Abstract
Background
A skeletal myopathy, perhaps attributable to neuro-endocrine excitation or disuse, has been described in heart failure (HF) patients, and is thought to contribute to their exercise limitation. Our purpose was to assess biochemical and morphometric characteristics of skeletal muscles of HF patients on optimal HF therapy. A secondary purpose was to explore the effects of clonidine, which interrupts the neuro-endocrine excitation, on these same muscle characteristics.
Methods and Results
Eleven HF patients (50.8±3.4 years, peak VO2 11.6±2.5 ml/kg/min) underwent two vastus lateralis biopsies (pre/post clonidine). Baseline values were compared to biopsies in 11 age-matched, healthy controls. Scatter plots of individual values for each mitochondrial enzyme revealed almost complete overlap between HF and control groups; mean values, although tending to be greater in controls versus HF patients, were not significantly different. The proportion of type 1 fibers was diminished in 10 of 11 patients. There was no difference in any of the variables after 3 months clonidine versus placebo.
Conclusion
In HF patients treated with optimal medical and device therapy, characteristic abnormalities of mitochondrial enzyme activity are not found, but muscle fiber type shifts are present. The remaining severe impairment in exercise capacity cannot be attributed to mitochondrial abnormalities.
Introduction
Patients with chronic systolic heart failure (HF) typically experience decreased exercise tolerance, early fatigue, and dyspnea, despite optimal medical and device therapy. Exercise in these patients appears to be limited not by central hemodynamic factors, but largely by peripheral, skeletal muscle, changes [1–10]. In fact, a skeletal myopathy of chronic HF has been described, and is characterized by fiber atrophy and shift from type 1 to type 2 fibers, capillary rarefaction, and decreased mitochondrial enzymes and volume [4,11–13]. The mechanisms underlying the skeletal myopathy of HF are not wholly defined, but include disuse, as well as chronic neuro-endocrine activation and inflammation [14–17].
Interestingly, many of the studies in which the skeletal myopathy was first reported and described predate the full use of currently available pharmacological and electrophysiological therapies to counter the neuro-endocrine activation that characterizes chronic HF. Indeed, Mettauer and colleagues[18], in a more recent report, studied mitochondrial respiration in situ in skeletal muscle biopsies from HF patients, all of whom were treated with angiotensin-converting enzyme inhibitors (ACEI), and found that muscle mitochondrial respiration was not diminished compared to that of sedentary controls, calling into question the persistence of metabolic abnormalities in the era of neuro-endocrine inhibition. However, in this study, neuro-endocrine blockade was incomplete, since HF patients were not taking beta-adrenergic blockers, and none had a biventricular pacemaker. The purpose of the current study was to examine the biochemical and morphometric characteristics of skeletal muscle biopsies from advanced HF patients optimized on medical therapy, including ACEI or angiotensin receptor blockers (ARB), carvedilol, aldosterone antagonists, and cardiac resynchronization therapy (in those meeting QRS criteria) to determine if the features of the skeletal myopathy of HF described in earlier studies were still present in HF patients on therapies targeting neuro-endocrine activation.
Increased sympathetic nerve activity (SNA) has been hypothesized to be a significant contributor to the skeletal myopathy and dysfunction in HF, directly, through a vasoconstrictor effect [19, 20], and indirectly, through inflammation [17, 21]. Central sympathetic outflow is consistently elevated in chronic HF patients, and clonidine has been shown to decrease resting SNA by approximately 30% [22]. Therefore, a secondary purpose was to perform an exploratory study of the effect of 3 months of a clonidine patch, as a further means to decrease central sympathetic outflow directed to muscle (MSNA), in a double-blinded controlled study on these same muscle characteristics, and on MSNA.
Materials and Methods
Study population
Advanced HF patients, New York Heart Association class II–III, followed in the Ahmanson-UCLA Cardiomyopathy Center by a multidisciplinary team of HF specialists, meeting the following criteria were eligible for the study: 1) age 21–65 years, 2) left ventricular ejection fraction ≤35%, 3) HF duration ≥ 6 months, 4) no active ischemia or ischemic event within 3 months, 5) not involved in a formal exercise training program, 6) on stable HF medications including ACEI or ARB, beta-blockers, and aldosterone antagonists for ≥ 3 months, 7) cardiac resynchronization therapy, when clinically indicated, inserted ≥ 6 months before enrollment, 8) BMI<35 kg/m2, 9) no contraindication to clonidine patch therapy, and 10) not on anticoagulation. The study was approved by the UCLA Human Subjects Protection Committee and was performed in accordance with the ethical standards laid down in the 1984 Declaration of Helsinki and later amendments. The participants gave their written informed consent. A subset of this study population was included in a previous report[23]. This trial was registered at http://www.clinicaltrials.gov (NCT00858845).
Tissue collection and processing
In the UCLA Out-Patient Surgery Center, following sterile preparation and anesthesia with 1% lidocaine of the thigh, an incision was made into the right or left lateral thigh approximately 20 cm superior to the patella. Two to three samples of right vastus lateralis muscle measuring approximately 1 by 4 cm were obtained, and the incision site was then closed and dressed. Specimens were immediately delivered to the College of American Pathologists-accredited UCLA Neuropathology Laboratory. Fresh muscle was quenched in isopentane cooled to −180° C in liquid nitrogen. A portion was stored at −80° C for biochemical studies. A small fascicle was removed prior to quenching, placed on filter paper, and fixed in 3% buffered glutaraldehyde. Blocks were prepared, processed, and cut transversely at 1μm prior to staining with toluidine blue for morphometric analysis of capillary density.
Biochemical analysis
Small aliquots (70–125 mg) of the frozen muscle were homogenized in approximately 1.5 ml Chappel-Perry medium [24] in a glass-glass homogenizer and spun at 600 g for 8 minutes to obtain the S1 fraction. A small aliquot was saved for citrate synthase determinations and the remainder spun at 12,000rpm to obtain the crude mitochondrial enriched fraction which was resuspended in Chappell medium giving the P2 fraction. NADH:O oxidoreductase activity was measured without sonification in the P2 with and without addition of rotenone plus KCN in the presence of 1 mg. bovine serum albumin and 10uM cytochrome c[25]. Succinate: cytochrome c reductase was estimated at 550nm after the manner of Sottacasa and colleagues [26] containing 2mM KCN, 2ug/ml rotenone and 50 um cytochrome c. Activity was determined in the presence and absence of 5mM malonate and the activity expressed as the difference. Rotenone sensitive NADH:cytochrome c oxidoreductase was determined in the presence of 5ug/ml rotenone after the method of Fischer[25]. Cytochrome c oxidase and citrate synthase were estimated as described by Cooperstein and Lazarow[27] and Srere[28] respectively. Activities were expressed in terms of non-collagen protein determined after alkaline digestion using the Biorad procedure.
Morphometric analysis
Eight micron frozen sections in OCT were serially examined with modified Gomori trichrome, adenosine triphosphatase (ATPase) at pH 9.4, 4.6 and 4.3, succinic dehydrogenase and oil red O reactions according to standard techniques[29]. Morphometric analysis of the muscle biopsy findings following the selected histoenzymatic procedures were performed on digital photo images analyzed in the Image Pro/Plus, version IV computer program. From this data the percentage of fiber types and average of fiber diameter were obtained. These parameters were obtained from the muscle fiber type specificity (1, 2A, 2X) indicated by the ATPase reactions. There was inconsistency in the reaction for the 2A and 2X, so these fiber populations were grouped and expressed as fiber type 2. The density of type 1 and type 2 fibers were assessed visually at 10× on a grid field. A total of 3–5 fields were assessed allowing for the enumeration and fiber typing of 240–550 fibers. Minimum fiber diameters were determined from the computer images under standard calibration. A minimum of 60 fibers of each type were assessed and mean fiber diameter determined.
Microvascular density was determined on 1 micron plastic sections[30]. Three blocks were selected for analysis and capillary counts performed at 40× on a standard grid. Contiguous fields were counted in 2 blocks which allowed for a total of 20–35 grid fields to be evaluated depending on section size. Vascular index was expressed as number of capillaries/mm2.
Microneurography to record muscle sympathetic nerve activity (MSNA)
Microneurography studies were performed in the Human Physiology Laboratory in the General Clinical Research Center at UCLA. MSNA was recorded using microneurography from the peroneal nerve as previously described[31–33]. Lead II of the ECG was recorded simultaneously with the neurogram using a multichannel digital data recorder (LabChart6 Pro, AD Instruments, Inc). Blood pressure was recorded during MSNA recordings using an automated pressure cuff placed on the upper arm, and heart rate was monitored continuously from the electrocardiogram patch electrodes. MSNA was identified using previously described methods[31–33], and a satisfactory neurogram exhibited a signal to noise ratio >3:1. Sympathetic bursts were determined by visual inspection by a single investigator (H.R.M.) without knowledge of randomization group (clonidine vs placebo). MSNA was expressed as burst frequency (bursts/minute) and bursts per 100 heart beats (bursts/100 HB).
Study protocol
HF patients were randomized in a double-blind fashion (1:1) to either clonidine patch (0.1 mg/weekly) or matching placebo for a treatment period of 3 months. This dose and duration of clonidine patch has been shown to be tolerated in HF patients, and to reduce resting MSNA by approximately 30%[22]. Patients underwent MSNA recording and vastus lateralis muscle biopsy (described above) on Day 0 of the study, and during week 12 of the study. Patients were asked to abstain from caffeine on the morning of the microneurography session, and were studied at approximately the same time of day for both sessions. Patients were positioned supinely for microneurography, and the ECG electrodes and blood pressure cuff were placed. After an adequate nerve recording site was obtained and a short rest period, 10 minutes of resting MSNA and HR were recorded. Blood pressure was recorded during the last 2 minutes. The microneurography study was then over, and the patient then underwent muscle biopsy as described above.
Statistical Methods
Statistical analysis was performed using SAS software (SAS 9.2, SAS Inc., Cary, NC). The primary end point of the study was the value of each mitochondrial enzyme (e.g. citrate synthase, cytochrome C oxidase, succinate cytochrome C reductase, and NADH:O) compared to age and gender-matched controls previously measured in the College of American Pathologists(CAP)-accredited UCLA Neuropathology Laboratory. The secondary endpoint was the muscle fiber type, size and vascular index compared to established normal values in the CAP-accredited UCLA Neuropathology Lab. Baseline continuous variables were calculated using independent two-tailed t-tests. The Bonferroni correction was used to correct for multiple comparisons. The primary endpoint of the exploratory clonidine study was change in citrate synthase activity as the estimate of mitochondrial oxidative enzyme activity. Secondary endpoints included changes in other mitochondrial enzymes, muscle fiber types and size, vascular index, and resting MSNA. Power analysis was based on the study by Tyni-Lenne and colleagues [34] in which a sample size of 5 per group provided 80% power to detect a mean difference of citrate synthase activity between groups of 30%. Two-way analysis of variance was used to compare mean difference of change between clonidine and placebo groups. Tukey criteria were used to compare adjusted p values that correct for multiple comparisons. Results are reported as means ± SEM. A p value <0.05 was considered statistically significant.
Results
Study population characteristics
Of 44 HF patients expressing interest in this study, 25 were ineligible (on-going medication adjustments (6), “too well” with either left ventricular ejection fraction >35% (3) or NYHA Class I (2), transportation issues (4), morbidly obese (3), enrolled in exercise program (2), other (5)), and of the remaining 19 patients, 8 ultimately declined. Eleven patients with advanced HF NYHA class II–III, were enrolled. Study population characteristics are displayed in Table 1.
Table 1.
Study Population Characteristics
| Heart Failure Patients N=11 |
|
|---|---|
| Age (years) | 50.8± 3.4 |
| BMI (kg/m2) | 27.9±1.7 |
| Female | 4 |
| Duration of HF (years) | 9.3±1.9 |
| Peak VO2 (ml/kg/min) | 11.6±2.5 |
| LVEF (%) | 23.3±2.5 |
| Diabetes mellitus | 4 |
| Hypertension | 4 |
| Etiology of HF | |
| CAD | 6 |
| Idiopathic | 3 |
| Alcohol | 1 |
| Noncompaction | 1 |
| Familial | 0 |
| Medications | |
| Beta-blockers | 10 |
| ACEI | 10 |
| ARB | 1 |
| Statin | 6 |
| Aldosterone antagonist | 9 |
| Aspirin | 6 |
| Clopidogrel | 1 |
| Furosemide | 9 |
| Digoxin | 5 |
| Amiodarone | 2 |
| Biventricular pacemaker | 6 |
| MSNA (bursts/min) | 40.9±3.4 |
ACEI=angiotensin inhibitor, ARB=angiotensin receptor blocker, BMI=body mass index, CAD=coronary artery disease, HF=heart failure, LVEF=left ventricular ejection fraction, MSNA=muscle sympathetic nerve activity, peak VO2=peak oxygen consumption. Values are means±SEM.
Biochemical and morphometric analysis
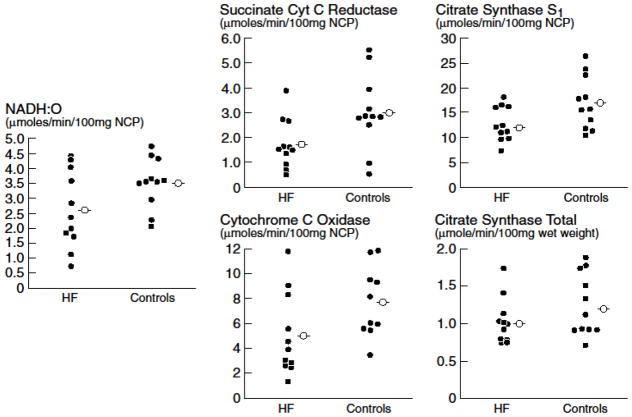
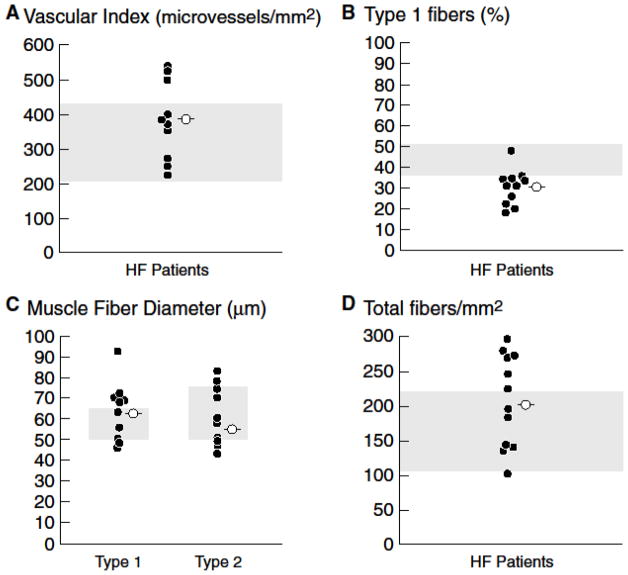
Scatter plots of individual mitochondrial enzymes from 11 advanced HF patients optimized on medical and device therapy compared with 11 previously collected controls with normal muscle biopsies (mean age 50±3.7 years) are displayed in Figure 1. Scatter plots of individual values of each type of mitochondrial enzyme reveal complete overlap between the groups. Although mean values tend to be greater in controls compared to HF patients, none of the mean values is significantly different between the groups. As previously reported in a subset of these HF patients [23], vascular index was within or above the normal range [30,35] in all HF patients (Figure 2A), but the proportion of type 1 fibers compared to type 2 fibers was diminished in 10 of 11 patients, consistent with a fiber shift from oxidative type 1 fibers to glycolytic type 2 fibers [29,30,35] (Figure 2B). The overall mean fiber 1 and 2 diameters (Figure 2C) and the mean total number of fibers per mm2 were within the normal range (Figure 2D) [29,36].
Figure 1.
Scatter plots of individual values (filled circles) of mitochondrial enzymes from 11 HF patients and 11 age-matched controls. Mean values (open circles) of each enzyme is lower in the HF versus control group, but not significantly so. There is almost complete overlap of individual values between the groups. NCP=non-collagen protein
Figure 2.
Scatter plots of individual measures (filled circles) and mean values (open circles) of morphometry in 11 HF patients. Panel A. Vascular index was within or above the normal range in all HF patients. Panel B. The proportion of type 1 fibers was diminished in 10 of 11 HF patients, consistent with a fiber shift from oxidative type 1 fibers to glycolytic type 2 fibers. Panel C. The overall mean fiber diameter is within the normal range in HF patients on optimal medical and device therapy. Panel D. Total number of fibers per mm2 is within the normal range in HF patients on optimal medical and device therapy. Shaded area represents the normal range established in prior published studies from the UCLA Neuropathology Lab [26] and in the literature [25,31,32].
Effect of clonidine. (Table 2)
Table 2.
Impact of 3 months Clonidine versus Placebo
| Clonidine | Placebo | |
|---|---|---|
| MSNA (bursts/min) | −4.3± 2.4 | 4.4±4.4 |
| MSNA (bursts/100HB) | −3.1±3.1 | 4.1±8.3 |
| Heart rate (bpm) | −3.5±1.5 | 0.6±5.0 |
| MAP (mmHg) | −1.6±12.0 | −10.6±5.5 |
| Citrate synthase total μmole/min/wet weight | −0.3±0.09 | 0.07±0.07 |
| Citrate synthase S1 μmole/min/100 NCP | −3.6±2.4 | 2.8±0.9 |
| Succinate cytochrome C reductase μmole/min/100 NCP | −0.5±0.5 | 0.6±0.2 |
| Cytochrome C oxidase μmole/min/100 NCP | 0.9±0.8 | 1.3±1.2 |
| NADH:O μmole/min/100 NCP | 0.3±0.2 | 2.5±1.6 |
| Fiber type 1 (%) | −4.6±3.8 | −5.6±1.9 |
| Vascular index microvessels/mm2 | −50.8±69.2 | −49.9±29.1 |
| Fiber type 1 diameter(μm) | −8.7±10.1 | 3.2±2.5 |
| Fiber type 2 diameter (μm) | −5.1±7.3 | −1.5±2.2 |
| Total fibers/mm2 | 2.5±30.8 | 10.1±9.3 |
Values are the difference between 3 months on drug or placebo and baseline. HB=heart beats, MSNA = muscle sympathetic nerve activity, NCP=non-collagen protein. Values are means±SEM. Differences were not significant for any variable.
Six patients were randomized to placebo, and 5 were randomized to clonidine patch. One patient randomized to placebo underwent urgent orthotopic heart transplantation before her second biopsy could be obtained. MSNA was not different between the groups at baseline, and tended to decrease on clonidine and increase on placebo, although the difference between groups was not significant. (Table 2) There was no difference in any of the biochemical or morphometric variables on clonidine compared to placebo. (Table 2)
Discussion
The novel findings of this study of muscle biopsies from the vastus lateralis muscle in advanced HF patients treated by a comprehensive HF team according to guideline recommended pharmacological and device therapy were that: 1) the mean activity of several different mitochondrial enzymes were not different between HF patients and healthy controls, and, in fact, the individual values were almost completely overlapping, 2) vascular index was similarly normal, but 3) a fiber shift from type 1 to 2 fibers, as reported previously, was present. A controversy in HF is whether the skeletal myopathy is attributable to neuro-endocrine activation or disuse(14–16). In an attempt to address this controversy, an exploratory, double-blind, placebo controlled study of the impact of clonidine, which inhibits central sympathetic outflow [22], on the biochemical or morphometric profile of the skeletal muscle was performed, and revealed no impact of clonidine compared with placebo on any parameter.
Older reports describing the skeletal myopathy of HF patients were largely published before the availability and widespread use of ACEI, ARBs, aldosterone antagonists, and/or beta-adrenergic blockade with carvedilol[6–9]. In addition to the favorable hemodynamic and cardiac remodeling effects of these medications, they also have beneficial effects on skeletal muscle in HF. Vescovo and colleagues[37] found that 6 months of ACEI or ARB therapy significantly increased exercise capacity, as measured by peak VO2 or ventilatory threshold. Importantly, therapy with ACEI and ARB was associated with a significant increase in the proportion of type 1, oxidative fibers. Unfortunately, biochemical analysis of mitochondrial enzymes was not done in this study. In a later study by the same investigative group, the impact of carvedilol, which has anti-oxidative effects, on oxidative damage in skeletal muscle from an animal model of heart failure, was tested [38]. Carvedilol compared with bisoprolol, a beta-adrenergic blocker without anti-oxidative effects, improved force production in isolated muscles, whereas bisoprolol did not. This improved muscle function was accompanied by a significantly decreased degree of myofibrillar peroxidation. The impact of carvedilol on mitochondrial enzymes was also not measured in this study.
Mettauer and colleagues [18] specifically assessed the mitochondrial oxidative capacity of skeletal muscle in 15 patients with HF, who were all treated with ACEI, but not carvedilol or biventricular pacemakers. Uniquely, parameters of mitochondrial respiration were studied in situ, as well as by quantification of individual mitochondrial enzyme activities. Interestingly, the maximal oxidative phosphorylation capacity of the mitochondria in the presence of saturating amounts of ADP was similar in HF patients and sedentary controls. Surprisingly, activity of citrate synthase was significantly lower in HF patients compared with sedentary controls. This finding differs from our study of HF patients on ACEI/ARB, carvedilol, and aldosterone inhibitors, in whom activity of citrate synthase, and that of several other mitochondrial enzymes, was within the normal range. Two additional studies demonstrated findings similar to the current study. First, in a rat infarct model of HF, ACEI compared to no therapy were found to prevent mitochondrial dysfunction following coronary ligation, and interestingly, a decrease in muscle activity of citrate synthase was also prevented by ACEI(39). Finally, Toth and colleagues[40] studied HF patients on ACEI and carvedilol, and reported that the activities of cytochrome c oxidase and citrate synthase were within the normal range. Unlike our study, however, these investigators reported that there was not a fiber type switch from 1 to 2 fibers.
Despite the presence of mitochondrial enzymes within the normal range, the HF patients in the present study had severely limited exercise capacity as estimated by peak VO2. Potential mechanisms underlying this exercise limitation include decreased muscle mass, decreased vasodilatory response and impaired O2 delivery, attenuated metaboreceptor activation during exercise leading to less efficient distribution of blood flow during exercise, and impaired muscle energetic[4,6,41]. The observation that the vascular index was within the normal range in our study does not exclude the possibility that oxygen delivery is decreased. Esposito and colleagues [10] studied the mechanisms of exercise dysfunction in optimally treated HF patients using knee extensor exercise, which does not tax the cardiac capacity, thereby isolating peripheral factors from central (cardiac) ones. They found that the skeletal muscle structural features and metabolic reserve were intact in their small cohort of HF patients compared to healthy controls. Hyperoxia only minimally increased peak leg VO2, thus these investigators postulated over-activation of the SNA may underlie the persistent decrease in peak leg VO2 and decreased exercise capacity in their optimally treated HF patients. Application of investigative techniques, such as P31 NMR spectroscopy to evaluate phosphocreatine stores and cellular pH, in patients optimized on medical and device therapy, in whom exercise dysfunction persists, might be illuminating [7]. Additionally, the relatively unexplored contribution of abnormal excitation-contraction (E-C) coupling in patients with HF may be rewarding. Abnormal or inefficient E-C coupling may contribute to the decreased exercise capacity in skeletal muscles, just as it does in the failing myocardium [42,43]. Interestingly, in a separate paper in a subset of these HF patients on optimal medical and device therapy, we have recently reported that the presence of significant abnormalities of calcium handling proteins involved in E-C coupling [23].
In our exploratory, double blind, placebo-controlled, clonidine study, we found no effect of clonidine on mitochondrial enzyme activity, including citrate synthase activity, our primary endpoint. Of course, the mitochondrial enzyme activities were already within the normal range at baseline on optimal medical and device therapy, so perhaps it is not surprising that we did not see “improvement.” However, we did not see an effect on the morphometric parameters either, although at baseline, these were abnormal. The fiber shift from type 1 to type 2 fibers did not reverse on clonidine. This small, exploratory study suggests, but was likely underpowered to say definitively, that the interruption of the neuro-endocrine activity with clonidine does not impact the fiber shift in skeletal muscle of advanced HF patients already on optimal medical and device therapy.
It is debated whether the skeletal myopathy described in HF is a distinct entity that is part of the HF syndrome, resulting from neuro-endocrine activation/inflammation, or whether it is a result of disuse and deconditioning[13–15]. The answer is likely “both.” Since many of the previously described abnormalities in mitochondrial enzymes are no longer present in these HF patients on optimal medical (ACEI, ARB, beta-blockers and aldosterone inhibitors) and device therapy, which counteract neurohumoral activation, the argument for an important role for neuro-endocrine activation in leading to mitochondrial dysfunction is strengthened. On the other hand, we have found that the fiber shift from type 1 to 2 persists. Disuse is similarly characterized by a fiber shift from type 1 to 2 fibers, and may explain the presence of this shift in HF despite optimal therapy. The fact remains, however, that despite optimal medical and device therapy, exercise capacity is markedly diminished in these advanced HF patients. Whether this reflects continued abnormalities in the skeletal muscles other than those localized to the mitochondria, including muscle atrophy, impaired O2 delivery or transport [10], impaired muscle energetics, and/or abnormal E-C coupling, warrants further study.
Study Limitations
We recognize several limitations in our study. First, it would have been ideal to have a muscle biopsy before HF patients were optimized on medical and device therapy, and compare that to the steady state on therapy. However, most patients presenting to our clinic are at least partially treated with standard HF medical and device therapy at the time of presentation. Further, assessing daily activity level in HF patients before and after medical optimization would have been illuminating. We only assessed mitochondrial enzyme activity and morphometric parameters, but it would have been of interest to use electron microscopy to measure mitochondrial volume, P31 NMR spectroscopy to measure high energy phosphate profiles during exercise, and additional biochemical assays to assess markers of inflammation. Not all of the mitochondrial enzymes were measured, thus we cannot say definitively that all are within the normal range, but we did test the relevant enzymes that had been reported as abnormal in HF patients. Of course, the sample size in the clonidine study, although theoretically adequate to assess difference in citrate synthase activity, would have been inadequate to make all these additional comparisons. The HF study population was heterogeneous in this study, as it is in virtually all studies of human HF. Nonetheless, the severity of exercise dysfunction and the characteristics of the skeletal myopathy have been reported to be independent of the etiology of HF[9,44].
Conclusions
In summary, in HF patients optimally treated with HF medical and device therapy, characteristic abnormalities of mitochondrial enzyme activity are not found, but muscle fiber type shift from oxidative type 1 fibers to glycolytic type 2 fibers persists. The remaining severe impairment in exercise capacity in HF patients on optimal therapy cannot be attributed to mitochondrial abnormalities and warrants further investigation.
Acknowledgments
Funding Sources
This work was supported by NIH-RO1 HL084525 (H.R.M.), and the University of California, Los Angeles, General Clinical Research Center NIH-MO1-RR00865.
Footnotes
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Franciosa JA, Park M, Levine TB. Lack of correlation between exercise capacity and indices of resting left ventricular performance in heart failure. Am J Cardiol. 1991;47:33–9. doi: 10.1016/0002-9149(81)90286-1. [DOI] [PubMed] [Google Scholar]
- 2.Higginbotham M, Morris KG, Conn EH, et al. Determinants of variable exercise performance among patients with severe left ventricular dysfunction. Am J Cardiol. 1983;51:51–2. doi: 10.1016/s0002-9149(83)80010-1. [DOI] [PubMed] [Google Scholar]
- 3.Maskin CS, Forman R, Sonnenblick EH, et al. Failure of dobutamine to increase exercise capacity despite hemodynamic improvement in severe chronic heart failure. Am J Cardiol. 1983;51:177–82. doi: 10.1016/s0002-9149(83)80032-0. [DOI] [PubMed] [Google Scholar]
- 4.Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail. 2010;3:537–46. doi: 10.1161/CIRCHEARTFAILURE.109.903773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington D, Anker SD, Chua TP, et al. Skeletal muscle function and its relation to exercise tolerance in chronic HF. J Am Coll Cardiol. 1997;30:1758–64. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan MJ, Green HJ, Cobb FR. Altered skeletal muscle metabolic response to exercise in chronic heart failure. Circulation. 1991;84:1597–1607. doi: 10.1161/01.cir.84.4.1597. [DOI] [PubMed] [Google Scholar]
- 7.Massie BM, Conway M, Rajagopalan B, et al. Skeletal muscle metabolism during exercise under ischemic conditions in congestive heart failure: Evidence for abnormalities unrelated to blood flow. Circulation. 1988;78:320–26. doi: 10.1161/01.cir.78.2.320. [DOI] [PubMed] [Google Scholar]
- 8.Poole-Wilson PA, Ferro R. Role of skeletal muscle in the syndrome of chronic heart failure. J Mol Cell Cardiol. 1996;28:2275–85. doi: 10.1006/jmcc.1996.0220. [DOI] [PubMed] [Google Scholar]
- 9.Mancini DM, Coyle E, Coggan A, et al. Contribution of intrinsic skeletal muscle changes to 31P NMR skeletal muscle metabolic abnormalities in patients with chronic heart failure. Circulation. 1989;80:1338–46. doi: 10.1161/01.cir.80.5.1338. [DOI] [PubMed] [Google Scholar]
- 10.Espoisto F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010;55:1945–54. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lunde PK, Sjaastad I, Schiotz Thorud HM, et al. Skeletal muscle disorders in heart failure. Acta Physiol Scand. 2001;171:277–294. doi: 10.1046/j.1365-201x.2001.00830.x. [DOI] [PubMed] [Google Scholar]
- 12.Drexler H, Riede U, Munzel T, et al. Alterations of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–59. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81:518–27. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 14.Rehn TA, Munkvik M, Lunde PK, et al. Intrinsic skeletal muscle alterations in chronic heart failure patients: a disease specific myopathy or a result of deconditioning? Heart Fail Rev. 2012;17:421–36. doi: 10.1007/s10741-011-9289-4. [DOI] [PubMed] [Google Scholar]
- 15.Vescovo G, Serfini F, Facchin LL. Specific changes in skeletal muscle myosin heavy chain composition in cardiac failure: differences compared with disuse atrophy as assessed on microbiopsies by high resolution electrophoresis. Heart. 1996;76:337–343. doi: 10.1136/hrt.76.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duscha BD, Annex BH, Green HJ, et al. Deconditioning fails to explain peripheral skeletal muscle alterations in men with chronic heart failure. J Am Coll Cardiol. 2002;39:1170–74. doi: 10.1016/s0735-1097(02)01740-0. [DOI] [PubMed] [Google Scholar]
- 17.Gielen S, Adams V, Möbius-Winkler S, et al. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol. 2003;42:861–8. doi: 10.1016/s0735-1097(03)00848-9. [DOI] [PubMed] [Google Scholar]
- 18.Mettauer B, Zoll J, Sanchez H, et al. Oxidative capacity of skeletal muscle in heart failure patients versus sedentary or active control subjects. J Am Coll Cardiol. 2001;38:947–54. doi: 10.1016/s0735-1097(01)01460-7. [DOI] [PubMed] [Google Scholar]
- 19.Negrao CE, Rondon MUPB, Tinucci T, Alves MJNA, Roveda F, Braga AMW, Reis SF, Nastari L, Barretto ACP, Krieger EM, Middlekauff HR. Abnormal neurovascular control during exercise is linked to heart failure severity. Am J Physiol Heart. 2001;280:H1286–H1292. doi: 10.1152/ajpheart.2001.280.3.H1286. [DOI] [PubMed] [Google Scholar]
- 20.Coates AJ, Clark AL, Piepoli M, Volterrani M, Poole-Wilson PA. Symptoms and quality of life in heart failure: the muscle hypothesis. Brit Heart J. 1994;72(Suppl):S36–S39. doi: 10.1136/hrt.72.2_suppl.s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller-Wedan U, Wedan K. Immune modulation by catecholamines- a potential mechanism of cytokine release in HF? Herz. 2000;25:271–273. doi: 10.1007/s000590050019. [DOI] [PubMed] [Google Scholar]
- 22.Grassi G, Turri C, Seravalle G, et al. Effects of chronic clonidine administration on sympathetic nerve traffic and baroreflex function in heart failure. Hypertension. 2001;38:286–291. doi: 10.1161/01.hyp.38.2.286. [DOI] [PubMed] [Google Scholar]
- 23.Middlekauff HR, Vigna C, Verity MA, et al. Abnormalities of calcium handling proteins in skeletal muscle mirror those of the heart in humans with heart failure: a shared mechanism? J Card Fail. 2012;9:724–733. doi: 10.1016/j.cardfail.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verity MA, Turnbull DM. Assay of acyl-CoA dehydrogenase activity in frozen muscle biopsies: application to medium-chained acyl-CoA dehydrogenase deficiency. Biochem Med Metab Biol. 1993;49:351–62. doi: 10.1006/bmmb.1993.1036. [DOI] [PubMed] [Google Scholar]
- 25.Fischer JC, Ruitenbeek W, Trijbels JM, et al. Estimation of NADH oxidation in human skeletal muscle mitochondria. Clin Chim Acta. 1986;155:263–73. doi: 10.1016/0009-8981(86)90246-9. [DOI] [PubMed] [Google Scholar]
- 26.Sottocasa GL, Kuylenstierna B, Ernster L, et al. An electron-transport system associated with the outer membrane of liver mitochondria. a biochemical and morphological study. J Cell Biol. 1967;32:415–38. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cooperstein SJ, Lazarow A. A microspectrophotometric method for the determination of the cytochrome oxidase. J Biol Chem. 1951;189:665–70. [PubMed] [Google Scholar]
- 28.Srere PA, Kosicki GW. The purification of citrate-condensing enzyme. J Biol Chem. 1961;236:2557–59. [PubMed] [Google Scholar]
- 29.Dubowitz V, Sewry CA. Muscle Biopsy: A practical approach. 3. Philadelphia: Saunders; 2006. [Google Scholar]
- 30.Charles-Schoeman S, Verity MA. Nicotinamide adenine dinucleotide tetrazolium reductase identifies microvasculature activation in muscle from adult patients with dermatomyositis. J Rheum. 2012;39:94–9. doi: 10.3899/jrheum.110739. [DOI] [PubMed] [Google Scholar]
- 31.Wallin BG, Fagius J. Peripheral sympathetic neural activity in conscious humans. Ann Rev Physiol. 1988;50:565–76. doi: 10.1146/annurev.ph.50.030188.003025. [DOI] [PubMed] [Google Scholar]
- 32.Delius W, Hagbarth KE, et al. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand. 1972;84:65–81. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- 33.Park J, Middlekauff HR. Altered pattern of sympathetic activity with the ovarian cycle in female smokers. Am J Physiol Heart Circ Physiol. 2009;297:H564–H568. doi: 10.1152/ajpheart.01197.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyni-Lenné R, Gordon A, Europe E, et al. Exercise-based rehabilitation improves skeletal muscle capacity, exercise tolerance, and quality of life in both women and men with chronic heart failure. J Card Fail. 1998;4:9–17. doi: 10.1016/s1071-9164(98)90503-6. [DOI] [PubMed] [Google Scholar]
- 35.Ingjer F. Effects of endurance training on muscle fibre type ATPase activity, capillary supply and mitochondrial content in man. J Physiol. 1979;294:419–32. doi: 10.1113/jphysiol.1979.sp012938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brooke MH, Engel WK. The histographic analysis of human muscle biopsies with regard to fiber types: Adult male and female. Neurology. 1969;19:221–33. doi: 10.1212/wnl.19.3.221. [DOI] [PubMed] [Google Scholar]
- 37.Vescovo G, Dalla Libera L, Serafini F, et al. Improved exercise tolerance after losartan and enalapril in heart failure: correlation with changes in skeletal muscle myosin heavy chain composition. Circulation. 1998;98:1742–49. doi: 10.1161/01.cir.98.17.1742. [DOI] [PubMed] [Google Scholar]
- 38.Dalla Libera L, Ravara B, Gobbo V, et al. Skeletal muscle myofibrillar protein oxidation in heart failure and the protective effect of carvedilol. J Molec and Cell Cardiol. 2005;38:803–7. doi: 10.1016/j.yjmcc.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 39.Zoll J, Monassier L, Garnier A, et al. ACE inhibition prevents myocardial infarction-induced skeletal muscle mitochondrial dysfunction. J Appl Physiol. 2006;101:385–91. doi: 10.1152/japplphysiol.01486.2005. [DOI] [PubMed] [Google Scholar]
- 40.Toth MJ, Miller MS, Ward KA, et al. Skeletal muscle mitochondrial density, gene expression and enzyme activities in human heart failure: minimal effects of the disease and resistance training. J Appl Physiol. 2012;112:1864–74. doi: 10.1152/japplphysiol.01591.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sterns DA, Ettinger SM, Gray KS, et al. Skeletal muscle metaboreceptor exercise responses are attenuated in heart failure. Circulation. 1991;84:2034–9. doi: 10.1161/01.cir.84.5.2034. [DOI] [PubMed] [Google Scholar]
- 42.Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res. 1998;37:279–89. doi: 10.1016/s0008-6363(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 43.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001. [Google Scholar]
- 44.Whellan DJ, Nigam A, Arnold M, et al. Benefit of exercise therapy for systolic heart failure in relation to disease severity and etiology – findings from the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training study. Am Heart J. 2011;162:1003–10. doi: 10.1016/j.ahj.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]