Abstract
RAI14 (retinoic acid induced protein 14) is an actin-binding protein first identified in the liver. In the testis, RAI14 is expressed by both Sertoli and germ cells in the seminiferous epithelium. Besides binding to actin in the testis, RAI14 is also a binding protein for palladin, an actin cross-linking and bundling protein. A recent report has shown that RAI14 displays stage-specific and spatiotemporal expression at the ES [ectoplasmic specialization, a testis-specific filamentous (F)-actin-rich adherens junction] in the seminiferous epithelium of adult rat testes during the epithelial cycle of spermatogenesis, illustrating its likely involvement in F-actin organization at the ES. Functional studies in which RAI14 was knocked down by RNAi in Sertoli cells in vitro and also in testicular cells in vivo have illustrated its role in conferring the integrity of actin filament bundles at the ES, perturbing the Sertoli cell tight junction (TJ)-pemeability barrier function in vitro, and also spermatid polarity and adhesion in vivo, thereby regulating spermatid transport at spermiation. Herein, we critically evaluate these earlier findings and also provide a likely hypothetic model based on the functional role of RAI14 at the ES, and how RAI14 is working with palladin and other actin regulatory proteins in the testis to regulate the transport of (1) spermatids and (2) preleptotene spermatocytes across the seminiferous epithelium and the blood-testis barrier (BTB), respectively, during spermatogenesis. This model should serve as a framework upon which functional experiments can be designed to better understand the biology of RAI14 and other actin-binding and regulatory proteins in the testis.
Keywords: testis, RAI14, F-actin, spermatogenesis, ectoplasmic specialization, adherens junction, blood-testis barrier, spermatid adhesion
Introduction
RAI14 (retinoic acid induced protein 14) is a 110 kDa adaptor protein and a member of the growing RAI protein family. RAI1 (retinoic acid induced 1) is the first member and the best studied gene/protein of this growing family identified in the early 2000s.1,2 In humans, RAI1 gene is located on chromosome 17 at 17p11.2, inducible by retinoic acid and highly expressed in neuronal tissues.3,4 Based on its primary sequence, RAI1 does not contain potential membrane-spanning hydrophobic domains and as such, it is a cytosolic protein; however, it has a distinctive polymorphic polyglutamine tract near its NH2- terminus.3,4 Its mutation and/or deletion in humans leads to a complex neurobehavioral disorder known as Smith-Magenis syndrome (SMS),4,5 and also associated with schizophrenia2 and spinocerebellar ataxia type 2 (SCA2),6 whereas its duplication causes autism7 and Potocki-Lupski syndrome,8 illustrating its unique and physiological significance in the brain and neuronal function.
In early 2000s, RAI14 was independently found in retinal pigment epithelium designated NORPEG (novel retinal pigment epithelial cell protein) whose function was not known at the time;9,10 and also in the liver named ankycorbin (ankyrin repeat and coiled-coil structure-containing protein).11 RAI14 is an actin-binding protein in the liver.11 This information thus provides us with a clue on the likely function of this member in the RAI family proteins. RAI14 was subsequently found in human testes and highly expressed by human spermatozoa.12 Other studies have shown that RAI14 is expressed in many mammalian tissues and cells, but most predominantly in retina, placenta and testes.9,10 In humans, RAI14 has three isoforms produced by alternative splicing. Unlike RAI1, RAI14 serves as an adaptor and scaffold protein, associated with cortical actin cytoskeleton, F-actin stress fibers and the cell-cell adhesion sites.11 It has three ankyrin repeat domains near its NH2-terminus. An ankyrin repeat is a 33-residue motif composed of two α helices separated by loops, and these ankyrin domains are crucial to induce protein-protein interactions and signal transduction.13-15 These findings thus illustrate that RAI14 has the ability to recruit multiple signaling and regulatory proteins to F-actin via its ankyrin repeat domains to regulate multiple cellular functions, in particular F-actin cytoskeleton dynamics. In fact, RAI14 is a putative binding partner of 14-3-3, and this protein complex together with hundreds of partner proteins are known to be involved in cytoskeletal regulation and cellular organization,16 since 14-3-3 was shown to have an extensive list of binding partners.41
In this commentary, we critically evaluate the function of RAI14 in maintaining the homeostasis of F-actin network in the testis via its effects on a testis-specific and F-actin-rich ultrastructure known as ectoplasmic specialization (ES) in the seminiferous epithelium during the epithelial cycle of spermatogenesis. We also evaluate the likely role of RAI14 in coordinating with other actin regulatory proteins in F-actin organization at the Sertoli-Sertoli and Sertoli-spermatid interface during spermatogenesis based on a recently published report.17
RAI14 is an integrated component of the ectoplasmic specialization (ES)
In the testis, cell-cell interface between Sertoli cells in the seminiferous epithelium near the basement membrane creates a unique cell-tissue barrier known as the blood-testis barrier (BTB).18-20 The BTB is one of the tightest blood-tissue barriers since its discovery more than a century ago (for reviews, see refserences 19‒21). Subsequent studies have shown that the BTB, unlike all other blood-tissue barriers (e.g., the blood-brain and the blood-retina barriers) are composed of tight junctions (TJ), which are reinforced by bundles of actin filaments that are tightly packed and sandwiched in-between cisternae of endoplasmic reticulum and the Sertoli cell plasma membrane, and lying perpendicular to the plasma membrane.22 This unique F-actin-rich ultrastructure that co-exists with TJ and gap junction is the ectoplasmic specialization (ES), which together with the desmosome, create the BTB (for reviews, see references 20 and 23). Furthermore, ES was also found at the Sertoli-spermatid interface in the rodent testis but limited only to step 8–19 spermatids in the rat testis,24,25 which shares almost identical morphological features of the ES at the BTB. Thus, the ES at the Sertoli-spermatid and at the Sertoli cell-cell interface was designated apical and basal ES, respectively,26,27 since the former is restricted to the apical (adluminal) and the latter is only found at the basal compartment of the seminiferous epithelium. The only ultrastructural difference between the basal and the apical ES is that the actin filament bundles are found on both sides of the adjacent Sertoli cells at the basal ES, whereas these actin filament bundles are limited to the Sertoli cell at the apical ES. Since the discovery of the ES in the testis in the late 1970s, there are virtually no functional studies in the field to explore the role of the F-actin network at the ES except it was shown that these actin filaments confer the unusual adhesive strength to the ES28 and it is necessary for germ cell transport across the epithelium.29 However, it is conceivable that the F-actin network must undergo continuous re-organization from their “bundled” to their “de-bundled/branched” configuration to facilitate spermatid transport across the epithelium during spermiogenesis, as well as the transport of preleptotene spermatocytes across the BTB at stage VIII of the epithelial cycle.
Studies in the past decade have demonstrated the presence of a number of actin regulatory proteins at the ES (Table 1). Thus, actin filaments can be rapidly re-organized from their “bundled” to their “debundled” configuration and vice versa via the concerted efforts of these regulatory proteins mediated by their intrinsic activities (Table 1). In short, it is now generally accepted that the actin filament bundles at the basal ES and the apical ES can be rapidly converted from a “bundled” to a “de-bundled/branched” configuration30,31 via the intrinsic activity of Eps8 (epidermal growth factor receptor pathway substrate 8, an actin barbed end capping and bundling protein)32 and palladin (an actin cross-linking and bundling protein)33 versus Arp3 [actin-related protein 3, which together with Arp2 forms the Arp2/3 complex and when this complex is activated by N-WASP (neuronal Wiskott-Aldrich syndrome protein), it induces branched actin polymerization, causing barbed end branching of an existing actin filament to generate a branched network, effectively “de-bundling” actin filaments].34
Table 1. Actin-binding and regulatory proteins in the rat testis.
| Name of protein | Mr (kDa) | Function(s) | Expression by | Stage-specific expression in | Reference | |||
|---|---|---|---|---|---|---|---|---|
| |
|
|
SC |
GC |
PMO |
Apical ES |
Blood-testis barrier (Basal ES) |
|
| RAI14 |
110 |
Actin binding |
+ |
+ |
+ |
Low in VI, highest in VII, diminished in VIII, moderate in IX-XIV |
VIII-XII |
Qian et al. 201317 |
| Palladin |
95 |
Actin cross-linking / bundling |
+ |
+ |
+ |
Low in I-III, high in IV-VII, diminished in VIII, low in IX-XIV |
High in V-VI, diminished in VII-VIII |
Qian et al. 201333 |
| Eps8 |
97 |
Actin barbed end capping and bundling |
+ |
+ |
- |
High in V-VII, diminished in VIII |
High in V-VI, diminished in VII-VIII |
Lie et al. 200932 |
| Arp3 |
45 |
Actin nucleation and branching |
+ |
+ |
- |
Highest in VI-VII diminished in VIII |
Barely detected in VI-VII, abundant in VIII |
Lie et al. 201034 |
| Drebrin E |
110 |
Actin and Arp3 binding protein |
+ |
- |
- |
Low in V-VI, highest in VII, diminished in VIII |
Highest in V-VI, diminished in VII-VIII |
Li et al. 201136 |
| Filamin A |
280 |
Actin cross-linking/ branching |
+ |
- |
+ |
Not detectable |
Predominantly expressed at the BTB during its postnatal assembly |
Su et al. 201239 |
| Bcrp | 70 | Eps8/Arp3/Actin binding protein | + | + | + | Only in VI-VIII, highest in VII | Not present at the SC BTB | Qian et al. 201340 |
SC, Sertoli cells; GC, germ cells; PMO, peritubular myoid cells; ES, ectoplasmic specialization; BTB, blood-testis barrier; +, presence; -, absence.
To this growing list of actin binding and regulatory proteins shown in Table 1, RAI14 is an important player in regulating F-actin organization at the ES in the adult rat testis.17 RAI14 displays spatiotemporal and stage-specific expression at the apical and the basal ES in the seminiferous epithelium.17 RAI14 is not detectable in other parts of the seminiferous epithelium except the ES; however, it is not expressed at the apical ES at stage I‒VI of the cycle. At stage VII, it is highly expressed at the apical ES, intensely localized to the tip of the spermatid head, and co-localized with F-actin.17 Its expression is also detectable at the basal ES in the BTB, but at a level considerably less than the apical ES at stage VII; however, RAI14 expression is considerably high at the basal ES and co-localized with F-actin at the BTB in stage VIII tubules.17 Interestingly, its expression at the apical ES begins to diminish considerably at stage VIII of the epithelial cycle, no longer tightly restricted to the tip of the spermatid head but it gradually diffuses away and only partially co-localized with F-actin at the apical ES in early stage VIII of the epithelial cycle.17
RAI14 is involved in maintaining the actin filament bundles at the ES during the epithelial cycle of spermatogenesis
This pattern of restrictive localization and expression of RAI14, most notably at the apical and the basal ES in stage VII‒VIII tubules, plus its co-localization with F-actin at these sites, strongly suggest a role of RAI14 on F-actin organization at the ES. It is apparent that RAI14 exerts its effects in maintaining the organization of the F-actin network at the ES. This conclusion is reached based on the following observations. First, RAI14 was found to structurally interact with palladin, an actin cross-linking and bundling protein,17,33,35 but not Arp3, Eps8 or drebrin E (an actin-binding protein that recruits Arp3 to the ES in rat testes36),17 illustrating it may recruit palladin to the specific cellular domain to regulate the integrity of the actin filament bundles at the ES. Furthermore, RAI14 localized almost superimposable with F-actin filaments in Sertoli cells cultured in vitro,17 possibly by recruiting palladin to maintain the homeostasis of actin filament bundles at the ES. In fact, RAI14 was found to co-localize almost superimposable with palladin, but only partially with Arp3 and drebrin E, at the apical ES in stage VII tubules.17 Second, the notion that RAI14 is involved in regulating actin filament bundles at the ES is supported by findings that the knockdown of RAI14 in Sertoli cells cultured in vitro with an established TJ-permeability barrier that mimicked the BTB in vivo was shown to induce a dis-organization of F-actin in these cells, impeding the distribution of the basal ES proteins β- and γ-catenins, thereby perturbing the Sertoli cell TJ-permeability barrier.17 Lastly, a significant downregulation on the expression of RAI14 at the apical ES was detected in the seminiferous epithelium from rats treated with adjudin, a potential male contraceptive known to induce spermatid loss from the epithelium by disrupting apical ES,37 which was accompanied by a significant decline in the association of RAI14 with actin when examined by co-immunoprecipitation.17 Taken collectively, these observations thus demonstrate unequivocally that RAI14 is an integrated component of the ES, and it is involved in maintaining the integrity of the actin filament bundles at the ES since its loss, either induced by RNAi or adjudin, leads to a disruption of spermatid or Sertoli cell adhesion because the “de-bundled” actin filaments at the ES no longer support cell adhesive function at the apical or basal ES.
RAI14 regulates spermatid polarity and spermatid transport during spermiogenesis
As summarized above, RAI14 regulates ES function via its promoting effects on the integrity of the actin filament bundles at the ES, perhaps mediated by its binding partner palladin. The concept is further supported by studies in vivo by silencing RAI14 using RNAi.17 For instance, a knockdown of RAI14 at the apical ES led to a reduced F-actin at the site in stage VII tubules when F-actin was visualized by FITC-phalloidin, and the remaining F-actin no longer restricted tightly surrounding the tip of spermatid heads, instead, it was shifted to the convex (dorsal) side of spermatid heads and toward the base of spermatid heads, nearing their mid-piece.17 Furthermore, palladin was also mis-localized and its expression was downregulated at the apical ES, no longer restricted to the tip of spermatid heads but localized diffusely, covering other parts of the spermatid head.17 These changes thus led to a loss of spermatid polarity and the heads of many spermatids no longer pointed toward the basement membrane, instead they aligned randomly, deviated by as much as 90° to 180° from their intended orientation.17 Furthermore, many spermatids were found to remain entrapped deep inside the seminiferous epithelium in stage VIII tubules when they should have been aligned at the luminal edge near the tubule lumen to prepare for spermiation, leading to defects in spermiation.17 These latter findings also support the notion that the underlying mechanism(s) that regulates spermatid transport has been compromised due to a defect in F-actin re-organization at the apical ES following the knockdown of RAI14 in the testis in vivo, such that the timely conversion of the actin filament bundles at the apical ES from their “bundled” to their “de-bundled/branched” configuration was disrupted.
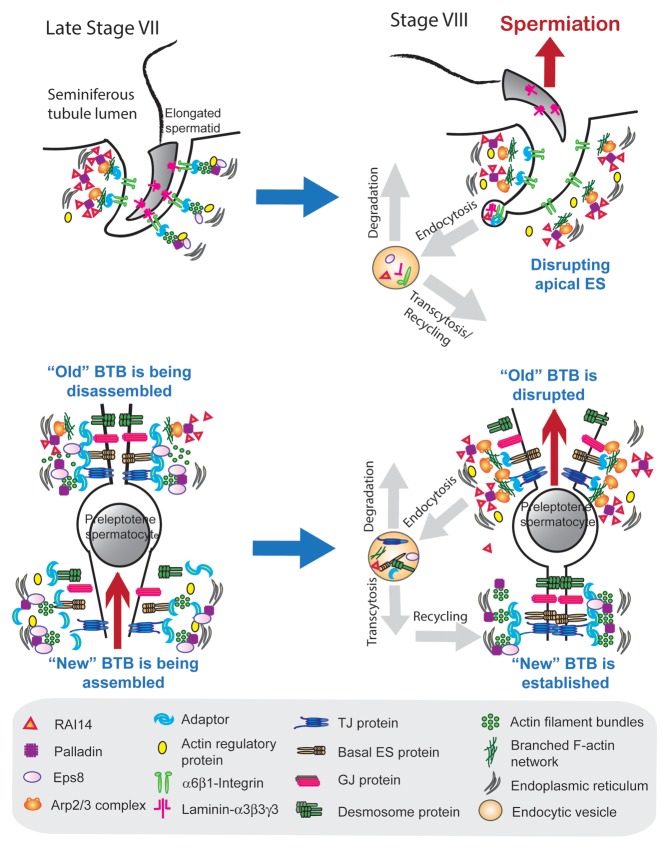
Collectively, these findings thus prompt us to conclude that RAI14 is working in concert with its binding partner palladin, an actin cross-linking and bundling protein, to maintain the dynamics of actin filament bundles at the ES. The RAI14/palladin complex is crucial to give the F-actin network its plasticity by facilitating its conversion between its “bundled” and “de-bundled” configuration via the intrinsic activity of palladin during the epithelial cycle to facilitate the transport of spermatids across the epithelium as well as the transport of preleptotene spermatocytes across the BTB at the apical and the basal ES, respectively. Thus, RAI14, besides working with its partner palladin, is likely to work in concert with other actin-binding and regulatory proteins at the ES (Table 1) to facilitate these events (Fig. 1). For instance, during the disruption of the apical and the basal ES at spermiation and the transit of preleptotene spermatocytes at stage VIII, respectively, the stage-specific and spatiotemporal expression of RAI14 at these sites that binds to palladin can either pull palladin away from these sites and/or by limiting the intrinsic actin filament cross-linking/bundling activity of palladin (Fig. 1). In short, palladin can no longer be used to maintain the actin filament bundles at the ES. This thus facilitates the Arp2/3 complex to exert its barbed end actin nucleation activity to convert actin filaments from their bundled to a de-bundled/branched configuration, destabilizing the ES (Fig. 1). However, when the apical and basal ES are intact, such as in early stage VII and other stages, the integrity of actin filament bundles at the ES is maintained via the intrinsic activity of palladin and Eps8. It is likely that during the transport of elongating spermatid across the seminiferous epithelium at spermiogenesis, RAI14 is working with palladin, and also in concert with Eps8, and the Arp2/3 complex to induce changes in F-actin organization, converting actin filaments from their bundled to their debundled/branched configuration at the apical ES to facilitate spermatid transport.
Figure 1. A schematic model illustrating the role of RAI14, an actin-binding protein, in F-actin organization during the epithelial cycle of spermatogenesis. RAI14 is an F-actin binding protein, and it also structurally interacts with palladin (an actin filament cross-linking and bundling protein). In short, RAI14 is a binding partner of palladin in the testis. In late stage VII (upper left panel), RAI14 is highly expressed on the concave side of the spermatid head where endocytic vesicle-mediated protein trafficking takes place. It is like that the binding of RAI14 with palladin pulls palladin away from the site (or blocking the intrinsic activity of palladin) to facilitate barbed end nucleation of existing actin filaments, converting actin filament bundles to a branched actin network via the action of Arp2/3 complex. This thus destabilizes the apical ES to facilitate endocytosis, transcytosis and recycling of “old” apical ES proteins to newly formed apical ES at the interface of step 8 spermatid-Sertoli cell at stage VIII. These changes also spread to the convex side of the spermatid head to facilitate spermiation (upper right panel). At the BTB in late stage VII (see lower left panel), RAI14 binds to palladin at the “old” BTB site above the preleptotene spermatocyte in transit to pull palladin away from the site (or blocking the intrinsic activity of palladin) to initiate the disassembly of the “old” BTB which will take place at stage VIII (lower right panel). Due to the absence of functional palladin, Arp3 can exert its intrinsic activity to induce barbed end nucleation of the existing actin filaments, converting the bundled actin filaments to a branched network to facilitate “old” BTB disassembly (lower righty panel). Furthermore, palladin maintains the actin filament bundles behind the transiting preleptotene spermatocyte at the “new” BTB site via its intrinsic actin bundling activity. It is likely that via such a mechanism, the integrity of the BTB can be maintained during the transport of preleptotene spermatocytes across the immunological barrier.
In this context, it is of interest to note that in late stage VII of the cycle, the concave side of the spermatid head undergoes endocytic vesicle-mediated protein trafficking facilitated by the conversion of “bundled” actin filaments to their “de-bundled/branched” configuration to prepare for spermiation that takes place at stage VIII of the cycle (seeFig. 1). Interestingly, the expression of RAI1417 and also palladin33 are high at the apical ES at stage VII, concomitant with a surge in p-FAK-Tyr407 expression at the same site.38 It is thus possible that p-FAK-Tyr407 may activate the RAI14/palladin complex by phosphorylating either RAI14, palladin or both proteins. This either inactivates the intrinsic actin bundling activity of palladin, or the activated palladin can recruit more Arp2/3 complex (but less Eps8) to the site to induce de-bundling of actin filaments to facilitate protein endocytosis since Arp3 and Eps8 are binding partners of palladin,33 or both. The net result thus destabilizes the apical ES, and similar changes can take place at the “old” BTB site above the transiting preleptotene spermatocytes to prepare for their transport across the BTB. These possibilities can now be carefully evaluated in future experiments.
Concluding remarks and future perspectives
The hypothetical model shown in Figure 1 has illustrated the likely role of RAI14 in conferring the plasticity of actin filament bundles at the ES, so that the F-actin network can be efficiently altered from its “bundled” to its “de-bundled/branched” configuration to facilitate the transport of spermatids and preleptotene spermatocytes across the seminiferous epithelium and the BTB, respectively. This is made possible via the intrinsic function of RAI14/palladin complex by binding to both Eps8 and Arp3 of the Arp2/3 complex since Eps8 and Arp3 are the putative binding partners of palladin.33 For instance, “new” BTB can be assembled behind the transiting preleptotene spermatocytes at the BTB via the recruitment of Eps8 by RAI14/palladin at the site (Fig. 1). Furthermore, “old” BTB can be disassembled above the transiting preleptotene via the recruitment of Arp3 by RAI14/palladin at the site which induces debundling of the actin filament bundles at the site (Fig. 1). It is obvious that this model will be rapidly updated when more data are available in future studies. For instance, the underlying mechanism(s) by which RAI14, palladin and Eps8 vs. the Arp2/3 complex are being recruited to the apical and/or basal ES during the epithelial cycle so that these proteins can exert their intrinsic activities to confer changes in the actin filament bundles at the ES remains to be elucidated. Does FAK and/or its activated p-FAK-Tyr397 or p-FAK-Tyr407 form recently shown to regulate ES dynamics38 play a role in recruiting these proteins to the ES? What is the role of c-Yes and c-Src in these events since these nonreceptor protein tyrosine kinases are likely working with FAK39 to mediate these changes?
Acknowledgments
This work was supported in part by grants from the National Institutes of Health, NICHD R01 HD056034 to C.Y.C. and U54 HD029990 Project 5 to C.Y.C.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/24824
References
- 1.Seranski P, Hoff C, Radelof U, Hennig S, Reinhardt R, Schwartz CE, et al. RAI1 is a novel polyglutamine encoding gene that is deleted in Smith-Magenis syndrome patients. Gene. 2001;270:69–76. doi: 10.1016/S0378-1119(01)00415-2. [DOI] [PubMed] [Google Scholar]
- 2.Toulouse A, Rochefort D, Roussel J, Joober R, Rouleau GA. Molecular cloning and characterization of human RAI1, a gene associated with schizophrenia. Genomics. 2003;82:162–71. doi: 10.1016/S0888-7543(03)00101-0. [DOI] [PubMed] [Google Scholar]
- 3.Elsea SH, Williams SR. Smith-Magenis syndrome: haploinsufficiency of RAI1 results in altered gene regulation in neurological and metabolic pathways. Expert Rev Mol Med. 2011;13:e14. doi: 10.1017/S1462399411001827. [DOI] [PubMed] [Google Scholar]
- 4.Gropman AL, Elsea SH, Duncan WCJ, Jr., Smith AC. New developments in Smith-Magenis syndrome (del 17p11.2) Curr Opin Neurol. 2007;20:125–34. doi: 10.1097/WCO.0b013e3280895dba. [DOI] [PubMed] [Google Scholar]
- 5.Elsea SH, Girirajan S. Smith-Magenis syndrome. Eur J Hum Genet. 2008;16:412–21. doi: 10.1038/sj.ejhg.5202009. [DOI] [PubMed] [Google Scholar]
- 6.Hayes S, Turecki G, Brisebois K, Lopes-Cendes I, Gaspar C, Riess O, et al. CAG repeat length in RAI1 is associated with age at onset variability in spinocerebellar ataxia type 2 (SCA2) Hum Mol Genet. 2000;9:1753–8. doi: 10.1093/hmg/9.12.1753. [DOI] [PubMed] [Google Scholar]
- 7.Nakamine A, Ouchanov L, Jiménez P, Manghi ER, Esquivel M, Monge S, et al. Duplication of 17(p11.2p11.2) in a male child with autism and severe language delay. Am J Med Genet A. 2008;146A:636–43. doi: 10.1002/ajmg.a.31636. [DOI] [PubMed] [Google Scholar]
- 8.Potocki L, Bi W, Treadwell-Deering D, Carvalho CM, Eifert A, Friedman EM, et al. Characterization of Potocki-Lupski syndrome (dup(17)(p11.2p11.2)) and delineation of a dosage-sensitive critical interval that can convey an autism phenotype. Am J Hum Genet. 2007;80:633–49. doi: 10.1086/512864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutty RK, Kutty G, Samuel W, Duncan T, Bridges CC, El-Sherbeeny A, et al. Molecular characterization and developmental expression of NORPEG, a novel gene induced by retinoic acid. J Biol Chem. 2001;276:2831–40. doi: 10.1074/jbc.M007421200. [DOI] [PubMed] [Google Scholar]
- 10.Kutty RK, Chen S, Samuel W, Vijayasarathy C, Duncan T, Tsai JY, et al. Cell density-dependent nuclear/cytoplasmic localization of NORPEG (RAI14) protein. Biochem Biophys Res Commun. 2006;345:1333–41. doi: 10.1016/j.bbrc.2006.04.184. [DOI] [PubMed] [Google Scholar]
- 11.Peng YF, Mandai K, Sakisaka T, Okabe N, Yamamoto Y, Yokoyama S, et al. Ankycorbin: a novel actin cytoskeleton-associated protein. Genes Cells. 2000;5:1001–8. doi: 10.1046/j.1365-2443.2000.00381.x. [DOI] [PubMed] [Google Scholar]
- 12.Yuan W, Zheng Y, Huo R, Lu L, Huang XY, Yin LL, et al. Expression of a novel alternative transcript of the novel retinal pigment epithelial cell gene NORPEG in human testes. Asian J Androl. 2005;7:277–88. doi: 10.1111/j.1745-7262.2005.00040.x. [DOI] [PubMed] [Google Scholar]
- 13.Cockman ME, Webb JD, Ratcliffe PJ. FIH-dependent asparaginyl hydroxylation of ankyrin repeat domain-containing proteins. Ann N Y Acad Sci. 2009;1177:9–18. doi: 10.1111/j.1749-6632.2009.05042.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferreiro DU, Komives EA. Molecular mechanisms of system control of NF-kappaB signaling by IkappaBalpha. Biochemistry. 2010;49:1560–7. doi: 10.1021/bi901948j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tee JM, Peppelenbosch MP. Anchoring skeletal muscle development and disease: the role of ankyrin repeat domain containing proteins in muscle physiology. Crit Rev Biochem Mol Biol. 2010;45:318–30. doi: 10.3109/10409238.2010.488217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin J, Smith FD, Stark C, Wells CD, Fawcett JP, Kulkarni S, et al. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr Biol. 2004;14:1436–50. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 17.Qian X, Mruk DD, Cheng CY. Rai14 (retinoic Acid induced protein 14) is involved in regulating f-actin dynamics at the ectoplasmic specialization in the rat testis. PLoS One. 2013;8:e60656. doi: 10.1371/journal.pone.0060656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.França LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol. 2012;763:237–59. [PubMed] [Google Scholar]
- 19.Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Setchell BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. Adv Exp Med Biol. 2008;636:212–33. doi: 10.1007/978-0-387-09597-4_12. [DOI] [PubMed] [Google Scholar]
- 22.Fawcett DW, Leak LV, Heidger PM., Jr. Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil Suppl. 1970;10:105–22. [PubMed] [Google Scholar]
- 23.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 24.Russell LD, Clermont Y. Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat Rec. 1976;185:259–78. doi: 10.1002/ar.1091850302. [DOI] [PubMed] [Google Scholar]
- 25.Russell LD. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell. 1977;9:475–98. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- 26.Russell LD. Morphological and functional evidence for Sertoli-germ cell relationships. in The Sertoli Cell. (eds. Russell, L.D. & Griswold, M.D.) 365-390 (Cache River Press, Clearwater, 1993). [Google Scholar]
- 27.Russell LD. Form, dimensions, and cytology of mammalian Sertoli cells. in The Sertoli Cell. (eds. Russell, L.D. & Griswold, M.D.) 1-37 (Cache River Press, Clearwater, 1993). [Google Scholar]
- 28.Russell LD, Goh JC, Rashed RMA, Vogl AW. The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol Reprod. 1988;39:105–18. doi: 10.1095/biolreprod39.1.105. [DOI] [PubMed] [Google Scholar]
- 29.Russell LD, Saxena NK, Turner TT. Cytoskeletal involvement in spermiation and sperm transport. Tissue Cell. 1989;21:361–79. doi: 10.1016/0040-8166(89)90051-7. [DOI] [PubMed] [Google Scholar]
- 30.Cheng CY, Lie PPY, Wong EWP, Mruk DD. Focal adhesion kinase and actin regulatory/binding proteins that modulate F-actin organization at the tissue barrier - Lession from the testis. Tissue Barriers. 2013;1:e24252. doi: 10.4161/tisb.24252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng CY, Lie PPY, Wong EWP, Mruk DD, Silvestrini B. Adjudin disrupts spermatogenesis via the action of some unlikely partners: Eps8, Arp2/3 complex, drebrin E, PAR6 and 14-3-3. Spermatogenesis. 2011;1:291–7. doi: 10.4161/spmg.1.4.18393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–67. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in adult rat testes. Endocrinology. 2013;154:1907–20. doi: 10.1210/en.2012-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–6. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian X, Mruk DD, Cheng YH, Cheng CY. Actin cross-linking protein palladin and spermatogenesis. Spermatogenesis. 2013;3:e23473. doi: 10.4161/spmg.23473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li MWM, Xiao X, Mruk DD, Lam YL, Lee WM, Lui WY, et al. Actin-binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis. 2011;1:123–36. doi: 10.4161/spmg.1.2.16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng CY, Mruk D, Silvestrini B, Bonanomi M, Wong CH, Siu MK, et al. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: a review of recent data. Contraception. 2005;72:251–61. doi: 10.1016/j.contraception.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, Cheng CY. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA. 2012;109:12562–7. doi: 10.1073/pnas.1202316109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao X, Mruk DD, Cheng CY. Intercellular adhesion molecules (ICAMs) and spermatogenesis. Hum Reprod Update. 2013;19:167–86. doi: 10.1093/humupd/dms049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian X, Mruk DD, Wong EWP, Cheng CY. Breast cancer resistance protein regulates apical ectoplasmic specialization dynamics stage specifically in the rat testis. Am J Physical Endocrinol Metab. 2013;304:E757–69. doi: 10.1152/ajpendo.00645.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun S, Wong EWP, Li MWM, Lee WM, Cheng CY. 14-3-3 and its binding partners are regulators of protein-protein interactons during spermatogenesis. J Endocr. 2009;202:327–36. doi: 10.1677/JOE-09-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]