Abstract
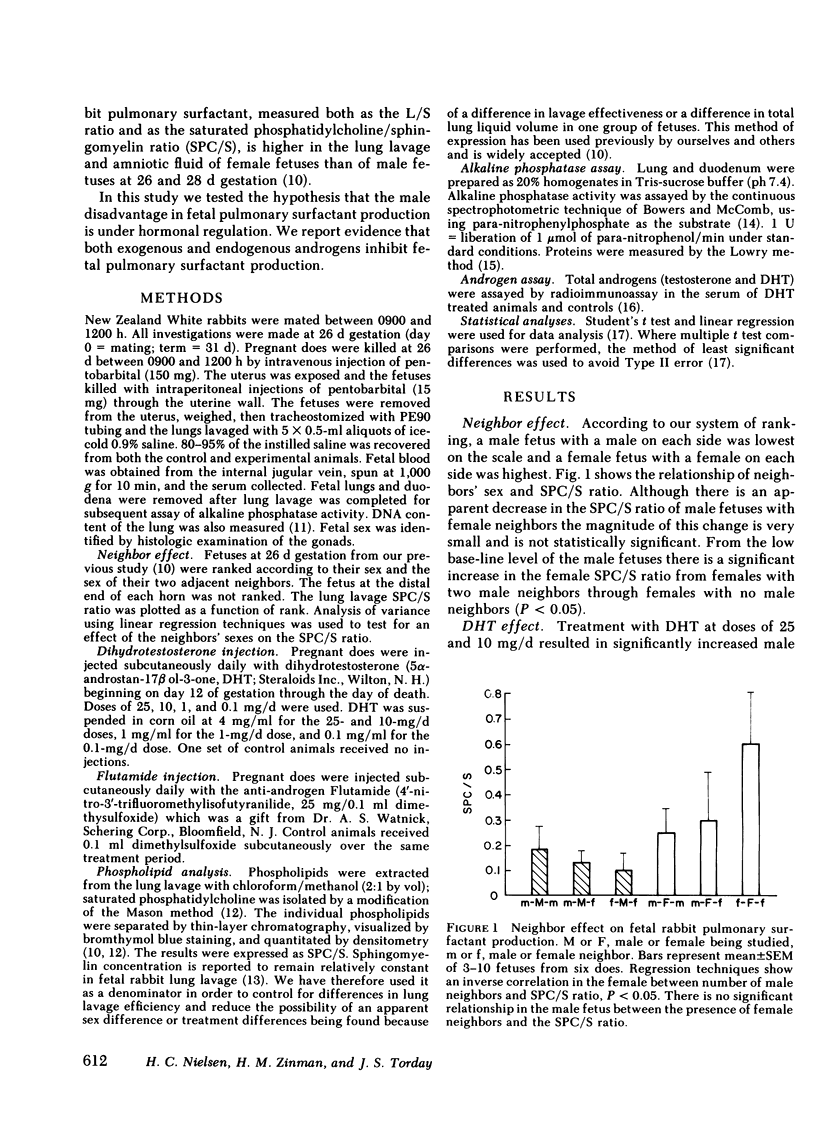
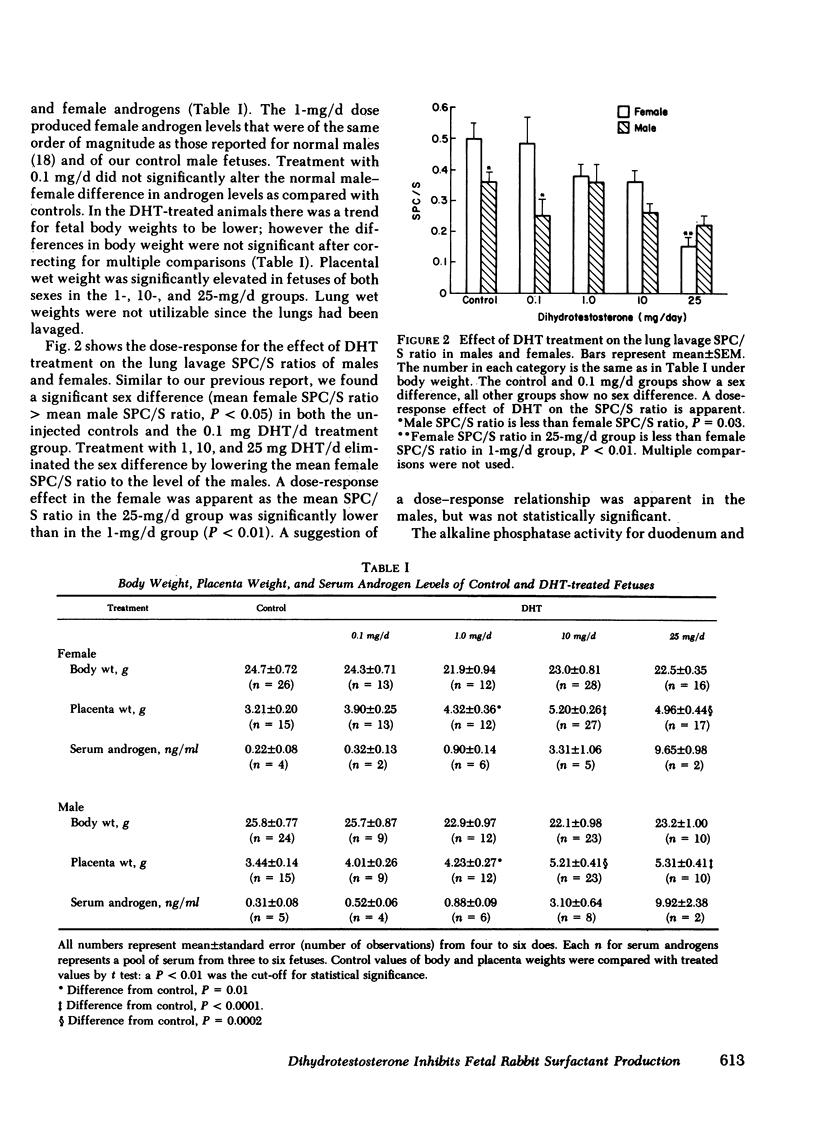
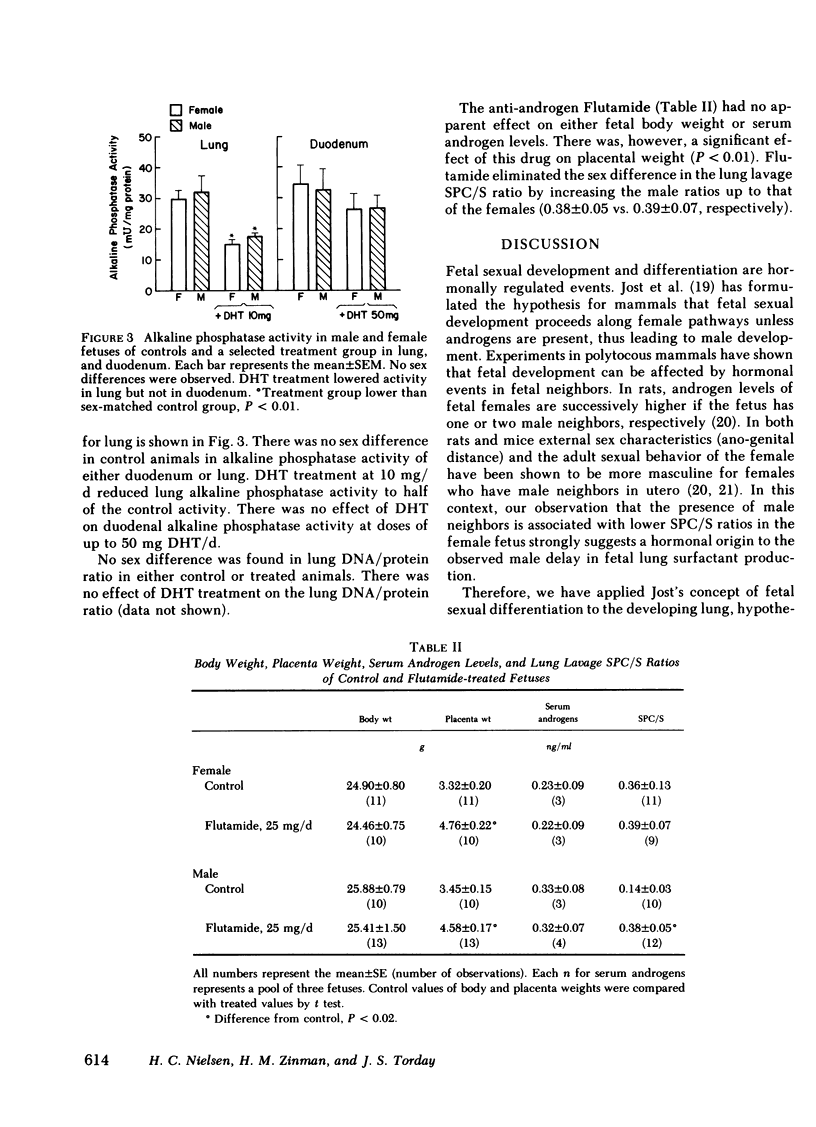
Males have a higher morbidity and mortality for neonatal respiratory distress syndrome (RDS) than females, and respond less well to hormone therapy designed to prevent RDS by stimulating fetal pulmonary surfactant production. We have shown that male fetuses exhibit delayed production of pulmonary surfactant. We tested the hypothesis that the sex difference in fetal pulmonary surfactant production is under hormonal control. Pulmonary surfactant was measured as the saturated phosphatidylcholine/sphingomyelin ratio (SPC/S) in the lung lavage of fetal rabbits at 26 d gestation. There was an association between the sex of neighboring fetuses and the SPC/S ratio of the female fetuses, such that with one or two male neighbors, respectively, females had decreasing SPC/S ratios (P < 0.05). We injected dihydrotestosterone (DHT) into pregnant does from day 12 through day 26 of gestation in doses of 0.1, 1.0, 10, and 25 mg/d, and measured the SPC/S ratio in fetal lung lavage on day 26. In groups with the normal sex difference in fetal serum androgen levels (controls, 0.1 mg DHT/d) the normal sex difference in the SPC/S ratio was also present (females > males, P = 0.03). In the 1-mg/d group there was no sex difference in androgen levels and the sex difference in the SPC/S ratio was also eliminated as the female values were lowered to the male level. Higher doses of DHT (10, 25 mg/d) further reduced the SPC/S ratios. We injected the anti-androgen Flutamide (25 mg/d) from day 12 through day 26 of gestation. This treatment eliminated the normal sex difference in the lung lavage SPC/S ratio by increasing the male ratios to that of the females. We conclude that androgens inhibit fetal pulmonary surfactant production. An understanding of the mechanism of the sex difference in surfactant production may allow development of therapy that is as effective in males as in females for preventing RDS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard P. L., Ballard R. A., Granberg J. P., Sniderman S., Gluckman P. D., Kaplan S. L., Grumbach M. M. Fetal sex and prenatal betamethasone therapy. J Pediatr. 1980 Sep;97(3):451–454. doi: 10.1016/s0022-3476(80)80204-6. [DOI] [PubMed] [Google Scholar]
- Bowers G. N., Jr, McComb R. B. A continuous spectrophotometric method for measuring the activity of serum alkaline phosphatase. Clin Chem. 1966 Feb;12(2):70–89. [PubMed] [Google Scholar]
- Cox M. A., Torday J. S. Pituitary oligopeptide regulation of phosphatidylcholine synthesis by fetal rabbit lung cells: lack of effect with prolactin. Am Rev Respir Dis. 1981 Feb;123(2):181–184. doi: 10.1164/arrd.1981.123.2.181. [DOI] [PubMed] [Google Scholar]
- Farrell P. M., Avery M. E. Hyaline membrane disease. Am Rev Respir Dis. 1975 May;111(5):657–688. doi: 10.1164/arrd.1975.111.5.657. [DOI] [PubMed] [Google Scholar]
- Gandelman R., vom Saal F. S., Reinisch J. M. Contiguity to male foetuses affects morphology and behaviour of female mice. Nature. 1977 Apr 21;266(5604):722–724. doi: 10.1038/266722a0. [DOI] [PubMed] [Google Scholar]
- Hartiala J. Testosterone metabolism in rabbit lung in vitro. Steroids Lipids Res. 1974;5(2):91–95. [PubMed] [Google Scholar]
- Jost A., Vigier B., Prépin J., Perchellet J. P. Studies on sex differentiation in mammals. Recent Prog Horm Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- Khosla S. S., Rooney S. A. Stimulation of fetal lung surfactant production by administration of 17beta-estradiol to the maternal rabbit. Am J Obstet Gynecol. 1979 Jan 15;133(2):213–216. doi: 10.1016/0002-9378(79)90479-4. [DOI] [PubMed] [Google Scholar]
- Kotas R. V., Avery M. E. The influence of sex on fetal rabbit lung maturation and on the response to glucocorticoid. Am Rev Respir Dis. 1980 Feb;121(2):377–380. doi: 10.1164/arrd.1980.121.2.377. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller H. C., Futrakul P. Birth weight, gestational age, and sex as determining factors in the incidence of respiratory distress syndrome of prematurely born infants. J Pediatr. 1968 May;72(5):628–635. doi: 10.1016/s0022-3476(68)80005-8. [DOI] [PubMed] [Google Scholar]
- Morishige W. K., Uetake C. A. Receptors for androgen and estrogen in the rat lung. Endocrinology. 1978 Jun;102(6):1827–1837. doi: 10.1210/endo-102-6-1827. [DOI] [PubMed] [Google Scholar]
- Naeye R. L., Freeman R. K., Blanc W. A. Nutrition, sex, and fetal lung maturation. Pediatr Res. 1974 Mar;8(3):200–204. doi: 10.1203/00006450-197403000-00008. [DOI] [PubMed] [Google Scholar]
- Nielsen H. C., Torday J. S. Sex differences in fetal rabbit pulmonary surfactant production. Pediatr Res. 1981 Sep;15(9):1245–1247. doi: 10.1203/00006450-198109000-00004. [DOI] [PubMed] [Google Scholar]
- Pearson R. Letter: Lecithin/sphingomyelin ratios and sex incidence of respiratory-distress syndrome. Lancet. 1976 May 1;1(7966):960–960. doi: 10.1016/s0140-6736(76)92733-1. [DOI] [PubMed] [Google Scholar]
- Shimazaki J., Matsushita I., Furuya N., Yamanaka H., Shida K. Reduction of 5 alpha-position of testosterone in the rat ventral prostate. Endocrinol Jpn. 1969 Aug;16(4):453–458. doi: 10.1507/endocrj1954.16.453. [DOI] [PubMed] [Google Scholar]
- Sultan C., Migeon B. R., Rothwell S. W., Maes M., Zerhouni N., Migeon C. J. Androgen receptors and metabolism in cultured human fetal fibroblasts. Pediatr Res. 1980 Jan;14(1):67–69. doi: 10.1203/00006450-198001000-00016. [DOI] [PubMed] [Google Scholar]
- Torday J. S., Nielsen H. C., Fencl M. de M., Avery M. E. Sex differences in fetal lung maturation. Am Rev Respir Dis. 1981 Feb;123(2):205–208. doi: 10.1164/arrd.1981.123.2.205. [DOI] [PubMed] [Google Scholar]
- Torday J., Carson L., Lawson E. E. Saturated phosphatidylcholine in amniotic fluid and prediction of the respiratory-distress syndrome. N Engl J Med. 1979 Nov 8;301(19):1013–1018. doi: 10.1056/NEJM197911083011901. [DOI] [PubMed] [Google Scholar]
- Veyssiére G., Berger M., Jean-Faucher C., de Turckheim M., Jean C. Evolution de la testostéronémie chez le foetus de Lapin au cours de l'organogenèse sexuelle. C R Seances Acad Sci D. 1980 Feb 25;290(8):583–586. [PubMed] [Google Scholar]
- vom Saal F. S., Bronson F. H. Sexual characteristics of adult female mice are correlated with their blood testosterone levels during prenatal development. Science. 1980 May 9;208(4444):597–599. doi: 10.1126/science.7367881. [DOI] [PubMed] [Google Scholar]



