Abstract
Adenosine A1 receptor (A1AR) activation contracts smooth muscle, although signaling mechanisms aren’t thoroughly understood. Activation of A1AR leads to metabolism of arachidonic acid, including the production of 20-hydroxyeicosatetraenoic acid (20-HETE) by cytochrome P4504a (CYP4a). 20-HETE can activate protein kinase C-α (PKC-α) which crosstalks with extracellular signal-regulated kinase (ERK1/2) pathway. Both these pathways can regulate smooth muscle contraction, we tested the hypothesis that A1AR contracts smooth muscle through a pathway involving CYP4a, PKC-α, and ERK1/2. Experiments included isometric tension recordings of aortic contraction and Western blots of signaling molecules in wild type (WT) and A1AR knockout (A1KO) mice. Contraction to the A1-selective agonist CCPA was absent in A1KO mice aortae, indicating the contractile role of A1AR. Inhibition of CYP4a (HET0016) abolished CCPA-induced contraction in WT aortae, indicating a critical role for 20-HETE. Both WT and A1KO mice aortae contracted in response to exogenous 20-HETE. Inhibition of PKC-α (Gö6976) or ERK1/2 (PD98059) attenuated 20-HETE-induced contraction equally, suggesting that ERK1/2 is downstream of PKC-α. Contractions to exogenous 20-HETE were significantly less in A1KO mice; reduced protein levels of PKC-α, p-ERK1/2, and total ERK1/2 supported this observation. Our data indicate that A1AR mediates smooth muscle contraction via CYP4a and a PKC-α-ERK1/2 pathway.
Keywords: Cytochrome P4504a, Protein Kinase C-α, Extracellular Regulated Kinase, 20-HETE
Introduction
Adenosine is an extracellular signaling molecule that affects heart rate, coronary blood flow, and blood pressure (11). Adenosine alters vascular tone by binding to one of the four G-protein coupled receptors: A1, A2A, A2B and A3. Activation of A1 and A3 receptors contracts vascular smooth muscle, in part, by inhibiting adenylyl cyclase through pertussis toxin-sensitive Gi protein (1, 18, 40). In contrast, activation of A2A and A2B receptors relaxes smooth muscle, in part, by stimulating adenylyl cyclase (1). In the case of A2A, receptors, this process occurs through Gs and Golf proteins, whereas A2B receptors utilize Gs and Gq proteins (1). In addition to G protein signaling, activation of adenosine receptors also leads to the metabolism of arachidonic acid and the production of myriad signaling molecules (7).
Metabolites of arachidonic acid regulate smooth muscle tone, including prostaglandins, thromoboxanes, leukotrienes, and hydroxyeicosatetraenoic acids (HETEs) (17, 24, 33). Two subfamilies of cytochrome P450 (CYP) enzymes metabolize arachidonic acid: CYP-epoxygenases and ω-hydroxylases (24). The CYP-epoxygenases produce epoxyeicosatrienoic acids (EETs), which relax smooth muscle. In contrast, the ω-hydroxylases such as CYP4a and CYP4f produce 20 HETE, which contracts smooth muscle (15, 19, 25, 29, 45). The mechanisms linking CYP4a to smooth muscle contraction, however, are not entirely clear, especially in regard to adenosine A1 receptor activation.
20-HETE-induced contraction of smooth muscle appears to involve protein kinase C (PKC), Src-type tyrosine kinase, rho kinase and mitogen activated protein kinase (MAPK) pathways (19, 27, 38, 43). We have demonstrated previously that A1 receptor stimulation is linked to PKC-α and ERK1/2 activation in coronary artery smooth muscle (5). Gaps in our knowledge remain to be addressed, the most important of which pertain to whether A1-dependent activation of PKC-α and ERK1/2 results in smooth muscle contraction. Thus, the aim of the present study was to delineate mechanisms downstream of A1 receptor activation that lead to smooth muscle contraction. We tested the hypothesis that adenosine A1 receptors contract smooth muscle through a pathway involving CYP4a, PKC-α, and ERK1/2.
Methods
Animals
Some of the A1KO mice were obtained from Dr. Jurgen Schnermann (NIDDK, NIH) on C57BL/6 background. C57BL/6 (WT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Equal number of males and females of 14-18 weeks of age were used in our studies, as no gender differences were observed in the responses to the pharmacological agents used. The Institutional Animal Care and Use Committee of West Virginia University approved this study.
Isometric tension
Following anesthesia with pentobarbital sodium (100 mg/kg, i.p.), the aorta was removed and cut into 3-4 mm rings. Rings were mounted on stainless steel wires and suspended in 10 ml organ baths filled with Krebs-Henseleit buffer containing (in mM): 118 NaCl, 4.8 KCl, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 11 glucose and 2.5 CaCl2. Baths were maintained at 37°C and bubbled with 95% O2 and 5% CO2 (pH 7.4). Rings were equilibrated for 90 min under a resting tension of 1 g. Rings were contracted with 50 mM KCl twice to check viability. Rings were then treated with submaximal phenylephrine (PE; 1 μM) to obtain a stable contraction and integrity of the vascular endothelium was confirmed by relaxation to acetylcholine (1 μM). Tension was monitored continuously with a digital acquisition system and analyzed using Acknowledge 3.5.7 software (BIOPAC). Our laboratory previously validated all methods (4-5, 28, 40).
Concentration-response curves for 2-chloro-N (6) cyclo-pentyl-adenosine (CCPA; 10 pM to 10 μM) were run in parallel in aortic rings from WT and A1KO mice. In all experiments, drugs were administered to yield the next higher concentration only when the previous response reached steady state. 20-HETE (Cayman Chemical; Ann Arbor, MI) concentration-response curves were constructed, but in some experiments a single concentration (100 nM) was used as described previously (2, 27, 36, 44). Inhibitors of CYP4a (HET0016; Cayman Chemical), PKC-α, (Gö6976; Calbiochem; La Jolla, CA), and ERK1/2 (PD98059; Calbiochem) were added 30 min prior to contraction with PE (10-6M) and present throughout the experiments. Inhibitor concentrations were determined by others and us (3, 5-6, 8, 10, 22-23, 25, 29, 34). Acetylcholine and PE were dissolved in distilled water, while CCPA, HET0016, Gö6976, and PD98059 were dissolved in DMSO. We have shown that previously these vehicles have no effect on smooth muscle contraction (4). 20-HETE was dissolved in ethanol and had no effect on the vasoconstrictive properties of the drug. Percentage of contraction was determined as a percent change from the maximum phenylephrine (PE) response. If A = maximum total tension in the presence of PE; B = maximum total tension when a drug is added after PE; C = minimum passive tension determined after the experiment, after a final wash; D = PE-induced active tension = A minus C Thus, % contraction = [(B − A) / D] × 100%
Immunoblots
Aortae from WT and A1KO mice were homogenized with 150 μL radio-immuno precipitation assay buffer (RIPA, Cell Signaling Technology; 20 mM Tris-HCl (pH 7.5),(150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin), vortexed, and centrifuged for 10 min at 13,800 g at 4°C. Supernatants were stored at -80°C. Protein was measured using the Bradford dye procedure with bovine serum albumin (BSA) as a standard (Bio-Rad Laboratories; Hercules, CA). The protein extract was divided into aliquots and stored at -80°C. Samples (25 μg of total protein) were loaded on slab gels (10% acrylamide; 1 mm thick), separated by SDS-PAGE, and transferred to nitrocellulose membranes (Hybond-ECL). Protein transfer was confirmed by visualization of prestained molecular weight markers (Bio-Rad). Membranes were blocked with 5% nonfat dry milk and incubated with primary antibody. 1:1000 dilutions were used for CYP4a (Abcam; Cambridge MA) and PKC-α (BD Transduction Labs; San Diego, CA) antibodies, while 1:500 dilutions were used for total ERK1/2 and p-ERK1/2 (both from Santa Cruz Biotechnology; Santa Cruz, CA). The phospho-ERK1/2 blots were stripped at room temperature for 15 minutes and re-probed for total-ERK1/2. All membranes were stripped and probed for β-actin (Santa Cruz); this served as an internal control to normalize protein expression in each lane. Secondary antibodies were horseradish peroxidase-conjugated. Membranes were developed using enhanced chemiluminescence (AmershamBioSciences) and X-ray film.
Statistical Analysis
Data are expressed as mean ± SEM from n number of mice. Concentration-response curves and 20-HETE/inhibitor experiments were analyzed by 2-way analysis of variance (ANOVA). Comparisons of two densitometry values were made with unpaired t-tests. P < 0.05 was considered significant in all tests.
Results
Role of A1 receptor and CYP4a product in adenosine-induced smooth muscle contraction
CCPA, an adenosine A1-selective agonist, contracted aortic rings from WT mice (Fig. 1A, open circles). In contrast, CCPA-induced smooth muscle contraction was absent in aortic rings from A1KO mice (Fig. 1A, closed circles). ACh-induced response in WT (47 ± 2%) was not significantly different from A1KO (51 ± 3%, p>0.05). HET0016, an inhibitor of CYP4a, attenuated CCPA-induced smooth muscle contraction in aortic rings from WT mice (Fig. 1A, open squares). In contrast, HET0016 had no effect on CCPA responses in aortic rings from A1KO mice (Fig. 1A, closed squares). There is a significant difference (p<0.05) in PE-induced aortic contraction between WT and A1KO mice (WT: 0.21 ± 0.01 g; A1KO: 0.18 ± 0.03 g). However, HET0016 had no significant effect on PE-induced contraction of aortic smooth muscle between WT groups (control: 0.21 ± .01 g vs. treated: 0.2 ± .02 g) and A1KO mice (control: 0.18 ± .03 g vs. treated: 0.17 ± .02 g). CYP4a protein was expressed in aortae from WT and A1KO mice (Fig. 1B); however, the expression of CYP4a was lower (by 25%) in A1KO mice (p<0.05).
Fig. 1. Effect of A1 Adenosine Receptor-induced smooth muscle contraction in WT and A1 KO aortae.
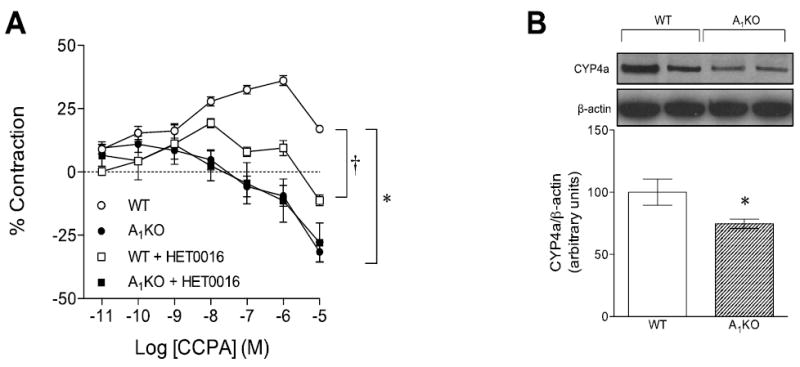
(A) The A1-selective agonist CCPA-mediated contractions with CYP4a inhibitor (10 μM HET0016), *p < 0.05 between WT and A1KO and †p < 0.05 between WT and WT + HET0016 (n=4-12). (B) Western blots for CYP4a expression in the aorta. *p < 0.05 between WT and A1KO (n=6).
Role of 20-HETE in vascular smooth muscle contraction
CYP4a is downstream of adenosine A1 receptor activation (29), therefore, we determined the contractile responses to its product 20-HETE (0.01-1 μM). Aortic rings from WT and A1KO mice both contracted in response to exogenous 20-HETE (Fig. 2B and 2C); however, 20-HETE-induced contraction was less in aortae from A1KO mice (Fig. 2C and 2D). We observed an unexpected increase in the contraction on addition of lowest concentration of 10-11M 20-HETE in both WT and A1KO (Fig. 2B and 2C).
Fig. 2. Effect of the CYP4a product, 20-HETE on aortic smooth muscle contraction from WT and A1KO.
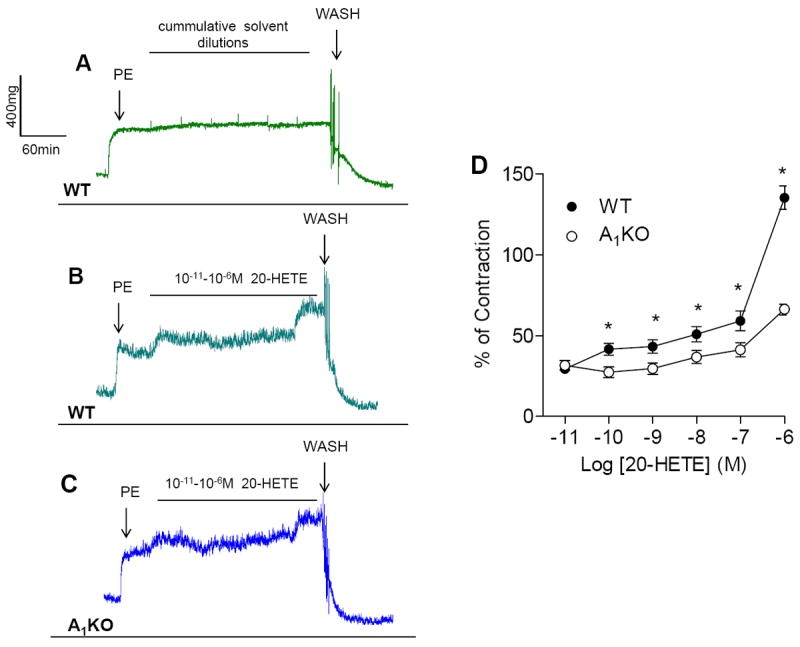
(A) Representative traces for concentration response curve for solvent (ethanol) in WT (n=4). (B) Representative traces for concentration response curve for 20-HETE in WT. (C) Representative traces for concentration response curve for 20-HETE in A1KO. (D) Exogenous 20-HETE contracts aortic smooth muscle in a concentration-dependent manner. *p < 0.05 between WT and A1KO mice (n=8-12)
Role of PKC-α in 20-HETE-induced contraction
20-HETE (100 nM), a CYP4a product, contracted aortae from WT and A1KO mice (Fig. 3A); however, the contractile response was less in aortic rings from A1KO mice. Inhibition of PKC-α (100 nM Gö6976) reduced 20-HETE-induced contraction in aortic rings from both WT (by 37%) and A1KO (by 29%) mice (Fig. 3A). The relative inhibitory effect of Gö6976 was the same in aortic rings from WT and A1KO mice (Fig. 3B). PKC-α protein was expressed in aortae from WT and A1KO mice (Fig. 3C); however, the expression of PKC-α was lower (by 47%) in A1KO mice (p<0.05).
Fig. 3. 20-HETE-induced smooth muscle contraction and its signaling through PKC-α and ERK.
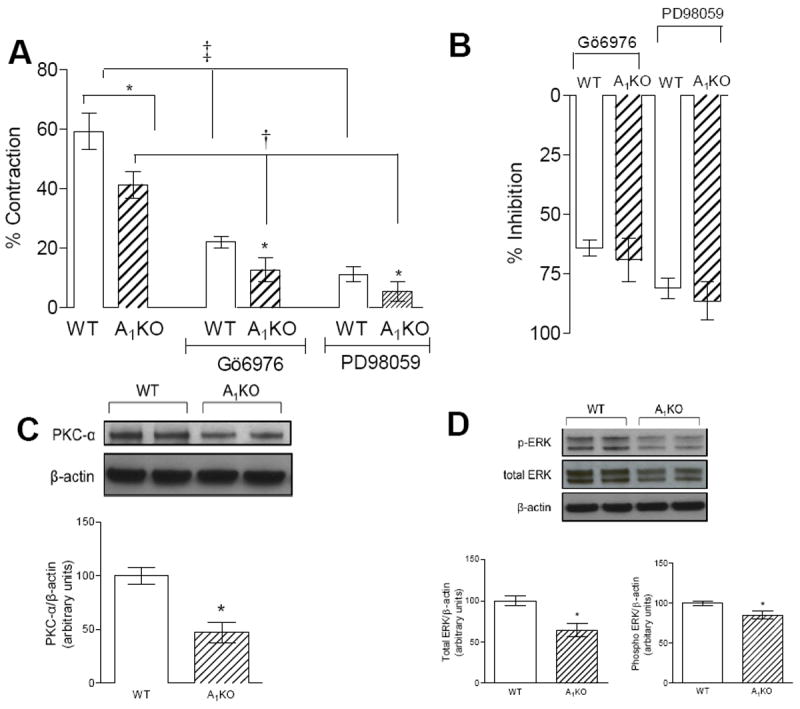
(A) Aortic smooth muscle contractions in response to 100 nM 20-HETE in WT and A1KO mice with or without PKC-α (100 nM Gö6976) and ERK1/2 kinase (1 μM PD98059) inhibitors, *p < 0.05 between A1KO(control/treated) and its respective control; †p < 0.05, WT, A1KO vs. WT + Gö6976 & A1KO+ Gö6976 respectively; ‡p < 0.05, WT, A1KO vs. WT + PD98059 & A1KO+ PD98059, respectively, n=8-16. The dagger (†) and double dagger (‡)indicate significant effects of Gö6976 and PD98059 in A1KO and WT mice, respectively. (B) The inhibitory effect of Gö6976 and PD98059 on 20-HETE-induced contractions are equivalent in WT and A1KO mice. *p < 0.05 between WT and A1KO (n=6-8) (C) Western blots for PKC-α expression in the aorta from WT and A1KO. (D) Western blots for total ERK1/2, phospho-ERK1/2 expression in the aorta from WT and A1KO mice.*p < 0.05 between WT and A1KO (n=6-8)
Role of ERK1/2 kinase in 20-HETE-induced contraction
The CYP4a product 20-HETE (100 nM) contracted aortic rings from both WT and A1KO mice (Fig. 3A); however, the contractile response was less in aortae from A1KO mice. Inhibition of ERK1/2 (1 μM PD98059) reduced 20-HETE-induced contraction in aortae from WT (by 48%) and A1KO (by 36%) mice (Fig. 3A). The relative inhibitory effect of PD98059 was similar in aortic rings from WT and A1KO mice (Fig. 3B). ERK1/2 protein was expressed in aortae from WT and A1KO mice (Fig. 3D); however, the expression of total ERK (by 35%) and phospho-ERK (by 15%) were lower in A1KO mice (p<0.05).
Discussion
We tested the hypothesis that adenosine A1 receptors contract smooth muscle through a pathway involving CYP4a, PKC-α, and ERK1/2. We used WT and A1KO mice, isometric tension recordings of aortic contraction, and Western blots of signaling molecule expression to address this. Our findings regarding an A1AR → CYP4a → PKC-α → ERK pathway were four-fold. First, CCPA-induced contraction was absent in aortae from A1KO mice, clearly supporting the idea that adenosine A1 receptors link to smooth muscle contraction. Second, when CYP4a was inhibited with HET0016, CCPA-induced contraction in WT aortae was abolished. These data uphold the notion that 20-HETE is a critical signaling molecule linking adenosine A1 receptors to smooth muscle contraction. Further, aortae from both WT and A1KO mice contracted in response to exogenous 20-HETE. Third, inhibition of PKC-α with Gö6976 attenuated contraction in response to 20-HETE, supporting the idea that PKC-α transduces the signal for contraction. Fourth, inhibition of ERK1/2 with PD98059 attenuated 20-HETE-induced contraction. This provides support for suggesting that ERK1/2 is downstream of PKC-α. Overall, these data indicate that adenosine A1 receptors mediate smooth muscle contraction via CYP4a (20-HETE) and a PKC-α-ERK1/2 pathway (Fig. 4).
Fig. 4. Adenosine A1 receptors link to smooth muscle contraction via CYP4a, PKC-α, and ERK1/2.
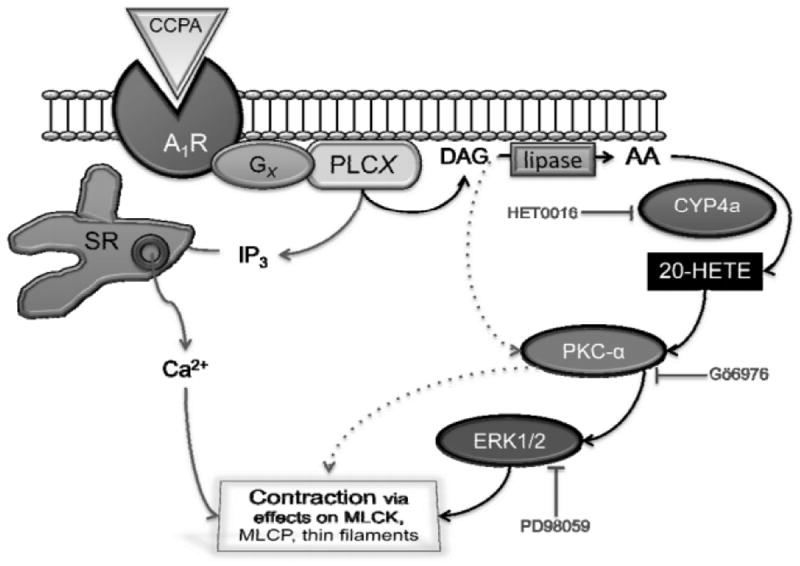
This figure illustrates our understanding of signaling from the A1 receptor to smooth muscle contraction. Adenosine A1 receptors (A1R) are sensitive to CCPA and coupled to phospholipase C (PLCX) by G-proteins (GX). Candidates for PLCX include PLCβ1, PLCβ3, and PLCγ1, while candidates for GX include Gi, Go, Gq, G11 as well as G-βγ subunits (5, 18). PLCX produces two second messengers from phosphatidylinositol 4,5 bisphosphate: diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3, which releases Ca2+ from the sarcoplasmic reticulum; SR). DAG can be metabolized by di- and monoacylglycerol lipases to produce arachidonic acid (AA). CYP4a metabolizes AA into 20-HETE, which activates PKC-α (DAG is also an activator; PKC- β and γ isoforms are also expressed (5). The ERK1/2 pathway is activated downstream of PKC-α. The culmination of this signaling is to contract smooth muscle through effects on, e.g., myosin light chain kinase (MLCK), myosin light chain phosphatase (MLCP), and thin filament regulatory proteins. Inhibiting CYP4a with HET0016 blocks CCPA-induced smooth muscle contraction (Fig. 1). Gö6976 and PD98095, which inhibit PKC-α and ERK1/2, inhibit smooth muscle contraction in response to 20-HETE (Fig. 3).
CCPA-induced contraction was absent in A1KO mice (Fig. 1A); this was not surprising, as others and we have reported it (40, 42). Smooth muscle contractions elicited by A1 receptors are similar to those found in a wide variety of systems like mouse afferent arterioles (14), human cultured prostatic stromal cells (31), cat esophageal smooth muscle cells (35), guinea pig aorta (12) and mouse coronary artery cells and carotid artery (5, 30, 39). Importantly, however, we observed some unexpected differences between WT and A1KO mice downstream of the A1 receptor. We did not have a reason to predict that in A1KO mice, CYP4a expression would be lower (Fig. 1B) and that responses to exogenous application of the CYP4a metabolite, 20-HETE would be attenuated (Fig. 2). Nor could we have predicted that PKC-α and ERK1/2 (Fig. 3) expression would be reduced in the aortae of A1KO mice. These findings suggest that genetic ablation of the A1 receptor leads to some changes in the entire signaling cascade. This is the converse of conditions where A1 receptors are upregulated and associated with increased expression of PKC and p42/44 ERK (9, 20).
Smooth muscle contraction mediated by A1 receptors depends almost entirely upon CYP4a products, as HET0016 blocked CCPA-induced contraction (Fig. 1A; Fig. 4). In contrast, HET0016 had no effect on aortae from A1KO mice (Fig. 1A). These findings suggest that the CYP4a product 20-HETE plays a major role in A1 receptor signaling. This meshes well with previous studies reported from this laboratory (26), including results obtained by others from rat renal interlobar arteries (37) as well as human and rat cerebral arteries (41). 20-HETE is a potent vasoconstrictor of renal, mesenteric, skeletal and cerebral arterioles in several species (33). In vascular smooth muscle, 20-HETE functions as a second messenger to promote Ca2+ influx by depolarization, resulting in contraction (24). Our data show that administration of exogenous 20-HETE contracted aortic rings from both WT and A1KO mice at low concentrations (Fig. 2B, C and D), with no effect of the solvent. This is concordant with the data shown from other labs as well (13, 15) and underscores that 20-HETE is a potent vasoconstrictor.
The 20-HETE-induced contraction was lower in A1KO mice, suggesting reduced expression of signaling components downstream of CYP4a. 20-HETE has been shown to signal through PKC in cerebral vascular smooth muscle, renal arterioles and porcine coronary arteries (19, 32, 38). Further, previous studies from our lab have shown that in coronary smooth muscle activation of A1 receptors is linked to phospholipase C (PLC), PKC-α, and p-ERK1/2 signaling ((5); Fig. 4). We investigated this potential pathway in the aortae of WT and A1KO mice.
Inhibition of PKC-α (with the isoform-selective Gö6976) attenuated 20-HETE contractions in the aortae of both WT and A1KO mice (Fig. 3A). Contractions in response to 20-HETE were less in aortic rings from A1KO mice; however, the relative inhibitory effect of Gö6976 on 20-HETE induced contractions was similar in WT and A1KO mice (Fig. 3B). This, coupled with reduced expression of PKC-α protein (Fig. 3C), suggests a similar relative role of PKC-α in the contraction of aortae from WT and A1KO mice. A similar pattern of 20-HETE-induced contractile responses, sensitivity to pharmacological inhibition, and signaling molecule expression was found for ERK1/2 (Fig. 3). Inhibition of ERK1/2 (PD98059) attenuated 20-HETE contractions in the aortae of both WT and A1KO mice (Fig. 3A). The relative inhibitory effect of PD98059 on 20-HETE induced contractions was similar in WT and A1KO mice (Fig. 3B). This coupled with reduced expression of phospho-ERK and total ERK (Fig. 3D), suggests similar relative roles of ERK1/2 in the contraction of smooth muscle WT and A1KO mice. Inhibition of ERK1/2 was more efficacious than inhibition of PKC-α in blocking 20-HETE-induced contraction. This may indicate that the MAPK pathway integrates the input of several relevant signaling pathways including PKC, rho kinases, Ras, and/or tyrosine kinases. There has been no direct evidence showing that PKCα, ERK1/2 and CYP4a are transcriptionally regulated by A1AR. However, there is evidence showing that stimulation of adenosine A1 receptors activates c-fos in Chinese Hamster ovary cells expressing the human A1 receptor. The transcription factor c-fos regulates the expression of several genes including ERK1/2, PKC-α and PKC-μ (16). Similarly, in human embryonic kidney (HEK-29) cells, stimulation of A1 receptor coupled to Gα induced ERK and STAT1 phosphorylation through a c-fos dependent manner (21). Thus, we can speculate that A1 receptor may be involved at the transcriptional level as well, but more work is needed to confirm this.
Our current understanding of signaling for A1 → CYP4a → PKC-α → ERK pathway is summarized in Fig. 4. A1 receptor activation with, e.g., CCPA, activates CYP4a which metabolizes AA into 20-HETE. CCPA-induced contractions are almost entirely dependent upon signaling through CPY4a, as inhibition with HET0016 abrogates contraction (Fig. 1A). 20-HETE activates PKC-α and this is integral to contraction, as Gö6976 blocks the response (Fig. 3). The ERK1/2 pathway is activated downstream of PKC-α, as Gö6976 blocks ERK activation (5) and PD98059 blocks the contraction (Fig. 3). ERK1/2 integrates the signaling for contraction and transduces it to, e.g., myosin light chain kinase (MLCK), myosin light chain phosphatase (MLCP), and thin filament regulatory proteins.
In summary, we have shown that A1 receptor activation leads to smooth muscle contraction via CYP4a, PKC-α, and ERK 1/2. Because A1 receptors and 20-HETE are implicated in a variety of cardiovascular disorders, a better understanding of this pathway will be important for identifying therapeutic targets and treatment opportunities.
Acknowledgments
Our work was supported by start up funds from West Virginia University and NIH grants HL094447, HL027339 (SJM).
References
- 1.Abbracchio MP, Brambilla R, Ceruti S, Kim HO, von Lubitz DK, Jacobson KA, Cattabeni F. G protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol Pharmacol. 1995;48:1038–1045. [PubMed] [Google Scholar]
- 2.Alonso-Galicia M, Sun CW, Falck JR, Harder DR, Roman RJ. Contribution of 20-HETE to the vasodilator actions of nitric oxide in renal arteries. Am J Physiol. 1998;275:F370–378. doi: 10.1152/ajprenal.1998.275.3.F370. [DOI] [PubMed] [Google Scholar]
- 3.Ansari HR, Husain S, Abdel-Latif AA. Activation of p42/p44 mitogen-activated protein kinase and contraction by prostaglandin F2alpha, ionomycin, and thapsigargin in cat iris sphincter smooth muscle: inhibition by PD98059, KN-93, and isoproterenol. J Pharmacol Exp Ther. 2001;299:178–186. [PubMed] [Google Scholar]
- 4.Ansari HR, Nadeem A, Talukder MA, Sakhalkar S, Mustafa SJ. Evidence for the involvement of nitric oxide in A2B receptor-mediated vasorelaxation of mouse aorta. Am J Physiol Heart Circ Physiol. 2007;292:H719–725. doi: 10.1152/ajpheart.00593.2006. [DOI] [PubMed] [Google Scholar]
- 5.Ansari HR, Teng B, Nadeem A, Roush KP, Martin KH, Schnermann J, Mustafa SJ. A(1) adenosine receptor-mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2009;297:H1032–1039. doi: 10.1152/ajpheart.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang K, Xiao D, Huang X, Longo LD, Zhang L. Chronic hypoxia increases pressure-dependent myogenic tone of the uterine artery in pregnant sheep: role of ERK/PKC pathway. Am J Physiol Heart Circ Physiol. 2009;296:H1840–1849. doi: 10.1152/ajpheart.00090.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng MK, Doumad AB, Jiang H, Falck JR, McGiff JC, Carroll MA. Epoxyeicosatrienoic acids mediate adenosine-induced vasodilation in rat preglomerular microvessels (PGMV) via A2A receptors. Br J Pharmacol. 2004;141:441–448. doi: 10.1038/sj.bjp.0705640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cogolludo A, Moreno L, Bosca L, Tamargo J, Perez-Vizcaino F. Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: role of protein kinase Czeta. Circ Res. 2003;93:656–663. doi: 10.1161/01.RES.0000095245.97945.FE. [DOI] [PubMed] [Google Scholar]
- 9.Coulson R, Proch PS, Olsson RA, Chalfant CE, Cooper DR. Upregulated renal adenosine A1 receptors augment PKC and glucose transport but inhibit proliferation. Am J Physiol. 1996;270:F263–274. doi: 10.1152/ajprenal.1996.270.2.F263. [DOI] [PubMed] [Google Scholar]
- 10.Ding Y, Schwartz D, Posner P, Zhong J. Hypotonic swelling stimulates L-type Ca2+ channel activity in vascular smooth muscle cells through PKC. Am J Physiol Cell Physiol. 2004;287:C413–421. doi: 10.1152/ajpcell.00537.2003. [DOI] [PubMed] [Google Scholar]
- 11.Drury AN, Szent-Gyorgyi A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J Physiol. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford WR, Broadley KJ. Effects of adenosine receptor agonists on induction of contractions to phenylephrine of guinea-pig aorta mediated via intra- or extracellular calcium. Gen Pharmacol. 1999;33:143–150. doi: 10.1016/s0306-3623(98)00279-1. [DOI] [PubMed] [Google Scholar]
- 13.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87:60–65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- 14.Hansen PB, Castrop H, Briggs J, Schnermann J. Adenosine induces vasoconstriction through Gi-dependent activation of phospholipase C in isolated perfused afferent arterioles of mice. J Am Soc Nephrol. 2003;14:2457–2465. doi: 10.1097/01.asn.0000086474.80845.25. [DOI] [PubMed] [Google Scholar]
- 15.Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB, Roman R. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266:H2098–2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- 16.Hill KJ, Webber AC, Hill SJ. A role of protein kinase C mu in signalling from the human adenosine A1 receptor to the nucleus. Br J Pharmacol. 2003;139:721–732. doi: 10.1038/sj.bjp.0705294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoagland KM, Maier KG, Moreno C, Yu M, Roman RJ. Cytochrome P450 metabolites of arachidonic acid: novel regulators of renal function. Nephrol Dial Transplant. 2001;16:2283–2285. doi: 10.1093/ndt/16.12.2283. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lange A, Gebremedhin D, Narayanan J, Harder D. 20-Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272:27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- 20.Lee HT, Emala CW. Characterization of adenosine receptors in human kidney proximal tubule (HK-2) cells. Exp Nephrol. 2002;10:383–392. doi: 10.1159/000065306. [DOI] [PubMed] [Google Scholar]
- 21.Lo RK, Wong YH. Transcriptional activation of c-Fos by constitutively active Galpha(16)QL through a STAT1-dependent pathway. Cell Signal. 2006;18:2143–2153. doi: 10.1016/j.cellsig.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Loufrani L, Lehoux S, Tedgui A, Levy BI, Henrion D. Stretch induces mitogen-activated protein kinase activation and myogenic tone through 2 distinct pathways. Arterioscler Thromb Vasc Biol. 1999;19:2878–2883. doi: 10.1161/01.atv.19.12.2878. [DOI] [PubMed] [Google Scholar]
- 23.Matsumoto T, Tostes RC, Webb RC. Uridine adenosine tetraphosphate-induced contraction is increased in renal but not pulmonary arteries from DOCA-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;301:H409–417. doi: 10.1152/ajpheart.00084.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyata N, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J Smooth Muscle Res. 2005;41:175–193. doi: 10.1540/jsmr.41.175. [DOI] [PubMed] [Google Scholar]
- 25.Nayeem MA, Poloyac SM, Falck JR, Zeldin DC, Ledent C, Ponnoth DS, Ansari HR, Mustafa SJ. Role of CYP epoxygenases in A2A AR-mediated relaxation using A2A AR-null and wild-type mice. Am J Physiol Heart Circ Physiol. 2008;295:H2068–2078. doi: 10.1152/ajpheart.01333.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nayeem MA, Ponnoth DS, Boegehold MA, Zeldin DC, Falck JR, Mustafa SJ. High-salt diet enhances mouse aortic relaxation through adenosine A2A receptor via CYP epoxygenases. Am J Physiol Regul Integr Comp Physiol. 2009;296:R567–574. doi: 10.1152/ajpregu.90798.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obara K, Koide M, Nakayama K. 20-Hydroxyeicosatetraenoic acid potentiates stretch-induced contraction of canine basilar artery via PKC alpha-mediated inhibition of KCa channel. Br J Pharmacol. 2002;137:1362–1370. doi: 10.1038/sj.bjp.0704960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponnoth DS, Nadeem A, Mustafa SJ. Adenosine-mediated alteration of vascular reactivity and inflammation in a murine model of asthma. Am J Physiol Heart Circ Physiol. 2008;294:H2158–2165. doi: 10.1152/ajpheart.01224.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponnoth DS, Nayeem MA, Kunduri SS, Tilley SL, Zeldin DC, Ledent C, Mustafa SJ. Role of omega-hydroxylase in adenosine-mediated aortic response through MAP kinase using A2A-receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2012;302:R400–408. doi: 10.1152/ajpregu.00481.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prentice DJ, Kelly MD, Ledent C, Hourani SM. Relaxation of the mouse isolated aorta and carotid artery in response to adenosine analogues in genetically-modified mice lacking the adenosine A(2A) receptor. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:127–133. doi: 10.1007/s00210-002-0581-7. [DOI] [PubMed] [Google Scholar]
- 31.Preston A, Frydenberg M, Haynes JM. A1 and A2A adenosine receptor modulation of alpha 1-adrenoceptor-mediated contractility in human cultured prostatic stromal cells. Br J Pharmacol. 2004;141:302–310. doi: 10.1038/sj.bjp.0705535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randriamboavonjy V, Kiss L, Falck JR, Busse R, Fleming I. The synthesis of 20-HETE in small porcine coronary arteries antagonizes EDHF-mediated relaxation. Cardiovasc Res. 2005;65:487–494. doi: 10.1016/j.cardiores.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 33.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 34.Sakwe AM, Rask L, Gylfe E. Protein kinase C modulates agonist-sensitive release of Ca2+ from internal stores in HEK293 cells overexpressing the calcium sensing receptor. J Biol Chem. 2005;280:4436–4441. doi: 10.1074/jbc.M411686200. [DOI] [PubMed] [Google Scholar]
- 35.Shim JO, Shin CY, Lee TS, Yang SJ, An JY, Song HJ, Kim TH, Huh IH, Sohn UD. Signal transduction mechanism via adenosine A1 receptor in the cat esophageal smooth muscle cells. Cell Signal. 2002;14:365–372. doi: 10.1016/s0898-6568(01)00270-4. [DOI] [PubMed] [Google Scholar]
- 36.Singh TU, Choudhury S, Parida S, Maruti BS, Mishra SK. Arachidonic acid inhibits Na-K-ATPase via cytochrome P-450, lipoxygenase and protein kinase C-dependent pathways in sheep pulmonary artery. Vascul Pharmacol. 2012;56:84–90. doi: 10.1016/j.vph.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Sodhi K, Wu CC, Cheng J, Gotlinger K, Inoue K, Goli M, Falck JR, Abraham NG, Schwartzman ML. CYP4A2-induced hypertension is 20-hydroxyeicosatetraenoic acid- and angiotensin II-dependent. Hypertension. 2010;56:871–878. doi: 10.1161/HYPERTENSIONAHA.110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun CW, Falck JR, Harder DR, Roman RJ. Role of tyrosine kinase and PKC in the vasoconstrictor response to 20-HETE in renal arterioles. Hypertension. 1999;33:414–418. doi: 10.1161/01.hyp.33.1.414. [DOI] [PubMed] [Google Scholar]
- 39.Talukder MA, Morrison RR, Mustafa SJ. Comparison of the vascular effects of adenosine in isolated mouse heart and aorta. Am J Physiol Heart Circ Physiol. 2002;282:H49–57. doi: 10.1152/ajpheart.2002.282.1.H49. [DOI] [PubMed] [Google Scholar]
- 40.Tawfik HE, Schnermann J, Oldenburg PJ, Mustafa SJ. Role of A1 adenosine receptors in regulation of vascular tone. Am J Physiol Heart Circ Physiol. 2005;288:H1411–1416. doi: 10.1152/ajpheart.00684.2004. [DOI] [PubMed] [Google Scholar]
- 41.Toth P, Rozsa B, Springo Z, Doczi T, Koller A. Isolated human and rat cerebral arteries constrict to increases in flow: role of 20-HETE and TP receptors. J Cereb Blood Flow Metab. 2011 doi: 10.1038/jcbfm.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Yang JN, Arner A, Boels PJ, Fredholm BB. Adenosine A(1) receptors and vascular reactivity. Acta Physiol (Oxf) 2010;199:211–220. doi: 10.1111/j.1748-1716.2010.02093.x. [DOI] [PubMed] [Google Scholar]
- 43.Williams JM, Murphy S, Burke M, Roman RJ. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol. 2010;56:336–344. doi: 10.1097/FJC.0b013e3181f04b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu CC, Schwartzman ML. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat. 2011;96:45–53. doi: 10.1016/j.prostaglandins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. 20-HETE is an endogenous inhibitor of the large-conductance Ca(2+)-activated K+ channel in renal arterioles. Am J Physiol. 1996;270:R228–237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]


