Time to first treatment has increased for those with colon and rectal cancers at VA Medical Centers. Patient, tumor, and hospital factors are associated with prolonged time to treatment.
Abstract
Purpose:
Timeliness of cancer treatment is an important aspect of health care quality. Veterans Affairs Medical Centers (VAMCs) are expected to treat a growing number of patients with cancer. Our objectives were to examine treatment times from diagnosis to first-course therapy for patients with colon and rectal cancers and assess factors associated with prolonged wait times.
Methods:
From the VA Central Cancer Registry, patients who underwent colon or rectal resection for cancer from 1998 to 2008 were identified. Time from diagnosis to definitive cancer-directed therapy was measured, and multivariable regression methods were used to determine predictors of prolonged wait times for colon (≥ 45 days) and rectal (≥ 60 days) cancers.
Results:
From 124 VAMCs, 14,097 patients underwent colectomy, and 3,390 underwent rectal resection for cancer. For colon cancer, the median time to treatment increased by 68% over time (P < .001). From 2007 to 2008, the median time to colectomy was 32 days. Predictors of prolonged wait times included age ≥ 55 years (v < 55 years), time period (2007 to 2008 v 1998 to 2000), black race (v white), marriage status (married v unmarried), high-volume center status (v low volume), and treatment at a different hospital (v same hospital as initial diagnosis; all P < .05). For rectal cancer, the overall median time to first-course treatment increased by 74% (P < .001). From 2007 to 2008, the median time to proctectomy was 47 days. Similar predictors of prolonged wait times were identified for rectal cancer.
Conclusion:
Time to first treatment has increased for patients with colon and rectal cancers at VAMCs. Patient, tumor, and hospital factors are associated with prolonged time to treatment.
Introduction
Colorectal cancer care requires the coordinated effort of all aspects of the health care system. As the complexity of multimodality cancer care increases, several weeks or even months may elapse as a patient moves through the initial cancer diagnosis to definitive therapy. Currently, Veterans Affairs Medical Centers (VAMCs) treat approximately 3% of cancers in the United States, of which 11% are colon or rectal malignancies.1 The compounding effects of the aging population, physician shortages, and increases in military personnel entering the VA health care system will likely considerably affect demands on VA health care resources.2,3
Little is known about the time interval from diagnosis to cancer-directed treatment at VAMCs. When examining lung cancer at VA hospitals using international studies as a comparison group, Schultz et al4 concluded that wait times were comparable at VA and non-VA centers based on historical controls (median, 22 v 24 days, respectively). Similarly, the VA Office of Quality and Performance did not estimate a considerable difference in the timeliness of treatment of patients with colorectal cancer.5 However, when comparing time from diagnosis to first cancer treatment by center type directly, more recent work suggests this interval may be considerably longer at VAMCs versus non-VA hospitals.6
Nevertheless, these conflicting reports were based on a sample of the VA population. To date, no study to our knowledge has evaluated colorectal cancer wait times for patients being treated at all cancer-treating VAMCs, and it is unknown what specific factors may lead to prolonged wait times within the VA health care system. Therefore, our objectives were: one, to examine wait times for definitive therapy for colon and rectal cancers; and two, to assess patient, tumor, and hospital factors associated with prolonged wait times at all cancer-treating VAMCs.
Methods
Data Source
Data were obtained from the VA Central Cancer Registry (VACCR). The VACCR was established in 1995 after a VA directive mandated all VAMCs performing cancer surgery submit treatment-, tumor-, and patient-level data to a central registry.7 Trained cancer registrars, who are periodically audited to ensure data reliability, perform data collection. The VACCR conforms to the data quality standards and reporting processes established by the Facility Oncology Registry Data Standards.8
Patient Selection and Exclusion
From the VACCR (1998 to 2008), 27,784 patients with colon or rectal cancer who underwent surgical resection were identified based on International Classification of Disease–Oncology codes.9 Patients were excluded from this study if they had documented stage IV disease (n = 3,284), staging information was missing (n = 2,138), or the date of diagnosis or treatment was missing (n = 182). In addition, because this study sought to evaluate treatment delays for elective colon and rectal surgeries, patients were excluded if the diagnosis and operation were on the same day, indicating it was an emergent case, or the cancer diagnosis was not known before the index surgery (n = 4,693). The final study cohort included 17,487 patients.
Time to Treatment
The VACCR requires that patients have an initial cancer diagnosis date recorded based on the first clinical or histologic confirmation or, if in retrospect, the patient had cancer at an earlier date of suspicious diagnostic findings. Time to treatment was calculated based on the number of days from diagnosis to definitive treatment. Time to surgery was calculated for both colon and rectal cancers, and time to neoadjuvant chemoradiotherapy was additionally evaluated for patients with rectal cancer. The date of radiation therapy initiation was not available, and therefore, time to neoadjuvant therapy was calculated based on the date of chemotherapy initiation. Only 2.4% of patients with rectal cancer undergoing resection were coded as receiving radiotherapy alone.
Hospital Classification
Average annual surgical volume was calculated for all VAMCs. Hospitals were ranked based on median surgical case volume and separated into quartiles of increasing average annual case-volume categories with equal numbers of patients in each group. The middle quartiles were combined, leaving three surgical volume groups for analysis: < 25, 25 to 50, and ≥ 50 cases per year.
Patients were additionally classified based on the location of diagnosis and treatment, specifically, whether the reporting facility diagnosed the index cancer, treated the index cancer, or both. Patients were categorized into two groups: one, diagnosis and treatment at the same hospital; and two, diagnosis at an outside facility and treatment at the reporting VAMC. Importantly, patients who did not undergo definitive treatment at a VAMC (eg, transferred to non-VA facility after diagnosis) were not included in the data set.
Patient-, Tumor-, and Treatment-Specific Variables
Variables collected by the VACCR are defined based on Facility Oncology Registry Data Standards.8 To evaluate changes in treatment trends over time, four time periods were created based on roughly equal numbers of patients (1998 to 2000, 2001 to 2003, 2004 to 2006, and 2007 to 2008). Patient characteristics included patient age (< 55, 55 to 64, 65 to 74, or ≥ 75 years), race/ethnicity (black, white, Hispanic, or other), and marital status. Tumor and treatment characteristics included American Joint Committee on Cancer stage,10 tumor grade (low, intermediate, or high), histologic subtype (adenocarcinoma, mucinous carcinoma, or other), surgical extent (hemicolectomy, total colectomy, sigmoid colectomy, proctectomy, total proctocolectomy, abdominoperineal resection, or pelvic exenteration), and neoadjuvant chemoradiotherapy use.
Statistical Analysis
Colon and rectal cancers were analyzed separately because they are clinically distinct diseases. Analysis was performed at the patient (patient as unit of analysis) and hospital (hospital as unit of analysis) levels. Multivariable analysis was performed to evaluate predictors of treatment delays with generalized linear mixed models, with the individual hospital as the random effect.11,12 Delay in treatment was defined as the upper quartile of wait times separately for colon (≥ 45 days) and rectal (≥ 60 days) cancers. These cut points are clinically reasonable because rectal cancers require additional multimodality evaluation (eg, endoscopic ultrasound, medical oncologist referral, discussion in multidisciplinary conference). Variables were chosen for initial entry into the models if they were thought to be clinically important or if they had a univariate association with the outcome variable at a level of P < .20. To further evaluate time trends, select variables that we believed were clinically relevant were tested for interaction with year of diagnosis, with similar results. We therefore report the full model using all years. Additional sensitivity analysis evaluated models using alternative time-to-treatment cut points, also with similar findings. Model fit was assessed based on the fixed effects with the c-statistic, Hosmer-Lemeshow χ2 statistic, and Akaike information criterion generated from standard logistic regression models.13,14 The threshold for statistical significance was set at P ≤ .05, and P values were based on two-sided tests. All statistical analysis was performed using SAS software (version 9.3; SAS Institute, Cary, NC). A collaborative institutional review board from Northwestern University, University of Illinois at Chicago, and Jesse Brown VAMC approved this study.
Results
From the VACCR, 17,487 patients who underwent surgical resection for colon (14,097 patients; 124 hospitals) and rectal cancers (3,390 patients; 118 hospitals) from 1998 to 2008 were identified. The median age at diagnosis for both cancers was 66 years, and the majority of patients were men (colon, 97.8%; rectal, 98.5%). Patients were evenly distributed throughout cancer stages, and most patients were diagnosed and treated at the same VAMC (colon, 90.7%; rectal, 84.1%). Table 1 lists these and other patient and treatment characteristics.
Table 1.
Demographic and Tumor Characteristics of Patients With Colon and Rectal Cancers Undergoing Resection at VA Hospitals (1998 to 2008)
| Characteristic | Colon Cancer (n = 14,097) |
Rectal Cancer (n = 3,390) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 66 | 66 | ||
| IQR | 59-74 | 59-74 | ||
| Female sex | 313 | 2.2 | 49 | 1.5 |
| Race | ||||
| White | 10,746 | 76.2 | 2,738 | 80.8 |
| Black | 2,331 | 16.5 | 391 | 11.5 |
| Hispanic | 710 | 5.0 | 177 | 5.2 |
| Other | 310 | 2.2 | 84 | 2.5 |
| Unmarried | 6,721 | 47.7 | 1,683 | 49.7 |
| Year of diagnosis | ||||
| 1998 to 2000 | 3,112 | 22.1 | 775 | 22.9 |
| 2001 to 2003 | 4,020 | 28.5 | 989 | 29.2 |
| 2004 to 2006 | 4,162 | 29.5 | 965 | 28.5 |
| 2007 to 2008 | 2,803 | 19.9 | 661 | 19.5 |
| AJCC stage | ||||
| I | 4,394 | 31.2 | 1,030 | 30.4 |
| II | 5,138 | 36.5 | 1,149 | 33.9 |
| III | 4,565 | 32.4 | 1,211 | 35.7 |
| Grade | ||||
| Low | 1,396 | 10.5 | 297 | 8.8 |
| Intermediate | 10,082 | 76.1 | 2,423 | 71.5 |
| High | 1,773 | 13.4 | 370 | 10.9 |
| Tumor histology | ||||
| Adenocarcinoma | 12,843 | 91.1 | 3,155 | 93.1 |
| Mucinous | 1,148 | 8.1 | 202 | 6.0 |
| Other | 106 | 0.8 | 33 | 1.0 |
| Location of diagnosis and treatment | ||||
| Same hospital | 12,782 | 90.7 | 2,850 | 84.1 |
| Different hospitals | 1,315 | 9.3 | 539 | 15.9 |
| Surgical extent | ||||
| Hemicolectomy | 12,590 | 89.3 | — | — |
| Total colectomy | 508 | 3.6 | — | — |
| Sigmoid colectomy | 999 | 7.1 | — | — |
| Proctectomy | — | — | 2,492 | 73.5 |
| Total proctocolectomy | — | — | 124 | 3.7 |
| APR | — | — | 715 | 21.1 |
| Pelvic exenteration | — | — | 59 | 1.7 |
| Hospital surgical volume, patient cases per year* | ||||
| < 25 | 2,777 | 19.7 | 597 | 17.6 |
| 25 to 50 | 8,388 | 59.5 | 2,069 | 61.3 |
| ≥ 50 | 2,932 | 20.8 | 724 | 21.4 |
NOTE. Missing values for tumor grade: n = 1,190; 6.6%.
Abbreviations: AJCC, American Joint Committee on Cancer; APR, abdominoperineal resection; IQR, interquartile range; VA, Veterans Affairs.
Average annual hospital-level colorectal surgical case volume.
Time to Treatment
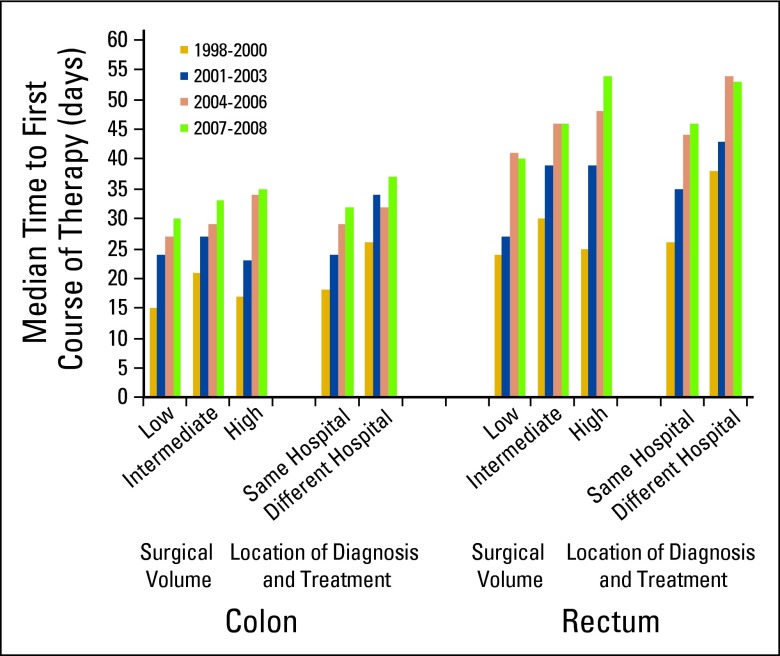
The number of days from diagnosis to treatment and trends over time were assessed (Appendix Table A1, online only). Among patients undergoing resection for colon cancer, the overall median time to treatment was 27 days, which increased from a median of 19 (1998 to 2000) to 32 days (2007 to 2008; P < .05). Overall, 25% of patients waited ≥ 45 days to colectomy (Appendix Fig A1, online only), whereas in the most recent time period (2007 to 2008), 32% of patients waited ≥ 45 days to colon surgery. When stratified by average annual surgical volume status and treating hospital, median wait times were greatest in the most recent time period (2007 to 2008) at high-volume hospitals and when patients were diagnosed and treated at separate facilities (Fig 1). Hospital-level time to first-course therapy was evaluated in the 124 VAMCs. For colon cancer, 27% of centers (33 of 124) had median wait times ≥ 30 days, whereas one center had a median wait time ≥ 45 days (Appendix Fig A2, online only).
Figure 1.
Median days to first-course therapy for patients with colon and rectal cancers undergoing surgical resection at Veterans Affairs hospitals by surgical volume and location of diagnosis and treatment (1998 to 2008).
Among patients with rectal cancer undergoing resection, the overall median time to treatment was 39 days, which increased from a median of 27 (1998 to 2000) to 47 days (2007 to 2008; P < .05; Appendix Table A1, online only). In patients undergoing surgery first, the median time to surgery was 47 days, whereas in patients undergoing chemoradiotherapy first, the median time to neoadjuvant therapy was 46 days. Overall, 25% of patients waited ≥ 60 days to first-course therapy (either surgery first or neoadjuvant chemoradiotherapy), whereas in the most recent time period (2007 to 2008), 34% of patients waited ≥ 60 days. Similar to colon cancer, after stratification by volume status and treating facility, median wait times were greatest at high-volume centers and when patients were diagnosed and treated at separate VAMCs (Fig 1). Hospital-level time to first-course therapy for rectal cancer was also evaluated; 72% of centers (85 of 118) had median wait times ≥ 30 days, 28% (33 of 118) had median wait times ≥ 45 days, and 5% (six of 118) had median wait times ≥ 60 days (Appendix Fig A2, online only).
Independent Predictors of Treatment Delays
Table 2 summarizes unadjusted and adjusted predictors of treatment delays for both colon and rectal cancers. The strongest factors independently associated with prolonged time to colectomy (≥ 45 days) were patient age ≥ 75 years (v < 55 years; odds ratio [OR], 1.60; 95% CI, 1.35 to 1.90), year of diagnosis in 2007 to 2008 (v 1998 to 2000; OR, 2.55; 95% CI, 2.34 to 2.92), treatment at a high-volume center (v low volume; OR, 1.59; 95% CI, 1.14 to 2.20), and diagnosis and treatment at different facilities (v same hospital; OR, 1.53; 95% CI, 1.34 to 1.75). Compared with patients with stage I cancers, prolonged time to first-course therapy was less likely for stages II (OR, 0.52; 95% CI, 0.47 to 0.58) and III tumors (OR, 0.51; 95% CI, 0.46 to 0.56). In addition, black race (v white) also increased the likelihood of prolonged time to surgery (OR, 1.16; 95% CI, 1.03 to 1.30), whereas being married was associated with decreased odds of prolonged time to treatment (OR, 0.87; 95% CI, 0.80 to 0.94). Other factors associated with decreased odds of prolonged wait time were high-grade tumors (v low grade), mucinous histologic subtype (v adenocarcinoma), and sigmoid colectomy procedures (v hemicolectomy).
Table 2.
Predictors of Treatment Delay From Diagnosis to First-Course Therapy Among Patients With Colon and Rectal Cancers Undergoing Surgical Resection at VA Hospitals (1998 to 2008)*
| Characteristic | Colon Cancer |
Rectal Cancer |
||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
|||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Age, years | ||||||||
| < 55 | 1.0† | 1.0† | 1.0† | 1.0† | ||||
| 55 to 64 | 1.47 | 1.24 to 1.73 | 1.43 | 1.20 to 1.70 | 1.45 | 1.12 to 1.89 | 1.46 | 1.10 to 1.94 |
| 65 to 74 | 1.35 | 1.14 to 1.59 | 1.50 | 1.27 to 1.79 | 1.20 | 0.92 to 1.57 | 1.42 | 1.06 to 1.91 |
| ≥ 75 | 1.43 | 1.22 to 1.68 | 1.60 | 1.35 to 1.90 | 1.28 | 0.97 to 1.69 | 1.54 | 1.13 to 2.09 |
| Year of diagnosis | ||||||||
| 1998 to 2000 | 1.0† | 1.0† | 1.0† | 1.0† | ||||
| 2001 to 2003 | 1.35 | 1.19 to 1.52 | 1.35 | 1.19 to 1.53 | 1.69 | 1.33 to 2.16 | 1.72 | 1.32 to 2.22 |
| 2004 to 2006 | 2.10 | 1.87 to 2.36 | 2.14 | 1.89 to 2.42 | 2.73 | 2.16 to 3.46 | 2.90 | 2.25 to 3.74 |
| 2007 to 2008 | 2.40 | 2.12 to 2.71 | 2.55 | 2.34 to 2.92 | 2.86 | 2.22 to 3.67 | 3.14 | 2.39 to 4.13 |
| Race | ||||||||
| White | 1.0† | 1.0† | 1.0† | 1.0† | ||||
| Black | 1.14 | 1.03 to 1.26 | 1.16 | 1.03 to 1.30 | 1.10 | 0.87 to 1.39 | 1.16 | 0.89 to 1.51 |
| Hispanic | 1.08 | 0.91 to 1.29 | 0.94 | 0.74 to 1.20 | 0.81 | 0.56 to 1.17 | 0.74 | 0.47 to 1.16 |
| Other | 1.24 | 0.96 to 1.59 | 1.24 | 0.95 to 1.63 | 1.32 | 0.83 to 2.10 | 1.22 | 0.73 to 2.05 |
| Married (v unmarried) | 0.88 | 0.81 to 0.94 | 0.87 | 0.80 to 0.94 | 0.79 | 0.68 to 0.92 | 0.78 | 0.66 to 0.92 |
| AJCC Stage | ||||||||
| I | 1.0† | 1.0† | 1.0† | 1.0† | ||||
| II | 0.52 | 0.47 to 0.57 | 0.52 | 0.47 to 0.58 | 0.71 | 0.59 to 0.85 | 0.72 | 0.58 to 0.89 |
| III | 0.50 | 0.45 to 0.54 | 0.51 | 0.46 to 0.56 | 0.69 | 0.58 to 0.83 | 0.69 | 0.56 to 0.84 |
| Tumor histology | ||||||||
| Adenocarcinoma | 1.0† | 1.0† | 1.0† | 1.0† | ||||
| Mucinous | 0.74 | 0.63 to 0.86 | 0.79 | 0.68 to 0.93 | 0.81 | 0.58 to 1.13 | 0.75 | 0.52 to 1.09 |
| Other | 1.76 | 1.18 to 2.60 | 1.29 | 0.84 to 1.99 | 1.78 | 0.88 to 3.60 | 1.36 | 0.62 to 1.97 |
| Grade | ||||||||
| Low | 1.0† | 1.0† | 1.0† | 1.0† | ||||
| Intermediate | 0.83 | 0.73 to 0.94 | 0.92 | 0.80 to 1.06 | 1.01 | 0.76 to 1.33 | 1.13 | 0.83 to 1.55 |
| High | 0.61 | 0.51 to 0.72 | 0.72 | 0.60 to 0.86 | 1.11 | 0.78 to 1.57 | 1.28 | 0.87 to 1.89 |
| Neoadjuvant CRT | — | — | 1.04 | 0.89 to 1.22 | 0.82 | 0.68 to 1.00 | ||
| Location of diagnosis and treatment | ||||||||
| Same hospital | 1.0† | 1.0† | 1.0† | 1.0† | ||||
| Different hospitals | 1.50 | 1.32 to 1.69 | 1.53 | 1.34 to 1.75 | 1.80 | 1.48 to 2.19 | 1.84 | 1.48 to 2.29 |
| Surgical extent | ||||||||
| Hemicolectomy | 1.0† | 1.0† | — | — | ||||
| Total colectomy | 1.08 | 0.88 to 1.32 | 1.13 | 0.91 to 1.40 | — | — | ||
| Sigmoid colectomy | 1.13 | 0.97 to 1.30 | 1.17 | 1.01 to 1.37 | — | — | ||
| Total proctocolectomy | — | — | 1.29 | 0.88 to 1.90 | 1.36 | 0.89 to 2.09 | ||
| Proctectomy | — | — | 1.0† | 1.0† | ||||
| APR | — | — | 0.90 | 0.74 to 1.09 | 0.99 | 0.80 to 1.22 | ||
| Pelvic exenteration | — | — | 0.84 | 0.46 to 1.55 | 1.03 | 0.53 to 1.99 | ||
| Hospital surgical volume, patient cases per year‡ | ||||||||
| < 25 | 1.0† | 1.0† | 1.0† | 1.0† | ||||
| 25 to 50 | 1.25 | 1.13 to 1.39 | 1.40 | 1.13 to 1.73 | 1.37 | 1.10 to 1.71 | 1.37 | 0.97 to 1.92 |
| > 50 | 1.43 | 1.27 to 1.62 | 1.59 | 1.14 to 2.20 | 1.57 | 1.22 to 2.03 | 1.63 | 1.01 to 2.64 |
Abbreviations: AJCC, American Joint Committee on Cancer; APR, abdominoperineal resection; CRT, chemoradiotherapy; OR, odds ratio; VA, Veterans Affairs.
Delay for colon cancer, ≥ 45 days; rectal cancer, ≥ 60 days.
Referent.
Average annual hospital-level colorectal surgical case volume.
Among patients with rectal cancer, the strongest predictors of prolonged time to first-course therapy (≥ 60 days) were year of diagnosis during 2007 to 2008 (v 1998 to 2000; OR, 3.14; 95% CI, 2.39 to 4.13), treatment at a high-volume center (v low volume; OR, 1.63; 95% CI, 1.01 to 2.64), and diagnosis and treatment at different hospitals (v same hospital; OR, 1.84; 95% CI, 1.48 to 2.29). No racial/ethnic differences were significantly associated with time to first-course therapy for rectal cancer; however, being married was associated with a reduced likelihood of prolonged wait time (OR, 0.78; 95% CI, 0.66 to 0.92). Patients with more-advanced tumors were also less likely to experience prolonged wait times.
Discussion
The Institute of Medicine identified the timeliness of care as one of six domains of health care quality.15 The number of patients requiring treatment is expected to expand, especially within the VA health care system, further increasing the demand for cancer care services.16 The timeliness of care may be a valuable indicator of hospital efficiency, resource use, and overall hospital quality. We directly studied timelines of colon and rectal cancer care and found that wait times increased markedly from 1998 to 2008 for both cancers, and patients treated at high-volume centers experienced particularly long treatment delays.
To our knowledge, no data exist regarding specific VAMC characteristics related to treatment wait times. Prior studies have primarily focused on center-type comparisons. For example, evidence suggests that National Cancer Institute Comprehensive Cancer Centers, which are primarily high-volume referral centers, have significantly longer wait times compared with community centers (colon cancer: OR, 2.01; 95% CI, 1.87 to 2.15; rectal cancer: OR, 2.43; 95% CI, 2.20 to 2.69). Patients treated at Commission on Cancer–accredited VAMCs had the greatest likelihood of experiencing treatment delays (colon: OR, 3.55; 95% CI, 3.12 to 4.04; rectal: OR, 4.28; 95% CI, 3.32 to 5.54).6 In a separate study from the VA, Jackson et al1 reported a median wait time from colorectal cancer diagnosis to treatment of 20 days, concluding that the VA performs well in meeting established benchmarks of care. This study was limited to 2,492 veterans from 2003 to 2006 and did not differentiate between colon and rectal cancers, which our study demonstrates is warranted given the clinically substantial differences in the timelines of care. Finally, Jackson et al used a coding algorithm that did not exclude patients who had wait times of 0 days. We specifically excluded these patient cases from analysis because they likely reflect urgent/emergent surgeries.
Among all cancer-treating VAMCs in the most recent time period (2007 to 2008), we found treatment delays were greatest at high-volume hospitals for both colon (median, 35 days) and rectal cancers (median, 54 days). Furthermore, there were substantial increases in treatment delays over time among all volume categories, especially at high-volume centers. For example, from 1998 to 2008, wait time increased by a median of 14 days for patients with colon cancer and nearly 30 days for those with rectal cancer treated at high-volume centers. On multivariable analysis, high-volume VAMCs were 59% and 63% more likely to have prolonged wait times compared with low-volume centers for colon and rectal cancers, respectively. Hence, given the growing and aging veteran population as well as efforts to regionalize cancer care to high-volume centers, treatment wait times at VAMCs will likely continue to increase in the future.3
Previous studies have identified a number of characteristics associated with prolonged wait times. In the largest study from the National Cancer Data Base, patient age ≥ 55 years, nonwhite race, and earlier tumor stage were associated with prolonged wait times. Other studies have documented similar factors associated with increasing time to treatment among surgically treated patients with cancer.6,17,18 The VA is a model for integrated care; however, assessing predictors of prolonged wait times, specifically at VAMCs, is less clear.19 Nevertheless, we identified a number of factors influencing wait time. First, cancer type was particularly associated with treatment delays. Patients with rectal cancer had substantially longer wait times across every tumor, treatment, and hospital characteristic, compared with colon cancer. This is not surprising given the multimodality diagnostic and treatment planning requirements. For example, it is now standard of care for patients with rectal cancer to undergo transrectal ultrasound.
Limited data exist regarding what is considered a reasonable treatment wait time after colon or rectal cancer diagnosis. In part because of the lack of high-level evidence that prolonged wait times lead to worse outcomes,20 no stakeholder organization within the United States has recommended specific maximum wait-time intervals. In the largest and most comprehensive study from the National Cancer Data Base, the median time to treatment was 12 days for colon cancer and 23 days for rectal cancer among patients diagnosed and treated at the same hospital.6 International organizations have provided additional guidance regarding appropriate treatment wait times. The United Kingdom National Health Service Cancer Plan recommends a maximum wait time of 1 month for all cancers,21 whereas the Canadian Society of Surgical Oncology recommends 2 weeks as the maximum wait-time interval.22 If these guidelines were applied to colorectal cancer wait times at VAMCs, only one in four patients would meet the 2-week benchmark, and half would meet the 1-month benchmark. Moreover, if the number of median days to first-course treatment were considered at the hospital level, only 6% of VAMCs (seven of 124) would meet the 2-week benchmark, and 60% of VAMCs (74 of 124) would meet the 1-month benchmark.
Although the exact reasons for increasing wait times cannot be explicitly determined from these data, the answer is likely multifactorial. Extended wait times may be related to limited hospital resources in the face of a growing patient population within the VA system, the fragmentation and lack of coordination of specialized care, the increasing complexity and demands of care, and the lack of systems to improve efficiency. Most cancer care is currently discipline rather than disease based, and improving multidisciplinary systems of care may help reduce wait times. In addition, certain patient populations are particularly vulnerable to inherent inefficiencies in the current multimodality nature of complex cancer care.
This study should be considered with respect to certain limitations. First, this study assumed accurate and standardized dates of diagnosis for colon and rectal cancers. Although there may be some miscoding, we examined hospital-level inconsistencies and believe the data presented are valid. Furthermore, intermittent audits are performed to ensure data reliability and standardization. Second, this is a retrospective observational study, and therefore, we were only able to detect associations and were unable to control for unmeasured confounding factors. A number of predictors such as race and marriage status were found to be statically significant; however, clinical significance is not known. Third, although this report identifies substantial treatment delays at VAMCs relative to other center types and international comparisons, this study was not able to evaluate if prolonged wait times influenced short- or long-term outcomes. Nevertheless, extended wait times to first-course cancer treatment have been associated with worse outcomes for other cancers, namely breast cancer.23 In addition, patients experience considerable anxiety while awaiting cancer treatment, an important but often underappreciated aspect of health care quality.24 Finally, we were limited by the data collected in the VACCR. For example, information on patient comorbidities was not available for analysis. Nevertheless, we were able to account for patient age, which is a good surrogate for comorbidity burden and likely explains much of the variation.25 In addition, studies have demonstrated that comorbidity burden may in fact be decreased at high-volume hospitals.26–28
For patients with colon or rectal cancer treated at VAMCs, wait times for treatment have significantly increased over time. Patient and hospital factors are associated with prolonged treatment times, and opportunities exist to increase the quality of cancer care for veterans with colorectal cancer. Future work determining the specific and modifiable components that prolong wait times may prompt individual VAMCs with longer treatment times to focus on improving the timeliness of their care.
Acknowledgment
Supported by a Career Development Award from the Health Services Research Division, Department of Veterans Affairs (D.J.B.).
Appendix
Table A1.
Median No. of Days From Diagnosis to First-Course Therapy by Patient, Tumor, and Hospital Characteristics
| Characteristic | Colon Cancer (n = 14,097) |
Rectal Cancer |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 3,390) |
Surgery First (n = 2,147) |
Chemoradiation First (n = 1,243) |
||||||||
| Median No. of Days | IQR | ≥ 45 Days (%) | Median No. of Days | IQR | ≥ 60 Days (%) | Median No. of Days | IQR | Median No. of Days | IQR | |
| All patients | 27 | 13-44 | 24.6 | 39 | 23-61 | 26.6 | 35 | 20-61 | 44 | 29-61 |
| Age, years | ||||||||||
| < 55 | 22 | 9-39 | 19.2 | 35 | 21-55 | 22.2 | 31 | 16-55 | 39 | 27-55 |
| 55 to 64 | 27 | 14-45 | 25.8 | 41 | 26-64 | 29.3 | 37 | 21-66 | 45 | 30-63 |
| 65 to 74 | 27 | 14-44 | 24.2 | 39 | 22-60 | 25.6 | 35 | 20-60 | 45 | 28-61 |
| ≥ 75 | 27 | 12-45 | 25.3 | 37 | 21-62 | 26.7 | 35 | 19-61 | 43 | 31-63 |
| Year of diagnosis | ||||||||||
| 1998 to 2000 | 19 | 9-35 | 16.4 | 28 | 15-46 | 15.5 | 27 | 14-43 | 34 | 21-50 |
| 2001 to 2003 | 25 | 12-41 | 20.9 | 36 | 20-57 | 23.7 | 33 | 19-57 | 41 | 27-57 |
| 2004 to 2006 | 30 | 15-49 | 29.2 | 45 | 28-68 | 33.4 | 44 | 26-72 | 47 | 34-66 |
| 2007 to 2008 | 32 | 17-51 | 32.0 | 47 | 31-69 | 34.3 | 47 | 29-78 | 46 | 33-64 |
| Race | ||||||||||
| White | 27 | 13-43 | 24.0 | 39 | 23-61 | 26.5 | 35 | 20-60 | 45 | 30-62 |
| Black | 27 | 11-47 | 26.5 | 39 | 21-67 | 28.4 | 35 | 19-71 | 42 | 25-59 |
| Hispanic | 27 | 14-45 | 25.5 | 39 | 25-56 | 22.6 | 37 | 21-61 | 41 | 31-55 |
| Other | 28 | 14-48 | 28.1 | 41 | 28-65 | 32.1 | 50 | 29-67 | 39 | 22-62 |
| Married | ||||||||||
| Yes | 27 | 13-43 | 23.4 | 39 | 23-58 | 24.4 | 35 | 21-58 | 42 | 30-59 |
| No | 27 | 12-46 | 25.9 | 40 | 23-64 | 28.9 | 36 | 19-65 | 45 | 28-64 |
| AJCC stage | ||||||||||
| I | 34 | 19-53 | 33.6 | 42 | 26-68 | 31.7 | 41 | 25-67 | 48 | 33-69 |
| II | 23 | 11-41 | 20.8 | 38 | 22-59 | 24.6 | 31 | 17-56 | 42 | 28-61 |
| III | 23 | 10-40 | 20.0 | 37 | 21-58 | 24.3 | 31 | 16-55 | 43 | 30-61 |
| Grade | ||||||||||
| Low | 29 | 15-48 | 27.7 | 40 | 24-60 | 25.6 | 34 | 22-61 | 43 | 31-59 |
| Intermediate | 27 | 12-44 | 24.1 | 39 | 23-60 | 25.7 | 35 | 20-60 | 44 | 29-61 |
| High | 23 | 10-38 | 18.8 | 35 | 21-61 | 27.6 | 31 | 16-61 | 42 | 31-64 |
| Tumor histology | ||||||||||
| Adenocarcinoma | 27 | 13-44 | 24.9 | 39 | 23-61 | 26.8 | 35 | 20-62 | 44 | 29-61 |
| Mucinous | 24 | 11-40 | 19.6 | 37 | 21-56 | 22.8 | 35 | 20-52 | 41 | 26-59 |
| Other | 37 | 18-57 | 36.8 | 50 | 34-87 | 39.4 | 41 | 34-87 | 78 | 40-92 |
| Location of diagnosis and treatment | ||||||||||
| Same hospital | 26 | 12-43 | 23.8 | 38 | 22-59 | 24.7 | 34 | 20-59 | 42 | 28-59 |
| Different hospital | 31 | 15-52 | 31.9 | 47 | 28-71 | 37.1 | 44 | 24-74 | 49 | 33-69 |
| Surgical extent | ||||||||||
| Hemicolectomy | 26 | 12-44 | 24.4 | — | — | — | ||||
| Total colectomy | 28 | 13-47 | 25.8 | — | — | — | ||||
| Sigmoid colectomy | 30 | 17-47 | 26.6 | — | — | — | ||||
| Proctectomy | — | 39 | 23-61 | 26.9 | 35 | 20-61 | 45 | 31-62 | ||
| Total proctocolectomy | — | 45 | 23-71 | 32.3 | 44 | 17-78 | 46 | 31-60 | ||
| APR | — | 38 | 24-59 | 24.9 | 35 | 20-64 | 41 | 28-58 | ||
| Pelvic exenteration | — | 32 | 15-57 | 23.7 | 17 | 8-40 | 46 | 26-63 | ||
| Hospital surgical volume, patient cases per year* | ||||||||||
| < 25 | 25 | 12-41 | 20.9 | 33 | 20-53 | 21.3 | 30 | 17-50 | 40 | 28-59 |
| 25 to 50 | 27 | 13-44 | 24.8 | 39 | 24-62 | 27.1 | 35 | 20-61 | 45 | 30-62 |
| > 50 | 27 | 12-47 | 27.4 | 42 | 25-65 | 29.8 | 41 | 21-72 | 43 | 29-61 |
Abbreviations: AJCC, American Joint Commission on Cancer; APR, abdominoperineal resection; IQR, interquartile range.
Average annual hospital-level colorectal surgical case volume.
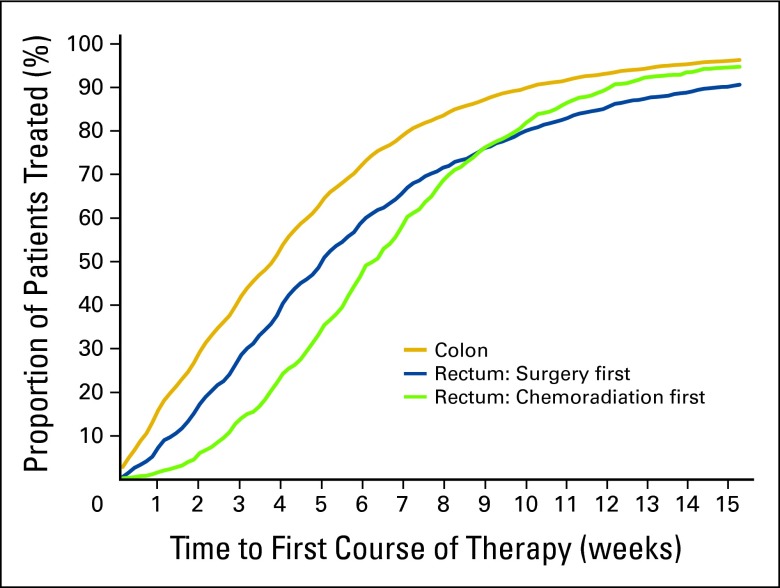
Figure A1.
Number of weeks from diagnosis to first-course therapy for patients with stages I to III colon and rectal cancers undergoing surgical resection at Veterans Affairs hospitals (1998 to 2008).
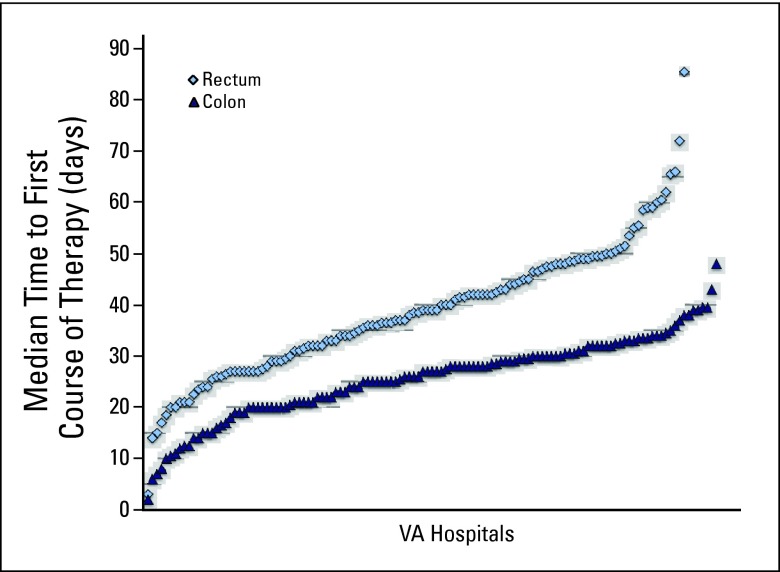
Figure A2.
Hospital-level median number of days to first-course therapy for patients with colon and rectal cancers undergoing surgical resection at Veterans Affairs (VA) hospitals (1998 to 2008).
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Ryan P. Merkow, Karl Y. Bilimoria, Martin D. McCarter, David J. Bentrem
Financial support: David J. Bentrem
Provision of study materials or patients: David J. Bentrem
Collection and assembly of data: Ryan P. Merkow, Karl Y. Bilimoria, Karen L. Sherman, Howard S. Gordon, David J. Bentrem
Data analysis and interpretation: Ryan P. Merkow, Karl Y. Bilimoria, Karen L. Sherman, Howard S. Gordon, David J. Bentrem
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Jackson GL, Melton LD, Abbott DH, et al. Quality of nonmetastatic colorectal cancer care in the Department of Veterans Affairs. J Clin Oncol. 2010;28:3176–3181. doi: 10.1200/JCO.2009.26.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yancik R, Ries LA. Aging and cancer in America: Demographic and epidemiologic perspectives. Hematol Oncol Clin North Am. 2000;14:17–23. doi: 10.1016/s0889-8588(05)70275-6. [DOI] [PubMed] [Google Scholar]
- 3.Yancik R. Population aging and cancer: A cross-national concern. Cancer J. 2005;11:437–441. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Schultz EM, Powell AA, McMillan A, et al. Hospital characteristics associated with timeliness of care in veterans with lung cancer. Am J Respir Crit Care Med. 2009;179:595–600. doi: 10.1164/rccm.200806-890OC. [DOI] [PubMed] [Google Scholar]
- 5.Office of Quality and Performance, Veteran Health Affairs. Washington, DC: Department of Health and Human Services; 2009. Quality of Colorectal Cancer Care in the VA 2003-2006: National and VISN Results of Office of Quality and Performance Special Study; pp. 1–10. [Google Scholar]
- 6.Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States: Trends and predictors of delays. Ann Surg. 2011;253:779–785. doi: 10.1097/SLA.0b013e318211cc0f. [DOI] [PubMed] [Google Scholar]
- 7.Veterans Health Administration. VHA Directive 2003-034, National Cancer Strategy June 20, 2003. Washington, DC: Department of Veterans Affairs; 2003. [Google Scholar]
- 8.American College of Surgeons. Commission on Cancer Program and Data Standards: Facility Oncology Registry Data Standards. http://www.facs.org/cancer/ncdb/cocmanuals.html.
- 9.Fritz A, Jack A, Parkin DM, et al., editors. International Classification of Diseases for Oncology. ed 3. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 10.Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. ed 7. Chicago, IL: Springer; 2010. [Google Scholar]
- 11.Greenland S. Principles of multilevel modelling. Int J Epidemiol. 2000;29:158–167. doi: 10.1093/ije/29.1.158. [DOI] [PubMed] [Google Scholar]
- 12.Moore L, Hanley JA, Turgeon AF, et al. Evaluating the performance of trauma centers: Hierarchical modeling should be used. J Trauma. 2010;69:1132–1137. doi: 10.1097/TA.0b013e3181cc8449. [DOI] [PubMed] [Google Scholar]
- 13.Hosmer DW, Lemeshow S. Applied Logistic Regression. ed 2. New York, NY: John Wiley and Sons; 2000. [Google Scholar]
- 14.Merkow RP, Hall BL, Cohen ME, et al. Relevance of the c-statistic in risk-adjustment models in surgery. J Am Coll Surg. 2012;214:822–830. doi: 10.1016/j.jamcollsurg.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001. [PubMed] [Google Scholar]
- 16.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 17.Gwyn K, Bondy ML, Cohen DS, et al. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100:1595–1604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 18.Bardell T, Belliveau P, Kong W, et al. Waiting times for cancer surgery in Ontario: 1984-2000. Clin Oncol (R Coll Radiol) 2006;18:401–409. doi: 10.1016/j.clon.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Kizer KW, Dudley RA. Extreme makeover: Transformation of the veterans health care system. Annu Rev Public Health. 2009;30:313–339. doi: 10.1146/annurev.publhealth.29.020907.090940. [DOI] [PubMed] [Google Scholar]
- 20.Ramos M, Esteva M, Cabeza E, et al. Lack of association between diagnostic and therapeutic delay and stage of colorectal cancer. Eur J Cancer. 2008;44:510–521. doi: 10.1016/j.ejca.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Department of Health. The NHS Cancer Plan: A Plan for Investment, a Plan for Reform. London, United Kingdom: Crown Copyright; 2000. [Google Scholar]
- 22.Simunovic M, Thériault ME, Paszat L, et al. Using administrative databases to measure waiting times for patients undergoing major cancer surgery in Ontario, 1993-2000. Can J Surg. 2005;48:137–142. [PMC free article] [PubMed] [Google Scholar]
- 23.Richards MA, Westcombe AM, Love SB, et al. Influence of delay on survival in patients with breast cancer: A systematic review. Lancet. 1999;353:1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 24.Jones RV, Greenwood B. Breast cancer: Causes of patients' distress identified by qualitative analysis. Br J Gen Pract. 1994;44:370–371. [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Wong ML, Hamilton N, et al. Impact of age and comorbidity on non–small-cell lung cancer treatment in older veterans. J Clin Oncol. 2012;30:1447–1455. doi: 10.1200/JCO.2011.39.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Refaie WB, Muluneh B, Zhong W, et al. Who receives their complex cancer surgery at low-volume hospitals? J Am Coll Surg. 2012;214:81–87. doi: 10.1016/j.jamcollsurg.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Bilimoria KY, Bentrem DJ, Talamonti MS, et al. Risk-based selective referral for cancer surgery: A potential strategy to improve perioperative outcomes. Ann Surg. 2010;251:708–716. doi: 10.1097/SLA.0b013e3181c1bea2. [DOI] [PubMed] [Google Scholar]
- 28.Paulson EC, Mitra N, Sonnad S, et al. National Cancer Institute designation predicts improved outcomes in colorectal cancer surgery. Ann Surg. 2008;248:675–686. doi: 10.1097/SLA.0b013e318187a757. [DOI] [PubMed] [Google Scholar]