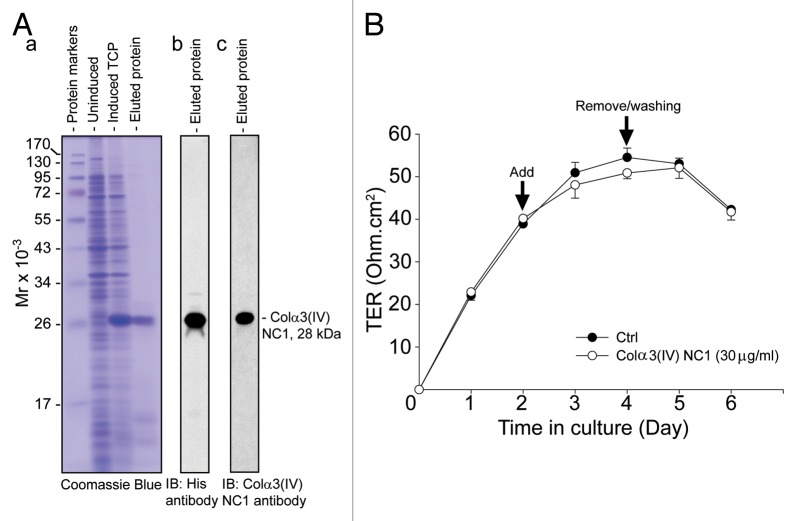
Figure 2. Expression of recombinant Colα3(IV)NC1 protein in bacteria, its purification, and its effects on the Sertoli cell TJ-permeability barrier function. (A) BL21 (DE3) competent E. coli cells transformed with pET46 Ek/LIC-Colα3(IV) NC1 vector was induced with 0.1 mM IPTG to express the recombinant protein. Strong expression of Colα3(IV) NC1 domain (28 kDa) was noted when compared the un-induced total cell protein (TCP) to induced TCP as shown in a representative Coomassie blue stained gel (A:a). Recombinant protein was purified by Ni2+-Sepharose chromatography in which the histidine (His, H) tag at the N-terminus of the recombinant protein specifically bound to the Ni-column. The eluted protein was purified to apparent homogeneity (A:b), and it was recognized by both anti-His (A:b) and anti-Colα3(IV) NC1 antibody (A:c) (see Table 1). (B) Primary Sertoli cells were plated at 1.2 × 106 cells/cm2 on Matrigel-coated bicameral units at time 0 to allow the assembly of a functional TJ-permeability barrier. Purified bacterial Colα3(IV) NC1 recombinant protein was refolded and dialyzed against PBS as described in Materials and Methods, and it was included in the F12/DMEM medium on day 2 at a concentration of 30 μg/ml (~1 µM). Mild but not statistically significant perturbation of the Sertoli cell BTB was observed when compared with PBS control (Ctrl) (Fig. 2B). Bacterial recombinant Colα3(IV) NC1 protein was removed 2 d after incubation. Each data point had n = 3 bicameral units. This experiment was repeated three times using different batches of Sertoli cells as well as recombinant protein, and yielded similar results.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.