Abstract
Adipocytes play important roles in lipid storage, energy homeostasis and whole body insulin sensitivity. Studies in the last two decades have identified the hormones and cytokines that activate specific STATs in adipocytes in vitro and in vivo. Five of the seven STAT family members are expressed in adipocyte (STATs 1, 3, 5A, 5B and 6). Many transcription factors, including STATs, have been shown to play an important role in adipose tissue development and function. This review will summarize the importance of adipocytes, indicate the cytokines and hormones that utilize the JAK-STAT signaling pathway in fat cells and focus on the identification of STAT target genes in mature adipocytes. To date, specific target genes have been identified for STATs, 1, 5A and 5B, but not for STATs 3 and 6.
Keywords: adipocytes, STAT, adipocyte, adipose tissue, transcription
Adipocytes
Adipocytes, or fat cells, are highly specialized cells that play a major role in energy homeostasis in vertebrates. Obesity is the primary disease of fat cells and the most common metabolic disorder in the industrial world. Obesity affects > 30% of the adult population in the United States and is a major risk factor for the development of Type 2 diabetes mellitus, cardiovascular disease and hypertension. Adipocytes have three primary functions: they are insulin-sensitive, they store lipid and they secrete hormones that act in distant tissues. The disruption of any one of these functions results in an unhealthy metabolic disease state. Recent studies suggest that obesity and its related disorders may be linked to a breakdown in the regulatory mechanisms that control the expression of a variety of genes in adipocytes. Significant advances toward an understanding of these regulatory processes have been made by studying the function of transcription factors, which regulate the differentiation of fat cells and are involved in the modulation of adipocyte gene expression. It is well recognized that several transcription factors are induced during adipocyte differentiation, play a critical role in the regulation of adipocyte gene expression and are altered in conditions of obesity and/or insulin resistance.1,2 Several laboratories have investigated the role of STATs (signal transducers and activators of transcription) in adipocyte development and function.
Signal Transducers and Activators of Transcriptions: STATs
There are seven signal transducers and activators of transcription (STAT) proteins that are designated STATs 1, 2, 3, 4, 5A, 5B and 6 that exhibit unique tissue distributions and regulate the expression of tissue specific genes.3 The expression and modulation of gene expression modulated by STATs can be cell specific, and transgenic knockout studies have shown critical roles for every member of the STAT family.3 STATs are primarily activated by cytokines and hormones. Ligand binding initiates a cascade that results in STAT tyrosine phosphorylation, dimerization and translocation to the nucleus where STATs modulate transcription.3,4 In addition to the canonical activation by tyrosine phosphorylation, STATs can undergo several other posttranslational modifications, including serine phosphorylation, acetylation, methylation and sumoylation.5 Of note, there is also substantial evidence that STATs can function in non-canonical mechanisms to modulate transcriptional activity and they can also function in chromatin organization and mitochondrial respiration in ways that appear to be independent of transcriptional regulation.5,6
STATs and Adipocytes
Studies by independent groups have revealed that STATs 1, 3, 5A, 5B and 6 are expressed in fat cells. As shown in Figure 1, STATs 1, 5A and 5B are substantially induced during 3T3-L1 adipocyte differentiation.7 Similar induction patterns of STATs 5A and 5B occur in human subcutaneous preadipocytes.8 The expression and activation of STAT6 is not changed during adipocyte development. Studies in both mouse and human cells have revealed a similar induction of STATs 3, 5A and 5B, but there was a difference in STAT1 induction in mouse and human cells. However, it is unlikely that STAT1 plays a critical role in adipocyte development because STAT1 knockout mice do not have any apparent body weight abnormalities.9 The significance of the differential expression of STAT1 during the adipogenic program remains unclear. However, there are numerous studies that establish a role of STATs 3, 5A and 5B in human and murine adipogenesis. STAT3 expression does increase during the proliferative phase that occurs during the adipogenesis of the murine 3T3-L1 cells.10 STAT3 expression is tightly regulated by PIAS3, protein inhibitor of activated STAT3.11 Inhibition of adipogenesis has also been observed with AG490, a JAK2 inhibitor, and STAT3 siRNAs.12 Furthermore, the ectopic expression of a dominant negative STAT3 suppresses adipocyte differentiation.13 Mice lacking STAT3 in adipose tissue were generated using the aP2 promoter and these mice exhibited higher body weights and increased adipocyte size compared with wild-type littermates.14 Collectively, these studies suggest a possible role for STAT3 in adipogenesis and body weight homeostasis. Nevertheless, additional studies are necessary to further clarify the contribution of STAT3 in adipocyte development and physiology.
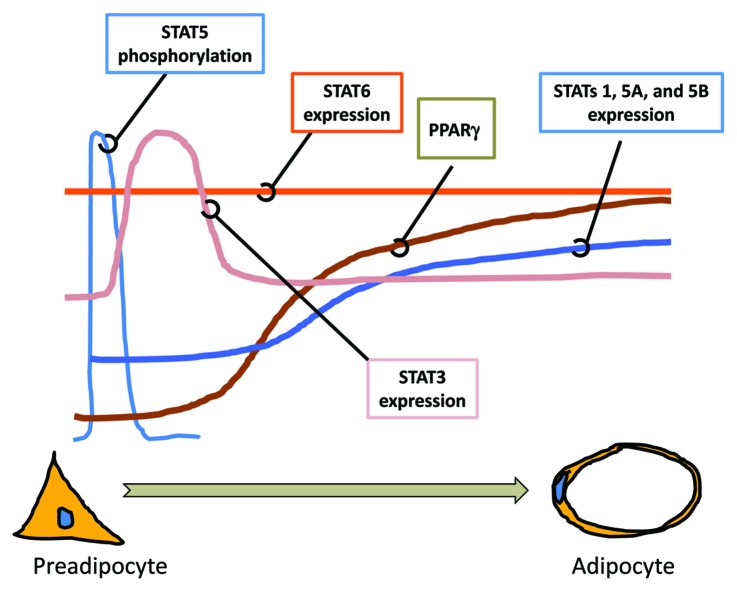
Figure 1. The expression and activation of STATs during adipocyte differentiation. Many studies have examined the expression and activation of STATs during the development of adipocytes from preadipocytes. As shown above, STAT5 proteins are tyrosine phosphorylated at the initiation of adipocyte differentiation. This activation precedes the increase in expression of STATs 1, 3, 5A and 5B, which is observed. STAT3 expression is increased during clonal expansion. The induction of the transcription factor PPARγ, a necessary factor for adipocyte development, precedes the increased expression of STATs, 1, 5A and 5B. The expression of STAT6 does not change during adipocyte development. Although the expression of several STATs is changed during adipocyte development, only STAT5 proteins have been shown to play a critical role in adipocyte development in vitro and in vivo.
The involvement of STAT5 in adipogenesis has been widely investigated. The ectopic expression of C/EBP transcription factors in non-precursor cells can induce adipogenesis15 in a mechanism that is accompanied by a substantial increase in STAT5A and STAT5B expression.16 PPARγ, a transcription factor critical for adipocyte development, has also been shown to increase the expression of both STATs 5A and 5B during adipocyte development.17 In preadipocytes, the control that growth hormone exerts on adipogenesis is inhibited by STAT5 antisense oligonucleotides.18 Also, constitutively active STAT5 can drive adipogenesis in preadipocytes.19 A variety of methods including transgenic knockout mice and ectopic expression studies have confirmed the physiological relevance of STAT5 proteins in adipogenesis. Ectopic expression of STAT5A induces adipogenesis in 3T3-L1 preadipocytes,20 and in several non-precursor cell lines.21 Unlike STAT5A, STAT5B does not display pro-adipogenic properties in non-precursor cells.21 Growth hormone activated STAT5 proteins have been shown to induce PPARγ expression in 3T3-L1 cells and C3H10T1/2 cells,22 suggesting a mechanism by which STAT5 proteins are able to promote adipocyte differentiation. Interestingly, transgenic knockout experiments have shown that disruption in either STAT5A or STAT5B or both genes resulted in abnormal adipose tissue and mice lacking both STAT5 proteins had fat pads one-fifth the normal size.23 To date, there are no studies on tissue-specific knockouts of STAT5 genes in adipocytes and the phenotype of the STAT5 null mice could be attributed to developmental effects of STAT5 that are independent of direct effects on preadipocyte differentiation. However, new studies have shown that ectopic STAT5A expression can confer adipogenesis in fibroblasts in vivo.24 In addition to demonstrating a direct role of STAT5A in preadipocyte differentiation in vivo, these studies also revealed the usefulness of athymic mice in studying the role of transcription factors in adipose tissue development. In summary, the importance of the STAT5 proteins in adipogenesis has been demonstrated in vitro and in the whole animal.
Activators of STATs in Adipocytes
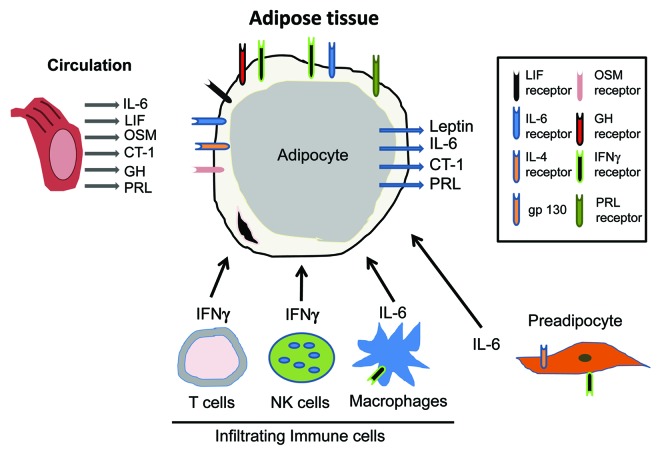
Studies by a variety of groups over the past 20 years have shown that adipocytes are responsive to several JAK-STAT activating cytokines and hormones including LIF, OSM, CT-1, interleukin (IL)-6, CNTF, NP, GH, PRL and IFNγ (Table 1). Most of these observations are based on vitro studies performed in cultured murine or human adipocytes. However, there is also sufficient and compelling evidence to show that adipose tissue in vivo is responsive to these JAK-STAT activators.25-27 As shown in Figure 2, adipocytes contain receptors for these ligands, most of which are present in circulation. An important function of adipocytes is the production of a variety of endocrine mediators. Of note, there are four JAK-STAT activating hormones which have been shown to be produced from adipocytes (Fig. 2). One of these STAT activators is leptin, an important endocrine hormone that serves as an adiposity signal and can affect food intake and energy expenditure. Of note, the majority of leptin is produced and secreted from adipocytes and the primary target tissue is the arcuate nucleus in the hypothalamus. Leptin binding to its receptor within this feeding center in the hypothalamus results in JAK2, STAT3 and STAT5 activation. In leptin receptor-deficient mice, analysis of mutant leptin receptor knock-in has revealed distinct roles of STAT3 and STAT5 in leptin action.28-31 Besides leptin, other JAK-STAT activating hormones have also been shown to be produced from adipocytes (refer to Fig. 2) including IL-6,32,33 CT-134 and PRL.35-38
Table 1. STAT activators in adipocytes.
| STATs | Activator | Reference(s) |
|---|---|---|
| STAT1 |
Cardiotrophin-1 |
64 |
| Growth hormone |
56 and 65 |
|
| Interferon γ |
46, 56 and 57 |
|
| Interleukin-11 |
66 |
|
| Leukemia inhibitory factor |
56, 57 and 67 |
|
| Oncostatin M |
56 and 57 |
|
| STAT3 |
Cardiotrophin-1 |
27 and 64 |
| Ciliary neurotrophic factor |
27, 68 and 69 |
|
| Growth hormone |
65 |
|
| Interferon γ |
46, 56 and 57 |
|
| Interleukin-6 |
56, 70–72 |
|
| Interleukin-11 |
66 |
|
| Leukemia inhibitory factor |
56, 57, 67 and 71 |
|
| Neuropoietin |
73–75 |
|
| Oncostatin M |
25, 56, 57 and 71 |
|
| STAT5A and 5B |
Growth hormone |
22, 26, 56, 65, 76 and 77 |
| Prolactin |
76 |
|
| STAT6 | Unknown | — |
Figure 2. Adipocytes are central players in responding to and producing STAT activating hormones. Adipose tissue is largely comprised of adipocytes but also contains preadipocytes and infiltrating immune cells, including NK cells, T cells and macrophages. Adipocytes are highly responsive to many hormones and growth factors that utilize the JAK-STAT pathway. The receptors for these ligands are indicated in the diagram. Infiltrating immune cells also produce cytokines that act in a paracrine fashion to activated STAT signaling in adipocytes. Adipocytes also have important endocrine properties and four JAK-STAT activating hormones have been shown to be produced from adipocytes.
Adipose tissue is largely comprised of adipocytes, but like other tissues contains endothelial cells, connective tissue and other types of stromal cells. The presence of infiltrating immune cells, such as macrophages and T cells is well documented and studies in the last decade suggest that these cells are modulated in conditions of obesity and Type 2 diabetes. IFNγ is produced from both natural killer (NK) cells39 and T cells40-43 present in adipose tissue. IFNγ can inhibit the differentiation of preadipocytes,44,45 induce insulin resistance in mature adipocytes46,47 and decrease PPARγ expression in adipocytes.48 It is highly likely that the production of IFNγ from infiltrated immune cells acts in a paracrine fashion on adjacent adipocytes to result in insulin resistance. As clearly indicated in Figure 2, the JAK-STAT signaling pathways play an important role in the majority of cell types that are found in adipose tissue. Although endothelial cells have endocrine functions, there is no evidence of JAK-STAT producing hormones from these cells. However, it is probable that the production of JAK-STAT ligands from adipocytes and infiltrating immune cells likely impacts the functions of endothelial cells that reside in adipose tissue.
STAT Target Genes in Mature Adipocytes
The tissue distribution of each STAT is unique, and it is widely accepted that STAT proteins have cell-specific functions. The modulation of tissue-specific genes has been shown to be a physiological role of STAT proteins in a variety of cell types, including adipocytes. Target genes for STATs 1 and 5 in adipocytes have been identified. These adipocyte STAT target genes code for proteins that regulate adipocyte development, insulin action and fat and carbohydrate metabolism. As summarized in this article, numerous investigations have revealed the importance of STAT5 proteins during adipogenesis in vitro and in vivo.21,23 As shown in Figure 1, STAT 5 proteins are activated early during adipocyte differentiation.21 Studies have also shown that STAT5 proteins are capable of directly binding the PPARγ3 promoter49 and can transactivate the PPARγ2 and PPARγ3 promoters.22,49 Although a number of transcription factors have profound effects on adipocyte development, PPARγ is a critical transcriptional regulator that is absolutely required for fat cell differentiation. PPARγ is a STAT5 target during adipocyte development and its modulation by STAT5 likely plays a role in the ability of STAT5 to promote adipocyte differentiation in vitro and in vivo. However, the majority of studies on STAT target genes have focused on fully differentiated adipocytes. The identification of STAT target genes in mature fat cells will be summarized below.
In adipocytes, studies have shown that PPARγ is also a STAT1 target gene. Initially, a probable STAT1 binding site was identified in the PPARγ2 promoter based on the consensus sequence of an interferon-γ-activated site (GAS) element that is known to mediate IFNγ-sensitive regulation in a STAT-dependent manner. In vitro studies revealed that STAT1 homodimers bind to an IFNγ responsive site within the PPARγ2 promoter in 3T3-L1 adipocytes.50 These data suggest that IFNγ-induced repression of PPARγ2 was mediated by the direct action of STAT1 on the PPARγ2 promoter. Modulation of both PPARγ activation pathways and IFNγ signaling has been associated with the development of insulin resistance.46,47,51 Accordingly, STAT1 likely mediates the ability of IFNγ to induce insulin resistance46,47,52,53 and block adipogenesis44,45 via transcriptional regulation of PPARγ levels. An IFNγ-sensitive binding site for STAT1 was also discovered in the murine lipoprotein lipase (LPL) promoter.54 LPL is the rate-limiting enzyme that catalyzes the hydrolysis of serum triglycerides from lipoproteins into free fatty acids for uptake and storage in adipose tissue. In murine adipocytes, IFNγ-activated STAT1 binds to the LPL promoter in a manner that is consistent with IFNγ-induced repression of LPL expression and inhibition of LPL activity.44,55 While STAT3 also exhibits tyrosine phosphorylation and nuclear translocation in response to IFNγ, STAT1 is a more robust mediator of IFNγ signaling in murine and human adipocytes.46,56,57 In these studies, STAT3 was unable to bind to the identified STAT1 binding sites within the PPARγ promoter,50 and LIF, a potent STAT3 activator, did not confer binding of STAT3 to the IFNγ sensitive region of the LPL promoter.54 In summary, both PPARγ and LPL have been shown to be STAT1 target genes in murine fat cells.
STAT3 is abundantly expressed in adipocytes7,8 and mediates the action of numerous cytokines in fat cells (Table 1). As reviewed in the previous section, STAT3 may play a role in adipogenesis. However, with the exception of C/EBPβ as a potential STAT3 gene target activated early in the adipogenic program,12 to date no adipocyte-specific direct target genes have been identified for STAT3. Although STAT6 is equivalently expressed in preadipocytes and throughout fat cell differentiation,7 only IL-4 has been shown to activate this transcription factor in 3T3-L1 preadipocytes but not in adipocytes.58 Thus, activators, functions and gene targets of STAT6 in both preadipocytes and adipocytes remain to be elucidated. Overall, there is almost nothing known about the identity of STAT3 and STAT6 target genes in adipocytes.
The majority of studies on STATs in adipocytes have focused on the identification of STAT5 target genes in mature fully differentiated adipocytes. Since STAT5 proteins are activated early during adipocyte differentiation and have been shown to play such a key role in adipocyte development, it is not surprising that most studies have focused on the functions of STAT5 proteins in mature adipocytes. The promoter for acyl CoA oxidase (AOX), the rate limiting enzyme in peroxisomal fatty acid β-oxidation, contains a STAT5 binding site that modulates its gene expression in fat cells.59 Transfection studies have shown that the promoter activity of aP2, an abundantly expressed lipid binding protein in fat cells, can be activated by STAT5.60 Conversely, STAT5 mediates the inhibition of aP2 expression in rat primary preadipocytes,61 which was the first study to suggest that STAT5 proteins could act as transcriptional repressors. Since that time, our own research has revealed that STAT5A can act as a transcriptional repressor in adipocytes. A STAT5A binding site in the murine fatty acid synthase (FAS) promoter mediates the repression of FAS transcription that occurs with growth hormone (GH) or prolactin (PRL) treatment.62 FAS catalyzes the production of long chain fatty acids and is a crucial enzyme involved in de novo lipogenesis. In addition to modulation of genes associated with lipid metabolism such as AOX and FAS, STAT5 can also increase the transcription of pyruvate dehydrogenase kinase (PDK)-4, a known regulator of glycolysis, that is highly induced in adipocytes by PRL or GH in a STAT5 dependent manner.63 Under these conditions, insulin resistance accompanies the induction of PDK4. It is well known that PRL and GH are important modulators of lipid metabolism and are also potent inducers of STAT5 in adipocytes.56,60 Hence, many of the metabolic actions of these anterior pituitary hormones could be mediated by STAT5′s direct modulation of target genes. Although relatively few STAT5 target genes have been identified in adipocytes, we hypothesize that several other STAT5A target genes that play a role in lipid or glucose metabolism will be identified. Figure 3 illustrates that STAT5 can modulate genes that are associated with all of the critical functions of adipocytes. Our unpublished data indicate that STAT5 proteins also substantially regulate the expression of adiponectin, an important adipocyte hormone that modulates insulin sensitivity. Another fat secreted hormone, lipocalin-2, appears to be regulated by STAT1. As indicated in Table 2, a collection of studies reveal that STATs 1 and 5 are important transcriptional regulators in adipocytes that contribute to hormone induced modulation of genes that contribute to lipid and glucose metabolism, insulin sensitivity and the endocrine properties of adipocytes.
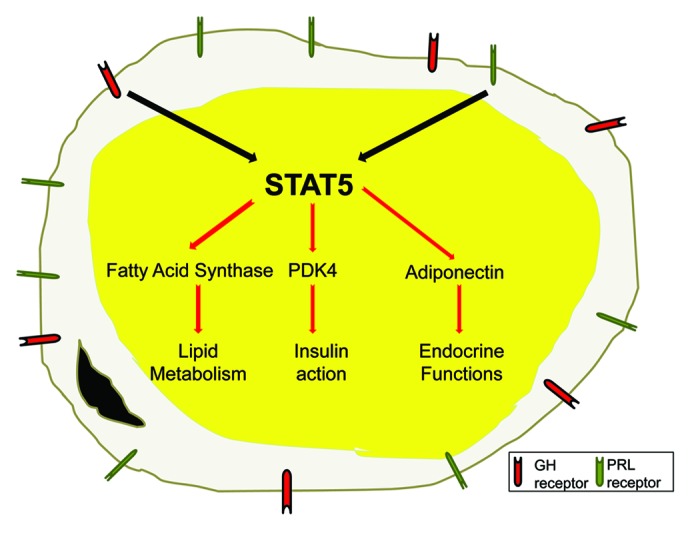
Figure 3. Growth hormone and prolactin induce STAT5 to modulate key genes associated with adipocyte function. To date, only growth hormone (GH) and prolactin (PRL) have been shown to be physiological activators of STATs 5A and B in adipocytes. Adipocytes have several key functions that include lipid accumulation, carbohydrate and lipid metabolism, insulin sensitivity and endocrine functions. Recent studies have shown that STAT5 proteins can directly modulate the transcription of genes that contribute to all of these critical fat cell functions.
Table 2. STAT target genes in mature adipocytes.
| Cytokine or hormone | STAT transcription factor | Target gene |
|---|---|---|
| GH, PRL |
STAT5 |
FAS |
| GH |
STAT5A |
aP2 |
| GH |
STAT5A |
AOX |
| GH, PRL |
STAT5 |
PDK4 |
| GH |
STAT5 |
Adiponectin |
| IFNγ |
STAT1 |
LPL |
| IFNγ | STAT1 | Lipocalin-2 |
Summary and Future Outlook
Overall, relatively few STAT-regulated genes have been identified in adipocytes. Nonetheless, the STAT 1, 3 and 5 target genes identified thus far encode proteins that are important for fat cell development and for adipocyte-specific functions, such as insulin sensitivity and lipid and carbohydrate metabolism. Although STATs were originally identified as positive regulators of transcription, they act as both transcriptional activators12,49,59,60,63,64 and repressors50,54,61,62 in fat cells. Additional studies in both cultured adipocytes and in adipose tissue are needed to reveal the complete regulatory potential of the STAT family members in adipocytes. Future studies are also necessary to determine how tyrosine phosphorylation of STATs that is typically associated with STATs ability to modulate transcription is affected by other covalent modifications such as serine phosphorylation, acetylation, methylation and sumoylation. In addition, the non-canonical mechanisms and functions of STATs in adipocytes have not been investigated. Other studies that indicate that STATs can participate in chromatin organization and mitochondrial respiration in ways that are independent of transcriptional regulation65 will likely be an intense area of investigation in adipocyte biology in the near future.
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/23092
References
- 1.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–34. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarjeant K, Stephens JM. Adipogenesis. Cold Spring Harb Perspect Biol. 2012;4:a008417. doi: 10.1101/cshperspect.a008417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darnell JE., Jr. STATs and gene regulation. Science. 1997;277:1630–5. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 4.Schindler C, Darnell JE., Jr. Transcriptional responses to polypeptide ligands: the JAK-STAT pathway. Annu Rev Biochem. 1995;64:621–51. doi: 10.1146/annurev.bi.64.070195.003201. [DOI] [PubMed] [Google Scholar]
- 5.Stark GR, Darnell JE., Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36:503–14. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wegrzyn J, Potla R, Chwae YJ, Sepuri NB, Zhang Q, Koeck T, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–7. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stephens JM, Morrison RF, Pilch PF. The expression and regulation of STATs during 3T3-L1 adipocyte differentiation. J Biol Chem. 1996;271:10441–4. doi: 10.1074/jbc.271.18.10441. [DOI] [PubMed] [Google Scholar]
- 8.Harp JB, Franklin D, Vanderpuije AA, Gimble JM. Differential expression of signal transducers and activators of transcription during human adipogenesis. Biochem Biophys Res Commun. 2001;281:907–12. doi: 10.1006/bbrc.2001.4460. [DOI] [PubMed] [Google Scholar]
- 9.Meraz MA, White JM, Sheehan KC, Bach EA, Rodig SJ, Dighe AS, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–42. doi: 10.1016/S0092-8674(00)81288-X. [DOI] [PubMed] [Google Scholar]
- 10.Deng J, Hua K, Lesser SS, Harp JB. Activation of signal transducer and activator of transcription-3 during proliferative phases of 3T3-L1 adipogenesis. Endocrinology. 2000;141:2370–6. doi: 10.1210/en.141.7.2370. [DOI] [PubMed] [Google Scholar]
- 11.Deng J, Hua K, Caveney EJ, Takahashi N, Harp JB. Protein inhibitor of activated STAT3 inhibits adipogenic gene expression. Biochem Biophys Res Commun. 2006;339:923–31. doi: 10.1016/j.bbrc.2005.10.217. [DOI] [PubMed] [Google Scholar]
- 12.Zhang K, Guo W, Yang Y, Wu J. JAK2/STAT3 pathway is involved in the early stage of adipogenesis through regulating C/EBPβ transcription. J Cell Biochem. 2011;112:488–97. doi: 10.1002/jcb.22936. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Zhou Y, Lei W, Zhang K, Shi J, Hu Y, et al. Signal transducer and activator of transcription 3 (STAT3) regulates adipocyte differentiation via peroxisome-proliferator-activated receptor gamma (PPARgamma) Biol Cell. 2010;102:1–12. doi: 10.1042/BC20090070. [DOI] [PubMed] [Google Scholar]
- 14.Cernkovich ER, Deng J, Bond MC, Combs TP, Harp JB. Adipose-specific disruption of signal transducer and activator of transcription 3 increases body weight and adiposity. Endocrinology. 2008;149:1581–90. doi: 10.1210/en.2007-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996;16:4128–36. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephens JM, Morrison RF, Wu Z, Farmer SR. PPARgamma ligand-dependent induction of STAT1, STAT5A, and STAT5B during adipogenesis. Biochem Biophys Res Commun. 1999;262:216–22. doi: 10.1006/bbrc.1999.0889. [DOI] [PubMed] [Google Scholar]
- 17.Stewart WC, Morrison RF, Young SL, Stephens JM. Regulation of signal transducers and activators of transcription (STATs) by effectors of adipogenesis: coordinate regulation of STATs 1, 5A, and 5B with peroxisome proliferator-activated receptor-gamma and C/AAAT enhancer binding protein-alpha. Biochim Biophys Acta. 1999;1452:188–96. doi: 10.1016/S0167-4889(99)00129-9. [DOI] [PubMed] [Google Scholar]
- 18.Yarwood SJ, Sale EM, Sale GJ, Houslay MD, Kilgour E, Anderson NG. Growth hormone-dependent differentiation of 3T3-F442A preadipocytes requires Janus kinase/signal transducer and activator of transcription but not mitogen-activated protein kinase or p70 S6 kinase signaling. J Biol Chem. 1999;274:8662–8. doi: 10.1074/jbc.274.13.8662. [DOI] [PubMed] [Google Scholar]
- 19.Shang CA, Waters MJ. Constitutively active signal transducer and activator of transcription 5 can replace the requirement for growth hormone in adipogenesis of 3T3-F442A preadipocytes. Mol Endocrinol. 2003;17:2494–508. doi: 10.1210/me.2003-0139. [DOI] [PubMed] [Google Scholar]
- 20.Nanbu-Wakao R, Morikawa Y, Matsumura I, Masuho Y, Muramatsu MA, Senba E, et al. Stimulation of 3T3-L1 adipogenesis by signal transducer and activator of transcription 5. Mol Endocrinol. 2002;16:1565–76. doi: 10.1210/me.16.7.1565. [DOI] [PubMed] [Google Scholar]
- 21.Floyd ZE, Stephens JM. STAT5A promotes adipogenesis in nonprecursor cells and associates with the glucocorticoid receptor during adipocyte differentiation. Diabetes. 2003;52:308–14. doi: 10.2337/diabetes.52.2.308. [DOI] [PubMed] [Google Scholar]
- 22.Kawai M, Namba N, Mushiake S, Etani Y, Nishimura R, Makishima M, et al. Growth hormone stimulates adipogenesis of 3T3-L1 cells through activation of the Stat5A/5B-PPARgamma pathway. J Mol Endocrinol. 2007;38:19–34. doi: 10.1677/jme.1.02154. [DOI] [PubMed] [Google Scholar]
- 23.Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, et al. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–50. doi: 10.1016/S0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 24.Stewart WC, Pearcy LA, Floyd ZE, Stephens JM. STAT5A expression in Swiss 3T3 cells promotes adipogenesis in vivo in an athymic mice model system. Obesity (Silver Spring) 2011;19:1731–4. doi: 10.1038/oby.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White UA, Stewart WC, Stephens JM. Gp130 cytokines exert differential patterns of crosstalk in adipocytes both in vitro and in vivo. Obesity (Silver Spring) 2011;19:903–10. doi: 10.1038/oby.2010.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zvonic S, Story DJ, Stephens JM, Mynatt RL. Growth hormone, but not insulin, activates STAT5 proteins in adipocytes in vitro and in vivo. Biochem Biophys Res Commun. 2003;302:359–62. doi: 10.1016/S0006-291X(03)00179-7. [DOI] [PubMed] [Google Scholar]
- 27.Zvonic S, Baugh JE, Jr., Arbour-Reily P, Mynatt RL, Stephens JM. Cross-talk among gp130 cytokines in adipocytes. J Biol Chem. 2005;280:33856–63. doi: 10.1074/jbc.M508020200. [DOI] [PubMed] [Google Scholar]
- 28.Bates SH, Kulkarni RN, Seifert M, Myers MG., Jr. Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab. 2005;1:169–78. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Buettner C, Pocai A, Muse ED, Etgen AM, Myers MG, Jr., Rossetti L. Critical role of STAT3 in leptin’s metabolic actions. Cell Metab. 2006;4:49–60. doi: 10.1016/j.cmet.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong Y, Ishida-Takahashi R, Villanueva EC, Fingar DC, Münzberg H, Myers MG., Jr. The long form of the leptin receptor regulates STAT5 and ribosomal protein S6 via alternate mechanisms. J Biol Chem. 2007;282:31019–27. doi: 10.1074/jbc.M702838200. [DOI] [PubMed] [Google Scholar]
- 31.Piper ML, Unger EK, Myers MG, Jr., Xu AW. Specific physiological roles for signal transducer and activator of transcription 3 in leptin receptor-expressing neurons. Mol Endocrinol. 2008;22:751–9. doi: 10.1210/me.2007-0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flower L, Gray R, Pinkney J, Mohamed-Ali V. Stimulation of interleukin-6 release by interleukin-1beta from isolated human adipocytes. Cytokine. 2003;21:32–7. doi: 10.1016/S1043-4666(02)00495-7. [DOI] [PubMed] [Google Scholar]
- 33.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50. doi: 10.1210/jc.83.3.847. [DOI] [PubMed] [Google Scholar]
- 34.Natal C, Fortuño MA, Restituto P, Bazán A, Colina I, Díez J, et al. Cardiotrophin-1 is expressed in adipose tissue and upregulated in the metabolic syndrome. Am J Physiol Endocrinol Metab. 2008;294:E52–60. doi: 10.1152/ajpendo.00506.2007. [DOI] [PubMed] [Google Scholar]
- 35.Brandebourg T, Hugo E, Ben-Jonathan N. Adipocyte prolactin: regulation of release and putative functions. Diabetes Obes Metab. 2007;9:464–76. doi: 10.1111/j.1463-1326.2006.00671.x. [DOI] [PubMed] [Google Scholar]
- 36.Hugo ER, Brandebourg TD, Comstock CE, Gersin KS, Sussman JJ, Ben-Jonathan N. LS14: a novel human adipocyte cell line that produces prolactin. Endocrinology. 2006;147:306–13. doi: 10.1210/en.2005-0989. [DOI] [PubMed] [Google Scholar]
- 37.Hugo ER, Borcherding DC, Gersin KS, Loftus J, Ben-Jonathan N. Prolactin release by adipose explants, primary adipocytes, and LS14 adipocytes. J Clin Endocrinol Metab. 2008;93:4006–12. doi: 10.1210/jc.2008-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McFarland-Mancini M, Hugo E, Loftus J, Ben-Jonathan N. Induction of prolactin expression and release in human preadipocytes by cAMP activating ligands. Biochem Biophys Res Commun. 2006;344:9–16. doi: 10.1016/j.bbrc.2006.03.168. [DOI] [PubMed] [Google Scholar]
- 39.O’Rourke RW, Metcalf MD, White AE, Madala A, Winters BR, Maizlin II, et al. Depot-specific differences in inflammatory mediators and a role for NK cells and IFN-gamma in inflammation in human adipose tissue. Int J Obes (Lond) 2009;33:978–90. doi: 10.1038/ijo.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P, et al. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol. 2009;29:1608–14. doi: 10.1161/ATVBAHA.109.192583. [DOI] [PubMed] [Google Scholar]
- 41.Rocha VZ, Folco EJ, Sukhova G, Shimizu K, Gotsman I, Vernon AH, et al. Interferon-gamma, a Th1 cytokine, regulates fat inflammation: a role for adaptive immunity in obesity. Circ Res. 2008;103:467–76. doi: 10.1161/CIRCRESAHA.108.177105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strissel KJ, DeFuria J, Shaul ME, Bennett G, Greenberg AS, Obin MS. T-cell recruitment and Th1 polarization in adipose tissue during diet-induced obesity in C57BL/6 mice. Obesity (Silver Spring) 2010;18:1918–25. doi: 10.1038/oby.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang H, Youm YH, Vandanmagsar B, Ravussin A, Gimble JM, Greenway F, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J Immunol. 2010;185:1836–45. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grégoire F, De Broux N, Hauser N, Heremans H, Van Damme J, Remacle C. Interferon-gamma and interleukin-1 beta inhibit adipoconversion in cultured rodent preadipocytes. J Cell Physiol. 1992;151:300–9. doi: 10.1002/jcp.1041510211. [DOI] [PubMed] [Google Scholar]
- 45.Keay S, Grossberg SE. Interferon inhibits the conversion of 3T3-L1 mouse fibroblasts into adipocytes. Proc Natl Acad Sci U S A. 1980;77:4099–103. doi: 10.1073/pnas.77.7.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGillicuddy FC, Chiquoine EH, Hinkle CC, Kim RJ, Shah R, Roche HM, et al. Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. J Biol Chem. 2009;284:31936–44. doi: 10.1074/jbc.M109.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waite KJ, Floyd ZE, Arbour-Reily P, Stephens JM. Interferon-gamma-induced regulation of peroxisome proliferator-activated receptor gamma and STATs in adipocytes. J Biol Chem. 2001;276:7062–8. doi: 10.1074/jbc.M007894200. [DOI] [PubMed] [Google Scholar]
- 48.Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem. 2002;277:4062–8. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- 49.Meirhaeghe A, Fajas L, Gouilleux F, Cottel D, Helbecque N, Auwerx J, et al. A functional polymorphism in a STAT5B site of the human PPAR gamma 3 gene promoter affects height and lipid metabolism in a French population. Arterioscler Thromb Vasc Biol. 2003;23:289–94. doi: 10.1161/01.ATV.0000051382.28752.FE. [DOI] [PubMed] [Google Scholar]
- 50.Hogan JC, Stephens JM. The identification and characterization of a STAT 1 binding site in the PPARgamma2 promoter. Biochem Biophys Res Commun. 2001;287:484–92. doi: 10.1006/bbrc.2001.5606. [DOI] [PubMed] [Google Scholar]
- 51.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–3. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 52.Koivisto VA, Pelkonen R, Cantell K. Effect of interferon on glucose tolerance and insulin sensitivity. Diabetes. 1989;38:641–7. doi: 10.2337/diabetes.38.5.641. [DOI] [PubMed] [Google Scholar]
- 53.Shiba T, Higashi N, Nishimura Y. Hyperglycaemia due to insulin resistance caused by interferon-gamma. Diabet Med. 1998;15:435–6. doi: 10.1002/(SICI)1096-9136(199805)15:5<435::AID-DIA566>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 54.Hogan JC, Stephens JM. STAT 1 binds to the LPL promoter in vitro. Biochem Biophys Res Commun. 2003;307:350–4. doi: 10.1016/S0006-291X(03)01198-7. [DOI] [PubMed] [Google Scholar]
- 55.Doerrler W, Feingold KR, Grunfeld C. Cytokines induce catabolic effects in cultured adipocytes by multiple mechanisms. Cytokine. 1994;6:478–84. doi: 10.1016/1043-4666(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 56.Balhoff JP, Stephens JM. Highly specific and quantitative activation of STATs in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1998;247:894–900. doi: 10.1006/bbrc.1998.8890. [DOI] [PubMed] [Google Scholar]
- 57.Stephens JM, Lumpkin SJ, Fishman JB. Activation of signal transducers and activators of transcription 1 and 3 by leukemia inhibitory factor, oncostatin-M, and interferon-gamma in adipocytes. J Biol Chem. 1998;273:31408–16. doi: 10.1074/jbc.273.47.31408. [DOI] [PubMed] [Google Scholar]
- 58.Deng J, Hua K, Lesser SS, Greiner AH, Walter AW, Marrero MB, et al. Interleukin-4 mediates STAT6 activation in 3T3-L1 preadipocytes but not adipocytes. Biochem Biophys Res Commun. 2000;267:516–20. doi: 10.1006/bbrc.1999.1993. [DOI] [PubMed] [Google Scholar]
- 59.Coulter AA, Stephens JM. STAT5 activators modulate acyl CoA oxidase (AOX) expression in adipocytes and STAT5A binds to the AOX promoter in vitro. Biochem Biophys Res Commun. 2006;344:1342–5. doi: 10.1016/j.bbrc.2006.04.071. [DOI] [PubMed] [Google Scholar]
- 60.Nanbu-Wakao R, Fujitani Y, Masuho Y, Muramatu M, Wakao H. Prolactin enhances CCAAT enhancer-binding protein-beta (C/EBP beta) and peroxisome proliferator-activated receptor gamma (PPAR gamma) messenger RNA expression and stimulates adipogenic conversion of NIH-3T3 cells. Mol Endocrinol. 2000;14:307–16. doi: 10.1210/me.14.2.307. [DOI] [PubMed] [Google Scholar]
- 61.Richter HE, Albrektsen T, Billestrup N. The role of signal transducer and activator of transcription 5 in the inhibitory effects of GH on adipocyte differentiation. J Mol Endocrinol. 2003;30:139–50. doi: 10.1677/jme.0.0300139. [DOI] [PubMed] [Google Scholar]
- 62.Hogan JC, Stephens JM. The regulation of fatty acid synthase by STAT5A. Diabetes. 2005;54:1968–75. doi: 10.2337/diabetes.54.7.1968. [DOI] [PubMed] [Google Scholar]
- 63.White UA, Coulter AA, Miles TK, Stephens JM. The STAT5A-mediated induction of pyruvate dehydrogenase kinase 4 expression by prolactin or growth hormone in adipocytes. Diabetes. 2007;56:1623–9. doi: 10.2337/db06-1286. [DOI] [PubMed] [Google Scholar]
- 64.Zvonic S, Hogan JC, Arbour-Reily P, Mynatt RL, Stephens JM. Effects of cardiotrophin on adipocytes. J Biol Chem. 2004;279:47572–9. doi: 10.1074/jbc.M403998200. [DOI] [PubMed] [Google Scholar]
- 65.Smit LS, Meyer DJ, Billestrup N, Norstedt G, Schwartz J, Carter-Su C. The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH. Mol Endocrinol. 1996;10:519–33. doi: 10.1210/me.10.5.519. [DOI] [PubMed] [Google Scholar]
- 66.Tenney R, Stansfield K, Pekala PH. Interleukin 11 signaling in 3T3-L1 adipocytes. J Cell Physiol. 2005;202:160–6. doi: 10.1002/jcp.20100. [DOI] [PubMed] [Google Scholar]
- 67.Hogan JC, Stephens JM. Effects of leukemia inhibitory factor on 3T3-L1 adipocytes. J Endocrinol. 2005;185:485–96. doi: 10.1677/joe.1.05980. [DOI] [PubMed] [Google Scholar]
- 68.Ott V, Fasshauer M, Dalski A, Klein HH, Klein J. Direct effects of ciliary neurotrophic factor on brown adipocytes: evidence for a role in peripheral regulation of energy homeostasis. J Endocrinol. 2002;173:R1–8. doi: 10.1677/joe.0.173R001. [DOI] [PubMed] [Google Scholar]
- 69.Zvonic S, Cornelius P, Stewart WC, Mynatt RL, Stephens JM. The regulation and activation of ciliary neurotrophic factor signaling proteins in adipocytes. J Biol Chem. 2003;278:2228–35. doi: 10.1074/jbc.M205871200. [DOI] [PubMed] [Google Scholar]
- 70.Andersson CX, Sopasakis VR, Wallerstedt E, Smith U. Insulin antagonizes interleukin-6 signaling and is anti-inflammatory in 3T3-L1 adipocytes. J Biol Chem. 2007;282:9430–5. doi: 10.1074/jbc.M609980200. [DOI] [PubMed] [Google Scholar]
- 71.Miyaoka Y, Tanaka M, Naiki T, Miyajima A. Oncostatin M inhibits adipogenesis through the RAS/ERK and STAT5 signaling pathways. J Biol Chem. 2006;281:37913–20. doi: 10.1074/jbc.M606089200. [DOI] [PubMed] [Google Scholar]
- 72.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–84. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 73.White UA, Stewart WC, Mynatt RL, Stephens JM. Neuropoietin attenuates adipogenesis and induces insulin resistance in adipocytes. J Biol Chem. 2008;283:22505–12. doi: 10.1074/jbc.M710462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.White UA, Stephens JM. Neuropoietin activates STAT3 independent of LIFR activation in adipocytes. Biochem Biophys Res Commun. 2010;395:48–50. doi: 10.1016/j.bbrc.2010.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.White UA, Stephens JM. The gp130 receptor cytokine family: regulators of adipocyte development and function. Curr Pharm Des. 2011;17:340–6. doi: 10.2174/138161211795164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fleenor D, Arumugam R, Freemark M. Growth hormone and prolactin receptors in adipogenesis: STAT-5 activation, suppressors of cytokine signaling, and regulation of insulin-like growth factor I. Horm Res. 2006;66:101–10. doi: 10.1159/000093667. [DOI] [PubMed] [Google Scholar]
- 77.Story DJ, Stephens JM. Modulation and lack of cross-talk between signal transducer and activator of transcription 5 and Suppressor of cytokine signaling-3 in insulin and growth hormone signaling in 3T3-L1 adipocytes. Obesity (Silver Spring) 2006;14:1303–11. doi: 10.1038/oby.2006.148. [DOI] [PubMed] [Google Scholar]