Drug shortages have substantial economic costs and mandate treatment changes that may affect efficacy and toxicity.
Abstract
Purpose:
Cancer drug shortages have increased considerably over the past 5 years, but quantitative analyses of the scope and effects are limited. We assessed the effects of drug shortages on outpatient medication use in a single New York City university hospital.
Methods:
We examined pharmacy records for drug shortages, as defined by the American Society of Health-System Pharmacists. We assessed outpatient records for all patients with cancer treated with infusional antineoplastic medications from April 2010 to September 2010 and April 2011 to September 2011.
Results:
Twelve medications were in shortage in 2010 and 22 in 2011. Drugs in shortage were used for 170 patients (50.8%) in 2010 and 241 patients (63.6%) in 2011 (P < .001). Of 235 patients treated in August-September 2011, there were 23(9.8%) documented therapy changes due to shortages, compared with zero changes in August-September 2010 (P < .001). Among patients treated in August-September 2010, 24 (11.4%) received paclitaxel and 19 (9.0%) received docetaxel. Among patients treated in August-September 2011, 11 (4.7%) received paclitaxel and 38 (16.2%) received docetaxel, a 69% decrease for paclitaxel and 80% increase for docetaxel from 1 year prior (P = .009, and P = .024, respectively). The estimated cost of a single treatment with paclitaxel for one patient with body-surface area 1.75 was $47.59 versus $858.39 for docetaxel, a 1,704% increase. Surveyed physicians frequently reported lower level evidence (30.4%) and increased risk of toxicity (34.8%) with alternative therapy in drug shortage cases.
Conclusion:
Oncology drug shortages affected the majority of patients in our center and increased at an alarming rate. Drug shortages have substantial economic costs and mandate treatment changes that may affect efficacy and toxicity.
Introduction
The US Food and Drug Administration (FDA) recorded 61 medication shortages in 2005 and almost three times as many, 178, in 2010.1 The impact of these shortages on patient care remains unknown despite widespread attention to the problem in popular media.2,3 In an early attempt to evaluate the impact of drug shortages, investigators at Johns Hopkins conducted a survey of 370 pharmacists in 2003. Nine drug shortages were reported in the study, and these shortages affected between 31% and 96% of hospitals surveyed.4 No cancer chemotherapy shortages were reported. The majority of respondents agreed that medication shortages had both changed practice and compromised patient care. A recent survey of 353 members of the American Society of Health System Pharmacists (ASHP) reported that pharmacists were spending between 8 and 12 hours each week and physicians were spending between 0 and 2 hours each week managing the medication shortages.5 Only four of the 30 medications addressed were antineoplastics or cancer supportive care medications. This study did not attempt to quantify the impact of the drug shortages on patient care. Attempts to study the drug shortages are hindered by a lack of consensus on the definition of a drug shortage, inconsistent reporting of drug shortages, and geographic variability in medication supply. We examined outpatient oncology medication use at a single New York City university hospital between April and September 2010, and between April and September 2011 to evaluate changes in medication use influenced by drug shortages. We planned a subset analysis of treatment in August-September 2011, the height of medication supply problems at our hospital. We surveyed treating physicians on the efficacy and toxicity of alternative medications that were used in place of medications in shortage. We also calculated changes in cost of treatment caused by medication substitutions in cases of drug shortage.
We hypothesized that the number of chemotherapeutic agents in shortage would increase, and that more patients would be affected in 2011 compared with 2010. We also hypothesized that the drug shortages would decrease the quality of cancer care, either by affecting efficacy or by causing increased toxicity. Finally, we anticipate that the drug shortages would increase the cost of cancer care at our institution.
Methods
Drug Shortage Data
We defined a shortage in accordance with the ASHP definition of a “supply issue that affects how the pharmacy prepares or dispenses a drug product or influences patient care when prescribers must use an alternative agent.”5 We determined drug shortages experienced at our institution by means of pharmacy records over the time periods reviewed. We defined a shortage as occurring when the wholesale oncology pharmacy under contract with our cancer center could not supply the quantity of medication requested in our order. In these instances, the wholesale distributor either completed the order with an alternative product or was unable to provide the quantity requested. Continuum Cancer Center pharmacy staff confirmed with the wholesale pharmacy that the occurrence was related to a disruption in their supply. The investigators then confirmed that the medications listed in shortage by our pharmacy were also in the FDA database of drug shortages. We determined drug pricing from pharmacy purchasing records.
Inclusion Criteria
We reviewed the patient visits to the outpatient infusion suite at the Continuum Cancer Center at Roosevelt Hospital, documented in the IDX Webframe scheduling system from April 1, 2010, until September 30, 2010, and from April 1, 2011, until September 30, 2011. The subjects were identified by a search of the IDX scheduling database for patients who had a visit with a hematologist or oncologist that was coded for infusional therapy. We further selected only those patients with a primary or secondary diagnosis of a malignant neoplasm, and included all patients treated in the specified date range.
We recorded regimens used, additional patient information, and disease characteristics from chart review. We further reviewed charts for explicit documentation of changes in therapy because of drug shortage. In cases of documented shortage-related treatment change, we e-mailed questionnaires to the treating physicians at the completion of the data collection in October 2011. The physicians were asked if, at the initiation of alternative therapy due to the drug shortage, they anticipated (1) the efficacy of the alternative medication to be better, equivalent, or worse than the unavailable drug; and (2) the toxicity of the alternative medication to be better, equivalent, or worse than the unavailable drug.
The study was approved by the institutional review board of St. Luke's Roosevelt Hospital Center (IRB #11-187).
Variables
The demographic data collected included patient age, sex, and disease. Treatment data collected included chemotherapy medications used.
Outcomes
The primary end points are proportion of cancer medications in shortage, proportion of patients treated with a medication in shortage, and proportion of chemotherapy regimens changed as a result of drug shortages. The secondary end points include proportion of treatment regimens changed to chemotherapy with lower level evidence or increased toxicity. We performed a preplanned analysis of the same end points in August-September 2010 and August-September 2011.
Statistical Analyses
We computed descriptive statistics, including means, medians, standard deviations, and interquartile ranges for baseline characteristics of the patients in the sample. We computed proportions of patients receiving each of the chemotherapy drugs, limited by disease and treatment year, and compared proportions in 2010 with proportions in 2011 using Fisher's exact test. All P values are two sided.
Results
Three hundred thirty-five patients received infusional therapy from April 2010 to September 2010, and 379 patients from April 2011 to September 2011. The baseline characteristics were similar in both groups (Table 1). The median age of patients was 61 years and was the same in both years. Seventy one percent (237) of the patients treated in 2010 were female, and 74% (279) in 2011. The site of cancer treated was also comparable between years 2010 and 2011: breast (37.0% and 40.6%), GI (18.5% and 16.6%), genitourinary (9.8% and 11.1%), gynecologic (7.5% and 5.8%), lymphoma (11.6% and 10.3%), and lung (8.1% and 7.1%).
Table 1.
Baseline Patient Characteristics
| Characteristic | 2010 |
2011 |
P (for comparisons between years) | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| No. treated | 335 | 379 | NS | ||
| Age, years | NS | ||||
| Median | 61 | 61 | |||
| Range | 22-92 | 24-92 | |||
| Sex | NS | ||||
| Male | 98 | 29.3 | 100 | 26.4 | |
| Female | 237 | 70.7 | 279 | 73.6 | |
| Site of cancer | NS | ||||
| Breast | 37.0 | 40.6 | |||
| GI | 18.5 | 16.6 | |||
| GU | 9.8 | 11.1 | |||
| GYN | 7.5 | 5.8 | |||
| Lymphoma | 11.6 | 10.3 | |||
| Lung | 8.1 | 7.1 | |||
| Others | 7.5 | 8.4 | |||
| Drugs in shortage | 12 of 44 | 27.3 | 22 of 43 | 51.2 | .029 |
| No. of patients treated with drugs in shortage | 170 | 50.8 | 241 | 63.6 | < .001 |
Abbreviations: GU, genitourinary; GYN, gynecologic; NS, not significant.
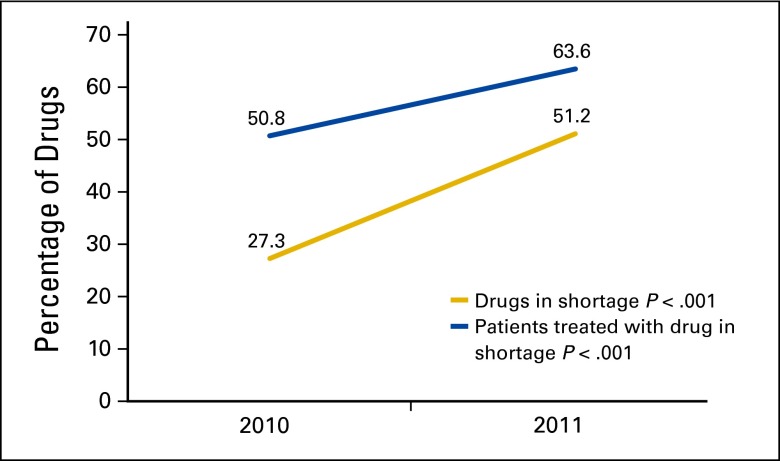
Of 44 chemotherapy drugs used in 2010, 12 (27.3%) met the criteria for drug shortage. Twenty-two (51.2%) of 43 chemotherapy drugs used in 2011 were in shortage (P = .029). All drugs reported in shortage by our cancer center pharmacy were available in the FDA drug shortage registry. The proportion of patients receiving a shortage drug increased from 50.8% (170 of 335) in 2010 to 63.6% (241 of 379) in 2011 (P < .001; Figure 1). The drugs in shortage are presented in Table 2.
Figure 1.
Clinical impact of drug shortages in 2010 and 2011.
We performed a preplanned subset analysis for the months of August-September 2011, the peak of medication availability problems at Continuum Cancer Center, and compared it with August-September 2010. The baseline characteristics of these patients were similar between the two years and comparable to the overall patient data. No shortage-related changes in administered chemotherapy were documented among 211 patients treated in August-September 2010. Liposomal doxorubicin, fluorouracil, and paclitaxel were all unavailable during parts of August and September 2011. Twenty three (9.8%) of 235 patients treated in August-September 2011 received alternative therapy because of a drug shortage (P < .001). Women (87%) were affected disproportionately, and the median age of affected patients was 57 years (Table 3). Of the 23 patients, 11 had breast cancer, six had gynecological malignancies, three had lung cancer, two had Kaposi's sarcoma, and one had pancreatic cancer. Although leucovorin never became completely unavailable during the time periods studied, we were aware of leucovorin dosage changes in 2011, which we were unable to capture in this data set.
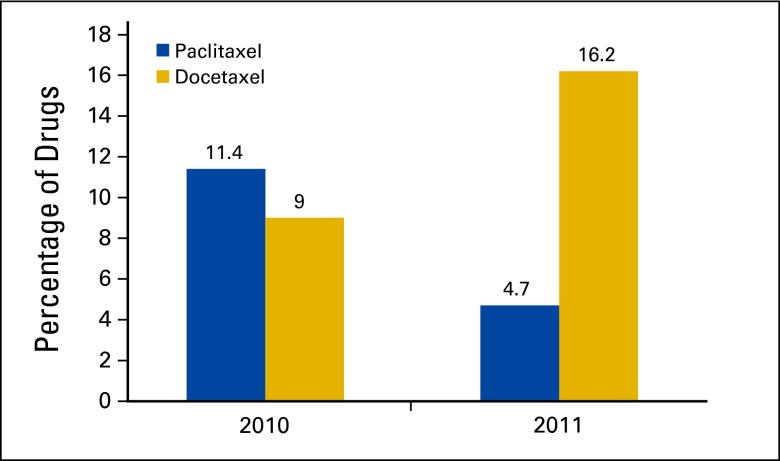
Changes from paclitaxel to docetaxel accounted for 17 of 23 (73.9%) therapy changes. The use of paclitaxel decreased from 11.4% (24 of 211) in August-September 2010 to 4.7% (11 of 235) in August-September 2011, a 69% decrease in the use of paclitaxel from the year prior (P = .009; Figure 2). Similarly, the use of docetaxel increased from 9.0% (19 of 211) in August-September 2010 to 16.2% (38 of 235) in August-September 2011, an 80% increase in use from the previous year (P = .024).The estimated cost of a single treatment with paclitaxel for one patient with body surface area 1.75 was $47.59 versus $858.39 for docetaxel, a 1,704% increase.
Figure 2.
Use of taxanes in August-September 2010 and 2011.
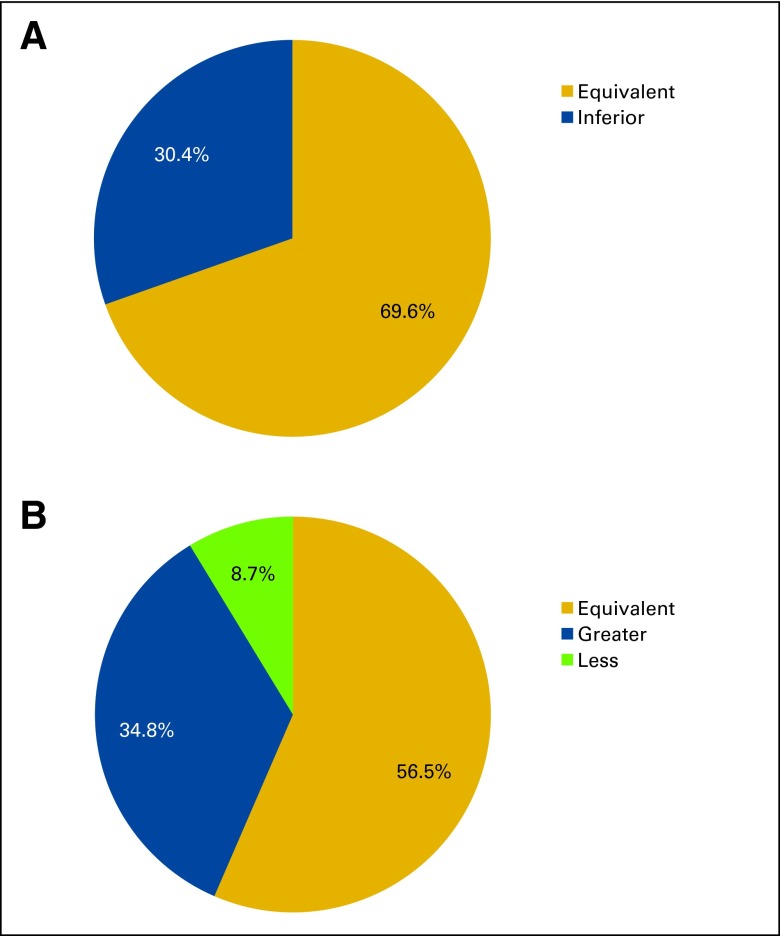
We administered a questionnaire to the treating physicians regarding the relative efficacy and toxicity of the alternative medications used in the 23 therapy changes. Physicians were asked their opinions regarding the efficacy and toxicity of the unavailable and alternative medications at the start of the alternative therapy. The return rate on the 23 questionnaires was 100%, and the answer rate for the questions was 100%. Surveyed physicians reported that the efficacy of the alternative regimen was equivalent to that of the unavailable medication in 69.6% of cases, and inferior in 30.4%. Physicians also reported that the toxicity of the alternative regimen was likely to be greater than that of the unavailable medication in 34.8% of the cases, likely to be less in 8.9% of cases, and likely to be equivalent in 56.5% of cases (Appendix Figure A1, online only).
Discussion
Injectable chemotherapeutics are particularly susceptible to supply problems. The manufacture of sterile injectables is complex, and few companies make the medications, leaving little room for disturbances in supply or demand.6 Reimbursement by the Centers for Medicare and Medicaid Services is fixed at 6% above the average sales price, which can limit the profitability of generic medications.7 The US Department of Health and Human Services Office of the Assistant Secretary for Planning and Evaluation prepared a report in October 2011 that attributed the drug shortages to rapid expansion of the volume of oncology sterile injectables related to new products and products coming off of patent.8 The widespread drug shortages warranted action by the federal government, and President Obama signed the Food and Drug Administration Safety and Innovation Act into law on July 9, 2012.1 Among the 72 active drug shortages reported by the FDA on October 10, 2011, 22 were for antineoplastic or oncology supportive medications. These included mainstays of clinical practice: bleomycin, carboplatin, cisplatin, cyclophosphamide, cytarabine, docetaxel, doxorubicin, epirubicin, etoposide, fluorouracil, gemcitabine, ifosfamide, irinotecan, leucovorin, methotrexate, mitoxantrone, paclitaxel, vincristine, and vinorelbine.1 Despite widespread media coverage on the drug shortages, there is a dearth of published data on the scope and effects of the oncology drug shortages. We found a rapid increase in the proportion of patients treated with medications in shortage between 2010 and 2011. In our cancer center, the proportion of chemotherapeutic agents considered in shortage almost doubled between 2010 and 2011, leading to a significant increase in the proportion of patients being treated with a shortage drug. Our experience is consistent with the FDA record of reported drug shortages and highlights the severity of the problem in a university hospital with substantial resources.1 To our knowledge, this is the first report to describe the impacts of the drug shortages on individual patient therapies.
Inconsistent definitions of shortages add to the complexity of studying medication supply. In a 2003 survey, the authors defined a drug shortage “as any occurrence in which it was necessary to purchase or use an alternative medication because of the lack of availability of the drug of choice at the time of patient care.”4(p2016) In a 2011 survey, the authors cite the ASHP definition of shortage as “a supply issue that affects how the pharmacy prepares or dispenses a drug product or influences patient care when prescribers must use an alternative agent.”9 These varied definitions attest to a growing recognition that the effects of a medication shortage reverberate throughout the health care system. Even in cases in which patients ultimately receive the appropriate medications, the resources devoted to maintaining standard care can be onerous.
Attempts to evaluate the effect of the shortages on patient care are further complicated by the highly variable availability of medications on the FDA shortage list, with significant changes between institutions and over short time periods. In 2003, the Drug Information Service at the University of Utah Hospitals and Clinics reported findings from 7 years of monitoring 224 drug shortages.10 In 54% of shortages, pharmacy staff anticipated that clinicians would be unfamiliar with the alternative medication in terms of mechanism, adverse effects, or interactions. There was no direct evaluation of effects of the shortages on patient care. A survey of 1,800 health care workers, (64% pharmacists) by the Institute for Safe Medication Practices noted that approximately 20% of respondents reported at least one adverse patient outcome related to a medication shortage within the previous year.11 An unpublished Premier Healthcare Alliance survey of 311 pharmacy experts representing 288 health care facilities reported that 89% of respondents experienced a medication shortage that may have caused a medication safety issue or an error in patient care.12 Much of the available data report on conjecture about patient effects. Almost no information is available on documented changes in patient treatment related to the drug shortages.
In our study, approximately one of 10 patients treated in August-September 2011 experienced a change in their treatment regimen as a result of a drug shortage. Treating physicians considered the alternative medication less efficacious that the unavailable medication in 30.4% of cases and considered the toxicity of the alternative medication greater in 34.8% of cases, although we cannot rule out that physician bias contributed to this assessment. Perhaps even more worrisome than the number of changes of therapy is the staggering 63.8% of all patients in 2011 who received a medication considered in shortage. If more of the drugs in shortage become unavailable, the effects would likely have profound impact on cancer care in the United States.
Any estimate of the economic cost of the medication shortages is necessarily handicapped by all of the challenges previously mentioned. The 2011 ASHP survey estimated the annual labor cost of managing the drug shortages at $216 million, by multiplying worker's hours devoted to handling the shortage by national average salaries and applying those data to the 2011 national number of health systems.5 Premier Healthcare Alliance, a for-profit organization of more than 2,700 hospitals and health care systems, estimated the annual impact of drug shortages in oncology to be $10.5 million, on the basis of a member survey in early 2011. They included increased costs only in cases where generic equivalent alternatives are available.12 A recently published analysis estimated an additional cost of $11,168 for each patient with ovarian cancer treated with docetaxel instead of paclitaxel during a drug shortage.13 The authors estimated a national excess yearly cost of greater than $100 million for newly diagnosed ovarian cancer cases during a paclitaxel shortage affecting half of all patients. In our center, we noted an 80% increase in the use of docetaxel in August-September 2011 compared with 2010. The estimated cost of a single dose of paclitaxel for one patient with body-surface area 1.75 was $47.59 versus $858.39 for docetaxel, a 1,704% increase. We recognize that any extrapolation of data from a 2-month sample at the peak of the shortages is problematic. Nonetheless, many of the drugs in shortage are generics, and the economic consequences of substitutions can be severe.
We recognize several limitations of our study. The sample size is relatively small and entirely from a single hospital. Rather than using the FDA-defined registry of drug shortages, we chose to limit our drug shortage definition to only those shortages that were directly affecting our pharmacy. We consider this a conservative estimate of the number of drug shortages that allows a more direct exploration of the effects on patients. Other hospitals likely have very different experiences of the drug shortages, and our results may not be generalizable. Interhospital variability is, however, a fundamental feature of the current drug shortages. Review of drug shortage effects in any large database is limited because participating institutions are likely to have highly variable drug shortage rosters at any time and over any time period. Generalizable data likely will come from a better estimation of the real variability in the problem, that is, repeated sampling of individual institutions. Our study can serve as a beginning to further exploration at other institutions and a comparator for alternative methods of drug shortage study. Even if our study represents only a small sample of patients over a short interval, it begins to address the issue of the severity of drug shortages, which should itself be an impetus for change.
We ascribed the change in taxane prescribing patterns predominantly to the drug shortages. It is possible that treating physicians changed practice in response to data on the relative benefits of docetaxel and paclitaxel. We do not, however, think that the shift is largely related to emerging data because the majority of the cases had documentation of treatment changes in response to drug shortage. In addition, we are unaware of significant new clinical data occurring during that time period that should have triggered a change in drug therapy. Our physician survey was administered retrospectively, and physicians were aware of the ongoing study of drug shortages. It is possible that bias was introduced through physician perception of study goals, emerging national discussion of the drug shortages, selective recall, and knowledge of actual patient adverse medication effects. We have been cautious to interpret the survey results as exploratory and not definitive because of this limitation.
In conclusion, we found that drug shortages increased significantly between 2010 and 2011 and mandated substantial numbers of treatment changes for the first time in 2011. Our treating physicians frequently reported that the available alternatives increased the risk of toxicity and had a weaker evidence base than the medications in shortage. We also documented a large increase in drug cost attributable to the drug shortage. We hope that our study will support the ongoing national discussion about the scope of, and solutions to, the drug shortage crisis.
Acknowledgment
Presented as a poster session at the Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 2-4, 2012.
Appendix
Table A1.
Drugs in Shortage
| 2010 | 2011 |
|---|---|
| Bleomycin | Bleomycin |
| Carboplatin | Carboplatin |
| Cisplatinum | Cisplatinum |
| Cyclophosphamide | Cyclophosphamide |
| Cytarabine | Cytarabine |
| Dacarbazine | |
| Daunorubicin | Daunorubicin |
| Doxorubicin | Doxorubicin |
| Liposomal doxorubicin | Liposomal doxorubicin |
| Epirubicin | Epirubicin |
| Etoposide | |
| Flouoruracil | |
| Gemcitibine | |
| Ifosfamide | |
| Irinotecan | |
| Leucovorin | Leucovorin |
| Mesna | |
| Methotrexate | |
| Mitomycin | Mitomycin |
| Mitoxantrone | |
| Paclitaxel | |
| Vincristine | |
| Vinorelbine |
Table A2.
Characteristics of Patients Who Had a Change in Treatment Regimen in 2011 As a Result of Drug Shortage
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 57 | |
| Range | 35-75 | |
| Sex | ||
| Male | 3 | 13 |
| Female | 20 | 87 |
| Site of cancer | ||
| Breast | 11 | 47.8 |
| Gynecolgic | 6 | 26.1 |
| Kaposi sarcoma | 2 | 13 |
| Lung | 3 | 8.7 |
| Pancreas | 1 | 4.3 |
| Shortage drug | ||
| Paclitaxel | 17 | 73.9 |
| Liposomal doxorubicin | 5 | 21.7 |
| Flourouracil | 1 | 4.3 |
Figure A1.
Survey results showing physician reports on (A) efficacy and (B) toxicity risk of alternate regimens used in cases of drug shortages.
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Daniel J. Becker, Ronald H. Blum, Michael L. Grossbard
Administrative support: Molly Thorn, Louis B. Harrison
Provision of study materials or patients: Daniel J. Becker, Molly Thorn, Janna Roitman, Louis B. Harrison, Michael L. Grossbard
Collection and assembly of data: Daniel J. Becker, Sumit Talwar, Benjamin P. Levy, Molly Thorn, Janna Roitman, Michael L. Grossbard
Data analysis and interpretation: All authors
Manuscript writing: Daniel J. Becker, Sumit Talwar, Benjamin P. Levy, Ronald H. Blum, Louis B. Harrison, Michael L. Grossbard
Final approval of manuscript: All authors
References
- 1.US Food and Drug Administration. FDA and manufacturers work to prevent drug shortages. http://www.fda.gov/Drugs/DrugSafety/DrugShortages/ucm257746.htm.
- 2.Allen J. Chemo drug Taxol shortage puts cancer patients at risk. ABC News [serial online] 2011. http://abcnews.go.com/Health/CancerPreventionAndTreatment/chemo-drug-taxol-shortage-leaves-doctors-scrambling-cancer/story?id=13906891.
- 3.Harris G. U.S. scrambling to ease shortage of vital medicine. The New York Times. 2011:A1. [Google Scholar]
- 4.Baumer AM, Clark AM, Witmer DR, et al. National survey of the impact of drug shortages in acute care hospitals. Am J Health Syst Pharm. 2004;61:2015–2022. doi: 10.1093/ajhp/61.19.2015. [DOI] [PubMed] [Google Scholar]
- 5.Kaakeh R, Sweet BV, Reilly C, et al. Impact of drug shortages on U.S. health systems. Am J Health Syst Pharm. 2011;68:1811–1819. doi: 10.2146/ajhp110210. [DOI] [PubMed] [Google Scholar]
- 6.Jensen V, Rappaport BA. The reality of drug shortages—The case of the injectable agent propofol. N Engl J Med. 2010;363(9):806–807. doi: 10.1056/NEJMp1005849. [DOI] [PubMed] [Google Scholar]
- 7.Gatesman ML, Smith TJ. The shortage of essential chemotherapy drugs in the United States. N Engl J Med. 2011;365:1653–1655. doi: 10.1056/NEJMp1109772. [DOI] [PubMed] [Google Scholar]
- 8.Haninger K, Jessup A, Koehler K. Economic analysis of the causes of the drug shortages. 2011. http://aspe.hhs.gov/sp/reports/2011/DrugShortages/ib.pdf.
- 9.ASHP Expert Panel on Drug Product Shortages. Fox ER, Birt A, et al. ASHP guidelines on managing drug product shortages in hospitals and health systems. Am J Health Syst Pharm. 2009;66:1399–1406. doi: 10.2146/ajhp090026. [DOI] [PubMed] [Google Scholar]
- 10.Fox ER, Tyler LS. Managing drug shortages: Seven years' experience at one health system. Am J Health Syst Pharm. 2003;60:245–253. doi: 10.1093/ajhp/60.3.245. [DOI] [PubMed] [Google Scholar]
- 11.Johnson PE. Drug shortages: Impact and strategies. J Natl Compr Canc Netw. 2011;9:815–819. doi: 10.6004/jnccn.2011.0070. [DOI] [PubMed] [Google Scholar]
- 12.Cherici C. Navigating drug shortages in American healthcare: A Premier Healthcare Alliance analysis. http://www.premierinc.com/about/news/11-mar/drug-shortage-white-paper-3-28-11.pdf.
- 13.Havrilesky LJ, Garfield CF, Barnett JC, et al. Economic impact of paclitaxel shortage in patients with newly diagnosed ovarian cancer. Gynecol Oncol. 2012;125:631–634. doi: 10.1016/j.ygyno.2012.03.028. [DOI] [PubMed] [Google Scholar]