Abstract
Formaldehyde is a colorless, pungent gas commonly found in homes that is a respiratory irritant, sensitizer, carcinogen and asthma trigger. Typical household sources include plywood and particleboard, cleaners, cosmetics, pesticides, and others. Development of a fast and simple measurement technique could facilitate continued research on this important chemical. The goal of this research is to apply an inexpensive short-term measurement method to find correlations between formaldehyde sources and concentration, and formaldehyde concentration and asthma control. Formaldehyde was measured using 30-minute grab samples in length-of-stain detector tubes in homes (n=70) of asthmatics in the Boston, MA area. Clinical status and potential formaldehyde sources were determined. The geometric mean formaldehyde level was 35.1 ppb and ranged from 5–132 ppb. Based on one-way ANOVA, t-tests, and linear regression, predictors of log-transformed formaldehyde concentration included absolute humidity, season, and the presence of decorative laminates, fiberglass, or permanent press fabrics (p<0.05), as well as temperature and household cleaner use (p<0.10). The geometric mean formaldehyde concentration was 57% higher in homes of children with very poorly controlled asthma compared to homes of other asthmatic children (p=0.078). This study provides a simple method for measuring household formaldehyde and suggests that exposure is related to poorly controlled asthma.
Keywords: Formaldehyde, asthma, housing, colorimetric detector tubes, household chemical exposure, absolute humidity
Introduction
Formaldehyde has recently received public attention from both its classification as a known human carcinogen (NTP, 2011) and the health concerns raised after the use of contaminated trailers following Hurricane Katrina by the Federal Emergency Management Agency (FEMA) in 2005 and later (Maddalena et al., 2009). Much of the recent focus has been on formaldehyde levels indoors, where people spend 87% of their time (Klepeis et al., 2001) and where the concentrations are typically higher than outdoor concentrations (Zhang et al., 1994; Gonzalez-Flesca et al., 1999; Khoder et al., 2000). Children exposed to formaldehyde in excess of 49 ppb (60 µg/m3) in indoor air have an increased risk of childhood asthma (Rumchev et al., 2002), and a recent meta-analysis of several studies has shown a positive association between formaldehyde levels and childhood asthma (McGwin et al., 2009). Other health outcomes that may be associated with formaldehyde or the presence of particle board, a known formaldehyde source, in homes include chronic bronchitis, increased exhaled nitric oxide (marker of lower airway inflammation), increased wheeze, respiratory symptoms, adverse effects on lung function, atopy and increased risk of sensitization (Mendell, 2007). In addition, previous studies have shown that formaldehyde exposure in the home may increase asthma severity (Venn et al., 2003).
Formaldehyde is typically measured in the home by the reaction of 2,4-dinitrophenylhydrazine (DNPH) in a cartridge (Kuntz et al., 1980; Grosjean, 1991; EPA, 1999) or by the collection of formaldehyde in an impinger (CARB, 1991; Woo et al., 1998), in addition to a variety of other techniques (Li et al., 2001; Rocha et al., 2008; Salthammer et al., 2010). In the cartridge method, formaldehyde in the air reacts with DNPH on a sorbent to form the product 2,4-dinitrophenylhydrazone, which is then transported to a laboratory setting for analysis by high performance liquid chromatography (HPLC). This method is the most common in-home measurement method with a detection limit of 0.5 ppb (EPA, 1999). In the impinger method, formaldehyde in the air is trapped in a liquid, and is also analyzed by HPLC. Both methods require prolonged sampling time, expensive analytical equipment, and are prone to contamination during transportation and analysis. Other techniques such as the active diffusive sampling technique proposed by Rocha and colleagues (Rocha et al., 2008) also present various challenges, such as the necessity of constructing sampling devices that are not commercially available. Additionally, many techniques were developed for measuring formaldehyde in the workplace, where concentrations are relatively high (Triebig et al., 1989). Formaldehyde sampling in homes is often difficult due to the relatively low concentrations present, and only some of the available techniques have adequate sensitivity (Sandner et al., 2001). While many techniques have successfully been utilized in the past, overall these methods remain time-consuming, difficult, and/or expensive. The need for a fast and simple formaldehyde measurement technique with appropriate detection limits for household concentrations has been highlighted in a recent review (Salthammer et al., 2010). Here, this small study will also determine if a shorter-term measurement method could still be an indicator of asthma morbidity and also an indicator of potential household sources.
The establishment of a simple, quick, and inexpensive formaldehyde measurement technique for use in epidemiological studies conducting in-home sampling would encourage continued measurement of this important indoor air pollutant. While much literature on formaldehyde exists, formaldehyde measurement could attain more widespread use in epidemiological studies. The ability to easily obtain multiple measurements would also allow researchers to look at factors such as concentration trends over time as related to health outcomes. In addition, the availability of such a technique would allow for expanded applications of formaldehyde measurement, including as a part of public health interventions and preventative medicine. A recent review explored chemical exposures in the home as an important area for public health intervention, including avoidance of particle board products that emit formaldehyde (Sandel et al., 2010). Quantifying formaldehyde levels in the homes of asthmatic children is important because this group may be particularly vulnerable to this respiratory irritant (Krzyzanowski et al., 1990). In addition, a simple and quick measurement technique would allow for easy evaluation of a program by allowing for sampling both before and after an intervention with minimal time spent in the home.
Formaldehyde is often measured in the workplace with high formaldehyde levels for safety considerations using colorimetric gas detector tubes. Many of these tubes are designed to measure formaldehyde closer to the range of the Occupational Safety and Health Administration (OSHA) permissible exposure limit (PEL) of 750 ppb or the action level of 500 ppb. While the concentration range of many of these tubes is higher than levels typically found in homes, there is a commercially available option with the measurement range of 10–480 ppb. The required sampling time is 10–30 minutes, and no expensive analytical equipment is required. In addition, there is no potential for contamination from ambient formaldehyde during transport or analysis, because the tube is sealed prior to sampling and results are obtained immediately at the conclusion of the sampling period. The goals of this research are 1) to evaluate the use of a simple and inexpensive formaldehyde measurement method within the home environment, 2) to determine what housing factors affect formaldehyde concentration, and 3) to analyze the relationship between household formaldehyde exposure and asthma control level. Gaining information about this common indoor air pollutant can lead to improved knowledge of correlations between formaldehyde concentrations, formaldehyde sources and human health outcomes and help target interventions that hold promise for reducing formaldehyde exposure and improving associated asthma control.
Methods
Kitagawa 710 formaldehyde detector tubes (Komyo Rikagaku Kogyo K.K., Kawasaki-City, Kanagawa 213-0006, Japan) were used to measure formaldehyde according to manufacturer instructions, with one modification. Instead of using the manufacturer’s pump, we substituted a low-cost aquarium pump (Tetra Whisper® 100), which was modified by reversing the diaphragm to pull air through the tube. We choose a pump capable of achieving an approximately 300 mL/min flow rate that remains constant. In actual use, the average pump flow rate was 299 mL/min and ranged from 262–350 mL/min. This alternative pump drastically reduced the overall cost of sampling formaldehyde in this study of 70 homes to approximately $500 USD (cost of pump and tubes combined), with a savings around $3000 USD. The manufacturer reports a relative standard deviation of 10% and a detection limit of 5 ppb for this method.
Formaldehyde readings and pump performance were validated to ensure that the alternative pump did not affect the results. Formaldehyde measurement was checked in a laboratory setting by heating a permeation tube (Vici Metronics, Poulsbo, Washington, USA) in a furnace to 50°C to release a known concentration of formaldehyde into a vial. The permeation tube was inserted into the vial, and the air was pulled through the tube with the pump. We corrected for temperature and pressure as recommended by the manufacturer. The slope of the line between the expected formaldehyde reading and the actual formaldehyde reading was 0.998 with a standard error of 0.158 (R2=0.930). Pump performance was also validated by measuring the flow rate through the permeation tubes from four different pumps and from the same pump over time. The pump flow rate remained constant over the time period required for sampling. In addition, the slight variations in measured pump flow rate (262–350 mL/min) on different sampling dates in the field did not significantly affect the value of formaldehyde readings after sampling time correction to maintain a constant sampling volume (p=0.687).
Formaldehyde in the air was measured in 70 homes of primarily low-income asthmatic children and adults in the Boston, MA area as a part of the Boston Allergen Sampling Study (manuscript in preparation) between July 2008 and February 2010. The main objectives of this study were to determine which of three commonly used house dust allergen sampling methods, which sampling rooms, and which surfaces within rooms were most useful in predicting uncontrolled asthma in asthmatic children with documented sensitization to indoor allergens. Housing characteristics such as flooring type, number of occupants, ownership, heating type, number of windows, number of vents, condition of the walls, and building age were recorded via a structured interview with the resident and visual inspection as is common in many studies (Wilson et al., 2010). Residents were also asked about professional pesticide application within the last four months and about resident smoking habits. The visible presence of specific potential formaldehyde sources in the kitchen, including gas stoves, fingernail polish, fingernail hardener, latex paint, plywood and particle board, fiberglass, new carpet, decorative laminates, permanent press fabrics, grocery bags, and paper towels was also recorded. The amount was not quantified but rather any presence at all of an item was counted as “yes.” If an item was not visually present (or difficult to identify visually, such as with new carpet), the resident was asked to confirm its presence or absence. Residents were also asked about the use of ammonia-based cleaners in the past week, which should be sufficiently longer than the hours to days required for general cleaner concentration to diffuse (Zhu et al., 2001; Nazaroff, 2004). Ammonia can interfere with the chemical reaction inside the tubes, and cleaners themselves may also be a source of formaldehyde. Temperature and relative humidity were also recorded. Absolute humidity was calculated from relative humidity and temperature and assumed average sea-level pressure. In addition, the validated Asthma Control Test (ACT) (Nathan et al., 2004) for ages 12 and up was administered to the asthmatic resident. If the child was under 12, the parent or caregiver was asked to answer for the child.
The formaldehyde sampling was conducted in the kitchen on top of the refrigerator if possible. Previous studies have shown that formaldehyde concentration is typically consistent throughout a home (Clarisse et al., 2003; Marchand et al., 2008), so this location was chosen to avoid interference with the allergen measurement part of the study. Sampling location in the kitchen also did not affect the formaldehyde concentration (p=0.212) if it was not possible to place the sampling device on top of the refrigerator (n=9). The room was not altered prior to sampling (i. e. no windows or doors were closed), but the number of open windows and the presence of an exhaust fan venting outside were noted and each was not significantly associated with formaldehyde concentration (p>0.1 for both cases). The flow rate of the pump was measured prior to sampling to adjust sampling time to maintain a constant sampled air volume. Formaldehyde tubes remained sealed until immediately before sampling to prevent contamination. After the seal on the tube was broken on both ends using the manufacturer’s glass cutter, the tube was connected to the pump in a configuration to pull air through the tube and the apparatus was placed on top of the refrigerator. A nominal 10 minute sampling period measuring concentrations from 40–480 ppb was followed by an additional nominal 20 minutes of sampling time to measure formaldehyde in the range of 10–120 ppb. Formaldehyde readings at both time points were recorded, and the final reading was calculated from the 30 minute reading unless the concentration exceeded 120 ppb. The final reading was also adjusted for temperature as recommended by the manufacturer.
The manufacturer suggests that ammonia and NOx can interfere with formaldehyde readings if present at high concentrations of at least 500 ppb. Residents were asked if ammonia-based cleaners had been used in the week prior to sampling, and the presence of a gas stove was noted for potential NOx release. However, both cleaning products and gas stoves can also release formaldehyde (Hollowell et al., 1980; Colombo et al., 1991; Flyvholm and Andersen, 1993; Singer et al., 2006). While these are not the only potential ammonia sources, none of the colorimetric changes associated with ammonia exposure were observed. The presence of ammonia would be expected to falsely lower readings, and in fact houses where cleaners were used tended to have higher (Table 1) formaldehyde concentrations, suggesting a lack of ammonia interference. Analyses were conducted both including and excluding the 10 homes that used ammonia-based cleaners, and the lack of a substantial difference in the overall results prompted the inclusion of these 10 homes. Interference from NOx could falsely increase formaldehyde readings, but the presence of a gas stove did not have a significant effect on the concentration (Table S1 in Supporting Information). These results are consistent with a study in houses in Quebec, Canada that found gas stoves influence NO2 but not formaldehyde concentrations (Gilbert et al., 2006). This study also found the maximum in-home NO2 concentration to be 15 ppb (29.1 µg/m3) (Gilbert et al., 2006), which is substantially less than the 500 ppb interference limit, making interference in a typical home unlikely.
Table 1.
Descriptive statistics for at least marginally significant potential formaldehyde sources and environmental conditions (n=67).†
| Source or environmental condition | Yes # (%)‡ | GM Formaldehyde Concentration (ppb) |
p- value* |
|
|---|---|---|---|---|
| Yes | No | |||
| Absolute humidity (gm−3) | Average: 9.64±3.50 |
N/A | <0.001 | |
| Decorative laminates, fiberglass, or permanent press fabrics | 16 (24%) | 50.6 | 31.3 | 0.013 |
| Season | 0.044 | |||
| Winter | 17 (25%) | 27.0 | ||
| Spring | 9 (13%) | 34.9 | ||
| Summer | 17 (25%) | 52.5 | ||
| Fall | 24 (36%) | 32.8 | ||
| Permanent press fabrics | 6 (9%) | 56.0 | 33.5 | 0.079 |
| Ammonia-based cleaners used within the past week | 10 (15%) | 48.0 | 31.7 | 0.080 |
| Temperature | Average: 22.6±3.2°C |
N/A | 0.095 | |
See Table S1 in Supporting Information for information on all considered variables and missing data information.
For continuous variables, average ± standard deviation is reported.
The p-values were calculated using a t-test comparing “Yes” and “No” categories only for categorical variables, except for season which used ANOVA analysis. The p-values for continuous variables were calculated using linear regression analysis.
Statistical analysis was conducted in PASW (SPSS) version 19 (IBM, Chicago, IL, USA) using one-way ANOVA and t-tests for individual variables, and linear regression. Statistical significance was defined as p < 0.05 and significance values between 0.05 and 0.10 were considered marginally significant. Formaldehyde concentrations were log10 transformed for all analyses. Analyses were performed for environmental factors including temperature, absolute humidity, and seasons, and for the presence of formaldehyde-releasing products (plywood and particle board, fingernail polish, etc.). All considered variables are listed in Table S1, along with variable combinations that were used in the linear regression. If variables had less than 5 homes with a missing or unsure response, the answer was counted as “no.” For variables with 5 or more homes in the unsure/missing category, indicator variables were created. For linear regression analysis, variables were combined as follows: non-wood expected high-level emitters (nail polish and latex paint) were combined, expected medium-level formaldehyde emitters (permanent-press fabrics, fiberglass, and decorative laminates) were combined, and the presence of expected low-level formaldehyde emitters were summed (cardboard, grocery bags, and paper towels). All variables were added to the model initially, and the non-significant variables were removed sequentially to obtain the final model. Any missing data were excluded from the analysis, and the formaldehyde reading was not completed in 3 homes which were removed from the analysis (final n=67).
Independent sample 2-tailed t-tests were conducted for the asthma control analysis. Because of the small sample size, statistical analysis of the ACT included bivariate versions of both individual questions and the overall measure (score < 12). Only children under the age of 18 who completed the assessment were included in the health effects analysis (n=37). This analysis was restricted to children because i) children are the major age group in this study, ii) the effects of environmental exposures may differ between children and adults (Bearer, 1995), and iii) other asthma-related respiratory effects have been seen in children previously (Garrett et al., 1999; Rumchev et al., 2002; Venn et al., 2003; McGwin et al., 2009).
Results
Housing and population characteristics
Table S1 displays housing characteristics and all of the variables included in the statistical analysis (statistically significant and marginally significant variables appear in Table 1). Table 2 displays population characteristics of the asthmatic including age, race, and gender (demographic information for children included in the asthma control analysis appears in Table S2). The average age included in the study was 20.4 years, and ages ranged from 4.8 to 64.9 years. The average age for children was 10.5 years and the average age for adults was 43.2 years. There was an average of 3.8 people living in each unit, and an average of 6.6 dwelling units per building. Only 5 (7%) of the homes were single-family dwellings (one unit per building). Most units were constructed of wood (58%), and most of the remaining units were constructed of brick (36%). Over half (52%) of the units were built before 1950, a quarter (25%) were built between 1950–1978, and the remainder were built after 1978 (15%) or the date of construction was unknown. Residents were not asked about recent renovations in particular, but no units had a new carpet. In 15 (22%) homes, it was reported that at least one resident smokes indoors.
Table 2.
Demographics (n=67)*.
| Population information | Yes # (%) |
|---|---|
| Children (age under 18) | 46 (69%) |
| Female | 35 (52%) |
| Race | |
| African | 2 (3%) |
| African-American/Black | 40 (60%) |
| Cape Verdean | 1 (1%) |
| Caribbean | 3 (4%) |
| Hispanic | 8 (12%) |
| White | 5 (7%) |
| Other | 6 (9%) |
| Missing Race | 2 (3%) |
See Table S2 in Supporting Information for demographic information for the children included in the asthma control analysis.
Formaldehyde levels
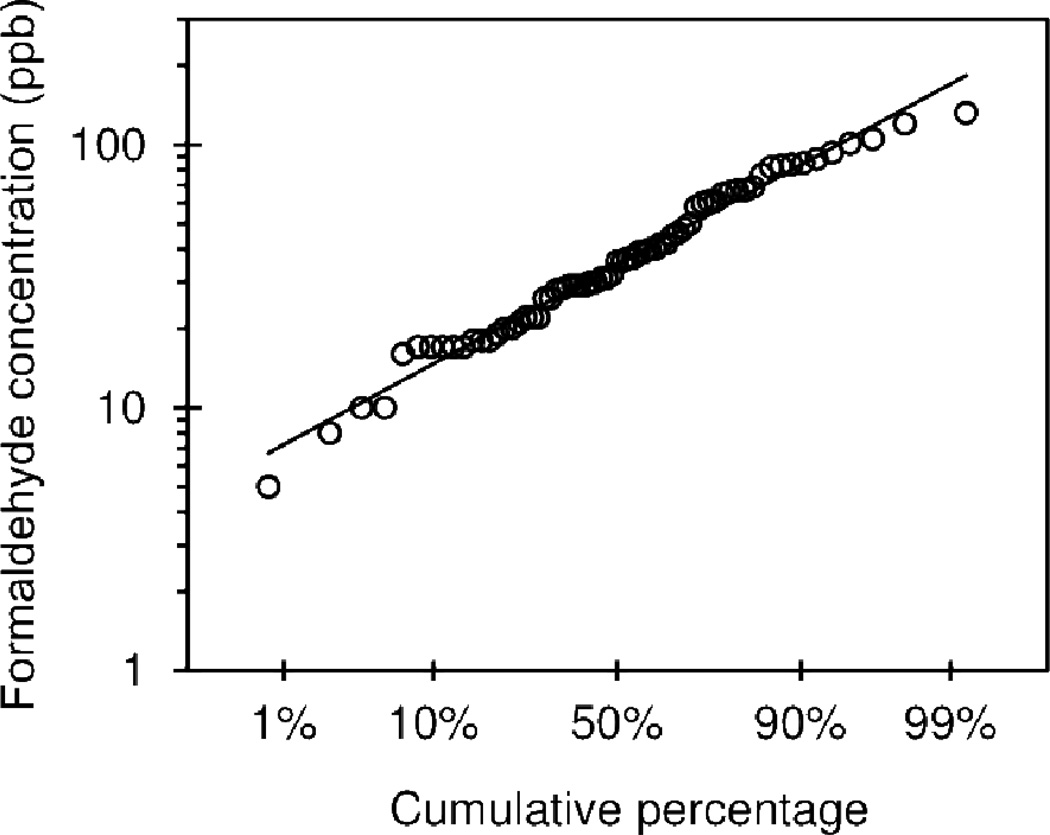
The cumulative distribution of formaldehyde concentration is shown in Figure 1. Formaldehyde was detected in all homes, and the concentrations ranged from 5–132 ppb. The data were log-normally distributed with a geometric mean of 35.1 ppb, and the geometric standard deviation was 1.98. The median was 36.0 ppb. The World Health Organization (WHO) indoor limit of 100ppb (WHO, 1989) was exceeded in 4 (6%) houses, and the National Institute for Occupational Safety and Health (NIOSH) recommended exposure limit (REL) over an 8-hour day is 16 ppb (NIOSH, 1981), which was exceeded in 62 (93%) houses, although this level was based on the limit of detection and not health effects data. The level found to increase the prevalence of asthma in children of 49 ppb (60µg/m3) (Rumchev et al., 2002) was exceeded in 21 (31%) houses.
Figure 1.
Cumulative distribution of the formaldehyde concentration (ppb) in 67 homes of primarily low-income asthmatics in the Boston, MA area.
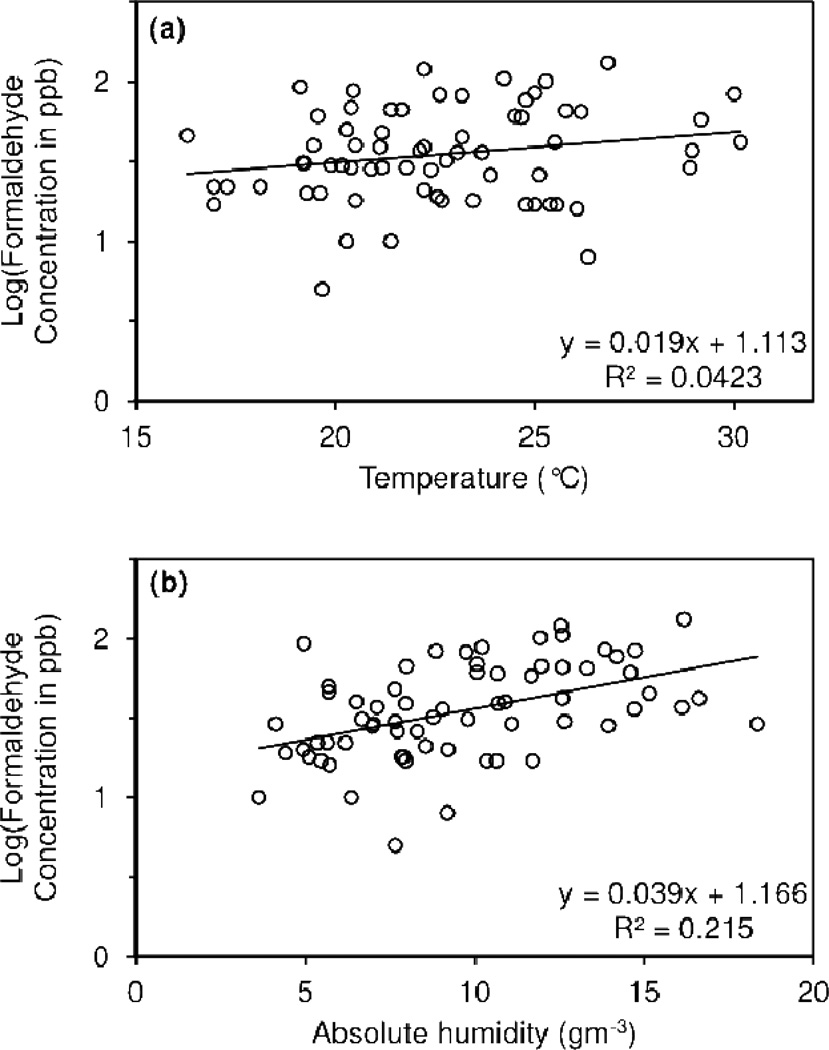
Table 1 and Table S1 also display geometric mean formaldehyde concentrations based on measured variables, as well as the associated p-value from a t-test. Formaldehyde concentration changed with temperature, absolute humidity, and season. Regression analysis revealed that temperature (Figure 2a) was marginally significant and absolute humidity (Figure 2b) was significant at predicting formaldehyde concentration. Formaldehyde concentrations were highest at high temperature and high absolute humidity. Changes in formaldehyde concentration with season were statistically significant (Table 1). Concentrations were highest in the summer, followed by fall, spring, and then winter.
Figure 2.
Linear regression analysis of (a) temperature (°C) and (b) absolute humidity (gm−3) as predictors of formaldehyde concentration.
Formaldehyde concentration also increased with cleaner use, the presence of permanent press fabrics, and the combination variable combining permanent press fabrics, decorative laminates, and fiberglass. While the formaldehyde concentration also changed with the presence of visible plywood and particle board, the formaldehyde concentration surprisingly appears to decrease when these pressed-wood products are present (Table S1). Pressed-wood products are known formaldehyde emitters (Myers, 1985; Zhang et al., 2007), but the formaldehyde release may be erratic, especially under non-steady-state conditions (Myers, 1985) and with other formaldehyde-emitters present (Pickrell et al., 1984). In addition to this potentially erratic behavior, it is likely that these pressed-wood products are present in nearly every house in the study but are simply not visible (such as plywood subflooring) and these invisible pressed-wood products were not included in this variable. Therefore, this variable was deemed to be a poor measure of plywood and particle board in a home and was excluded from Table 1 and further analysis. No houses in the study contained nail hardener or new carpet, so these variables were also excluded from all subsequent analysis.
Multiple linear regression modeling (Table 3) indicated that absolute humidity was the strongest predictor of formaldehyde concentration, and no other variables were significant in this model. A second multiple linear regression model (Table 4) showed that if absolute humidity was excluded, the presence of fiberglass, decorative laminates, or permanent-press fabrics led to statistically significant increases in formaldehyde concentration, while the use of cleaning products within the last week was marginally significant. Residuals were normally distributed in both models. All other variables in Table S1 did not significantly contribute to formaldehyde level in this study and were subsequently removed from the model. Season showed co-linear characteristics with absolute humidity, and was therefore removed from the model.
Table 3.
Log-linear regression model of environmental factors to predict formaldehyde concentration. S = 0.2652 R2 = 21.5% R2 (adjusted) = 20.3% p-value < 0.001.
| Predictor | Coefficient | Standard Error of Coefficient |
p- value |
|---|---|---|---|
| Intercept | 1.166 | 0.096 | <0.001 |
| Absolute Humidity (gm−3) | 0.039 | 0.009 | <0.001 |
Table 4.
Log-linear regression model of household items visibly present to predict formaldehyde concentration. S = 0.2805 R2 = 14.9% R2 (adjusted) = 10.8% p-value = 0.017.
| Predictor | Level (value) | Coefficient | Standard Error of Coefficient |
p-value |
|---|---|---|---|---|
| Intercept | - | 1.459 | 0.043 | <0.001 |
| Ammonia-based cleaners used within the past week | Yes (1)* | 0.183 | 0.097 | 0.064 |
| Unsure/Missing (2)* | 0.130 | 0.123 | 0.294 | |
| Fiberglass, permanent press fabrics, or decorative laminates | Yes (1)* | 0.198 | 0.082 | 0.019 |
versus No (0)
The ACT questionnaire was completed by the caregiver or child for 37 of the 46 children in the study. Table 5 displays the results of the questions on the ACT as compared to formaldehyde concentration. The study participants with asthma rated in the most severe category of each question were compared to the other study participants. For all five questions, the participants with the worst asthma control had a higher formaldehyde concentration in their homes. For the questions about interference with work or school and shortness of breath these results were marginally significant. The children in the most severe group for these questions had geometric mean formaldehyde levels that were higher than other participants by 66% and 81%, respectively. Participants with an overall ACT score < 12 (very poor control) also had formaldehyde concentrations that were 57% higher than other participants with a marginally significant p-value of 0.078.
Table 5.
Asthma Control Test (ACT) comparison of formaldehyde concentrations in most severe group of each question to the remaining groups*.
| Asthma Control Test (ACT) Question | Yes # (%) (in most Severe group) |
GM Formaldehyde Concentration (ppb) |
p-value | |
|---|---|---|---|---|
| Most Severe group |
All Other groups |
|||
| 1. In the past 4 weeks, how much of the time did your asthma keep you from getting as much done at work, school or at home? | 5 (14%) | 57.4 | 34.6 | 0.066 |
| 2. During the past 4 weeks, how often have you had shortness of breath? | 3 (8%) | 64.0 | 35.0 | 0.086 |
| 3. During the past 4 weeks, how often did your asthma symptoms (wheezing, coughing, shortness of breath, chest tightness or pain) wake you up at night or earlier than usual in the morning? | 4 (11%) | 53.3 | 35.5 | 0.184 |
| 4. During the past 4 weeks, how often have you used your rescue inhaler or nebulizer medication (such as albuterol)? | 4 (11%) | 46.5 | 36.1 | 0.409 |
| 5. How would you rate your asthma control during the past 4 weeks? | 3 (8%) | 60.2 | 35.5 | 0.128 |
| Overall ACT < 12 (very poor control) | 6 (16%) | 54.0 | 34.4 | 0.078 |
This analysis was restricted to children (age < 18) for which the ACT data was available (n=37).
Season was initially considered as a categorical variable in the statistical model since asthma severity can vary by season (Sears, 2008). The 37 samples were distributed fairly evenly among the seasons (range 8–11 per season), and children with very poorly controlled asthma were also distributed fairly evenly among the seasons (one in winter, two in spring, two in summer, and one in fall). Season was not found to be significant and was subsequently dropped from the model (p=0.23). However, this analysis was conducted on a small sample size of n=37 and season may be significant in subsequent, larger studies.
Discussion
This formaldehyde measurement method is simple, quick and inexpensive and was easily included in an existing asthma study. While the correlation coefficient of 0.964 and the detection limit of 5 ppb may not be as precise as the DNPH method (example values have been reported as 0.99998 and 0.09 ppb (0.07 µg/m3), respectively (Sandner et al., 2001)), the measurement range of 10–480 ppb and the precision are appropriate for in-home measurement. The equipment is all commercially available, and the process of incorporating formaldehyde measurement into an existing epidemiological study using this method requires minimal financing and effort. Total additional equipment costs for the sampling in this study of 70 homes including the pump and tubes were approximately $500 USD.
The measured formaldehyde concentrations with a geometric mean of 35.1 ppb are similar to other studies. For instance, some recent studies have found average formaldehyde levels in homes to be 12.6 ppb (Garrett et al., 1999), 22.8 ppb (Dingle and Franklin, 2002), 24.0 ppb (29.5 µg/m3) (Gilbert et al., 2006), 26 ppb (Krzyzanowski et al., 1990), 36 ppb (43.7 µg/m3) (Lindstrom et al., 1995) and 40 ppb (Hodgson et al., 2000). Previous studies have also found formaldehyde to be influenced by humidity but not temperature (Gilbert et al., 2006). Fiberglass, decorative laminates, permanent-press fabrics and cleaning products can be expected to increase formaldehyde concentration since they are known formaldehyde releasers.
This measurement method is appropriate when a short-term formaldehyde reading is desired due to the rapid sampling time of 30 minutes or less. Short sampling time provides the benefit of nearly immediate results, which could be provided to residents during the same visit if used in a public health intervention situation, allowing for education about the reading. In addition, short-term samples are not subject to tampering when left unattended for long time frames. The method described herein is economical and easy to use, which are key advantages as compared to longer-term sampling approaches. Further, because the method is relatively inexpensive, repeat samples could be collected on a seasonal or monthly basis if fluctuations in formaldehyde concentrations are suspected.
Previous studies have also used short-term sampling methods (Marchand et al., 2008) and the DNPH method was originally developed for an intended 2-hour sampling period (Kuntz et al., 1980). However, the formaldehyde concentration is likely constantly in flux, and even a week-long sample taken in the same dwelling did not correlate well to a week-long sample taken half of a year later (Sexton et al., 1989). However, formaldehyde concentrations have been shown to be fairly consistent within the same home over the course of a week with some diurnal variation (Stock, 1987). While the concentration in one manufactured home changed with season, it remained similar within the same season (Hodgson et al., 2004) and three other manufactured homes had consistent readings over time (Hodgson et al, 2000). Readings in this study are considered seasonally representative since conditions that alter readings on the short-term time scale were minimized, such as accounting for open windows. It is also noteworthy that despite the limitations of short-term sampling, correlations with asthma severity were still found.
In this study, the formaldehyde concentration was measured at the end of the period monitored for asthma severity and assumed to be an indicator of the longer-term formaldehyde concentration that is persistent in the home from at least the previous month. This analysis would not detect the effect of short-term formaldehyde spikes on day-to-day asthma control, but is likely indicative of long-term exposure trends. Future studies should follow-up on these results by measuring formaldehyde exposure followed by the ACT or other asthma severity measures. NOx has a known correlation with many of the same health outcomes that are likely to be associated with formaldehyde (Neas et al., 1991), such that any interference of NOx with the formaldehyde reading may confound results related to respiratory disease. However, typical NOx levels in homes (Gilbert et al., 2006) are much less than the manufacturer’s indicated interference limit, and no significant increase in formaldehyde concentration was seen with the presence of gas stoves, indicating that this interference was likely negligible in the household setting. In addition, the increases in formaldehyde readings due to humidity (Gilbert et al., 2006), fiberglass, decorative laminates, permanent-press fabrics and cleaning products were likely not due to increases in NOx, suggesting little to no interference in this study. In future epidemiological studies, it may be advisable to also consider measuring NOx, both because it is relevant to human health outcomes and to ensure that it does not interfere with formaldehyde readings, although this may increase the cost and effort associated with the study. Although not reported in the product documentation provided by the manufacturer, the manufacturer suggests that other aldehydes and ketones might also interfere to cause a higher reading (L. Kent, Kitagawa America, personal communication). Previous studies have found that formaldehyde is typically the compound with the highest in-house concentration among these chemicals (Hodgson et al., 2000; Jurvelin et al., 2001; Liu et al., 2006; Marchand et al., 2008; Zhang et al., 1994) such that while these readings may be artificially elevated slightly, the values are still likely to accurately reflect the formaldehyde concentration. However, future studies should also consider this potential interference. If these tubes are used for public health interventions and preventative medicine, it is also important to consider that many of these other chemicals may also be associated with negative health effects (Cassee et al., 1996; O’Brian et al., 2005) and may arise from similar sources as formaldehyde (Hodgson et al., 2000; Hodgson et al., 2002; Liu et al., 2006) such that a small interference from these compounds may be acceptable in these applications.
While the number of children in this study who completed the ACT test was small (n=37), Table 5 still shows a marginally significant trend toward decreased asthma control with increased formaldehyde concentration. One limitation of this study is that the formaldehyde readings were taken after the period used for asthma severity testing, but it is likely that these results are at least somewhat indicative of longer-term formaldehyde concentration trends in the home. Future studies that measure formaldehyde concentration before asthma severity should be conducted to confirm these results.
These asthma severity results for the overall score were not due to changes in absolute humidity (p=0.672). However, because absolute humidity is the best predictor of formaldehyde concentration, reducing the indoor humidity level may in turn reduce the formaldehyde concentration and help with relief of asthma symptoms. Other possible methods to reduce formaldehyde levels based on the results of this study include reducing temperature, reducing the use of formaldehyde-releasing cleaners, and removing potential formaldehyde sources such as decorative laminates, fiberglass, and permanent-press fabrics. These asthma control results are consistent with the epidemiological literature showing an association between formaldehyde exposure and respiratory effects (McGwin et al., 2009; Mendell, 2007; Venn et al., 2003). Reducing indoor exposure to formaldehyde may be an important component to help some asthma patients better control their asthma, but further study is needed to confirm these results. Formaldehyde exposure measurement and control might be considered a strong candidate for future public health interventions or preventive medicine, and the use of this simple and rapid method may help facilitate this work.
Nearly a third of the houses in this study exceeded the formaldehyde level of 49ppb (60µg/m3) found to increase the risk of childhood asthma (Rumchev et al., 2002). This is not surprising as all of the houses included in this study are home to at least one individual with asthma. However, this high percentage, coupled with the association between formaldehyde concentrations and asthma control, underscores the public health importance of measuring and understanding this respiratory irritant. It is our hope that this simple and inexpensive colorimetric detector tube technique will be incorporated into future epidemiological studies to strengthen associations between formaldehyde exposure and respiratory effects and also help to target home-based asthma interventions.
Conclusions
Formaldehyde measurement was successfully incorporated into an existing epidemiological allergy and asthma study by the utilization of commercially available colorimetric detector tubes. The simultaneous measurement of NOx may be advisable in future studies to both avoid this potentially interfering factor and also because it is an important indoor contaminate, although interference from this contaminate is likely minor if present at all. It is important to note that other aldehydes and ketones may also cause some interference, although formaldehyde is likely the dominant contributor to the measurement. The geometric mean value of formaldehyde in the homes of these asthmatics was consistent with literature values, and nearly a third of the homes exceeded a value previously shown to increase the risk of childhood asthma. Homes of children with very poorly controlled asthma showed a 57% increase in formaldehyde concentration. The results of this study support a new and convenient method for the measurement of formaldehyde in homes and underscore the importance of measuring this common respiratory irritant, both in research settings and as a part of public health interventions.
Supplementary Material
Practical Implications.
A simple, replicable model for formaldehyde testing in homes was developed. Formaldehyde is a ubiquitous chemical present in all homes in this study in Boston, MA. Concentration was dependent on absolute humidity, season, temperature, household cleaner use, and the presence of decorative laminates, fiberglass, or permanent press fabrics. Increased formaldehyde concentration showed an association with decreased asthma control, which suggests that decreasing formaldehyde concentration may improve asthma control.
Acknowledgements
Karen Dannemiller is supported by a National Science Foundation Graduate Research Fellowship. Initial funding for pump testing and device verification was provided by the Helen Terry MacLeod Research Grant from the Pembroke Center at Brown University and by the Superfund Research Program. Funding for the allergen portion of the study was provided by the U.S Department of Housing and Urban Development, Office of Healthy Homes and Lead Hazard Control (Grant # MALHH0163-07).
References
- Bearer CF. Environmental health hazards: How children are different from adults. Future Child. 1995;5:11–26. [PubMed] [Google Scholar]
- CARB. Determination of formaldehyde and acetaldehyde in emissions from stationary sources. Sacramento, CA: California Air Resources Board; 1991. Method 430. [Google Scholar]
- Cassee FR, Groten JP, Feron VJ. Changes in the nasal epithelium of rats exposed by inhalation to mixtures of formaldehyde, acetaldehyde, and acrolein. Fundam. appl. Toxicol. 1996;29:208–218. doi: 10.1006/faat.1996.0024. [DOI] [PubMed] [Google Scholar]
- Clarisse B, Laurent AM, Seta N, Le Moullec Y, El Hasnaoui A, Momas I. Indoor aldehydes: measurement of contamination levels and identification of their determinants in Paris dwellings. Environ. Res. 2003;92:245–253. doi: 10.1016/s0013-9351(03)00039-2. [DOI] [PubMed] [Google Scholar]
- Colombo A, De Bortoli M, Knöppel H, Schauenburg H, Vissers H. Small chamber tests and headspace analysis of volatile organic compounds Emitted from Household Products. Indoor Air. 1991;1:13–21. [Google Scholar]
- Dingle P, Franklin P. Formaldehyde levels and the factors affecting these levels in homes in Perth, Western Australia. Indoor Built Environ. 2002;11:111–116. [Google Scholar]
- EPA. Office of Research and Development. Center for Environmental Research Information. Cincinnati, OH: US Environmental Protection Agency; 1999. Determination of formaldehyde in ambient air using adsorbent cartridge followed by high performance liquid chromatography (HPLC) Compendium Method TO-11A. [Google Scholar]
- Flyvholm MA, Andersen P. Identification of formaldehyde releasers and occurrence of formaldehyde and formaldehyde releasers in registered chemical products. Am. J. Ind. Med. 1993;24:533–552. doi: 10.1002/ajim.4700240505. [DOI] [PubMed] [Google Scholar]
- Garrett MH, Hooper MA, Hooper BM, Rayment PR, Abramson MJ. Increased risk of allergy in children due to formaldehyde exposure in homes. Allergy. 1999;54:330–337. doi: 10.1034/j.1398-9995.1999.00763.x. [DOI] [PubMed] [Google Scholar]
- Gilbert NL, Gauvin D, Guay M, Héroux MÈ, Dupuis G, Legris M, Chan CC, Dietz RN, Lévesque B. Housing characteristics and indoor concentrations of nitrogen dioxide and formaldehyde in Quebec City, Canada. Environ. Res. 2006;102:1–8. doi: 10.1016/j.envres.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Flesca N, Cicolella A, Bates M, Bastin E. Pilot study of personal, indoor and outdoor exposure to benzene, formaldehyde and acetaldehyde. Environ. Sci. Pollut. R. 1999;6:95–102. doi: 10.1007/BF02987560. [DOI] [PubMed] [Google Scholar]
- Grosjean D. Ambient levels of formaldehyde, acetaldehyde and formic acid in southern California: results of a one-year baseline study. Environ. Sci. Technol. 1991;25:710–715. [Google Scholar]
- Hodgson AT, Rudd AF, Beal D, Chandra S. Volatile organic compound concentrations and emission rates in new manufactured and site-built houses. Indoor Air. 2000;10:178–192. doi: 10.1034/j.1600-0668.2000.010003178.x. [DOI] [PubMed] [Google Scholar]
- Hodgson AT, Beal D, McIlvaine JE. “Sources of formaldehyde, other aldehydes and terpenes in a new manufactures house.”. Indoor Air. 2002;12:235–242. doi: 10.1034/j.1600-0668.2002.01129.x. [DOI] [PubMed] [Google Scholar]
- Hodgson AT, Nabinger SJ, Persily AK. Volatile Organic Compound Concentrations and Emission Rates Measured over One Year in a New Manufactured House. LBNL-56272. 2004 Available at: http://www.bfrl.nist.gov/IAQanalysis/docs/LBNL-562722.pdf. [Google Scholar]
- Hollowell CD, Berk JV, Boegel ML, Miksch RR, Nazaroff WW, Traynor GW. Stud. Environ. Sci. Vol. 8. M. B. Michel, Elsevier; 1980. Building ventilation and indoor air quality; pp. 387–396. [Google Scholar]
- Jurvelin J, Vartiainen M, Jantunen M, Pasanen P. Personal Exposure Levels and Microenvironmental Concentrations of Formaldehyde and Acetaldehyde in the Helsinki Metropolitan Area, Finland. J. Air & Water Manage. Assoc. 2001;51:17–24. doi: 10.1080/10473289.2001.10464251. [DOI] [PubMed] [Google Scholar]
- Khoder MI, Shakour AA, Farag SA, Abdel Hameed AA. Indoor and outdoor formaldehyde concentrations in homes in residential areas in Greater Cairo. J. Environ. Monitor. 2000;2:123–126. doi: 10.1039/a908756g. [DOI] [PubMed] [Google Scholar]
- Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, Behar JV, Hern SC, Engelmann WH. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J. Expo. Anal. Env. Epid. 2001;11:231. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- Krzyzanowski M, Quackenboss JJ, Lebowitz MD. Chronic respiratory effects of indoor formaldehyde exposure. Environ. Res. 1990;52:117–125. doi: 10.1016/s0013-9351(05)80247-6. [DOI] [PubMed] [Google Scholar]
- Kuntz R, Lonneman W, Namie G, Hull LA. Rapid determination of aldehydes in air analyses. Anal. Lett. 1980;13:1409–1415. [Google Scholar]
- Li J, Dasgupta PK, Genfa Z, Hutterli MA. Measurement of atmospheric formaldehyde with a diffusion scrubber and light-emitting diode-liquid-core waveguide based fluorometry. Field Anal. Chem. Tech. 2001;5:2–12. doi: 10.1021/ac000611+. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Proffitt D, Fortune CR. Effects of modified residential construction on indoor air quality. Indoor Air. 1995;5:258–269. [Google Scholar]
- Liu W, Zhang J, Zhang L, Turpin BJ, Weisel CP, Morandi CP, Stock TH, Colome S, Korn LR. Estimating contributions of indoor and outdoor sources to indoor carbonyl concentrations in three urban areas in the United States. Atmos. Envion. 2006;40:2202–2214. [Google Scholar]
- Maddalena R, Russell M, Sullivan DP, Apte MG. Formaldehyde and Other Volatile Organic Chemical Emissions in Four FEMA Temporary Housing Units. Environ. Sci. Technol. 2009;43:5626–5632. doi: 10.1021/es9011178. [DOI] [PubMed] [Google Scholar]
- Marchand C, Le Calvé S, Mirabel P, Glasser N, Casset A, Schneider N, De Blay F. Concentrations and determinants of gaseous aldehydes in 162 homes in Strasbourg (France) Atmos. Environ. 2008;42:505–516. [Google Scholar]
- Mcgwin G, Jr, Lienert J, Kennedy JI., Jr Formaldehyde exposure and asthma in children: a systematic review. Environ. Health Perspect. 2009;118:313–317. doi: 10.1289/ehp.0901143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell MJ. Indoor residential chemical emissions as risk factors for respiratory and allergic effects in children: a review. Indoor Air. 2007;17:259–277. doi: 10.1111/j.1600-0668.2007.00478.x. [DOI] [PubMed] [Google Scholar]
- Myers GE. The effects of temperature and humidity on formaldehyde emission from UF-bonded boards: a literature critique. Forest. Prod. J. 1985;35:20–31. [Google Scholar]
- Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J. Allergy Clin. Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Nazaroff W. Cleaning products and air fresheners: exposure to primary and secondary air pollutants. Atmos. Environ. 2004;38:2841–2865. [Google Scholar]
- Neas LM, Dockery DW, Ware JH, Spengler JD, Speizer FE, Ferris BG. Association of indoor nitrogen dioxide with respiratory symptoms and pulmonary function in children. Am. J. Epidemiol. 1991;134:204–219. doi: 10.1093/oxfordjournals.aje.a116073. [DOI] [PubMed] [Google Scholar]
- NIOSH. Occupational safety and health guideline for formaldehyde. Atlanta, GA: National Institute for Occupational Safety and Health; 1988. pp. 81–123. Available: http://www.cdc.gov/niosh/docs/81-123/pdfs/0293.pdf. [Google Scholar]
- NTP. Report on Carcinogens Twelfth Edition. Research Triangle Park, NC: Department of Health and Human Services, Public Health Service, National Toxicology Program; 2011. [Google Scholar]
- O’Brian PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- Pickrell JA, Griffis LC, Mokler BV, Kanapilly GM, Hobbs CH. Formaldehyde release from selected consumer products: influence of chamber loading, multiple products, relative humidity, and temperature. Environ. Sci. Technol. 1984;18:682–686. [Google Scholar]
- Rocha FR, Coelho LHG, Lopes MLA, Carvalho LRF, Fracassi Da, Silva JA, Do Lago CL, Gutz IGR. Environmental formaldehyde analysis by active diffusive sampling with a bundle of polypropylene porous capillaries followed by capillary zone electrophoretic separation and contactless conductivity detection. Talanta. 2008;76:271–275. doi: 10.1016/j.talanta.2008.02.037. [DOI] [PubMed] [Google Scholar]
- Rumchev KB, Spickett JT, Bulsara MK, Phillips MR, Stick SM. Domestic exposure to formaldehyde significantly increases the risk of asthma in young children. Eur. Respir. J. 2002;20:403–408. doi: 10.1183/09031936.02.00245002. [DOI] [PubMed] [Google Scholar]
- Salthammer T, Mentese S, Marutzky R. Formaldehyde in the Indoor Environment. Chem. Rev. 2010;110:2536–2572. doi: 10.1021/cr800399g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandel M, Baeder A, Bradman A, Hughes J, Mitchell C, Shaughnessy R, Takaro TK, Jacobs DE. Housing interventions and control of health-related chemical agents: a review of the evidence. J. Public Health Manag. Pract. 2010;16:S24–S33. doi: 10.1097/PHH.0b013e3181e3cc2a. [DOI] [PubMed] [Google Scholar]
- Sandner F, Dott W, Hollander J. Sensitive indoor air monitoring of formaldehyde and other carbonyl compounds using the 2,4-dinitrophenylhydrazine method. Int. J. Hyg. Envir. Heal. 2001;203:275–279. doi: 10.1078/s1438-4639(04)70038-3. [DOI] [PubMed] [Google Scholar]
- Sears MR. Epidemiology of asthma exacerbations. J. Allergy Clin. Immunol. 2008;122:662–670. doi: 10.1016/j.jaci.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Sexton K, Petreas MX, Liu KS. Formaldehyde exposures inside mobile homes. Environ. Sci. Technol. 1989;23:985–988. [Google Scholar]
- Singer B, Coleman B, Destaillats H, Hodgson A, Lunden M, Weschler C, Nazaroff W. Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmos. Environ. 2006;40:6696–6710. doi: 10.1021/es052198z. [DOI] [PubMed] [Google Scholar]
- Stock TH. Formaldehyde Concentrations Inside Conventional Housing. J. Air Pollut. Control Assoc. 1987;37:913–918. doi: 10.1080/08940630.1987.10466284. [DOI] [PubMed] [Google Scholar]
- Triebig G, Schaller KH, Beyer B, Müller J, Valentin H. Formaldehyde exposure at various workplaces. Sci. Total Environ. 1989;79:191–195. doi: 10.1016/0048-9697(89)90362-8. [DOI] [PubMed] [Google Scholar]
- Venn AJ, Cooper M, Antoniak M, Laughlin C, Britton J, Lewis SA. Effects of volatile organic compounds, damp, and other environmental exposures in the home on wheezing illness in children. Thorax. 2003;58:955–960. doi: 10.1136/thorax.58.11.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Formaldehyde, Environmental Health Criteria. Vol. 89. Geneva, Switerland: World Health Organization; 1989. [Google Scholar]
- Wilson J, Dixon S, Breysse P, Jacobs DE, Adamkiewicz G, Chew GL, Dearborn D, Krieger J, Sandel M, Spanier A. Housing and Allergens: A Pooled Analysis of Nine US Studies. Env. Res. 2010;110:189–198. doi: 10.1016/j.envres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Woo CS, Barry SE, Zaromb S. Detection and estimation of part-per-billion levels of formaldehyde using a portable high-throughput liquid absorption air sampler. Environ. Sci. Technol. 1998;32:169–176. [Google Scholar]
- Zhang J, Lioy PJ, He Q. Characteristics of aldehydes: concentrations, sources, and exposures for indoor and outdoor residential microenvironments. Environ. Sci. Technol. 1994;28:146–152. doi: 10.1021/es00050a020. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Luo X, Wang X, Qian K, Zhao R. Influence of temperature on formaldehyde emission parameters of dry building materials. Atmos. Environ. 2007;41:3203–3216. [Google Scholar]
- Zhu J, Cao XL, Beauchamp R. Determination of 2-butoxyethanol emissions from selected consumer products and its application in assessment of inhalation exposure associated with cleaning tasks. Environ. Int. 2001;26:589–597. doi: 10.1016/s0160-4120(01)00046-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.