At one institution, approximately one half of primary prophylaxis pegfilgrastim was not indicated per published guidelines, highlighting a need to change prescribing practices, to reduce costs without harming patients.
Abstract
Purpose:
Pegfilgrastim reduces the risk of febrile neutropenia (FN) and is indicated as primary prophylaxis when the risk of FN approaches 20% in each chemotherapy cycle. There have been few reports evaluating the appropriate use of pegfilgrastim in comparison with published guidelines. We sought to determine possible over-prescribing as a way to maintain quality and reduce cost.
Methods:
A retrospective medical record review was performed to determine whether pegfilgrastim was used appropriately in the primary prophylaxis of FN in chemotherapy regimens with less than 20% risk of FN. Patients were identified by means of administrative records, and data were collected from the electronic medical record at an academic cancer center outpatient clinic serving approximately 13,000 patients per year.
Results:
Two hundred ninety-two patients were identified, of whom 124 were initially evaluated and 88 were included. Thirty-three patients (37%) had no risk factors, and 20 (22%) had one risk factor that would justify pegfilgrastim use with low- or intermediate-risk regimens. The most common cancer diagnosis of patients with zero or one risk factor was lymphoma, and the most common regimens with overuse of pegfilgrastim were doxorubicin-bleomycin-vinblastine-dacarbazine (ABVD) and ritux-imab-cyclophosphamide-doxorubicin-vincristine-prednisone (R-CHOP). One hundred eighty-four pegfilgrastim doses (46%) were classified as avoidable. The cost to the health system for unnecessary drug use was $712,264 in 1 year.
Conclusion:
At one institution, approximately one half of all primary prophylaxis pegfilgrastim was not indicated per published guidelines. This represents an excellent opportunity to change prescribing practices to reduce costs without harming patients.
Introduction
Bone marrow suppression is the most common dose-limiting toxicity of chemotherapy, and subsequent febrile neutropenia (FN) is associated with significant morbidity and mortality. Colony-stimulating factors (CSFs) have been shown to reduce the incidence of FN by approximately 50%. According to the 2006 ASCO WBC growth factor guidelines, CSFs are indicated for primary prophylaxis of FN with high-risk chemotherapy regimens, defined as an FN incidence of > 20%.1 Per ASCO and other national and European guidelines, CSFs are not indicated as primary prophylaxis for low-risk (< 10% incidence of FN) or intermediate-risk (10%–20% incidence of FN) chemotherapy regimens.1–3
In addition to regimen-specific FN risk, other patient-specific risk factors for developing FN may justify use of CSFs with low- or intermediate-risk regimens. Such risk factors include age ≥ 65 years, previous chemotherapy or radiotherapy, malignancy with bone marrow involvement, recent surgery, poor performance or nutritional status, liver dysfunction, and low baseline WBC counts.2
The use of pegfilgrastim in comparison to published guidelines for primary prophylaxis of FN has rarely been evaluated. Baker et al4 compared CSF use at a large, tertiary care medical center with the original ASCO guidelines published in 1994. At that time, the guidelines identified a 40% FN risk as an appropriate threshold for use of CSFs for primary prophylaxis.5 Baker et al reported that 12% of all CSF use at their institution was prescribed outside guideline recommendations. Potosky et al6 reported that 96% of CSF use in US patients occurred outside the current ASCO guidelines, as documented by registry data. Ramsey et al7 found that in 2,728 patients with cancer with a low risk of FN, 10% of breast, 7% of colorectal, and 21% of non–small-cell lung cancer patients received CSFs; most use did not conform to product labeling or practice guidelines.
Overuse of pegfilgrastim poses a significant safety and financial burden. Patient safety concerns include rare cases of fatal splenic rupture, possible tumor growth stimulatory effects, and decreased quality of life as a result of bone pain.8 In addition, the average wholesale price of $3,871 per dose deters its use.9–11 Identification of unnecessary use, as described by current ASCO guidelines, could provide an opportunity to decrease pegfilgrastim use without compromising patient outcomes. A medication use evaluation was performed to compare use of pegfilgrastim in the outpatient clinics of Virginia Commonwealth University Health System (VCUHS) to the 2006 ASCO guidelines and to identify opportunities to enhance patient care.
Methods
A 1-year retrospective medical record review was conducted to evaluate pegfilgrastim use at VCUHS. All patients ≥ 18 years of age who received a chemotherapy regimen with a known FN incidence of less than 20% and at least one dose of outpatient pegfilgrastim between July 1, 2009, and June 30, 2010, were included. The risk of FN was ascertained by review of the main articles for each regimen, and the compiled list in the ASCO and National Comprehensive Cancer Network (NCCN) guidelines.1,2 Patients with acute leukemia and those participating in a clinical trial were excluded.
The primary objective was to evaluate the use of pegfilgrastim as primary prophylaxis in ambulatory oncology patients receiving low- (FN risk of < 10%) or intermediate- (FN risk of 10%–20%) risk chemotherapy. Analysis of the study data was used to characterize the potential cost savings of optimizing pegfilgrastim use.
Results
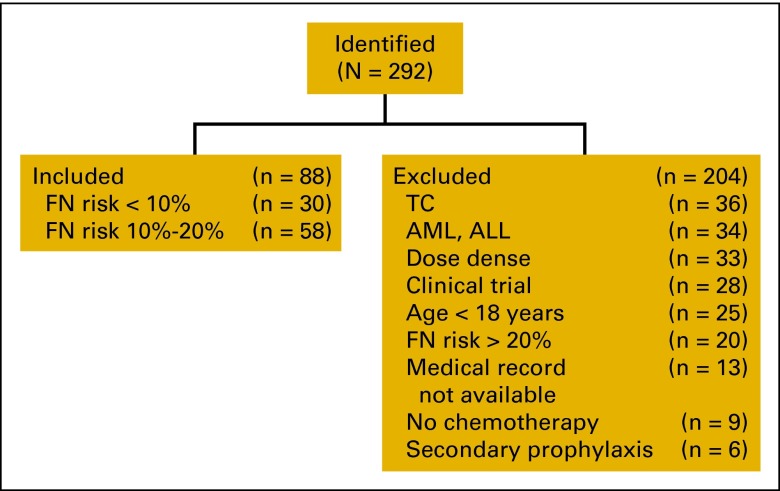
Of the 292 patients identified during the study period, 124 were initially included for evaluation. One hundred sixty-eight patients (58%) of the 292 identified were excluded for the following reasons: diagnosis of acute myeloid leukemia or acute lymphoblastic leukemia (n = 34), receipt of dose-dense chemotherapy (n = 33), participation in a clinical trial (n = 28), age less than 18 years (n = 25), receipt of chemotherapy associated with an FN incidence rate of > 20% (n = 20), lack of documentation (n = 13), no chemotherapy received (n = 9), or use of pegfilgrastim for secondary prophylaxis of FN rather than as primary prophylaxis (n = 6). An additional 36 patients who received docetaxel-cyclophosphamide (TC) were excluded because of the large variation of FN risk in published reports (4%–50%),12–14 leaving a total of 88 patients to be evaluated (Figure 1).
Figure 1.
Disposition of study patients. FN, febrile neutropenia; TC, docetaxel-cyclophosphamide; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia.
The baseline characteristics of the included patients are listed in Table 1. The most prevalent diagnoses were non-Hodgkin lymphoma (NHL) and breast cancer. Approximately half (52 of 88; 59%) of patients had a curative treatment goal as defined by the treating physician. Fifty-eight patients (66%) received intermediate FN–risk chemotherapy, and 30 patients (34%) received low FN–risk chemotherapy (Appendix Table A1, online only). Of the intermediate-risk regimens, the most commonly encountered were rituximab-cyclophosphamide-doxorubicin-vincristine– prednisone (R-CHOP; n = 15; 26%) and doxorubicin-bleomycin-vinblastine-dacarbazine (ABVD; n = 14; 24%). In those patients receiving low-risk chemotherapy, frequently encountered regimens included rituximab-cyclophosphamide-vincristine-prednisone (R-CVP; n = 6; 20%) and rituximab-bendamustine (n = 5, 17%).
Table 1.
Baseline Characteristics (N = 88)
| Characteristic | No. | % |
|---|---|---|
| Female sex | 43 | 49 |
| Age, years | ||
| Mean | 52 | |
| SD | 16 | |
| Cancer diagnosis | ||
| NHL | 29 | 33 |
| Breast | 17 | 19 |
| HL | 16 | 18 |
| Lung | 8 | 9 |
| Other | 18 | 21 |
| Treatment goal | ||
| Cure | 52 | 59 |
| Palliative* | 36 | 41 |
Abbreviations: HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma.
Identified by the prescriber as palliative, or for those patients with metastatic disease.
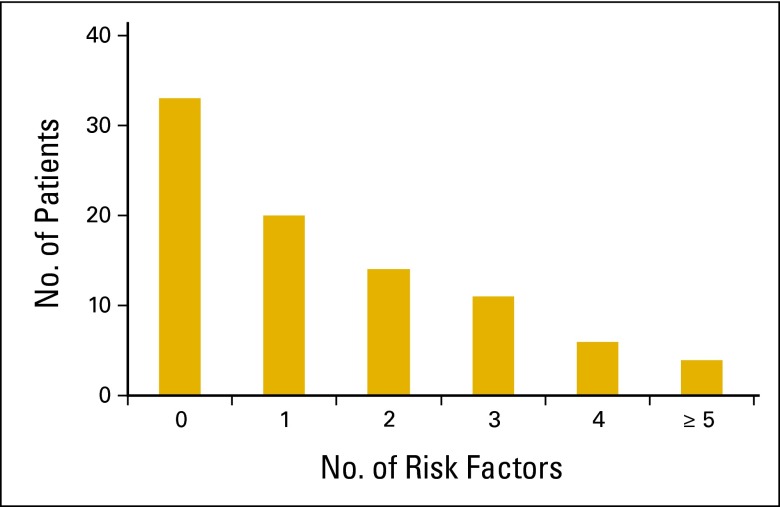
When documented in the electronic medical record (EMR), patient-specific risk factors for developing FN were assessed for each patient. Prior chemotherapy (n = 25; 28%), age > 65 years (n = 20; 23%), and advanced cancer (n = 19; 22%) were the most commonly identified risk factors. More than half (60%) of all patients had one or no patient-specific risk factors (Figure 2). Thirty-three patients (37%) had no risk factors, and 20 (23%) had one risk factor. Of the patients with no patient-specific risk factors, the most common diagnosis was HL (11 of 33; 33%), and the most common chemotherapy was ABVD (11 of 33; 33%). In patients with only one documented patient-specific risk factor, the most common diagnoses were NHL (7 of 20; 35%) and HL (5 of 20; 25%), and the most common chemotherapy regimens were R-CHOP (4 of 20; 20%) and ABVD (3 of 20; 15%). Among patients with one patient-specific risk factor, the most common reasons for pegfilgrastim use were advanced age (8 of 20; 40%), prior chemotherapy (3 of 20; 15%), and bone marrow involvement (3 of 20; 15%).
Figure 2.
Patient-specific risk factors.
A total of 399 doses of pegfilgrastim were administered to the 88 patients. To assess potential cost avoidance, “avoidable” doses were defined as those administered to patients receiving low- or intermediate-risk chemotherapy in the setting of no patient-specific risk factors. In the patients with no documented risk factors, the mean number of avoidable doses per patient was 5.6 (range, 1 to 12). The most common chemotherapy regimens with avoidable doses were ABVD (11 of 33; 33%), R-CHOP (6 of 33; 18%), and docetaxel-carboplatin-trastuzumab (4 of 33; 12%). The most common diagnoses of patients with avoidable doses were HL (11 of 33; 33%), breast cancer (10 of 33; 30%), and NHL (7 of 33; 21%). One hundred eighty-four pegfilgrastim doses (46%) were classified as avoidable. At an average wholesale cost per injection of $3,871, those avoidable doses cost $712,264.
Safety profile is also an important concern when using pegfilgrastim. In this review, 11 patients had documented adverse events attributed to pegfilgrastim. These events included joint pain (n = 6), musculoskeletal pain (n = 3), and rash (n = 2). Three patients (3.4%) experienced FN despite pegfilgrastim administration, with one of these patients reported to have two admissions as a result of FN.
Discussion
Bone marrow suppression is a common dose-limiting toxicity of chemotherapy and may result in life-threatening FN events. Pegfilgrastim reduces the incidence of FN significantly, but its use must be balanced against rare but serious adverse effects and the cost.11,15–22 Published guidelines support the use of prophylactic CSFs with low or intermediate FN risk as determined by the presence of patient-specific factors.
This study evaluated the ambulatory use of pegfilgrastim for primary prophylaxis of FN in comparison with published guidelines. Approximately one third of patients who received low or intermediate FN–risk chemotherapies had no documented patient-specific risk factors. The high proportion of pegfilgrastim use outside of published guidelines underscores the need for a risk stratification screening tool to facilitate optimal use of pegfilgrastim at our institution.
Based on an average wholesale cost of $3,871 per dose, the 184 potentially avoidable doses identified yield a total cost avoidance of $712,264 in a 1-year period. Other authors have postulated that financial incentives are a possible factor for overuse of CSFs, as use in health maintenance organizations was much lower.6 Amgen reported more than $5.2 billion in sales of pegfilgrastim and filgrastim in 2011 and has done intense marketing campaigns to oncologists.23 The revenue from pegfilgrastim to a typical practice is high, ranging from $141 to $1,312 per injection (P. Eisenberg, personal communication, January 2011). At VCUHS, oncologists receive no compensation on the basis of medication use, so there are no financial incentives for administering pegfilgrastim.
Approximately one third of the initial population assessed were patients diagnosed with breast cancer who were receiving TC. These patients were subsequently excluded as more recent data suggest that TC may confer greater FN risk than previously thought. The largest randomized US trial to date showed an FN rate of only 4% to 8%, but subsequent small Canadian studies have shown rates of 25% to 50%.12–14 Based on the reported higher FN rates, many practitioners are using CSFs as primary prophylaxis with TC.24 Further research is required to ascertain the true risk in clinical practice.
The palliative treatment goal in more than one third of patients merits discussion. Palliative care focuses on providing patients with relief from the symptoms, pain, and stress of a serious illness.25 Therefore, pegfilgrastim may not have been indicated, as CSF use has not increased cancer-specific survival in this population. As an alternative to using a CSF, dose modification is an acceptable strategy according to ASCO, NCCN, and European guidelines.1–3
A major strength of the present study is the focus on an area of clinical practice for which there are strong evidence-based recommendations regarding risk factors for FN and stratification of chemotherapy regimens, but a deficit of evidence-based data to guide decision making and prescribing of CSFs. At the time of this medical record review, no institution-specific alerts about appropriate pegfilgrastim use displayed in the electronic medical record.
There are limitations to this study, including the retrospective design of data collection, reliance on existing medical record availability, as well as potential inaccuracy and incompleteness of those medical records. Because this study relied on written documentation to identify additional patient-specific risk factors, patient FN risk is potentially underestimated.
In conclusion, this study demonstrated significant usage of pegfilgrastim outside published guideline recommendations, with a high cost to the patient and institution. There is significant opportunity for oncology providers and pharmacists to improve use of CSFs, which may result in improved patient care and a decreased financial burden.
Appendix
Table A1.
Regimen Febrile Neutropenia Risk Stratification
| Regimen | No. of Patients |
|---|---|
| Risk level < 10% (n = 30) | |
| Docetaxel-carboplatin-trastuzumab | 8 |
| R-CVP (rituximab, cyclophosphamide, vincristine, prednisone) | 6 |
| Rituximab-bendamustine | 5 |
| Bendamustine | 2 |
| Carboplatin-pemetrexed | 2 |
| MOPP (mechlorethamine, vincristine, procarbazine, prednisone) | 2 |
| Cisplatin-gemcitabine | 1 |
| Gemcitabine-docetaxel | 1 |
| Gemcitabine-oxaliplatin | 1 |
| Mitomycin-fluorouracil | 1 |
| Vinorelbine | 1 |
| Risk level 10%–20% (n = 58) | |
| R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) | 15 |
| ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) | 14 |
| Carboplatin-paclitaxel | 8 |
| R-EPOCH (rituximab, etoposide, prednisone, vincristine, doxorubicin, cyclosphosphamide) | 5 |
| AC (doxorubicin, cyclophosphamide) | 4 |
| FCR (fludarabine, cyclophosphamide, rituximab) | 3 |
| Azacitidine | 2 |
| Cisplatin-etoposide | 2 |
| Docetaxel | 2 |
| Carboplatin-etoposide | 1 |
| Cisplatin-fluorouracil | 1 |
| Gemcitabine-carboplatin | 1 |
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Geralyn E. Waters, Patricia Corrigan, Mandy Gatesman
Administrative support: Thomas J. Smith
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 2.Crawford J, Allen J, Armitage J, et al. Myeloid growth factors. J Natl Compr Canc Netw. 2011;9:914–932. doi: 10.6004/jnccn.2011.0075. [DOI] [PubMed] [Google Scholar]
- 3.Aapro MS, Bohlius J, Cameron DA, et al. European Organisation for Research and Treatment of Cancer. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Baker J, McCune JS, Harvey RD, 3rd, et al. Granulocyte colony stimulating factor use in cancer patients. Ann Pharmacother. 2000;34:851–857. doi: 10.1345/aph.19124. [DOI] [PubMed] [Google Scholar]
- 5.ASCO Ad Hoc Colony-Stimulating Factor Guidelines Expert Panel. American Society of Clinical Oncology recommendations for the use of hematopoietic colony-stimulating factors: Evidence-based, clinical practice guidelines. J Clin Oncol. 1994;12:2471–2508. doi: 10.1200/JCO.1994.12.11.2471. [DOI] [PubMed] [Google Scholar]
- 6.Potosky AL, Malin JL, Kim B, et al. Use of colony-stimulating factors with chemotherapy: Opportunities for cost savings and improved outcomes. J Natl Cancer Inst. 2011;103:979–982. doi: 10.1093/jnci/djr152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramsey SD, McCune JS, Blough DK, et al. Colony-stimulating factor prescribing patterns in patients receiving chemotherapy for cancer. Am J Manag Care. 2010;16:678–686. [PubMed] [Google Scholar]
- 8.Amgen. Neulasta [package insert] Thousand Oaks, CA: Amgen; 2010. [Google Scholar]
- 9.Ozer H, Mirtsching B, Rader M, et al. Neutropenic events in community practices reduced by first and subsequent cycle pegfilgrastim use. Oncologist. 2007;12:484–494. doi: 10.1634/theoncologist.12-4-484. [DOI] [PubMed] [Google Scholar]
- 10.Smith TJ, Khatcheressian JL. Neutropenic events in community practices reduced by first and subsequent cycle pegfilgrastim use. Oncologist. 2007;12:1465–1466. doi: 10.1634/theoncologist.12-12-1464. [DOI] [PubMed] [Google Scholar]
- 11.Red Book. Pharmacy's Fundamental Reference. Montvale, NJ: Thomson Reuters; 2010. p. 607. [Google Scholar]
- 12.Jones S, Holmes FA, O'Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of US Oncology Research Trial 9735. J Clin Oncol. 2009;27:1177–1183. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 13.Soong D, Haj R, Leung MG, et al. Editorial: High rate of febrile neutropenia in patients with operable breast cancer receiving docetaxel and cyclophosphamide. J Clin Oncol. 2009;27:e101–e102. doi: 10.1200/JCO.2009.23.0508. [DOI] [PubMed] [Google Scholar]
- 14.Vandenberg T, Younus J, Al-Khayyat Febrile neutropenia rates with adjuvant docetaxel and cyclophosphamide chemotherapy in early breast cancer: Discrepancy between published reports and community practice – a retrospective analysis. Curr Oncol. 2010;17:2–3. doi: 10.3747/co.v17i2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watring NJ, Wagner TW, Stark JJ. Spontaneous splenic rupture secondary to pegfilgrastim to prevent neutropenia in a patient with non-small-cell lung carcinoma. Am J Emerg Med. 2007;25:247–248. doi: 10.1016/j.ajem.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Hatzimichael E, Benetatos L, Stebbing J, et al. Spontaneous splenic haematoma in a multiple myeloma patient receiving pegfilgrastim support. Clin Lab Haematol. 2006;28:416–418. doi: 10.1111/j.1365-2257.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- 17.Kuendgen A, Fenk R, Bruns I, et al. Splenic rupture following administration of pegfilgrastim in a patient with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 2006;38:69–70. doi: 10.1038/sj.bmt.1705382. [DOI] [PubMed] [Google Scholar]
- 18.Nuamah NM, Goker H, Kilic YA, et al. Spontaneous splenic rupture in a healthy allogeneic donor of peripheral-blood stem cell following the administration of granulocyte colony-stimulating factor. A case report and review of the literature. Haematologica. 2006;91(suppl 5):ECR08. [PubMed] [Google Scholar]
- 19.Arshad M, Seiter K, Bilaniuk J, et al. Side effects related to cancer treatment: CASE 2. Splenic rupture following pegfilgrastim. J Clin Oncol. 2005;23:8533–8534. doi: 10.1200/JCO.2005.04.1012. [DOI] [PubMed] [Google Scholar]
- 20.Brown SL, Dale DC. Spontaneous splenic rupture following administration of granulocyte colony-stimulating factor (G-CSF): Occurrence in an allogeneic donor of peripheral blood stem cells. Biol Blood Marrow Transplant. 1997;3:341–343. [PubMed] [Google Scholar]
- 21.Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007;99:196–205. doi: 10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]
- 22.Citron ML. Dose-dense chemotherapy: Principles, clinical results and future perspectives. Breast Care. 2008;3:251–255. doi: 10.1159/000148914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amgen's full year 2011 revenue increased 4 percent to $15.6 billion and adjusted earnings per share increased 2 percent to $5.33. http://www.amgen.com/media/media_pr_detail.jsp?year=2012&releaseID=1653300.
- 24.Patt DA, Espirito JL, Turnwald B, et al. Primary and secondary pegfilgrastim utilization in adjuvant chemotherapy for breast cancer in the community. Presented at the Cancer Therapy and Research Center American Association of Cancer Research Breast Cancer Symposium; December 15, 2011; San Antonio, TX. [Google Scholar]
- 25.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol. 2012;30:880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]