Abstract
We show the technical feasibility of coating and micro patterning boron-doped ultrananocrystalline diamond (UNCD®) on metal microwires and of applying them as microsensors for the detection of dopamine in vivo using fast-scan cyclic voltammetry. UNCD electrode surface consistently generated electrochemical signals with high signal-to-noise ratio of >800 using potassium ferrocyanide-ferricyanide redox couple. Parylene patterned UNCD microelectrodes were effectively applied to detect dopamine reliably in vitro using flow injection analysis with a detection limit of 27 nM and in the striatum of the anesthetized rat during electrical stimulation of dopamine neurons.
Advances in neurochemical monitoring have driving progress in establishing brain-behavior relationships, a major goal of the neurosciences. In this regard, fast-scan cyclic voltammetry (FSCV) affords a superior combination of temporal and chemical resolution compared to other electroanalytical techniques.1, 2, 3, 4, 5, 6, 7, 8 While FSCV is clearly state-of-the-art, much work remains to identify an equally effective microsensor. The current gold standard is the carbon-fiber microelectrode (CFM).9 The small size (∼5–10 μm diameter) of the CFM affords spatially resolved measurements and limits tissue damage.13 When combined with extended-scan FSCV, a detection limit of ∼15 nM is obtained for dopamine in the brain.10 Unfortunately, this increased sensitivity comes at the expense of reduced response time.11, 12 To overcome the diminution of function with chronic implantation,16 the typical approach is to lower a fresh CFM.14, 15 Replacing borosilicate glass with polyimide-coated fused silica extends the lifetime of chronic measurements with the CFM,17 but repeated measurements over several months is technically challenging. Thus, there is a pressing need to develop neurochemical probes that match or exceed the key characteristics of the CFM (e.g., response time, spatial resolution, sensitivity, and minimal tissue disruption) but in the form of a chronically implantable microelectrode.
Emerging carbon nanomaterials such as nanotubes,18 nanofibers,19 and micro-nanocrystalline diamond20, 21, 22 have spurred renewed interest in investigating new electrode material technology. Among them, conductive boron-doped diamond (BDD) exhibits excellent chemical, electrochemical, and bio compatibility.23, 24, 25 Ultrananocrystalline diamond (UNCD) with its as-deposited near atomic-scale smoothness (e.g., Ra of ∼1–5 nm, Root-Mean-Square, rms) is an excellent choice for neurochemical monitoring. In spite of its many advantages, mass-manufacturing is greatly hindered by the general incompatibility of diamond with wafer-scale fabrication technologies. In this communication, we report UNCD microwire microelectrodes for in vivo detection of dopamine, an important neurotransmitter in the functions of motor control, motivation, and cognition.26
Hot Filament Chemical Vapor Deposition (HFCVD) technique was used to coat a 1.5 μm thick boron-doped UNCD27, 28 on two configurations of metal microwires, viz., 50 μm diameter tantalum “Ta” (Alfa Aesar, Ward Hill, MA) and 125-μm diameter tungsten “W” with pre-sharpened tip of 1-μm diameter (MicroProbes for Life Science, Gaithersburg, MD) (Figure 1a). The rationale for using Ta is its electrochemical inertness and W is its small size that would permit implantation between capillaries without altering local brain blood circulation. UNCD film was synthesized by CH4/H2 gaseous mixture with a ratio of ∼5%. The boron doping was realized by introducing Trimethylboron (TMB) gas with a fixed C/H ratio of ∼3000 ppm. The film resistivity (∼0.04 Ω·cm) was measured by a 4-point probe from witness wafer (Pro4, Lucas Labs, Gilroy, CA). UNCD microsensor fabrication involves the following major process steps: (i) coat UNCD on microwire, (ii) insulate coated microwires with a 3 μm-thick Parylene-C, and (iii) micro pattern parylene through a laser etching process to create UNCD microelectrode “windows” of 250 μm long at the microwire tip (Figure 1b).
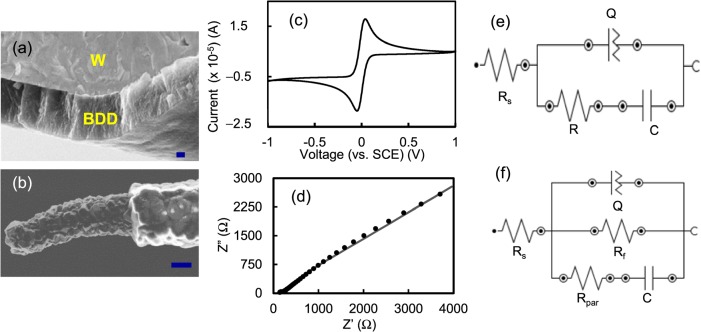
Figure 1.
SEM images of (a) Cross-section of BDD coated pre-sharpened tungsten microwire and (b) diamond microsensor after parylene coating and laser exposure. Scale bars: 200 nm and 10 μm, respectively. (c) Cyclic voltammogram of W-UNCD microelectrode (100 mV/s scan rate). ((d) and (e)) Nyquist plot of UNCD microelectrode (dotted curve-experimental and solid curve-fitted) and the equivalent circuit model. (f) Equivalent circuit fitted to EIS data of W-UNCD-parylene microelectrode after ECP.
Electrochemical properties of UNCD microsensor were evaluated using cyclic voltammetry (CV), differential pulse voltammetry (DPV), and electrochemical impedance spectroscopy (EIS) using an Autolab potentiostat (PGSTAT 302N, Metrohm, USA) in a two-electrode setup. A Pt coil (Alfa Aesar) was used as reference/counter electrode. The EIS spectra were recorded between 100 kHz and 100 mHz at 10 mV ac signal amplitude at OCP of 0.0 V. Measurements were carried out in a solution of 5 mM Fe(CN)63−/4− redox couple in 0.01 M PBS buffer unless otherwise specified. For flow injection analysis (FIA) of dopamine, measurements were collected in buffered physiological saline. The reference electrode was a chloridized silver wire (Ag/AgCl). For FSCV, a triangle wave from −0.4 to +1.0 V (normal scan) or −0.4 to +1.3 V (extended scan) and back was applied at a rate 300 V/s or 400 V/s every 100 ms. Injected at 0 s, the dopamine bolus was 1 or 10 μM for 10 s. Current was monitored across the peak oxidative potential for dopamine (+0.6 V). Buffer flow rate was 3 ml/min. For in vivo dopamine measurements in the urethane-anesthetized rat, all FSCV curves were collected in the striatum during electrical stimulation of ascending dopamine axons in the medial forebrain bundle. Data are collected from a single rat. Recordings at the CFM and UNCD microsensors are evoked by the same stimulus train and were collected concurrently at different striatal locations.
Cyclic voltammogram of as-deposited UNCD microwires showed a quasi-reversible behavior, where the separation in reduction-oxidation “redox” peak potential (ΔEp) of Fe(CN)63−/4− was 85 mV (Figure 1c), which is similar to literature values.27 The ΔEp is a measure of electron transfer rate and its value depends on the type of redox species. The ΔEp value of Fe(CN)63−/4− at the diamond surface is dependent on the amount of surface oxygen. The low value of 85 mV, (i.e., faster electron transfer rates) suggests that surface oxygen-containing groups such as hydroxyl or carboxyl groups are less. The electron transfer rate at the diamond electrodes is generally slower than that of a conventional carbon electrode for several analytes because BDDs mainly consists of sp3 bonded grains and have few oxygen-containing groups. The high rates observed at the UNCD surface suggests that it might be due to high boron doping used to grow the film or the surface contain non-diamond carbon impurities, primarily at the grain boundaries. The excellent signal-to-noise (S/N) ratio of >800 obtained from differential pulse voltammograms (data not shown) demonstrates that coating was highly conformal and pin-hole free (also confirmed from SEM images). EIS spectrum showed that the overall UNCD coating on W is uniform and homogenous (dotted curve, Figure 1d). Experimental data fit well to an equivalent circuit model [Rs(Q[RC])] that has one constant phase element CPE (Q) (Figure 1e). However, grain and grain boundary configuration of the UNCD film give rise to slight morphological variation on the electrode surface. Since there are differences in the physical and chemical properties of the grain-grain boundary, each gives rise to unique electrochemical properties, (i.e., grains showing a pure capacitive behavior and grain boundaries showing a CPE behavior).29 The solution resistance (Rs) is 106 Ω. The film resistance of 1 KΩ value is higher than that of the conventional electrode materials, but it is adequate for neurochemical sensing. The capacitance arising from diamond grains is ∼5.2 μF and the CPE values arising from grain boundaries are Y (admittance) = 252 μMho and N = 0.4.
The high S/N ratio observed on diamond surfaces was diminished when they were coated with parylene and micro patterned using the laser etch process to create the “windows” (e.g., the S/N ratio decreased to ∼6; data not shown). This might be due to several factors such as (i) the presence of “polymer” parylene residue that deactivates the electrode activity, (ii) surface damage that increases the film resistance, (iii) exposure of underlying metal substrate that increases the background current, or (iv) combination of the above. The electrode surface was electrochemically pretreated (Electrochemical Pretreatment, ECP, −5.0 V for 6 min in PBS buffer) to remove the residue. The ECP helped in increasing the S/N ratio increased from 6 to 25. But additional ECPs did not improve the ratio any further. The EIS studies confirm the influence of the above factors on electrode characteristics. The data were fitted to an equivalent circuit as shown in Figure 1f. The solution resistance is 1120 Ω. The diamond surface became more resistive (Rf, 750 KΩ), suggesting some surface damage during laser exposure. The CPE values arising from grain boundaries are Y = 13 nMho and N = 0.8. The high resistance (Rpar, 2430 KΩ) and low capacitance (C = 96 nF) confirms the presence of adequate insulation from parylene. Additional process steps such as optimizing ECP protocol, minimizing surface damage or diamond loss during laser etch and introducing post-plasma cleaning could further improve S/N ratio.
To investigate the electrode characteristics of UNCD in FIA and in vivo analysis, the general strategy used was to directly compare UNCD microelectrodes with a CFM. Initial testing used FIA, which is well established for high-throughput assessment of microsensor function in vitro. Fruitful in vitro testing of UNCD microelectrodes was followed up by measurements of dopamine in the brain of the urethane-anesthetized rat. Two different configurations of UNCD microelectrodes were tested with FIA. The first configuration was a UNCD microelectrode constructed on 50-μm diameter Ta wires with micro patterned parylene. This configuration provided adequate sensitivity to dopamine, because a bolus concentration of 1 μM was detected, and relatively featureless background cyclic voltammograms were obtained (data not shown). This configuration was also deemed suitable for implanting in the rat brain for measuring dopamine. In this procedure, microsensors are implanted in the dopamine-rich striatal region of the forebrain and a stimulation electrode is implanted in the medial forebrain bundle to electrically activate action potentials in dopamine neuronal axons. These action potentials are conducted to dopamine terminals in the striatum and cause the exocytotic release of dopamine into extracellular fluid. Initial experiments yielded no measurements of dopamine at UNCD microsensors, although a robust response was concurrently recorded at a CFM. However, considering that the large size of UNCD microsensors may produce trauma to the adjacent tissue and specifically prevent dopamine release locally, subsequent measurements in the presence of a drug to inhibit dopamine uptake, and promote dopamine diffusion from intact tissue into the traumatized tissue, yielded robust responses at both the CFM and 50-μm diameter Ta-UNCD-parylene microsensor (Figure 2). These in vitro and in vivo results demonstrate proof of concept for a UNCD microsensor for brain dopamine measurements. To address the issue of size, the second configuration used a UNCD-coated, pre-sharpened tungsten wire. As in the other configuration, parylene provided insulation and the UNCD surface was exposed by laser etching. This optimal configuration provided the best response to 1 μM dopamine with FIA of the two configurations tested. The un-optimized diamond microsensor demonstrates a detection limit of 67 nM (i.e., signal-to-noise ratio of 5:1). To further improve the detection sensitivity of diamond microsensors that can measure dopamine at physiologically relevant concentrations of >15 to 30 nM, the microelectrode was subjected to a UV treatment after ECP cycling (−1.0 to +2.0 V in 0.1 M sulfuric acid). This combination of ECP-UV treatment primarily reduces surface sp2 bonds and introduces oxygen-containing surface groups. It is known in the literature that ECP on graphite electrodes increased the π − π interaction between the sp2 bonds and polar aromatic dopamine, which improved dopamine adsorption and detection sensitivity.29 On the contrary; ECP on BDD increased ΔEp substantially because of a decrease in the amount of surface sp2 bonds. Interestingly, in this study, we found improvements in sensitivity after the ECP-UV treatment. There was a marginal increase in ΔEp of dopamine from 515 to 595 mV, but a significant raise in the oxidation peak current from 8.3 to 12.3 nA, i.e., a 45% increase in detection sensitivity and a substantial decrease in the background current (data not shown). These newly treated microelectrodes were then tested for dopamine monitoring with FSCV using FIA and compared directly to the CFM. As shown in Figure 3, the UNCD electrode and CFM exhibited similar detection limits for dopamine (∼27 nM) at normal scan parameters (−0.4 to 1.0 V at 300 V/s). Moreover, response times for the UNCD electrodes were vastly improved, with the CFM only slightly faster. Improved detection limits for dopamine are achieved at a CFM by using extended scan parameters (−0.4 to 1.3 V at 400 V/s) to over oxidize the carbon surface and render it more amenable to DA adsorption.11 Indeed, these parameters further reduced the detection limit for dopamine at the CFM to 11 nM. We have previously demonstrated that the extended scan does not improve the performance of the UNCD electrode and this was also the case here. However, with the improved surface treatment protocol, these data reduce the difference in detection limits for dopamine between the CFM and UNCD from 7.5-fold to only 2.5-fold (11 versus 27 nM, respectively). The current work in our laboratory is directed at eliminating this difference and perhaps exceeding the detection limit of the CFM by even more robust UV- and plasma treatment to render the electrode surface more amenable to dopamine adsorption in an analogous manner to that used for the CFM for which over oxidizing with the extended scan results in a CFM detection limit of ∼10 to 20 nM for dopamine. We believe a more robust UV- and plasma treatment should offer a cleaner diamond surface with further reductions in detection limit accompanied by improvements in response time.
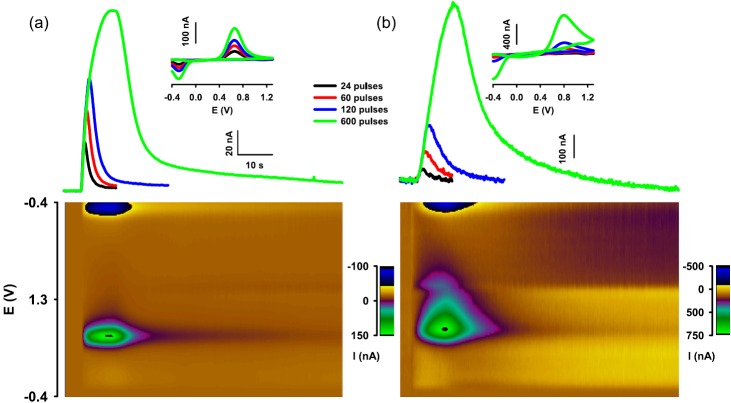
Figure 2.
Dopamine measurements in the anesthetized rat ((a)—CFM and (b)—UNCD)). Data are from a single rat after pharmacological inhibition of dopamine uptake with nomifensine (20 mg/kg i.p.). The pseduocolor plot underneath the curves shows all background subtracted voltammograms collected for the recording evoked by the 10 s stimulus train. Features indicate dopamine for both recordings. The UNCD microelectrodes was constructed from a 50 μm diameter Ta wire, insulated with parylene, and laser etched. Inset background-subtracted cyclic voltammograms.
Figure 3.
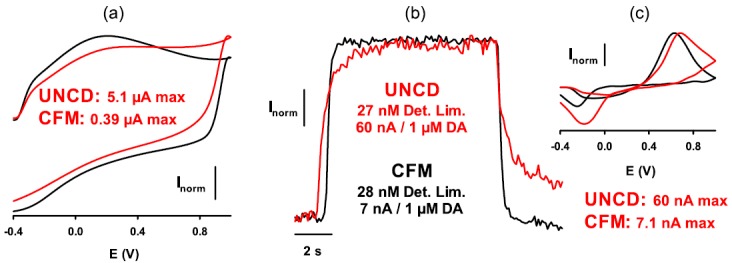
Comparison of the CFM (black lines and text) and UNCD microelectrode (red lines and text) for measuring dopamine (DA) with FIA using normal scan parameters. (a) Background cyclic voltammogram. Maximal (max) current for each voltammogram is given in μA. (b) Response to a 10 s, 1-μM injection of DA. The detection limit (Det. Lim.) in nM and sensitivity in nA/μM is given for each electrode. (c) Background-subtracted cyclic voltammogram for DA. Maximal (max) current for each voltammogram is given in nA. In all panels, current is normalized (Inorm) to maximal current.
In summary, we demonstrate a UNCD-coated, parylene-insulated tantalum metal microwire that is capable of measuring dopamine with FSCV. The sensing-UNCD surface was micro patterned by laser exposure. Moreover, this configuration for a UNCD microsensor was capable of monitoring extracellular dopamine in real-time in the rat striatum in vivo; therefore, it is viable as a brain-implantable sensor. Second, to reduce size, we also demonstrated a similar UNCD-coated, parylene-insulated, laser-exposed microsensor, but fashioned from a pre-sharpened tungsten microwire. This microsensor, with a form factor at its tip comparable to the CFM, exhibited a detection limit of 27 nM for dopamine, approaching that achieved with a CFM. These results therefore demonstrate proof of concept for realizing the analytical advantages of the CFM but in the form of a UNCD microsensor. Third, we addressed the manufacturability issue by developing a baseline process for reliably fabricating UNCD microsensors. The relative ease by which the proposed single UNCD microelectrodes can be configured as a microelectrode array akin to the highly popular microwire arrays used in neural stimulation and bioelectric recording represents a good first step to monitoring multiple neurochemicals in vivo.
Acknowledgments
This work was funded by National Institute of Mental Health (1R43MH094121-01 award to ADT, Inc., and ISU). We thank Grace Catausan and Dale McClure from ADT, Inc., for assisting in diamond coating.
References
- Sandberg S. G. and Garris P. A., “ Neurochemistry of addiction: Monitoring essential neurotransmitters of addiction,” in Advances in the Neuroscience of Addiction, edited by Kuhn C. M. and Koob G. F. (CRC Press, Boca Raton, FL, 2010), pp 101–136. [PubMed] [Google Scholar]
- Pihel K., Hsieh S., Jorgenson J. W., and Wightman R. M., Anal. Chem. 67, 4514 (1995). 10.1021/ac00120a014 [DOI] [PubMed] [Google Scholar]
- Baur J. E., Kristensen E. W., May L. J., Wiedemann D. J., and Wightman R. M., Anal Chem. 60, 1268 (1988). 10.1021/ac00164a006 [DOI] [PubMed] [Google Scholar]
- Borland L. M. and Michael A. C., “ An introduction to electrochemical methods in neuroscience,” in Electrochemical Methods for Neuroscience, edited by Michael A. C. and Borland L. M. (CRC Press, Boca Raton, 2007), pp 1–15. [Google Scholar]
- Cechova S. and Venton B. J., J. Neurochem. 105, 1253 (2008). 10.1111/j.1471-4159.2008.05223.x [DOI] [PubMed] [Google Scholar]
- Park J., Wheeler R. A., Fontillas K., Keithley K. B., Carelli R., and Wightman R. M., Biol. Psychiatry 71, 327 (2012). 10.1016/j.biopsych.2011.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips P. E., Stuber G. D., Heien M. L., Wightman R. M., and Carelli R., Nature 422, 614 (2003). 10.1038/nature01476 [DOI] [PubMed] [Google Scholar]
- Brown H. D., McCutcheon J. E., Cone J. J., Ragozzino M. E., and Roitman M. F., Eur. J. Neurosci. 34, 1997 (2011). 10.1111/j.1460-9568.2011.07914.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill P. S., Walker Q. D., Finnegan J. M., Mickelson G. E., Travis E. R., and Wightman R. M., Anal. Chem. 68, 3180 (1996). 10.1021/ac960347d [DOI] [PubMed] [Google Scholar]
- Robinson D. L. and Wightman R. M., “ Rapid dopamine release in freely moving rats,” in Electrochemical Methods for Neuroscience, edited by Michael A. C. and Borland L. M. (CRC Press, Boca Raton, 2007), pp. 17–34. [PubMed] [Google Scholar]
- Heien M. L., Phillips P. E., Stuber G. D., Seipel A. T., and Wightman R. M., Analyst 128, 1413 (2003). 10.1039/b307024g [DOI] [PubMed] [Google Scholar]
- Mitala C. M., Wang Y., Borland L. M., Jung M., Shand S., Watkins S., Weber S. G., and Michael A. C., J. Neurosci. Methods 174, 177 (2008). 10.1016/j.jneumeth.2008.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland L. M. and Michael A. C., J. Neurochem. 91, 220 (2004). 10.1111/j.1471-4159.2004.02708.x [DOI] [PubMed] [Google Scholar]
- Clapp-Lilly K. L., Roberts R. C., Duffy L. K., Irons K. P., Hu Y., and Drew K. L., J. Neurosci. Methods 90, 129 (1999). 10.1016/S0165-0270(99)00064-3 [DOI] [PubMed] [Google Scholar]
- Garris P. A., Kilpatrick M., Bunin M. A., Michael D., Walker Q. D., and Wightman R. M., Nature 398, 67 (1999). 10.1038/18019 [DOI] [PubMed] [Google Scholar]
- Kruk Z. L., Cheeta S., Milla J., Muscat R., Williams J. E., and Willner P., J. Neurosci. Methods 79, 9 (1998). 10.1016/S0165-0270(97)00156-8 [DOI] [PubMed] [Google Scholar]
- Clark J. J., Sandberg S. G., Wanat M. J., Gan J. O., Horne E. A., Hart A. S., Akers C. A., Parker J. G., Willuhn I., Martinez V., Evans S. B., Stella N., and Phillips P. E., Nat. Methods 7, 126 (2010). 10.1038/nmeth.1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs C. B., Vickrey T. L., and Venton B., Analyst 136, 3557 (2011). 10.1039/c0an00854k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehne J. E., Marsh M., Boakye A., Douglas B., Kim Y., Chang S.-Y., Jang D.-P., Bennet K. E., Kimble C., Andrews R., Meyyappan M., and Lee K. H., Analyst 136, 1802 (2011). 10.1039/c1an15025a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. B., Xie S., Shafer G., and Wilson C. G., Diamond Relat. Mater. 15, 225 (2006). 10.1016/j.diamond.2005.08.018 [DOI] [Google Scholar]
- Swain G. M., Park J., Mocko V. Q., Peckova K., Galligan J. J., and Fink G. D., Diamond Relat. Mater. 15, 761 (2006). 10.1016/j.diamond.2005.11.008 [DOI] [Google Scholar]
- Zhao H. X., Galligan J. J., and Swain G. M., Diamond Relat. Mater. 19, 182 (2010). 10.1016/j.diamond.2009.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvacka J., Quaiserova V., Park J., Show Y., Muck A., and Swain G. M., Anal. Chem. 75, 2678 (2003). 10.1021/ac030024z [DOI] [PubMed] [Google Scholar]
- Suzuki A., Ivandini T. A., Yoshimi K., Fujishima A., Oyama G., Nakazato T., Hattori N., Kitazawa S., and Einaga Y., Anal. Chem. 79, 8608 (2007). 10.1021/ac071519h [DOI] [PubMed] [Google Scholar]
- Yang W., Auciello O., Butler J. E., Cai W., Carlisle J. A., Gerbi J. E., Gruen D. M., Knickerbocker T., Lasseter T. L., Russell J. N., Smith L. M., and Hamers R. J., Nature Mater. 1, 253 (2002). 10.1038/nmat779 [DOI] [PubMed] [Google Scholar]
- Iversen S. D. and Iversen L. L., Trends Neurosci. 30, 188–193 (2007). 10.1016/j.tins.2007.03.002 [DOI] [PubMed] [Google Scholar]
- Granger M. C., Witek M., Xu J., Wang J., Hupert M., Hanks A., Miles M. D., Butler J. E., Lucazeau G., Mermoux M., Strojek J. W., and Swain G. M., Anal. Chem. 72(16), 3793 (2000). 10.1021/ac0000675 [DOI] [PubMed] [Google Scholar]
- Siddiqui S., Dai Z., Stavis C. J., Zeng H., Moldovan N., Hamers R. J., Carlisle J. A., and Arumugam P. U., Biosens. Bioelectron. 35, 284 (2012). 10.1016/j.bios.2012.03.001 [DOI] [PubMed] [Google Scholar]
- Sekioka N., Katob D., Uedab A., Kamatad T., Kuritab R., Umemurad S., Hironoe S., and Niwa O., Carbon 46, 1918 (2008). 10.1016/j.carbon.2008.08.006 [DOI] [Google Scholar]