Abstract
The blood-testis barrier (BTB) is an important ultrastructure for spermatogenesis. Delay in BTB formation in neonatal rats or its irreversible damage in adult rats leads to meiotic arrest and failure of spermatogonial differentiation beyond type A. While hormones, such as testosterone and FSH, are crucial to BTB function, little is known if there is a local regulatory mechanism in the seminiferous epithelium that modulates BTB function. Herein, we report that collagen α3(IV) chain, a component of the basement membrane in the rat testis, could generate a noncollagenous (NC1) domain peptide [Colα3(IV) NC1] via limited proteolysis by matrix metalloproteinase-9 (MMP-9), and that the expression of MMP-9 was upregulated by TNFα. While recombinant Colα3(IV) NC1 protein produced in E. coli failed to perturb Sertoli cell tight junction (TJ)-permeability barrier function, possibly due to the lack of glycosylation, Colα3(IV) NC1 recombinant protein produced in mammalian cells and purified to apparent homogeneity by affinity chromatography was found to reversibly perturb the Sertoli cell TJ-barrier function. Interestingly, Colα3(IV) NC1 recombinant protein did not perturb the steady-state levels of several TJ- (e.g., occludin, CAR, JAM-A, ZO-1) and basal ectoplasmic specialization- (e.g., N-cadherin, α-catenin, β-catenin) proteins at the BTB but induced changes in protein localization and/or distribution at the Sertoli cell-cell interface in which these proteins moved from the cell surface into the cell cytosol, thereby destabilizing the TJ function. These findings illustrate the presence of a local regulatory axis known as the BTB-basement membrane axis that regulates BTB restructuring during spermatogenesis.
Keywords: testis, non-collagenous domain, collagen, blood-testis barrier, Sertoli cell, ectoplasmic specialization, tight junction
Introduction
In the mammalian testis, the blood-testis barrier (BTB) located near to the basement membrane in the seminiferous epithelium is crucial for spermatogenesis.1-4 First, it divides the epithelium into the basal and adluminal compartments to establish a unique microenvironment in the adluminal compartment for spermiogenesis by limiting paracellular and transcellular transport of substances across the barrier by performing its gate-keeping function. This gatekeeping function of the BTB is manifested by the unique composition of proteins, ions, electrolytes, sugars and biomolecules (e.g., hormones) in the fluid of the seminiferous tubule lumen and the rete testis lumen vs. the blood plasma/serum.2,5-7 Second, the BTB also confers cell polarity by restricting the movement of membrane proteins and lipids between apical and basolateral membranes (i.e., fence function).8 Lastly, irreversible disruption of the BTB by treatment of adult rats with toxicants (e.g., glycerol, cadmium)9-14 is known to cause sterility. These findings collectively illustrate the significance of the BTB in spermatogenesis.
Most blood-tissue barriers (e.g., blood-brain barrier and blood-retinal barrier) are conferred by the endothelial tight junction (TJ) of microvessels, which is located at the apical region of the endothelial cells, furthest away from the basal lamina.15,16 The BTB, however, is constituted by co-existing TJ, basal ES [ectoplasmic specialization, a testis-specific filamentour (F)-actin-rich adherens junction (AJ)17], desmosome and gap junction, located adjacent to the basement membrane, which is a modified form of extracellular matrix (ECM).18 This unique morphological setting thus suggests that the basement membrane may modulate the BTB function during the epithelial cycle.19 In fact, studies have shown that a disruption of the basement membrane function via the use of antibodies impedes spermatogenesis and the Sertoli cell TJ-barrier,20-22 implicating there is a functional link between the BTB and the basement membrane.
In the mammalian testis, such as the testis in rodents and bulls, the basement membrane is composed of two major building blocks namely type IV collagen and laminins.19 Type IV collagen is a triple helical structure composed of three α chains of α1(IV) to α6(IV) chains with α3(IV) as the most predominant chain in the testis.23,24 Each collagen chain is characterized by an N-terminal noncollagenous 7S domain of ~15-amino acid residues, a middle collagenous domain of ~1,400 residues of G-X-Y repeats, and a C-terminal noncollagenous (NC1) domain of ~230 amino acid residues.19 Collagens are scaffolding proteins known to provide support to epithelial and endothelial cells. However, previous studies have shown that NC1 fragments of collagen chains generated by limited proteolysis by matrix metalloproteinases (MMPs), such as MMP-9, are physiologically active peptides.25 For instance, NC1 domain of collagen IV, also known as tumstatin, was shown to regulate cell adhesion, proliferation, angiogenesis and apoptosis in different cell types via its interactions with cell surface receptors, such as integrins.25-28 Furthermore, it was reported that using Sertoli cells cultured in vitro, TNFα, a cytokine produced by Sertoli and germ cells in the testis,3,29 was capable of inducing the production of activated MMP-9 by Sertoli cells,22 which might be used to induce limited proteolysis of collagen α3(IV) chains to generate the NC1 domain peptide—the biologically active peptide that possibly regulates Sertoli cell TJ-barrier function.22 Herein, we report findings that NC1 peptide derived from collagen α3(IV) chain was capable of perturbing Sertoli cell TJ function via its effects on protein localization at the Sertoli cell-cell interface, demonstrating unequivocally a functional relationship between the BTB and the basement membrane.
Results
Cleavage of Col IV to generate NC1 domain by MMP-9
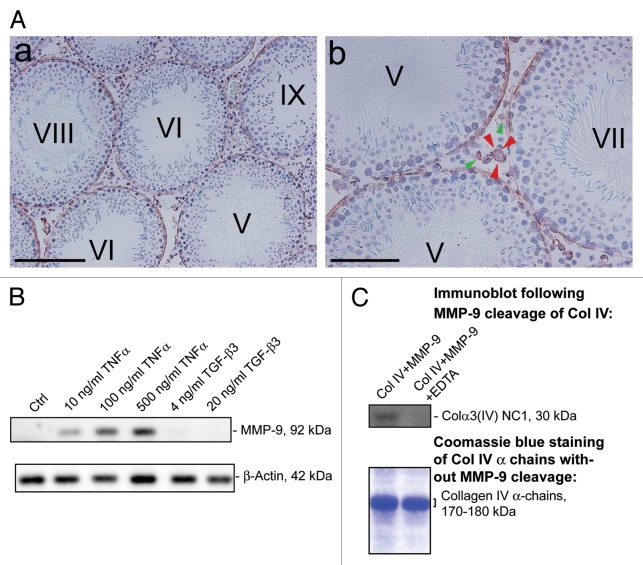
Using a specific anti-Col IV antibody (Table 1) for immunohistochemistry, Col IV was detected at the base of the seminiferous epithelium in seminiferous tubules of adult rat testes, consistent with its localization at the basement membrane (Fig. 1A). This finding is consistent with earlier studies showing that Col IV is one of the components of the basement membrane in mammalian testes including rodents,30-32 and α3, α4 and α5 chains of Col IV are recruited to the basement membrane of the tubules during postnatal development,23,30 and α3(IV) and α4(IV) chains of Col IV are known to constitute almost 80% of collagen chains in the basement membrane of tubules in bovine testes.24 In addition, no stage-specific expression of Col IV during the epithelial cycle was detected, consistent with its role as a structural basement membrane protein (Fig. 1A). Col IV was also detected in the interstitium in adult rat testes, associated with Leydig cells and endothelial cells of the microvessels (Fig. 1A, green and red arrowheads respectively), which is in agreement with previous studies. It is known that collagens including Col IV are expressed by Leydig cells in the mouse testis33 and endothelial cells as well as basement membrane of microvessels.34-37 Next, we investigated the upstream protease(s) that could possibly cleave the NC1 domain from Col IV in the basement membrane. MMP-9 is one of the best studied proteases known to cleave collagens.38,39 Also, MMP-9 was found near the basement membrane in the seminiferous epithelium and is a secretory product of Sertoli cells.22 To examine this possibility, Sertoli cells were cultured at low cell density (8 × 104 or 0.2 × 106 cells/cm2) on dishes without Matrigel. At this lower cell density, the amount of extracellular matrix substances (e.g., Col IV and nidogen) secreted by Sertoli cells and deposited on the dishes was sufficient to support their attachment and growth as earlier reported.40 Two days after isolation, 10–500 ng/ml human recombinant TNFα was added to F12/DMEM serum-free medium without bacitracin (which is a protease inhibitor and a polypeptide antibiotic). Sertoli cells were incubated with TNFα for 2 d before protein lysates, conditioned medium and extracellular matrix were collected. In line with our earlier results, TNFα was found to significantly induce the steady-state level of MMP-9 protein in the conditioned medium when cells were cultured at 8 × 104 cells/cm2 since MMP-9 is a secretory product of Sertoli cells (Fig. 1B).22 Similar effect was observed at higher cell density of 0.2 × 106 cells/cm2 (data not shown). A dose-dependent induction in MMP-9 by TNFα was detected and this effect was specific for TNFα because the inclusion of TGF-β3, another cytokine also known to disrupt the BTB, did not induce MMP-9 production by Sertoli cells (Fig. 1B). Using recombinant MMP-9 protein, it was shown that MMP-9 effectively cleaved Col IV to release the NC1 domain, which was readily detected by our antibody against Colα3(IV) NC1 (Fig. 1C, Table 1). This digestion was specific to MMP-9 because the presence of EDTA, an inhibitor of MMP-9, blocked the cleavage of Col IV to generate NC1 domain (Fig. 1C). We had tried to detect NC1 domain peptide in Sertoli cell lysates, conditioned medium and extracellular matrix by immunoblotting after treatment of TNFα using our anti-Colα3 (IV) NC1 antibody (Table 1). However, it is likely that due to the titer of the antibody, the level of endogenous NC1 that was cleaved by TNFα-induced MMP-9 was too low to be detected in these samples (data not shown).
Table 1. Antibodies used for different experiments in this report.
| Antibody | Vendor | Catalog no. | Applications/working dilution |
|---|---|---|---|
| Rabbit anti-Col IV |
Abcam |
ab6586 |
IHC* (1:100) |
| Rabbit anti-MMP-9 |
Abcam |
ab7299 |
IB (1:1000) |
| Rabbit anti-Colα3(IV) NC1 |
Wong & Cheng (current report) |
Not applicable |
IB (4 μg IgG/ml) |
| Rabbit anti-His |
Roche Applied Science |
04905318001 |
IB (1:1000) |
| Rabbit anti-Flag M2 |
Stratagene |
200470-21 |
IB (1:1000) |
| Rabbit anti-Occludin |
Life Technologies |
71-1500 |
IB (1:200) |
| Rabbit anti-CAR |
Santa Cruz Biotechnology |
sc-15405 |
IB (1:200), IF (1:50) |
| Rabbit anti-JAM-A |
Life Technologies |
36-1700 |
IB (1:150) |
| Rabbit anti-ZO-1 |
Life Technologies |
61-7300 |
IB (1:150) |
| Mouse anti-ZO-1 |
Invitrogen |
33-9100 |
IF (1:50) |
| Rabbit anti-N-cadherin |
Santa Cruz Biotechnology |
sc-7939 |
IB (1:200), IF (1:50) |
| Rabbit anti-α-catenin |
Santa Cruz Biotechnology |
sc-7894 |
IB (1:200) |
| Rabbit anti-β-catenin |
Santa Cruz Biotechnology |
sc-7199 |
IB (1:200) |
| Mouse anti-β-catenin |
Chemicon/EMD Millipore |
MAB2081 |
IF (1:50) |
| Goat anti-actin |
Santa Cruz Biotechnology |
sc-1616 |
IB (1:200) |
| Goat anti-rabbit IgG-Alexa Fluor 555 conjugated |
Invitrogen |
A21429 |
IF (1:250) |
| Goat anti-mouse IgG-Alexa Fluor 488 conjugated | Invitrogen | A11029 | IF (1:250) |
, IHC, immunohistochemistry; IB, immunoblotting; IF, immunofluorescence microscopy. It is noted that antibodies used for various experiments in this report cross-reacted with the corresponding proteins in the rat as indicated by the manufacturers.
Figure 1. TNFα upregulates MMP-9 to promote cleavage of collagen (Col) IV to generate Colα3(IV) NC1 domain. (A) Localization of Col IV in the seminiferous epithelium of adult rat testis was studied using frozen cross-sections fixed in ice-cold methanol and visualized with a specific anti-Col IV antibody (Table 1). Low magnification (A:a) micrograph illustrates that Col IV was expressed in the seminiferous epithelium of adult rat testes at all stages of the epithelial cycle. Higher magnified image shown in (A:b) illustrates that Col IV was restricted to the basal region of the seminiferous epithelium, consistent with its localization at the basement membrane. Immunoreactive Col IV was also found in Leydig cells (see green arrowheads in A:b), as well as endothelial cells and/or basal lamina of the microvessels (see red arrowheads in A:b) in the interstitium of adult rat testes. Scale bar, 100 µm and 25 µm in A:a and A:b, respectively. (B) Sertoli cells were cultured at 8 × 104 cells/cm2 on 6-well dishes without Matrigel. Different concentrations of TNFα and TGF-α3 were added 2 d after Sertoli cell isolation. Sertoli cell-conditioned medium was harvested 2 d thereafter, and 25 µg protein for each sample was used for SDS-PAGE and immunoblotting. TNFα, but not TGF-β3, was found to cause a dose-dependent increase in MMP-9 secretion by Sertoli cells as shown by immunoblotting (upper panel). The lower panel is the blot immunostained with an anti-β-actin antibody using corresponding Sertoli cell lysates (25 µg protein) to assess equal protein loading. (C) Recombinant MMP-9 (0.5 µg protein) was used to examine if it induced cleavage of recombinant mouse Col IV (BD Bioscience) (10 µg protein for each lane) contained mostly Col α3(IV) and Col α4(IV) of Mr 170–180 kDa) in a ratio of 1:20 (MMP9:Col IV) with or without EDTA (an inhibitor of MMP-9) to generate Colα3(IV) NC domain, which was detected by using a specific anti-Colα3(IV) NC domain antibody (Table 1) by immunoblotting [see upper panel in (C)]. The lower panel in (C) is the Coomassie blue-stained SDS-polyacrylamide gel of 10 µg purified recombinant Col IV protein per lane used for the experiment shown in the upper panel to demonstrate equal amount of Col IV was used for this experiment. Data shown herein are representative findings of an experiment. Each experiment, however, was repeated three times, excluding pilot experiments to optimize the experimental conditions, using testes of n = 3 rats (A), Sertoli cells isolated from different groups of 20-d-old male pups (B) or different batches of Col IV and MMP-9, respectively. Similar data were obtained in different experiments.
Production of mammalian recombinant Colα3(IV) NC1 domain protein
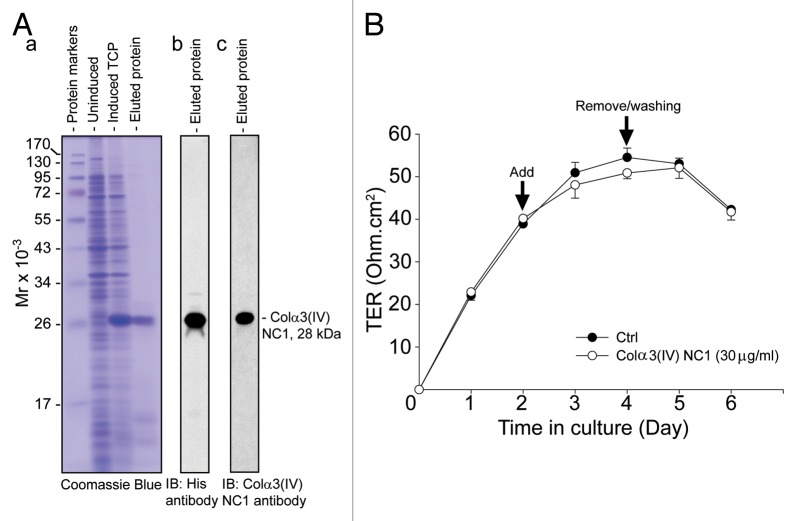
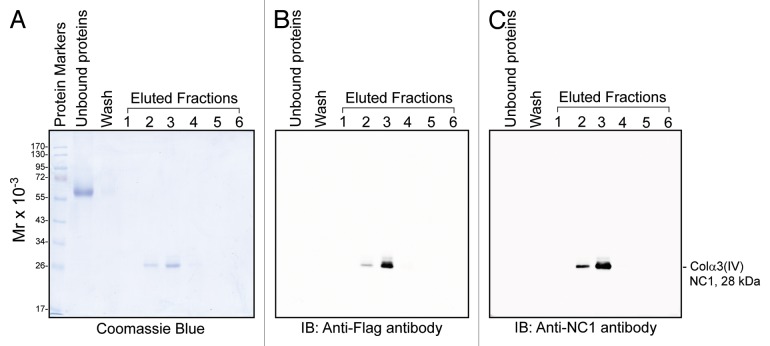
Since TNFα was found to induce MMP-9, which led to the cleavage of Col IV to generate NC1 domain (Fig. 1), these findings are consistent with earlier reports that Col IV is a putative substrate of MMP-9.38,39 Moreover, NC1 domain derived from this cleavage as shown in other epithelia was found to be biologically active, capable of modulating cell adhesion, cell proliferation and apoptosis;25 we thus sought to examine if NC1 domain has any biological effects on the BTB. Previous studies have shown that Colα3(IV) is one of the major chains expressed in the basement membrane of seminiferous tubules.30,32 We thus attempted to use bacterial cells to produce recombinant Colα3(IV) NC1 domain, which was purified to apparent homogeneity as shown in Figure 2A. The solubility and the yield of recombinant Colα3(IV) NC1 produced in bacteria were found to be poor, which may have resulted from the lack of glycosylation of the NC1 domain produced in E. coli. Nonetheless, we tested the ability of this recombinant protein in disrupting the Sertoli cell BTB by quantifying TER across the Sertoli cell epithelium with cells cultured onto Matrigel-coated bicameral units (Fig. 2B). Perhaps due to the lack of glycosylation of the recombinant NC1 domain protein, rendering it less effective to modulate the BTB function, we did not observe a statistically significant disruption on the TJ-permeability barrier in multiple experiments, one of which was shown herein (Fig. 2B). These findings also illustrate that recombinant Colα3(IV) NC1 domain proteins produced in E. coli based on three separate experiments with different batches of purified NC1 protein were not biologically active. We next switched to produce recombinant Colα3(IV) NC1 domain protein in mammalian 293T cells. BM40 signal peptide (SP) and Flag tag were genetically engineered at the 5′-end of Colα3(IV) NC1 domain using primers shown in Table 2 and described in Materials and Methods. The presence of the SP allowed the secretion of the recombinant protein into the conditioned medium, whereas the Flag tag facilitated its purification by using anti-Flag M2 agarose beads. Using this approach based on the use of secreted recombinant protein harvested in the conditioned medium vs. the use of cell lysates also provided the advantage of dealing with soluble protein, instead of insoluble protein which resulted from the E. coli system. After affinity chromatography using the anti-Flag M2 resin, recombinant Colα3(IV) NC1 protein was purified to apparent homogeneity as visualized by Coomassie blue stained gels (Fig. 3A). The purified protein was recognized by the anti-Flag antibody (Fig. 3B) and anti-Colα3(IV) NC1 antibody (Fig. 3C) (Table 1).
Figure 2. Expression of recombinant Colα3(IV)NC1 protein in bacteria, its purification, and its effects on the Sertoli cell TJ-permeability barrier function. (A) BL21 (DE3) competent E. coli cells transformed with pET46 Ek/LIC-Colα3(IV) NC1 vector was induced with 0.1 mM IPTG to express the recombinant protein. Strong expression of Colα3(IV) NC1 domain (28 kDa) was noted when compared the un-induced total cell protein (TCP) to induced TCP as shown in a representative Coomassie blue stained gel (A:a). Recombinant protein was purified by Ni2+-Sepharose chromatography in which the histidine (His, H) tag at the N-terminus of the recombinant protein specifically bound to the Ni-column. The eluted protein was purified to apparent homogeneity (A:b), and it was recognized by both anti-His (A:b) and anti-Colα3(IV) NC1 antibody (A:c) (see Table 1). (B) Primary Sertoli cells were plated at 1.2 × 106 cells/cm2 on Matrigel-coated bicameral units at time 0 to allow the assembly of a functional TJ-permeability barrier. Purified bacterial Colα3(IV) NC1 recombinant protein was refolded and dialyzed against PBS as described in Materials and Methods, and it was included in the F12/DMEM medium on day 2 at a concentration of 30 μg/ml (~1 µM). Mild but not statistically significant perturbation of the Sertoli cell BTB was observed when compared with PBS control (Ctrl) (Fig. 2B). Bacterial recombinant Colα3(IV) NC1 protein was removed 2 d after incubation. Each data point had n = 3 bicameral units. This experiment was repeated three times using different batches of Sertoli cells as well as recombinant protein, and yielded similar results.
Table 2. Primers used to construct pTracer-CMV2-Colα3(IV) NC1 expression vector.
| Sense primer 1* |
5’-AAGGATGATGACGACAAGACAAGAATGAGAGGCTTCATCT-3’ Flag NC1 |
| Sense primer 2 |
5’-CTGGCAGCCCCTCTGGCAGATTATAAGGATGATGACGACAAGA-3’ BM40 SP Flag NC1 |
| Sense primer 3 |
5’-TTTCTCCTTTGCCTGGCCGGGAGGGCCCTGGCAGCCCCT-3’ BM40 SP |
| Sense primer 4 |
5’-CAGATATC ATGGCTAGGGCCTGGATCTTCTTTCTCCTTTGCCTGGC-3’ BM40 SP |
| Anti-sense primer | 5’-CATCTAGATTAGTGTCTTTTCTTCATGCACAC-3’ |
, four overlapping PCRs were performed by using sense primer 1 and anti-sense primer in the first round and so on. Primer sequences were underlined to indicate the added BM40 signal peptide (SP) and Flag sequences at the 5’-end of Colα3(IV) NC1 coding sequence. The Flag encoding sequence is DYKDDDK while that for BM40 SP is MARAWIFFLLCLAGRALAAPLA. An extra Ala (A) amino acid was added after the start codon to form a Kozak sequence for high level of mRNA expression in Lenti-X 293T cells. Four more amino acids (APLA) from the rat BM40 coding sequence immediately after the SP were included to aid cleavage of the SP from the recombinant protein.
Figure 3. Production of recombinant Colα3(IV)NC1 domain protein in mammalian cells. Recombinant Colα3(IV)NC1 domain protein was expressed from pTracer-CMV2-Colα3(IV)NC1 stably transfected in Lenti-X 293T mammalian cells. Serum-free conditioned medium was harvested and Colα3(IV)NC1 domain was purified using anti-Flag M2 resin. Recombinant Colα3(IV)NC1 domain protein was detected in fractions 2 and 3 eluted from the affinity chromatography and it was purified to apparent homogeneity in a single step when its purity was analyzed by Coomassie blue stained SDS-polyacrylamide gel (A), and by immunoblotting using an anti-Flag (B) and an anti-Colα3(IV)NC1 antibody (C). These findings are representative data from three different experiments using different batches of recombinant protein, excluding pilot experiments to establish the optimal conditions, over a 2-y period which yielded similar results.
Colα3(IV) NC1 domain reversibly disrupts the Sertoli cell TJ-permeability barrier function
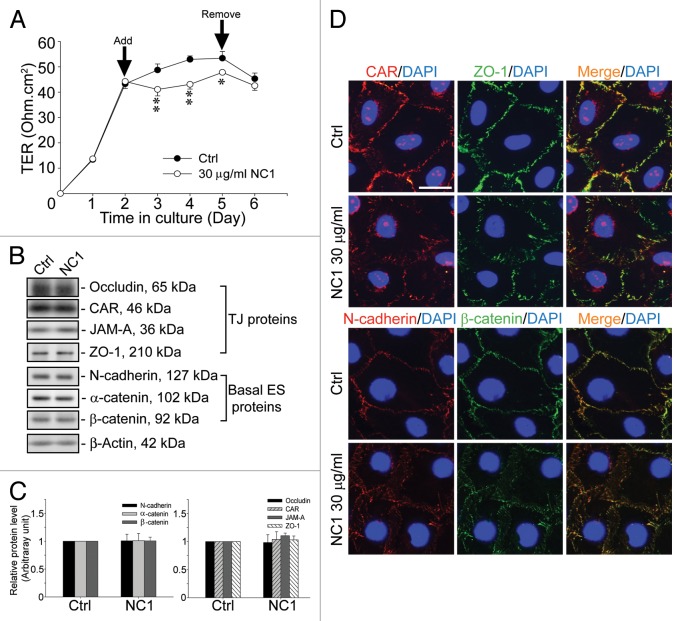
Sertoli cells were cultured alone for 2 d to allow the establishment of a functional TJ-permeability barrier, manifested by a relatively stable TER across the cell epithelium as shown in Figure 4A. The inclusion of purified recombinant Colα3(IV) NC1 protein at 30 µg/ml (1 µM) in F12/DMEM in the apical and basal compartment of the bicameral units was found to perturb the Sertoli TJ-permeability barrier (Fig. 4A). This disruptive effect was reversible since the removal of the recombinant protein by washing the Sertoli cell epithelium with fresh F12/DMEM without the recombinant protein allowed the “resealing” of the disrupted TJ-barrier (Fig. 4A). Interestingly, while the recombinant Colα3(IV) NC1 domain protein reversibly perturbed the TJ-barrier, it failed to downregulate the steady-state levels of both TJ and basal ES proteins at the Sertoli cell BTB, when cell cultures were terminated 2 d after addition of the recombinant protein in the medium for immunoblotting (Fig. 4B and C). We next assessed if there were any changes in the localization and/or distribution of junction proteins at the Sertoli cell-cell interface after treatment of Colα3(IV) NC1 (Fig. 4D). Treatment of Sertoli cells cultured on Matrigel-coated coverslips with 30 µg/ml Colα3(IV) NC1 for 2 d was found to induce mis-localization of junction proteins. For instance, TJ proteins (e.g., CAR and ZO-1) and basal ES proteins (e.g., N-cadherin and β-catenin) were no longer localized predominantly at the Sertoli cell-cell interface, instead these proteins moved away from the cell-cell interface and appeared to be in the cell cytosol (Fig. 4D). It is noted that some CAR staining was detected in the nucleus (Fig. 4D).
Figure 4. Disruption of the Sertoli cell TJ-permeability barrier function by recombinant Colα3(IV)NC1 domain protein mediated via changes in protein distribution and/or localization at the Sertoli cell-cell interface. (A) Sertoli cells were plated at 1.2 × 106 cells/cm2 on Matrigel-coated bicameral units with an established TJ-permeability barrier by day 2, and 30 µg/ml recombinant Colα3(IV) NC1 (1 µM) in PBS was included in the F12/DMEM. The presence of the NC1 domain recombinant protein was found to significantly perturb the TJ-barrier function vs. PBS control (Ctrl) until the recombinant protein was removed on day 5, and the partially disrupted TJ-barrier was “resealed.” *, p < 0.05; **, p < 0.01. (B) Sertoli cells were plated at 0.5 × 106 cells/cm2 for 2 d before 30 µg/ml (1 μM) of the recombinant NC1 domain was included in the F12/DMEM. Two d later, there was no significant change in steady-state levels of TJ and basal ES proteins when compared with PBS control (Ctrl). (C) This histogram summarizes results shown in (B) where protein levels from Ctrl were arbitrarily set at 1. Each bar is a mean ± SD of n = three independent experiments. (D) Sertoli cells were cultured at 0.045 × 106 cells/cm2 on Matrigel-coated coverslips, and treated with 30 µg/ml NC1 or PBS Ctrl for 2 d. Cells were fixed and stained for CAR (red) and ZO-1 (green) or N-cadherin (red) and β-catenin (green) by dual-labeled immunofluorescence analysis using the corresponding primary antibodies and Alexa Fluor 555- or 488-conjugated secondary antibodies (see Table 1). Sertoli cell nuclei were visualized by DAPI (blue). CAR, ZO-1, N-cadherin and β-catenin were all seen at the Sertoli cell-cell interface in Ctrl cells while treatment of cells with 30 µg/ml NC1 led to changes in distribution of these BTB-associated cell adhesion proteins, moving from the cell-cell interface into the cell cytosol. Scale bar, 5 µm, which applies to all micrographs. Findings of this dual-labeled immunofluorescence analysis experiment reported herein were results of a representative experiment, which was repeated three times using different Sertoli cell cultures and yielded similar results.
Discussion
Restructuring of the BTB that occurs near the basement membrane takes place simultaneously with spermiation that takes place at the adluminal edge of the seminiferous epithelium at stage VIII of the epithelial cycle. It is presently unknown how these two cellular events are being coordinated. An earlier study has shown that laminin fragments produced at the apical ES during spermiation via the action of MMP-2 were capable of perturbing the Sertoli cell BTB function near the basement membrane.41 A more recent report has identified the biologically active fragment of the laminin chain.42 Also, a knockdown of β1-integrin, an integrated component of the hemidesmosome in the testis by RNAi, was also found to perturb the Sertoli cell TJ barrier function.41 These findings thus support the notion that there is a local autocrine-based regulatory axis in the seminiferous epithelium, known as the “apical ES-BTB-hemidesmosome” axis, that regulates and/or coordinates cellular events across the epithelium during the epithelial cycle.41,42 The presence of this local functional axis was also supported by studies using a Sertoli cell injury model, based on the use of phthalates, which showed that a disruption of the apical ES involving cleavage of laminin-γ3 chains during spermatid loss also led to a BTB disruption.43-45
Herein, we report findings that support the presence of an autocrine-based axis between the BTB and the basement membrane. It is likely that biologically active Colα3(IV) NC1 domain peptides are generated at the basement membrane, plausibly via the action of TNFα-induced MMP-9 production that causes limited proteolysis of collagen α3(IV) chain in the basement membrane. More important, the perturbation of the Sertoli cell TJ-permeability barrier function by biologically active Colα3(IV) NC1 domain peptide is not mediated by downregulating the expression of integral membrane proteins nor their adaptors at the site since such changes would require minutes, if not hours, for changes in phenotypes. Instead, this biologically active collagen-based peptide is readily available to alter cellular localization and/or distribution at the Sertoli cell-cell interface rapidly. This, in turn, induces BTB restructuring at the site in response to the stages of the epithelial cycle during spermatogenesis and these changes are not mediated via de novo protein synthesis and/or gene expression as illustrated in this report. While the Colα3(IV) NC1 domain recombinant protein that was used in our studies to confirm its effects on the Sertoli cell TJ-permeability function and also protein distribution at the Sertoli cell-cell interface was purified to apparent homogeneity, it is still possible that other peptides and/or biomolecules can be produced in the basement membrane to affect the Sertoli cell TJ barrier function. Work is now in progress to examine this possibility.
It was noted that transient perturbation of the Sertoli cell TJ-permeability barrier induced by the Colα3(IV) NC1 domain peptide as reported herein was not as drastic as those induced by toxicants (e.g., cadmium),46,47 even though these changes were statistically significant. This is likely due to the possibility that Colα3(IV) NC1 domain peptide, which is released from the basement membrane via the action of MMP-9 during spermatogenesis, such as at stage VIII of the epithelial cycle, is not being used to “disrupt” the BTB analogous to the disruption induced by cadmium46,47 or bisphenol A (BPA).48 Instead, it is being used to disrupt the “old” BTB that are found above the preleptotene spermatocytes, which are located adjacent to the basement membrane as these germ cells are being transported across the BTB over a ~29 h period, the duration of stage VIII during the epithelial cycle;49,50 while “new” BTB is being assembled behind these spermatocytes. This possibility is supported by the findings that the steady-state protein levels of TJ (e.g., CAR, ZO-1)- and basal ES (e.g., N-cadherin, β-catenin)-proteins were not downregulated following treatment of the Sertoli cell epithelium with Colα3(IV) NC1 domain peptide as reported herein. Instead, this mild but statistically significant perturbation of the TJ-permeability barrier is mediated by changes in the localization of TJ- and basal ES-proteins at the Sertoli cell-cell interface, possibly via an increase in protein endocytosis at the “old” BTB, so that they can be transcytosed from the apical to the basal region of the preleptotene spermatocytes and these proteins can be recycled to assemble “new” BTB. This also represents a novel and physiologically efficient system to regulate BTB function during the transit of preleptotene spermatocytes across the BTB while the immunological barrier is still maintained.
It is noted that the TJ protein complex CAR-ZO-1 and the basal ES protein complex N-cadherin-β-catenin were co-localized almost to the same site at the Sertoli cell-cell interface as reported herein, which are also the structural protein complex of the BTB. This is not entirely unexpected since it is known that TJ coexists with basal ES, localized to the same site at the BTB in the testis when examined by electron microscopy.3,4,51-54 In this context, it is of interest to note that CAR, a TJ-integral membrane protein known to serve as a receptor for coxsackievirus and adenovirus55,56 to facilitate their crossing at the tissue barriers57,58 was also found in Sertoli cell nuclei besides its localization at the Sertoli cell-cell interface, corresponding to its localization at the TJ. A recent study has shown that CAR can indeed be internalized via protein endocytosis and associated with EEA1 (early endosome antigen 1, an early endosome marker), and it is involved in endocytic vesicle-mediated protein trafficking events such as protein endocytosis.59 Furthermore, an earlier study in human bladder cell lines has shown that CAR is also involved in cell cycle regulation in the nucleus to elicit its growth-inhibitory activity.60 Thus, it is not entirely unusual that CAR can be detected in the nucleus; however, it remains to be determined if the Colα3(IV) NC1 domain peptide is involved in regulating endocytic vesicle-mediated trafficking, and its functional relationship with CAR and other proteins at the BTB. Additional studies, such as the use of immunogold electron microscopy and/or biochemical assays of endocytosis and recycling, will need to be performed in future studies to assess the involvement of Colα3(IV) NC1 domain peptide in protein trafficking events.
As hemidesmosome is an integrated component at the Sertoli cell-basement membrane interface, we now rename this axis as the “apical ES-BTB-basement membrane” axis. This functional axis can now be vigorously investigated since components across this axis, including the crucial regulatory proteins, such as Colα3(IV) NC1 domain peptide reported herein, can now be examined in functional experiments. Thus, future studies should include a careful examination on the production of Colα3(IV) NC1 domain peptide during the epithelial cycle. Besides, the role of cytokines (e.g., TNFα), either alone or together with MMP-9 (and other MMPs) on its production should be evaluated. Furthermore, Colα3(IV) likely acts as a ligand, its receptor in Sertoli cells, such as integrins (and/or other molecules) should also be identified.
Materials and Methods
Animals and antibodies
Sprague-Dawley rats, 18-d-old pups or adult rats (~270–300 g b.w.) were obtained from Charles River Laboratories. The use of animals reported here was approved by the Rockefeller University Institutional Animal Care and Use Committee (Protocol Numbers 09-016 and 12-506). Antibodies used for different experiments reported herein were obtained commercially, unless otherwise specified, are listed in Table 1.
Production of antibody against Colα3(IV) NC1 domain
Recombinant Colα3(IV) NC1 domain was produced using the pET30 Ek/LIC vector kit in BL21 (DE3) bacteria (EMD Millipore). Colα3(IV) NC1 domain was amplified by sense 5′-GACGACGACAAGATCACAAGAATGAGAGGCTTCATCTTCA-3′ and antisense 5′-GAGGAGAAGCCCGGTGTGTCTTTTCTTCATGCACACCTGA-3′ primers using 90-d-old rat testis cDNA as template. This corresponded to the 4,321‒5,010 nt of rat full-length Colα3(IV) (NM_001135759), which is the NC1 domain. The stop codon in the original full-length sequence was omitted due to the presence of in frame stop codon in the vector. Purity of the recombinant protein expression in E. coli isolated by affinity chromatography was confirmed by SDS-PAGE with the gel stained by Coomassie blue. ~200 µg purified protein emulsified with Freund’s complete adjuvant was used to immunize a female white New Zealand rabbit, to be followed by two booster injection of purified protein (200 µg each) emulsified with Freund’s incomplete adjuvant. Blood (~20–50 ml) was collected ~4-wk thereafter, and then every 10-d over a ~10-wk period. Serum was obtained by centrifugation (2,000 g, 10 min, 4 °C, twice) after blood was allowed to clot overnight at 4 °C. IgG was isolated by sequential ammonium sulfate precipitation and DEAE affinity chromatography.61
Purification of Colα3(IV) NC1 domain recombinant protein produced in bacteria
Colα3(IV) NC1 was produced in bacteria using the pET46 Ek/LIC expression vector (EMD Millipore). Colα3(IV) NC1 coding sequence was amplified by sense primer: 5′-GACGACGACAAGATCACAAGAATGAGAGGCTTCATCTTCA-3′ and antisense primer: 5′-GAGGAGAAGCCCGGTTTAGTGTCTTTTCTTCATGCACA-3′ using pET30 Ek/LIC-Colα3(IV) NC1 as template. The authenticity of pET46 Ek/LIC-Colα3(IV) NC1 was confirmed by direct nucleotide sequencing (at Genewiz) and transformed into BL21 (DE3) (EMD Millipore) for recombinant protein production. Five ml overnight bacterial culture was used to inoculate 500 ml LB supplemented with 100 µg/ml carbenicillin (Life Technologies). Bacteria were allowed to grow until the OD600 reached ~0.65 and 0.1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) was used to induce production of recombinant protein for 3.5 h at 37°C. Bacteria were harvested at 6,000 × g 10 min. All bacterially expressed Colα3(IV) NC1 was found in the inclusion bodies (insoluble fraction). Bacteria were lysed and inclusion bodies were extracted by BugBuster Plus Lysonase kit (EMD Millipore) according to the manufacturer protocol. 6 M guanidine HCl, 0.1 M NaH2PO4 and 10 mM TRIS-HCl, pH 8.5 was used to dissolve inclusion bodies. Ni2+-Sepharose high performance resin (GE Healthcare) was used to purify the histidine (His)-tagged Colα3(IV) NC1 domain in a packed column (Bio-rad). To wash the resin, increasing amount of imidazole (10 mM and 25 mM) in 8 M urea, 0.1 M NaH2PO4 and 10 mM TRIS-HCl, pH 8 was used. Colα3(IV) NC1 domain was eluted with 250 mM imidazole, 8 M urea, 0.1 M NaH2PO4 and 10 mM TRIS-HCl, pH 8 and refolded according to Gu et al.62 Briefly, eluted protein was dialyzed against decreasing concentration of urea: 1) 4 M urea, 0.1 M NaCl and 20 mM TRIS-HCl, pH 6 overnight at 4°C; 2) 2 M urea, 0.1 M NaCl, 2 mM reduced glutathione, 0.2 mM oxidized glutathione, 1 mM EDTA and 20 mM TRIS-HCl, pH 6.5 for 8 h 4°C and 3) PBS (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4 and 1.47 mM KH2PO4) pH 7.4 overnight at 4°C.
Collagen (Col) IV cleavage by MMP-9
Murine MMP-9 (EMD Millipore) was first activated by treatment with 0.5 mg/ml TPCK-trypsin (Thermo Scientific) in an activation buffer (50 mM TRIS-HCl, 150 mM NaCl, 5 mM CaCl2 and 0.05% Brij-35, pH 7.5 at 22°C) for 30 min at 37°C. Thereafter, trypsin activity was inhibited by adding aprotinin (Sigma-Aldrich) (1 mg/ml) to activation buffer. 0.5 µg activated MMP-9 was then used to digest 10 g recombinant mouse Col IV [BD Biosciences, derived from Engelbreth-Holm-Swarm (EHS) lathrytic mouse tumor, a major component of the basement membrane which contained mostly Col α3(IV) and α4(IV) chains of Mr 170–180 kDa in a buffer of 1 M Tris (pH 8 at 22°C) with or without 200 mM EDTA, for 3 h at 37°C. The digested proteins were resolved by SDS-PAGE and probed for Colα3(IV) NC1 by immunoblotting.
Production of recombinant Colα3(IV) NC1 domain in mammalian cells
pTracer-CMV2 (Life Technologies) expression vector carrying rat Colα3(IV)NC1 domain was constructed by PCR using AccuPrime Pfx Taq (Life Technologies) and pET30 EK/LIC-Colα3(IV) NC1 as a template. Four sequential overlapping PCRs were performed to add a rat BM40 (NM_012656.1) signal peptide (SP) and Flag sequence at the 5′-end of Colα3(IV) NC1 coding sequence to allow extracellular secretion and aid purification. Sequences of the primers used for preparing the cDNA construct were listed in Table 2. The resulting DNA fragment was digested with EcoRV and XbaI for 7 h at 37°C and ligated into pTracer-CMV2 overnight at 15°C. Correct clone was confirmed by DNA sequencing (Genewiz). pTracer-CMV2-Colα3(IV) NC1 vector was linearized by ScaI for 16 h at 37°C. A human embryonic kidney cell line Lenti-X 293T cells (Clontech) was used to produce the recombinant Colα3(IV) NC1 domain protein. Cells were maintained in high glucose DMEM supplemented with 10% FBS (Life Technologies), 3.7 g/L NaHCO3, 1 mM sodium pyruvate, 100 units/ml penicillin, 100 g/ml streptomycin, 2 mM GlutaMAX and 0.1 mM MEM non-essential amino acids (Life Technologies), pH 7.4 at 37°C in humidified 10% CO2/95% air (v/v) atmosphere. Cells seeded at 4 × 104 cells/cm2 in 12-well dishes (with each well contained ~4-ml medium) were transfected with 1 g linearized DNA using Fugene HD reagent (Roche) according to manufacturer’s protocol. One day after transfection, cells were split 1:4 into 100-mm dish and 600 µg/ml zeocin (Life Technologies) were included in culture medium. Fresh medium was changed every 3 d until foci were formed. Individual colonies were trypsinized and cultured in 12-well dishes with 50 µg/ml zeocin. Positive clones were selected by RT-PCR using sense primer 1 and anti-sense primer (Table 2) as well as fluorescence microscopy to detect the GFP tag in the vector. A clone with the strongest Colα3(IV) NC1 domain expression was expanded into three 150-mm dishes, grown until confluency and replaced with serum-free high glucose DMEM. During the production of recombinant Colα3(IV) NC1 domain, zeocin was omitted. After 40 h of incubation, supernatant was collected and centrifuged at 2,000 g for 10 min to remove cell debris. Recombinant Colα3(IV)NC1 domain was purified using anti-Flag M2 affinity gel (Sigma-Aldrich) by incubating cleared supernatant with the resin at 4°C for 3 h in the presence of 0.2 M NaCl in a laboratory rocking platform (Clay Adams Nutator) to reduce non-specific binding. Resin was then packed into an EconoColumn (Bio-Rad), washed with TBS (50 mM TRIS-HCl, 150 mM NaCl, pH 7.4) and recombinant Colα3(IV) NC1 domain protein eluted with 0.1 M glycine HCl, pH 3.5. Eluted protein was neutralized with 1 M Tris, pH 8 and equilibrated in PBS (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4 and 1.47 mM KH2PO4, pH 7.4), and the purified was confirmed by SDS-PAGE using Coomassie blue-stained gel with an apparent Mr of 30 kDa. Four batches of recombinant proteins were produced over a 2 y period with consistent results.
Primary Sertoli cell cultures
Primary Sertoli cells were isolated from 20-d-old rat testes and cultured in serum-free F12/DMEM medium supplemented with insulin, transferrin, epidermal growth factor (EGF) and bacitracin as described.40 These Sertoli cells were differentiated and ceased to divide63 unless serum (such as 5% fetal calf serum) was present in the media.64 These cells were functionally indifferent from Sertoli cells isolated from adult rat testes based on earlier studies.65,66 But the purity of Sertoli cells isolated from adult testes was only ~85% reported in our earlier studies65,66 consistent with an earlier report.67 However, Sertoli cells isolated from 20-d-old rat testes had a purity of > 98% with negligible contamination of germ and Leydig cells when cell contaminants were assessed by RT-PCR and/or immunoblotting using the corresponding specific primers and/or specific antibodies against unique markers of these cells.40,68 Thus, Sertoli cells from 20-d-old rat testes were used in our experiments. To investigate the induction of MMP-9 by TNFα (R&D Systems, Minneapolis, MN) or TGF-α3 (Calbiochem-EMD Millipore), Sertoli cells were seeded at 8 × 104 or 0.2 × 106 cells/cm2 on 6-well-dishes (without Matrigel) for 2 d in F12/DMEM with other supplements but without bacitracin. Sertoli cell-conditioned medium and cell lysates were collected 2 d after treatment. To extract the extracellular matrix deposited by Sertoli cells, cells were seeded on 100-mm dishes without Matrigel and processed as earlier described69 with minor modifications. In brief, Sertoli cells were washed with PBS containing 1 mM phenylmethylsulfonyl fluoride and aminocaproic acid (1 mg/ml) for three times, to be followed by two successive incubations with the wash buffer and 5 mM EGTA for 15 min at 37°C to remove all Sertoli cells. Remaining cell debris was removed by three washes with PBS alone, to be followed by three washes of double distilled water. Four hundred µl of SDS sample buffer [0.125M Tris, pH 6.8 at 22°C containing 1% SDS (w/v), 1.6% 2-mercaptoethanol (v/v) and 10% glycerol (v/v)] was added and dishes were scraped thoroughly by using a rubber policeman. To access the effect of recombinant Colα3(IV) NC1 domain on the Sertoli cell BTB function, primary Sertoli cells were seeded at 0.5 × 106 cells/cm2 on Matrigel-coated 12-well dishes (for protein lysates preparation) or 1.2 × 106 cells/cm2 on Matrigel-coated bicameral unit [for transepithelial electrical resistant (TER) measurement] to assess the TJ-permeability barrier function. For cell staining, Sertoli cells at 4.5 × 104 cells/cm2 were seeded on Matrigel-coated coverslips, so that cell nuclei were evenly interspersed, and changes in the distribution and/or localization of BTB-proteins at the Sertoli cell-cell interface following treatment with Colα3(IV) NC1 domain recombinant protein could be easily assessed by dual-labeled immunofluorescence analysis. Recombinant Colα3(IV) NC1 domain protein in PBS (30 µg/ml, at 1 M) was added 2 d after cell isolation. PBS was used as a vehicle control. Recombinant protein was removed 2 d (for protein lysates and cell staining) or 3 d (for TER measurement to assess TJ function) after treatment.
Assessment of Sertoli cell TJ-permeability barrier function
The Sertoli cell TJ-permeability barrier function was monitored by quantifying the TER across the Sertoli cell epithelium as described.40,70 Sertoli cells were plated on Matrigel-coated bicameral units on day 0. Within ~2 d, Sertoli cells established a functional TJ-permeability barrier, with visible ultrastructures of TJ, basal ES, gap junction and desmosome under electron microscopy,71,72 which mimicked Sertoli BTB in vivo, and this system has been used by other investigators to study BTB regulation.46,73-78 Recombinant Colα3(IV) NC1 domain protein was added to apical and basal compartment of the bicameral units on day 2 at 1 µM (30 µg/ml). This concentration was selected based on pilot experiments. F12/DMEM was replaced daily and Colα3(IV) NC1 domain protein was also included in the replacement medium until day 5, when F12/MMEM without recombinant protein was used. Each time point has triplicate bicameral units in both the treatment and the control group. Each experiment was repeated at least three times using different batches of Sertoli cells.
Immunohistochemistry, immunofluorescence microscopy and immunoblotting
Frozen cross-sections of testes were obtained in a cryostat at -22°C, collected on poly-L-lysine-coated microscopic slides, fixed in methanol for 10 min at -20°C. Immunohistochemistry was performed using Histostain-SP kit (AEC, rabbit, Life Technologies) with 1:100 anti-Col IV (Abcam) (Table 1) as described.70 Immunofluorescence staining was performed as described.79 For cell staining, primary Sertoli cells were either fixed in methanol at -20°C for 10 min (for basal ES-proteins N-cadherin and β-catenin) or 4% paraformaldehyde/PBS (w/v) (for TJ-proteins CAR and ZO-1) at room temperature for 10 min, and then permeabilized with 0.2% Triton X-100 (v/v) for 5 min. Dual-labeled immunofluorescence analysis was performed using rabbit anti-CAR or rabbit anti-N-cadherin vs. mouse anti-ZO-1 or mouse anti-β-catenin to detect the corresponding TJ- and basal ES-proteins at the Sertoli cell-cell interface (see Table 1). Negative controls were performed using normal rabbit IgG, diluted in blocking solution to substitute the primary antibody. Cells were then incubated with Alexa Fluor-conjugated secondary antibodies of the corresponding species (Alexa Fluor 555 for red fluorescence; Alexa Fluor 488 for green fluorescence) (Table 1) to visualize the corresponding target proteins. Fluorescence microscopy was performed using an Olympus BX61 microscope equipped with DP71 12.5 megapixel digital camera, and images were acquired using MicrosuitFive software package. All images were obtained in TIFF image format and analyzed (such as image overlay) using Adobe PhotoShop. Immunoblotting was performed by using 25 µg protein and 5 µg protein of Sertoli cell lysates and Sertoli cell-conditioned media, respectively, as described.79 All the antibodies used herein are listed in Table 1. Results of immunohistochemistry, dual-labeled immunofluorescence analysis and immunoblotting reported herein were findings of a representative experiment. Each experiment was repeated at least four times, excluding pilot experiments to establish experimental conditions, using at least n = 3 rats or different batches of Sertoli cells over a 3 y period.
Acknowledgments
We thank Dr Weiliang Xia for his assistance in the preparation of the anti-rabbit anti-Colα3(IV) NC1 antibody which was used in this report.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Data acquisition and Statistical Analysis
Data presented here were results from at least three independent experiments using different batches of Sertoli cells and recombinant proteins. Statistical Analysis was performed by ONE-way ANOVA followed by Dunnett’s test using the GB-STAT software package (Version 7.0, Dynamic Microsystems).
Author Contributions
Conceived the ideas of this project: CYC. Designed experiments: EWPW, CYC. Performed the experiments: EWPW, CYC. Analyzed the data: EWPW, CYC. Prepared the reagents, materials and analysis tools: EWPW, CYC. Wrote the paper and final editing of the manuscript: EWPW, CYC.
Funding
This work was supported by grants from the National Institutes of Health (NICHD, R01 HD056034 to C.Y.C.; U54 HD-029990, Project 5, to C.Y.C.). E.W.P.W. was supported by a postdoctoral fellowship from the Noopolis Foundation. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Previously published online: www.landesbioscience.com/journals/spermatogenesis/article/25465
References
- 1.Setchell B. The functional significance of the blood-testis barrier. J Androl. 1980;1:3–10. [Google Scholar]
- 2.Setchell BP. Blood-testis barrier, junctional and transport proteins and spermatogenesis. Adv Exp Med Biol. 2008;636:212–33. doi: 10.1007/978-0-387-09597-4_12. [DOI] [PubMed] [Google Scholar]
- 3.Cheng CY, Mruk DD. The blood-testis barrier and its implications for male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Setchell BP, Waites GMB. The blood-testis barrier. in The Handbook of Physiology. Section 7, Vol. V. Male Reproductive System (eds. Hamilton, D.W. & Greep, R.O.) 143-172 (American Physiological Society, Washington, D.C., 1975). [Google Scholar]
- 6.Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–26. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- 7.Turner TT, Jones CE, Howards SS, Ewing LL, Zegeye B, Gunsalus GL. On the androgen microenvironment of maturing spermatozoa. Endocrinology. 1984;115:1925–32. doi: 10.1210/endo-115-5-1925. [DOI] [PubMed] [Google Scholar]
- 8.Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int Rev Cell Mol Biol. 2009;278:309–53. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiebe JP, Kowalik A, Gallardi RL, Egeler O, Clubb BH. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J Androl. 2000;21:625–35. [PubMed] [Google Scholar]
- 10.Wiebe JP, Barr KJ. The control of male fertility by 1,2,3-trihydroxypropane (THP;glycerol): rapid arrest of spermatogenesis without altering libido, accessory organs, gonadal steroidogenesis, and serum testosterone, LH and FSH. Contraception. 1984;29:291–302. doi: 10.1016/S0010-7824(84)80009-8. [DOI] [PubMed] [Google Scholar]
- 11.Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod. 1993;49:840–9. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- 12.Setchell BP, Waites GMH. Changes in the permeability of the testicular capillaries and of the ‘blood-testis barrier’ after injection of cadmium chloride in the rat. J Endocrinol. 1970;47:81–6. doi: 10.1677/joe.0.0470081. [DOI] [PubMed] [Google Scholar]
- 13.Parizek J, Zahor Z. Effect of cadmium salts on testicular tissue. Nature. 1956;177:1036–7. doi: 10.1038/1771036b0. [DOI] [PubMed] [Google Scholar]
- 14.Parizek J. Sterilization of the male by cadmium salts. J Reprod Fertil. 1960;1:294–309. doi: 10.1530/jrf.0.0010294. [DOI] [Google Scholar]
- 15.Campbell M, Humphries P. The blood-retina barrier: tight junctions and barrier modulation. Adv Exp Med Biol. 2012;763:70–84. [PubMed] [Google Scholar]
- 16.Easton AS. Regulation of permeability across the blood-brain barrier. Adv Exp Med Biol. 2012;763:1–19. doi: 10.1007/978-1-4614-4711-5_1. [DOI] [PubMed] [Google Scholar]
- 17.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dym M. Basement membrane regulation of Sertoli cells. Endocr Rev. 1994;15:102–15. doi: 10.1210/edrv-15-1-102. [DOI] [PubMed] [Google Scholar]
- 19.Siu MKY, Cheng CY. Dynamic cross-talk between cells and the extracellular matrix in the testis. Bioessays. 2004;26:978–92. doi: 10.1002/bies.20099. [DOI] [PubMed] [Google Scholar]
- 20.Lustig L, Denduchis B, González NN, Puig RP. Experimental orchitis induced in rats by passive transfer of an antiserum to seminiferous tubule basement membrane. Arch Androl. 1978;1:333–43. doi: 10.3109/01485017808988354. [DOI] [PubMed] [Google Scholar]
- 21.Denduchis B, Satz ML, Sztein MB, Puig RP, Doncel G, Lustig L. Multifocal damage of the testis induced in rats by passive transfer of antibodies prepared against non-collagenous fraction of basement membrane. J Reprod Immunol. 1985;7:59–75. doi: 10.1016/0165-0378(85)90021-X. [DOI] [PubMed] [Google Scholar]
- 22.Siu MKY, Lee WM, Cheng CY. The interplay of collagen IV, tumor necrosis factor-alpha, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–87. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 23.Gelly JL, Richoux JP, Leheup BP, Grignon G. Immunolocalization of type IV collagen and laminin during rat gonadal morphogenesis and postnatal development of the testis and epididymis. Histochemistry. 1989;93:31–7. doi: 10.1007/BF00266844. [DOI] [PubMed] [Google Scholar]
- 24.Kahsai TZ, Enders GC, Gunwar S, Brunmark C, Wieslander J, Kalluri R, et al. Seminiferous tubule basement membrane. Composition and organization of type IV collagen chains, and the linkage of alpha3(IV) and alpha5(IV) chains. J Biol Chem. 1997;272:17023–32. doi: 10.1074/jbc.272.27.17023. [DOI] [PubMed] [Google Scholar]
- 25.Ortega N, Werb Z. New functional roles for non-collagenous domains of basement membrane collagens. J Cell Sci. 2002;115:4201–14. doi: 10.1242/jcs.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339:269–80. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assadian S, Teodoro JG. Regulation of collagen-derived antiangiogenic factors by p53. Expert Opin Biol Ther. 2008;8:941–50. doi: 10.1517/14712598.8.7.941. [DOI] [PubMed] [Google Scholar]
- 28.Sudhakar A, Boosani CS. Inhibition of tumor angiogenesis by tumstatin: insights into signaling mechanisms and implications in cancer regression. Pharm Res. 2008;25:2731–9. doi: 10.1007/s11095-008-9634-z. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.O’Bryan MK, Hedger MP. Inflammatory networks in the control of spermatogenesis : chronic inflammation in an immunologically privileged tissue? Adv Exp Med Biol. 2008;636:92–114. doi: 10.1007/978-0-387-09597-4_6. [DOI] [PubMed] [Google Scholar]
- 30.Fröjdman K, Pelliniemi LJ, Virtanen I. Differential distribution of type IV collagen chains in the developing rat testis and ovary. Differentiation. 1998;63:125–30. doi: 10.1046/j.1432-0436.1998.6330125.x. [DOI] [PubMed] [Google Scholar]
- 31.Davis CM, Papadopoulos V, Sommers CL, Kleinman HK, Dym M. Differential expression of extracellular matrix components in rat Sertoli cells. Biol Reprod. 1990;43:860–9. doi: 10.1095/biolreprod43.5.860. [DOI] [PubMed] [Google Scholar]
- 32.Enders GC, Kahsai TZ, Lian G, Funabiki K, Killen PD, Hudson BG. Developmental changes in seminiferous tubule extracellular matrix components of the mouse testis: alpha 3(IV) collagen chain expressed at the initiation of spermatogenesis. Biol Reprod. 1995;53:1489–99. doi: 10.1095/biolreprod53.6.1489. [DOI] [PubMed] [Google Scholar]
- 33.Guittot SM, Verot A, Odet F, Chauvin MA, le Magueresse-Battistoni B. A comprehensive survey of the laminins and collagens type IV expressed in mouse Leydig cells and their regulation by LH/hCG. Reproduction. 2007;135:479–88. doi: 10.1530/REP-07-0561. [DOI] [PubMed] [Google Scholar]
- 34.Howard BV, Macarak EJ, Gunson D, Kefalides NA. Characterization of the collagen synthesized by endothelial cells in culture. Proc Natl Acad Sci USA. 1976;73:2361–4. doi: 10.1073/pnas.73.7.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsubara T, Trüeb B, Fehr K, Rüttner JR, Odermatt BF. The localization and secretion of type IV collagen in synovial capillaries by immunohistochemistry using a monoclonal antibody against human type IV collagen. Exp Cell Biol. 1984;52:159–69. doi: 10.1159/000163256. [DOI] [PubMed] [Google Scholar]
- 36.Kramer RH, Bensch KG, Davison PM, Karasek MA. Basal lamina formation by cultured microvascular endothelial cells. J Cell Biol. 1984;99:692–8. doi: 10.1083/jcb.99.2.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laurie GW, Leblond CP, Cournil I, Martin GR. Immunohistochemical evidence for the intracellular formation of basement membrane collagen (type IV) in developing tissues. J Histochem Cytochem. 1980;28:1267–74. doi: 10.1177/28.12.6164715. [DOI] [PubMed] [Google Scholar]
- 38.Shoji A, Kabeya M, Sugawara M. Real-time monitoring of matrix metalloproteinase-9 collagenolytic activity with a surface plasmon resonance biosensor. Anal Biochem. 2011;419:53–60. doi: 10.1016/j.ab.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 39.Gioia M, Monaco S, Van Den Steen PE, Sbardella D, Grasso G, Marini S, et al. The collagen binding domain of gelatinase A modulates degradation of collagen IV by gelatinase B. J Mol Biol. 2009;386:419–34. doi: 10.1016/j.jmb.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 40.Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods Mol Biol. 2011;763:237–52. doi: 10.1007/978-1-61779-191-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan HHN, Mruk DD, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:8950–5. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su L, Mruk DD, Lie PPY, Silvestrini B, Cheng CY. A peptide derived from laminin-γ3 reversibly impairs spermatogenesis in rats. Nat Commun. 2012;3:1185. doi: 10.1038/ncomms2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao PL, Lin YC, Richburg JH. TNF alpha-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol Reprod. 2009;80:581–9. doi: 10.1095/biolreprod.108.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao PL, Lin YC, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol Reprod. 2010;82:516–27. doi: 10.1095/biolreprod.109.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazaud-Guittot S. Dissecting the phthalate-induced Sertoli cell injury: the fragile balance of proteases and their inhibitors. Biol Reprod. 2011;85:1091–3. doi: 10.1095/biolreprod.111.095976. [DOI] [PubMed] [Google Scholar]
- 46.Janecki A, Jakubowiak A, Steinberger A. Effect of cadmium chloride on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment cultures--a new model for toxicological investigations of the “blood-testis” barrier in vitro. Toxicol Appl Pharmacol. 1992;112:51–7. doi: 10.1016/0041-008X(92)90278-Z. [DOI] [PubMed] [Google Scholar]
- 47.Siu ER, Wong EW, Mruk DD, Sze KL, Porto CS, Cheng CY. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009;150:3336–44. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li MWM, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: is this a suitable model for studying blood-testis barrier dynamics? Int J Biochem Cell Biol. 2009;41:2302–14. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol Rev. 2008;60:146–80. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–17. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- 51.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 52.França LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol. 2012;763:237–59. [PubMed] [Google Scholar]
- 53.Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/S0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- 54.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6:380–95. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–3. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 56.Cohen CJ, Shieh JT, Pickles RJ, Okegawa T, Hsieh JT, Bergelson JM. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc Natl Acad Sci USA. 2001;98:15191–6. doi: 10.1073/pnas.261452898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergelson JM. Receptors mediating adenovirus attachment and internalization. Biochem Pharmacol. 1999;57:975–9. doi: 10.1016/S0006-2952(98)00332-3. [DOI] [PubMed] [Google Scholar]
- 58.Freimuth P, Philipson L, Carson SD. The coxsackievirus and adenovirus receptor. Curr Top Microbiol Immunol. 2008;323:67–87. doi: 10.1007/978-3-540-75546-3_4. [DOI] [PubMed] [Google Scholar]
- 59.Su L, Mruk DD, Cheng CY. Regulation of the blood-testis barrier by coxsackievirus and adenovirus receptor. Am J Physiol Cell Physiol. 2012;303:C843–53. doi: 10.1152/ajpcell.00218.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okegawa T, Pong RC, Li Y, Bergelson JM, Sagalowsky AI, Hsieh JT. The mechanism of the growth-inhibitory effect of coxsackie and adenovirus receptor (CAR) on human bladder cancer: a functional analysis of car protein structure. Cancer Res. 2001;61:6592–600. [PubMed] [Google Scholar]
- 61.Cheng CY, Mathur PP, Grima J. Structural analysis of clusterin and its subunits in ram rete testis fluid. Biochemistry. 1988;27:4079–88. doi: 10.1021/bi00411a026. [DOI] [PubMed] [Google Scholar]
- 62.Gu Q, Zhang T, Luo J, Wang F. Expression, purification, and bioactivity of human tumstatin from Escherichia coli. Protein Expr Purif. 2006;47:461–6. doi: 10.1016/j.pep.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Orth JM. Proliferation of Sertoli cells in fetal and postnatal rats: a quantitative autoradiographic study. Anat Rec. 1982;203:485–92. doi: 10.1002/ar.1092030408. [DOI] [PubMed] [Google Scholar]
- 64.Ahmed EA, Barten-van Rijbroek AD, Kal HB, Sadri-Ardekani H, Mizrak SC, van Pelt AM, et al. Proliferative activity in vitro and DNA repair indicate that adult mouse and human Sertoli cells are not terminally differentiated, quiescent cells. Biol Reprod. 2009;80:1084–91. doi: 10.1095/biolreprod.108.071662. [DOI] [PubMed] [Google Scholar]
- 65.Li JCH, Lee TW, Mruk TD, Cheng CY. Regulation of Sertoli cell myotubularin (rMTM) expression by germ cells in vitro. J Androl. 2001;22:266–77. [PubMed] [Google Scholar]
- 66.Lui WY, Lee WM, Cheng CY. Transforming growth factor beta3 regulates the dynamics of Sertoli cell tight junctions via the p38 mitogen-activated protein kinase pathway. Biol Reprod. 2003;68:1597–612. doi: 10.1095/biolreprod.102.011387. [DOI] [PubMed] [Google Scholar]
- 67.Wright WW, Zabludoff SD, Erickson-Lawrence M, Karzai AW. Germ cell-Sertoli cell interactions. Studies of cyclic protein-2 in the seminiferous tubule. Ann N Y Acad Sci. 1989;564:173–85. doi: 10.1111/j.1749-6632.1989.tb25896.x. [DOI] [PubMed] [Google Scholar]
- 68.Lee NPY, Mruk DD, Conway AM, Cheng CY. Zyxin, axin, and Wiskott-Aldrich syndrome protein are adaptors that link the cadherin/catenin protein complex to the cytoskeleton at adherens junctions in the seminiferous epithelium of the rat testis. J Androl. 2004;25:200–15. doi: 10.1002/j.1939-4640.2004.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 69.Bafico A, Gazit A, Pramila T, Finch PW, Yaniv A, Aaronson SA. Interaction of frizzled related protein (FRP) with Wnt ligands and the frizzled receptor suggests alternative mechanisms for FRP inhibition of Wnt signaling. J Biol Chem. 1999;274:16180–7. doi: 10.1074/jbc.274.23.16180. [DOI] [PubMed] [Google Scholar]
- 70.Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–62. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong EWP, Lee WM, Cheng CY. Secreted Frizzled-related protein 1 (sFRP1) regulates spermatid adhesion in the testis via dephosphorylation of focal adhesion kinase and the nectin-3 adhesion protein complex. FASEB J. 2013;27:464–77. doi: 10.1096/fj.12-212514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–47. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 73.Janecki A, Jakubowiak A, Steinberger A. Effects of cyclic AMP and phorbol ester on transepithelial electrical resistance of Sertoli cell monolayers in two-compartment culture. Mol Cell Endocrinol. 1991;82:61–9. doi: 10.1016/0303-7207(91)90009-H. [DOI] [PubMed] [Google Scholar]
- 74.Janecki A, Steinberger A. Polarized Sertoli cell functions in a new two-compartment culture system. J Androl. 1986;7:69–71. doi: 10.1002/j.1939-4640.1986.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 75.Okanlawon A, Dym M. Effect of chloroquine on the formation of tight junctions in cultured immature rat Sertoli cells. J Androl. 1996;17:249–55. [PubMed] [Google Scholar]
- 76.Byers S, Hadley MA, Djakiew D, Dym M. Growth and characterization of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl. 1986;7:59–68. doi: 10.1002/j.1939-4640.1986.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 77.Chen J, Fok KL, Chen H, Zhang XH, Xu WM, Chan HC. Cryptorchidism-induced CFTR down-regulation results in disruption of testicular tight junctions through up-regulation of NF-κB/COX-2/PGE2. Hum Reprod. 2012;27:2585–97. doi: 10.1093/humrep/des254. [DOI] [PubMed] [Google Scholar]
- 78.Kaitu’u-Lino TJ, Sluka P, Foo CF, Stanton PG. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction. 2007;133:1169–79. doi: 10.1530/REP-06-0385. [DOI] [PubMed] [Google Scholar]
- 79.Wong EWP, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGF-beta3 is a Cdc42-dependent protein trafficking event. Proc Natl Acad Sci USA. 2010;107:11399–404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]