
Atlantic Canada (n = 10)‡ |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Compassionate access programs | ||||||||||
| Yes | 22 | 91.7 | 25 | 80.6 | 82 | 89.1 | 13 | 72.2 | 10 | 100 |
| No | 1 | 4.2 | 3 | 9.7 | 1 | 1.1 | 1 | 5.6 | 0 | 0.0 |
| Do not know | 1 | 4.2 | 3 | 9.7 | 9 | 9.8 | 4 | 22.2 | 0 | 0.0 |
| Special request forms to government agencies | ||||||||||
| Yes | 18 | 75.0 | 24 | 77.4 | 81 | 88.0 | 13 | 72.2 | 10 | 100 |
| No | 5 | 20.8 | 4 | 12.9 | 3 | 3.3 | 1 | 5.6 | 0 | 0.0 |
| Do not know | 1 | 4.2 | 3 | 9.7 | 8 | 8.7 | 4 | 22.2 | 0 | 0.0 |
| Enrolling patients onto clinical trials | ||||||||||
| Yes | 20 | 83.3 | 25 | 80.6 | 78 | 84.8 | 14 | 77.8 | 8 | 80.0 |
| No | 3 | 12.5 | 3 | 9.7 | 6 | 6.5 | 0 | 0.0 | 2 | 20.0 |
| Do not know | 1 | 4.2 | 3 | 9.7 | 8 | 8.7 | 4 | 22.2 | 0 | 0.0 |
| Shipping drugs from other countries | ||||||||||
| Yes | 5 | 20.8 | 5 | 16.1 | 15 | 16.3 | 2 | 11.1 | 2 | 20.0 |
| No | 18 | 75.0 | 22 | 80.0 | 65 | 70.7 | 12 | 66.7 | 7 | 70.0 |
| Do not know | 1 | 4.2 | 4 | 12.9 | 12 | 13.0 | 4 | 22.2 | 1 | 10.0 |
| Referring patients to other countries for treatment | ||||||||||
| Yes | 3 | 12.5 | 5 | 16.1 | 35 | 38.0 | 2 | 11.1 | 2 | 20.0 |
| No | 20 | 83.3 | 23 | 74.2 | 49 | 53.3 | 12 | 66.7 | 7 | 70.0 |
| Do not know | 1 | 4.2 | 3 | 9.7 | 8 | 8.7 | 4 | 22.2 | 1 | 10.0 |
| False claims on forms or dictations to fit funding criteria | ||||||||||
| Yes | 1 | 4.2 | 6 | 19.3 | 37 | 40.2 | 3 | 16.7 | 3 | 30.0 |
| No | 21 | 87.5 | 22 | 80.0 | 44 | 47.8 | 10 | 55.6 | 7 | 70.0 |
| Do not know | 2 | 8.3 | 3 | 9.7 | 11 | 12.0 | 5 | 27.8 | 0 | 0.0 |
| Using leftover drug supplies from other patients | ||||||||||
| Yes | 2 | 8.3 | 6 | 19.4 | 35 | 38.0 | 4 | 22.2 | 3 | 30.0 |
| No | 21 | 87.5 | 22 | 80.0 | 44 | 47.8 | 10 | 72.2 | 6 | 60.0 |
| Do not know | 1 | 4.2 | 3 | 9.7 | 13 | 14.1 | 4 | 5.6 | 1 | 10.0 |
No respondents were situated in Northern Canada (Yukon, Northwest Territories, and Nunavut).
Includes Alberta, Manitoba, and Saskatchewan.
Includes New Brunswick, Newfoundland and Labrador, Nova Scotia, and Prince Edward Island.
Figure A1.
Likelihood of survey respondents to use listed methods to improve patient access to oral chemotherapy medicine.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Muhammad Mamdani, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Hoffmann-La Roche, Novartis, Novo Nordisk, Pfizer Research Funding: None Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: Dolly Han, Martina Trinkaus, Muhammad Mamdani, Scott R. Berry, Raymond Jang, Jeffrey S. Hoch, Christine Simmons
Administrative support: Dolly Han, Martina Trinkaus, Sophie Hogeveen, Muhammad Mamdani, Jeffrey S. Hoch, Christine Simmons
Provision of study materials or patients: Dolly Han, Martina Trinkaus, Sophie Hogeveen, Muhammad Mamdani, Christine Simmons
Collection and assembly of data: Dolly Han, Sophie Hogeveen, Muhammad Mamdani, Christine Simmons
Data analysis and interpretation: Dolly Han, Martina Trinkaus, Muhammad Mamdani, Scott R. Berry, Raymond Jang, Jeffrey S. Hoch, Christine Simmons
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Canadian Cancer Society. Cancer Drug Access for Canadians, September 2009. http://www.cancer.ca/canada-wide/about%20us/media%20centre/cw-media%20releases/cw-2009/∼/media/CCS/Canada%20wide/Files%20List/English%20files%20heading/pdf%20not%20in%20publications%20section/CANCER%20DRUG%20ACCESS%20FINAL%20-%20English.ashx.
- 2.Berry SR, Hubay S, Soibelman H, et al. The effect of priority setting decisions for new cancer drugs on medical oncologists' practice in Ontario: A qualitative study. BMC Health Serv Res. 2007;7:193. doi: 10.1186/1472-6963-7-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Partridge AH, Avorn J, Wang PS, et al. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 4.O'Neill VJ, Twelves CJ. Oral cancer treatment: Developments in chemotherapy and beyond. Br J Cancer. 2002;87:933–937. doi: 10.1038/sj.bjc.6600591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan KK, Berry SR, Straus S, et al. Canadian medical oncologists' practice and perception on accessing new drugs for patients with metastatic colorectal cancer: A national survey. J Clin Oncol. 2008;26(suppl):361s. abstr 6600. [Google Scholar]
- 6.Bedell CH. A changing paradigm for cancer treatment: The advent of new oral chemotherapy agents. Clin J Oncol Nurs. 2003;7(suppl 6):5–9. doi: 10.1188/03.CJON.S6.5-9. [DOI] [PubMed] [Google Scholar]
- 7.Hartigan K. Patient education: The cornerstone of successful oral chemotherapy treatment. Clin J Oncol Nurs. 2004;7(suppl 6):21–24. doi: 10.1188/03.CJON.S6.21-24. [DOI] [PubMed] [Google Scholar]
- 8.Lonardi S, Bortolami A, Stefani M, et al. Oral anticancer drugs in the elderly an overview. Drugs Aging. 2007;24:395–410. doi: 10.2165/00002512-200724050-00004. [DOI] [PubMed] [Google Scholar]
- 9.Chafe R, Culyer A, Dobrow M, et al. Access to cancer drugs in Canada: Looking beyond coverage decisions. Healthc Policy. 2011;6:27–35. doi: 10.12927/hcpol.2011.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth CM, Dranitsaris G, Gainford MC, et al. External influences and priority-setting for anti-cancer agents: A case study of media coverage in adjuvant trastuzumab for breast cancer. BMC Cancer. 2007;7:110. doi: 10.1186/1471-2407-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoen C, Osborn R, Doty MM, et al. Toward higher-performance health systems: Adults' health care experiences in seven countries, 2007. Health Aff (Millwood) 2007;26:w717–w734. doi: 10.1377/hlthaff.26.6.w717. [DOI] [PubMed] [Google Scholar]
- 12.Thomson J, Schofield P, Mileshkin L, et al. Do oncologists discuss expensive anti-cancer drugs with their patients? Ann Oncol. 2005;17:702–708. doi: 10.1093/annonc/mdj136. [DOI] [PubMed] [Google Scholar]
- 13.Jefford M, Tattersall MH. Informing and involving cancer patients in their own care. Lancel Oncol. 2002;3:629–637. doi: 10.1016/s1470-2045(02)00877-x. [DOI] [PubMed] [Google Scholar]
- 14.Mileshkin L, Schofield PE, Jefford M, et al. To tell or not to tell: The community wants to know about expensive anticancer drugs as a potential treatment option. J Clin Oncol. 2009;27:5830–5837. doi: 10.1200/JCO.2009.22.7793. [DOI] [PubMed] [Google Scholar]
- 15.Canada's Public Policy Forum. Optimizing Access to Cancer Drugs for Canadians. http://www.ppforum.ca/publications/optimizing-access-cancer-drugs-canadians.
- 16.Public Policy Forum. Report on Second Annual Symposium on Cancer Drug Access; http://www.ppforum.ca/events/second-annual-symposium-cancer-drug-access. [Google Scholar]
- 17.Couper MP, Blair J, Triplett T. A comparison of mail and e-mail for a survey of employees in U.S. statistical agencies. J Off Stat. 1999;15:39–56. http://www.jos.nu/Articles/abstract.asp?article=15139. [Google Scholar]
- 18.Solomon DJ. Conducting web-based surveys. PARE. 2001;7:19. http://PAREonline.net/getvn.asp?v=7&n=19. [Google Scholar]
- 19.Nadler E, Eckert B, Neumann PJ. Do oncologists believe new cancer drugs offer good value? Oncologist. 2006;11:90–95. doi: 10.1634/theoncologist.11-2-90. [DOI] [PubMed] [Google Scholar]