Manually screening patient records increased enrollment to specific clinical trials. A screening intervention process, involving a dedicated screening coordinator, should be considered to improve clinical trial accrual.
Abstract
Purpose:
Low enrolment rates in clinical trials present a barrier to the development of novel cancer therapies. Currently, only 3% of patients with cancer participate, and many studies fail to achieve necessary enrolment. The objective of this study was to evaluate whether a screening intervention to identify potentially eligible patients (PEPs) would increase accrual rates.
Patients and Methods:
Over a 4-month intervention period, PEPs for 21 phase II-IV breast, gastrointestinal, genitourinary, gynecology, and lung cancer trials were identified by a screening coordinator. This individual reviewed the electronic medical records of patients attending outpatient clinics and flagged PEPs for 10 medical oncologists at the BC Cancer Agency. Patients who were already documented to be trial eligible by physicians were not flagged. Oncologists were surveyed regarding the helpfulness and accuracy of the intervention.
Results:
During the intervention period, 73 patients were enrolled, compared with 61 patients enrolled in the 4 months prior and 51 patients in the 4 months after. A total of 2,098 charts were reviewed, and 120 PEPs were identified during the intervention period, resulting in 19 PEPs who enrolled and four PEPs who declined a clinical trial. Relative accrual rates adjusted for oncologist appointments were 0.85 (P = .15) before and 0.70 (P < .005) after, relative to the intervention period. Oncologist-returned surveys indicated that 67% of flags were helpful, and 70% were accurate.
Conclusions:
In this study, manually screening patient records increased enrolment to specific clinical trials. A screening intervention process, involving a dedicated screening coordinator, should be considered to improve clinical trial accrual.
Introduction
Clinical trials are the gold standard for developing novel cancer therapeutics, determining their optimal delivery, and evaluating their efficacy in different subpopulations. Currently, more than 11,000 clinical trials listed by the National Cancer Institute (NCI) are accepting participants.1 However, only an estimated 2% to 4% of all patients with cancer participate in clinical trials.2–4 Low enrolment rates slow the progress of trials and may result in delayed study completion or even study closure.5–10 A recent NCI report found that 50% of all phase III NCI studies close prematurely, due in part to poor accrual, and just this year an analysis of 238 clinical trials across five US co-operative groups determined that 29% closed specifically as a result of poor accrual.11,12
Low enrolment rates in clinical trials have frequently been attributed to patient-related factors, such as lack of awareness, fear of being subjected to experimental maltreatment, desire to receive standard therapy, or geographic distance from centers that offer trials.13–15 Widespread efforts to improve patient perception and knowledge of clinical trials have led to better public awareness and perception of clinical trials, although these efforts have thus far yielded little improvement in overall clinical trial participation among patients with cancer.10,16,17
Recent studies have found that patient participation in clinical trials is strongly dependent on patients' perception of their physicians' reliability and attitudes toward clinical trials.3,14,18 For example, an online survey found that 73% of cancer survivors reported their primary physician as their main source for clinical trial information.3 Cancer survivors who felt that their physician spent a great deal of effort to find clinical trials suitable for them had an enrolment rate of 85%, compared with 39% and 23% of cancer survivors who believed their physicians spent only moderate or little effort, respectively. Another recent study found that although up to 83% of cancer survivors are potentially willing to participate in a randomized clinical trial, most are never offered a chance to do so.19 These findings suggest that physician-associated factors may significantly limit clinical trial recruitment.20
A survey of oncologists from the Eastern Cooperative Oncology Group examined physician factors that may discourage clinical trial participation. The most prominent factors identified were practical limitations such as excessive paperwork or additional time required to randomly assign patients.17,21 On the basis of these findings, we hypothesized that supportive interventions that help to reduce physician labor per patient accrued would significantly improve clinical trial accrual rates.
The objective of this study was to evaluate whether an intervention by a clinical trial screening coordinator to identify potentially eligible patients (PEPs) would improve accrual to selected phase II-IV clinical trials The patient screening protocol was designed to lighten physician workload by identifying PEPs for clinical trials. It was hypothesized that accrual rates would increase as a result of the screening intervention. A secondary objective of the study was to document medical oncologists' attitudes toward the screening intervention.
Patients and Methods
Site Description
The British Columbia Cancer Agency (BCCA) at the Vancouver Cancer Center (VCC) is a large, academic, urban cancer center located in Vancouver, Canada. Each year, the 26 medical oncologists associated with the VCC see more than 3,000 new patients. On average, 40 new clinical trials are opened every year and offered to patients with solid and lymphoid malignancies.
Study Design
Patient accruals were documented over a 12-month period between January 1 and December 31, 2011, and were separated into three 4-month periods: a preintervention phase (January-April), an intervention period during which chart screening was conducted (May-August), and a follow-up postintervention period without chart screening. This enabled the outcome of all patients screened during the study to be determined and accrual rates after discontinuation of the intervention to be documented.
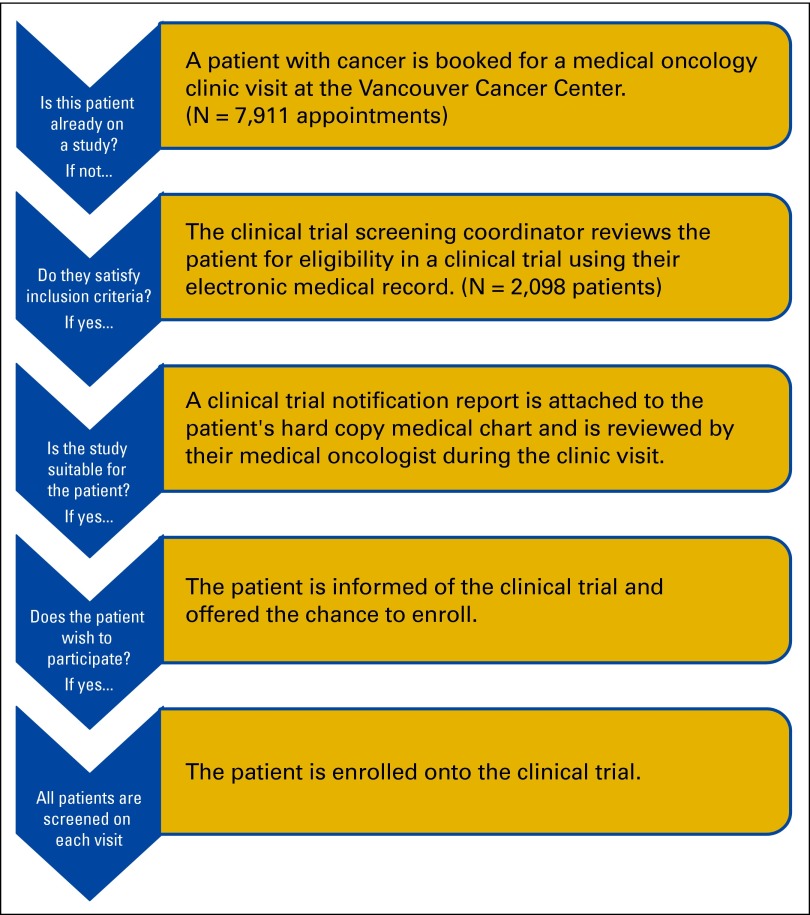
The screening intervention process is presented as a flowchart in Figure 1. The clinical trial screening coordinator identified patients scheduled for outpatient medical oncology visits and screened their electronic medical records (EMR) on the basis of trial protocol eligibility criteria. Patients who met the criteria were considered to be PEPs, and a one-page notification report was attached to their medical chart for in-clinic review by their medical oncologist. Patients who were not randomly assigned to a trial were rescreened during each subsequent appointment by the clinical trial screening coordinator. Members of the study team were given the opportunity to provide written feedback about the notification report by completing a survey at the end of the report.
Figure 1.
Identification and outcome of potentially eligible patients for clinical trials and flow chart of clinical trial recruitment process during chart screening implementation.
To address any potential breaches in policy or ethics resulting from the screening intervention, the BC Cancer Agency Research Ethics Board (REB) reviewed our final study design. Our study qualified as a quality improvement program under the Canadian Tri-Council Policy Statement, Chapter 2, Article 2.5.
Required Resources
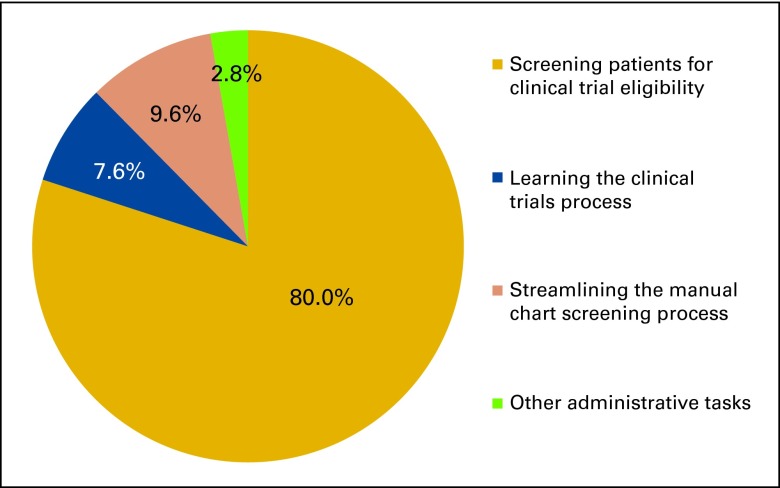
A 1.0 full-time equivalent clinical trial screening coordinator with no clinical experience and minimal previous knowledge about clinical trials was engaged for patient screening, notifying oncologists of identified PEPs, and surveying participating medical oncologists regarding the helpfulness and accuracy of the intervention for each PEP identified. The clinical trial screening coordinator was oriented on BCCA policies and procedures, including patient confidentiality and privacy procedures and trained on clinical trial–specific protocols by participating medical oncologists and other staff of the clinical trials department. After an initial 2- week learning period, the chart screening procedure was implemented, as shown in Figure 1. The coordinator spent 80% of time screening patients; other major activities are described in Appendix Figure A1 (online only).
Clinical Trial Selection and Inclusion Criteria
In total, 21 phase II-IV clinical trials were included in this intervention (Appendix Table A1, onlie only). Medical oncologists selected at least four clinical trials from each of five tumor groups: breast, GI, genitourinary (GU), gynecological, and lung cancers. In addition, we required the trials to have been open for at least 4 months before, and to remain open for 4 months after, the screening intervention.
Study-specific exclusion criteria varied between clinical trials, but general criteria included tumor site, stage of cancer, patient health performance (Eastern Cooperative Oncology Group performance status, Karnofsky performance score, or both), age, and line of therapy. Breast cancer trials were usually biomarker specific and restricted to patient subpopulations based on their expression of progesterone receptor, estrogen receptor, and human epidermal growth factor receptor 2. GI cancer trials for anti–epidermal growth factor receptor therapy required genetic screening for mutations in the KRAS gene. GU cancer trials tended to use increasing prostate-specific antigen levels as an additional method of detecting progressive disease among patients with prostate cancer. Some gynecological cancer trials required random assignment of patients within a set time after surgery. Finally, lung cancer trials offered at the BCCA mainly differentiated between small and non–small-cell types. All tumor groups tended to offer trials for advanced and/or metastatic disease. If patients satisfied all general inclusion criteria for the trial, then specific criteria such as hematology or other blood test results were matched against the patients' recent laboratory results.
A screening database was developed by the clinical trial screening coordinator for each tumor group. The database was used to record patient screens and was manually populated by the clinical trial screening coordinator to generate clinical trial notification reports for each PEP identified. The database was built using MS Access 2003.
Selection of Medical Oncologists
We approached 11 medical oncologists from the VCC who were distributed across tumor groups with a range of accrual rates, as determined by previous enrolment data collected by the VCC. Of these, 10 (90.9%) agreed to participate in the intervention, to be notified of any patients identified during the intervention, and to provide feedback regarding the screening intervention.
Patient Inclusion for Screening and Flagging
Patients included for this study were all those attending the outpatient clinics of ten medical oncologists at the BCCA from May to August 2011. Both current and new patients were considered.
Patients with upcoming appointments were identified by referring to the electronic clinic schedules of the participating medical oncologists. The EMRs of these patients were reviewed 1 business day before their clinic appointments by the clinical trial screening coordinator. Relevant screening information and results were manually extrapolated to the screening database for each screen. When a PEP was identified, the clinical trial screening coordinator flagged the patient using a notification report that included key eligibility criteria, and attached it to the hard-copy medical charts of the PEP for their medical oncologist to consider. We did not flag patients whose medical oncologists had explicitly noted their eligibility in the patients' EMR.
Statistical Analyses
Monthly accrual rates were collected for each of the study periods and converted to an accruals-per-clinic-appointment (APCA) ratio to control for variations in monthly clinic appointments. Comparisons were made between each study period using a negative-binomial regression model, which was generated with the APCA as the dependent variable and the three study periods as the predictor variable. Statistical analyses were performed using SAS v9.2.
Results
Screening
All 21 clinical trials included in the study remained open at least 4 months after the intervention period. Between May 1 and August 31, 2011, 2,098 patients were screened and 120 PEPs (5.7%) were identified and flagged for medical oncologists. By the end of the postintervention period, 15 PEPs were randomly assigned to a study identified by the flag, four were assigned to a different study, and four were offered but declined a referred study. In addition, several PEPs with breast cancer remained eligible for the referred trial, and may be offered those trials after completion of their adjuvant hormone therapy.
Overall Accrual
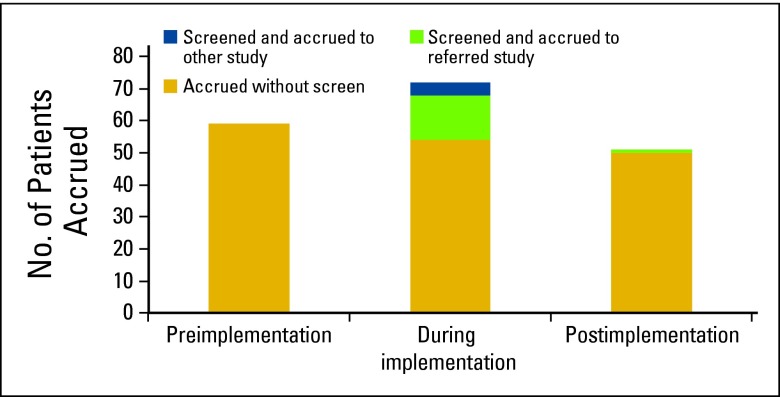
Four-month accruals for the 21 included clinical trials that occurred during the preintervention, intervention, and postintervention periods were 61, 73, and 51 patients respectively (Appendix Figure A2, online only). Of the 19 PEPs patients enrolled onto a clinical trial, 18 were accrued during the intervention period and one was accrued after the intervention.
Booking-adjusted accrual rates during each of the 4-month periods were: 0.00786 APCA preintervention, 0.00923 APCA during intervention, and 0.00641 APCA postintervention. Relative APCAs were 0.85 (95% CI, 0.67 to 1.06; P = .15) preintervention and 0.70 (95% CI, 0.54 to 0.90; P < .005) postintervention relative to the intervention period (Appendix Table A2, online only).
Physician Feedback
All participating medical oncologists responded to a survey during the intervention period, and a total of 33 completed surveys were returned (Table 1). Medical oncologists frequently reported that there was insufficient time to complete the surveys in clinic. On the basis of the survey results received, medical oncologists found that most of the PEP flags were accurate (n = 23; 69.7%) and helpful (n = 22; 66.7%). Medical oncologists also reported that they had previously considered the majority of identified PEPs (n = 24; 72.7%) for the recommended clinical trial. Medical oncologists found that most of the identified PEPs were suitable for the trial (n = 17; 51.5%); however, only a minority were initially identified as receptive (n = 5, 15.2%), and medical oncologists were unsure about how receptive most patients would be to trials (n = 27; 81.8%). Only one respondent (3%) identified the PEP as initially unreceptive to the recommended trial.
Table 1.
Questionnaire Results From Participating Oncologists
| Question and Answer | Frequency of Responses |
|
|---|---|---|
| No. | % | |
| Notification was accurate | ||
| Yes | 23 | 69.7 |
| No | 10 | 30.3 |
| Patient suitable for trial | ||
| Yes/potentially | 20 | 60.6 |
| No | 13 | 39.4 |
| Patient was considered before | ||
| Yes | 24 | 72.7 |
| No | 9 | 27.3 |
| Trial was described to patient by | ||
| Physician | 11 | 33.3 |
| None (yet) | 22 | 66.7 |
| Patient was receptive to trials | ||
| Yes | 5 | 15.2 |
| No | 1 | 3.0 |
| Maybe | 27 | 81.85 |
| Notification was helpful | ||
| Yes | 22 | 66.7 |
| No | 11 | 33.3 |
Discussion
During our 4-month intervention period of the patient chart screening intervention, the clinical trial screening coordinator manually screened 2,098 patient EMRs and identified 120 PEPs (5.7%) who had not yet been explicitly identified as eligible for clinical trials. Of the 19 PEPs enrolled onto a clinical trial, 18 were accrued during the intervention period, and one was enrolled in the 4 months after the end of the intervention. During the intervention period, 55 patients were enrolled without flagging; these patients were intentionally not flagged, as their medical oncologist had already demonstrated their awareness of the patient's eligibility for the study. A total of 18 additional patients were enrolled through flagging, which suggests that the intervention may have provided a relative improvement of up to 33% during the screening period. It should be noted that actual benefits as a result of the intervention may be more modest, as medical oncologists may already have identified some of the PEPs even before receiving the flag.
A portion of the PEPs identified were enrolled to studies other than the one identified by the flag; this may be attributed to patients being eligible for more than one open study. In these four cases, medical oncologists may have selected a different clinical trial for the patient on the basis of their own clinical experiences and careful consideration of the patient's disease and preferences. For the same reasons, the majority of the 97 patients who were flagged but not enrolled may have been unsuitable for the study identified (or clinical trials in general) for reasons not captured in the study eligibility criteria.
Our negative binomial regression found improved APCA during the intervention compared with the postintervention period (P = .05) and suggests a trend toward improved APCA during the preintervention period (P = .15). Over the three intervention periods, we also observed a decreasing trend for patients enrolled without requiring a flag (Appendix Figure A2). One feasible explanation was that the screening coordinator may have identified PEPs earlier than medical oncologists would have, which would result in a greater number of accruals during the intervention period, and fewer accruals during the postintervention period.
During the intervention period, all participating medical oncologists responded to the survey. As indicated by the feedback provided, oncologists were highly receptive to the screening intervention and reported that most of the notification reports were helpful and accurate. They also reported that most of the PEPs had been previously considered were suitable. At least one PEP was identified and enrolled during this intervention who was previously believed to be ineligible for the identified trial. Furthermore, some PEPs were identified who medical oncologists had not previously considered for the trial. Almost all PEPs identified were initially potentially receptive to clinical trials. Notifications identified as inaccurate were generally due to patients being flagged before they had experienced disease progression on their current line of therapy.
Some previous studies used automated e-Screening or clinical trial alert (CTA) systems to flag PEPs for noncancer trials.22–25 One such intervention targeted 10 endocrinologists and 104 general internists over 4 months.22 The CTA system increased accrual by up to 50% at some sites, but 90% of the 4,780 alerts generated were not acted on by physicians, and resulted in only 24 enrollments among 114 physicians; 0.5% of alerts led to enrollment, for an average of 0.21 patients enrolled per physician. During our manual chart screening intervention, 120 notifications for PEPs were issued to 10 oncologists, resulting in 19 enrolments; 15.8% of notifications led to patient enrolment, an average of 1.9 patients enrolled per-physician. Although these numbers are not directly comparable because of differences in physician specialization and study design, they suggest that the advantages of manual chart screening over CTA systems include greater numbers of patients enrolled per notification and greater improvements on the number of patients enrolled per physician. Disadvantages of manual chart screening compared with automated CTA systems include increased screening workload and reduced maximum capacity for number of patients screened per day.
The use of an e-Screening or CTA system in conjunction with manual chart screening may incorporate the advantages of both study designs. In our intervention, every patient with an upcoming appointment was manually screened. One recent study demonstrated that a well-designed e-Screening system could have a negative predictive value of 100%23; if a CTA system were used to exclude patients who do not meet basic inclusion criteria, this would reduce the number of charts for manual screening to manageable levels. The CTA system would allow the screening coordinator to identify more PEPs and include more clinical trials for manual screening. At the same time, because PEPs are still being manually identified, any notifications issued to physicians would retain significantly higher levels of accuracy and usefulness than alerts issued by the CTA system alone.
The major limitations of this study were its short duration and limited scale. Although this intervention would likely be applicable at most cancer care centers, we were unable to directly demonstrate this in our study. In addition, to ensure that we only included studies that were open throughout all three periods of our intervention, we could not include every trial available at the VCC. During our intervention, some trials closed, and others opened. This was anticipated, and these clinical trials were not included as part of our intervention. The duration of the intervention did not allow us to observe or adjust for any potential seasonal variations to accrual. The results of this study may also be influenced by physician selection bias and location effects. Although we selected physicians with a distribution of historical enrolment rates, our study did not analyze whether these enrollment rates were comparable to those of physicians from other centers. Similarly, there is no way for us to exclude center-specific effects, such as the availability of more clinical trials at BCCA, Vancouver Center relative to smaller centers.
On the basis of our experience with the screening intervention, we found that manually screening patient charts to determine patient eligibility for trials was labor intensive: even when excluding rescreens of patients who had previously been screened, the clinical trial screening coordinator would have screened an average of 125 charts every week over 4 months. Although medical oncologists found notifications for identified PEPs to be helpful and accurate, many considered the time required for this process to be prohibitive. Hiring a dedicated screener to screen patient charts for accrual to clinical trials has been shown to be effective and is likely to work in many different oncology clinic settings, including medical and radio-oncology contexts. The success of this small-scale pilot study demonstrates that a larger and more sophisticated chart screening intervention including both screening software and dedicated screening staff should be evaluated to confirm its effectiveness. This type of process will help to ensure that all eligible patients are offered the opportunity to participate in clinical studies.
Acknowledgment
Funding for this study was provided by the British Columbia Cancer Agency, Clinical Trials Unit. Presented in part at the American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 1-5, 2012.
We thank all clerks, data coordinators, and nurse coordinators who helped to introduce the clinical trial screening coordinator to clinical trial protocols. We would also like to offer special thanks to all 10 oncologists who participated and provided feedback for our intervention. Finally we would like to recognize and thank Vanessa Bernstein, MD, and Kim Chi, MD, Medical Director of Clinical Trials, Vancouver Center, BCCA, for their advice and input regarding the design of this intervention.
Appendix
Table A1.
Summary of the 21 Clinical Trials Included in the Study
| Cancer Stage and Study Phase | Study Phase | Line of Treatment | No. of Clinical Trials |
|---|---|---|---|
| Early stage | III | Second line only | 1 |
| Any line | 1 | ||
| Advanced | II | First line only | 3 |
| Second line only | 4 | ||
| Second line or later | 2 | ||
| Any line | 2 | ||
| III | First line only | 3 | |
| Second line only | 1 | ||
| Second line or later | 2 | ||
| IV | Second line only | 2 |
Table A2.
Accrual Rates by Period
| Intervention Period | No. of Accruals | NCA | APCA |
|---|---|---|---|
| Preintervention | 61 | 7,757 | 0.0079 |
| Intervention | 73 | 7,911 | 0.0092 |
| Postintervention | 51 | 7,951 | 0.0064 |
The total number of patients accrued to any of the 21 clinical trials included in this intervention at the VCC by the study period, relative to the number of bookings for eligible medical oncologists.
Abbreviations: APCA, accruals per clinic appointment; NCA, No. of clinic appointments.
Figure A1.
Distribution of the screening coordinator's time.
Figure A2.
Accrual outcomes by intervention period, showing the number of patients accrued to clinical trials.
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Leo Chen, Winson Y. Cheung, Hagen F. Kennecke
Financial support: Janice Grant, Hagen F. Kennecke
Administrative support: Janice Grant
Provision of study materials or patients: Winson Y. Cheung, Hagen F. Kennecke
Collection and assembly of data: Leo Chen
Data analysis and interpretation: Leo Chen, Winson Y. Cheung, Hagen F. Kennecke
Manuscript writing: Leo Chen, Winson Y. Cheung, Hagen F. Kennecke
Final approval of manuscript: All authors
References
- 1.National Cancer Institute. Clinical trials search results. //www.cancer.gov/clinicaltrials/search.
- 2.Go RS, Frisby KA, Lee JA, et al. Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer. 2005;106:426–433. doi: 10.1002/cncr.21597. [DOI] [PubMed] [Google Scholar]
- 3.Comis RL, Miller JD, Colaizzi DD, et al. Physician-related factors involved in patient decisions to enroll onto cancer clinical trials. J Clin Oncol. 2009;5:50–56. doi: 10.1200/JOP.0922001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 5.Ehrlich PF, Newman KD, Haase GM, et al. Lessons learned from a failed multi-institutional randomized controlled study. J Pediatr Surg. 2002;37:431–436. doi: 10.1053/jpsu.2002.30853. [DOI] [PubMed] [Google Scholar]
- 6.Haidich AB, Ioannidis JP. Effect of early patient enrolment on the time to completion and publication of randomized controlled trials. Am J Epidermiol. 2001;154:873–880. doi: 10.1093/aje/154.9.873. [DOI] [PubMed] [Google Scholar]
- 7.Padberg RM. Cancer clinical trial accrual: We have a problem. J Canc Educ. 2011;26:403–404. doi: 10.1007/s13187-011-0224-0. [DOI] [PubMed] [Google Scholar]
- 8.Kitterman DR, Cheng SK, Dilts DM, et al. The prevalence and economic impact of low-enrolling clinical studies at an academic medical center. Acad Med. 2011;86:1360–1366. doi: 10.1097/ACM.0b013e3182306440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20:2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 10.Lovato LC, Hill K, Hertert S, et al. Recruitment for controlled clinical trials: Literature summary and annotated biography. Lancet Oncol. 1997;18:328–352. doi: 10.1016/s0197-2456(96)00236-x. [DOI] [PubMed] [Google Scholar]
- 11.Scoggins JF, Ramsey SD. A national cancer clinical trials system for the 21st century: Reinvigorating the NCI cooperative group program. J Natl Canc Inst. 2010;102:1371. doi: 10.1093/jnci/djq291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroen AT, Petroni GR, Wang H, et al. Achieving sufficient accrual to address the primary endpoint in phase III clinical trials from U.S. Cooperative Oncology Groups. Clin Cancer Res. 2012;18:256–262. doi: 10.1158/1078-0432.CCR-11-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson SV, Momperousse D, Leventhal H. Physician perspectives on cancer clinical trials and barriers to minority recruitment. Cancer Contr. 2005;12(suppl 2):93–96. doi: 10.1177/1073274805012004S14. [DOI] [PubMed] [Google Scholar]
- 14.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: Identifying potential barriers to enrollment. J Clin Oncol. 2001;19:1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 15.Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: A meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7:141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 16.Umutyan A, Chiechi C, Beckett LA, et al. Overcoming barriers to cancer clinical trial accrual: Impact of a mass media campaign. Cancer. 2008;112:212–219. doi: 10.1002/cncr.23170. [DOI] [PubMed] [Google Scholar]
- 17.Benson AB, 3rd, Pregler JP, Bean JA, et al. Oncologists' reluctance to accrue patients onto clinical trials: An Illinois Cancer Center study. J Clin Oncol. 1991;9:2067–2075. doi: 10.1200/JCO.1991.9.11.2067. [DOI] [PubMed] [Google Scholar]
- 18.Ford E, Jenkins V, Fallowfield L, et al. Clinicians' attitudes towards clinical trials of cancer therapy. Br J Cancer. 2011;104:1535–1543. doi: 10.1038/bjc.2011.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins V, Farewell D, Batt L, et al. The attitudes of 1066 patients with cancer towards participation in randomized clinical trials. Br J Cancer. 2010;103:1801–1807. doi: 10.1038/sj.bjc.6606004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comis RL, Miller JD, Aldigé CR, et al. Public attitudes toward participation in clinical trials. J Clin Oncol. 2003;21:830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 21.Taylor KM, Feldstein ML, Skeel RT, et al. Fundamental dilemmas of the randomized clinical trial process: Results of a survey of the 1,737 Eastern Cooperative Oncology Group investigators. J Clin Oncol. 1994;12:1796–1805. doi: 10.1200/JCO.1994.12.9.1796. [DOI] [PubMed] [Google Scholar]
- 22.Embi PJ, Jain A, Clark J, et al. Effect of a clinical trial alert system on physician participation in trial recruitment. Arch Intern Med. 2005;165:2272–2277. doi: 10.1001/archinte.165.19.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thadani SR, Weng C, Bigger JT, et al. Electronic screening improves efficiency in clinical trial recruitment. J Am Med Inform Assoc. 2009;16:869–873. doi: 10.1197/jamia.M3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weng C, Batres C, Borda T, et al. A real-time screening alert improves patient recruitment efficiency. AMIA Annu Symp Proc. 2011;2011:1489–1498. [PMC free article] [PubMed] [Google Scholar]
- 25.Rollman BL, Fischer GS, Zhu F, Belnap BH. Comparison of electronic physician prompts versus waitroom case-finding on clinical trial enrolment. J Gen Intern Med. 2008;23(4):447–450. doi: 10.1007/s11606-007-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]