Abstract
During recent years a number of primary immunodeficiencies resulting from impaired function of JAK-STAT molecules have been described. One of these is the Hyper-IgE syndrome (HIES) characterized by elevated IgE levels, eczema, recurrent staphylococcal skin and pulmonary infections and pleiotropic somatic manifestations. In 2007 the genetic basis of HIES was revealed by identification of dominant negative STAT3 mutations in HIES patients. Subsequently impaired function of Tyk2 and DOCK8 have been implicated in milder forms of HIES. Since STAT3 acts as a central transcription factor downstream of multiple cytokine and growth factor receptors and thus regulates antimicrobial responses and cell survival, impaired STAT3 function results in immunodeficiency and in some cases tumorigenesis. However, as the immunological and molecular basis of HIES is being unraveled, important biological and immunological insight into JAK-STAT signaling is emerging that may have implications for our understanding of the pathogenesis and clinical management of patients with HIES.
Keywords: Hyper-IgE syndrome, STAT3, primary immunodeficiency, JAK-STAT signaling, chronic mucocutaneous candidiasis, Staphylococcus aureus, interleukin-6, Th17 response
Introduction
Insight into JAK-STAT signaling and function has increased dramatically within the past years, and a number of primary immunodeficiencies originating from impaired JAK-STAT signaling have been identified.1 Studies of the immunological and infectious phenotypes of patients with these primary immunodeficiencies most often confirm previous knowledge on the functions of these molecules. However, in certain cases, studying such patients may reveal novel and previously unknown immunological, cellular, developmental or endocrinological properties of the molecules and signaling pathways involved. This also holds true for HIES caused by impaired STAT3 signaling, which has revealed hitherto unknown immunological functions of this molecule in immunity and development. Indeed, it may seem surprising that impaired function of a single molecule can give rise to very heterogeneous immunological and somatic clinical pictures. At the same time, however, certain immunodeficiencies have a very specific phenotype and a very narrow infectious disease spectrum.
Within the JAK-STAT family of proteins, several primary immunodeficiencies have been described originating from specific mutations interfering with the expression, localization or function of a given molecule.1 These immunodeficiencies range from severe combined immunodeficiency (SCID) in the case of JAK3 mutations, over a moderately severe phenotype in the case of HIES involving STAT3 mutations, into a heterogeneous clinical presentation in STAT1 deficiency with Mendelian susceptibility to mycobacterial disease (MSMD), and finally to a relatively mild phenotype in the case of Tyk2 deficiency.1 The focus of the present review is primarily on STAT3 and HIES, whereas primary immunodeficiencies resulting from defects in other JAK-STAT molecules will be only briefly summarized here but have been recently review’ed by Casanova et al.1 In order to better understand the molecular and immunological basis of HIES, an introductory presentation of JAK-STAT signaling with special emphasis on STAT3 is given below.
The JAK-STAT Family of Proteins
The Janus kinase-signal transducers and activators of transcription (JAK-STAT) signaling pathway is evolutionarily conserved and mediates responses to several cytokines and growth factors.2 Cellular responses to JAK-STAT signaling are highly dependent on the specific cellular context and include proliferation, differentiation, migration, apoptosis and cell survival.3 This implies that JAK-STAT pathways are intimately involved in various physiological functions and processes, including innate and adaptive immune responses, hematopoiesis, stem cell maintenance, growth and development.4 Accordingly, mutations that interfere with JAK-STAT functions result in primary immunodeficiencies, hematological disorders, autoimmunity and cancer.2,3
The identification of the JAK-STAT pathway was the result of seminal work performed by Darnell, Stark, and Kerr in the search for mediators of interferon (IFN)-induced signaling.5 To date, four members of the JAK family (JAK 1, 2, 3 and Tyk 2) and seven members of the STAT family (STAT 1, 2, 3, 4, 5A, 5B and 6) have been identified.2 JAKs belong to a unique class of tyrosine kinases containing both a catalytic domain and a second kinase-like domain with autoregulatory function and derived their name from the two-headed Roman god Janus due to this duality.6 Subsequently, these kinases were functionally linked to the transcription factors STATs by functional genetic screening.5 Despite structural similarity of the STAT family of proteins they participate in quite different cellular processes.
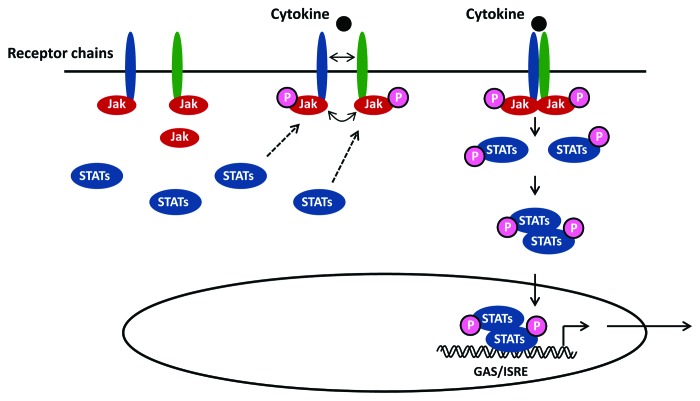
As illustrated in Figure 1, binding of an extracellular ligand, i.e., cytokine or growth factor, to either type I or II cytokine receptors leads to receptor homo- or hetero-dimerization resulting in recruitment and transactivation of receptor-associated tyrosine kinases (JAKs). This results in phosphorylation of certain domains within the intracellular region of the receptor, thus creating docking sites for recruited STAT molecules via their SH2 domain. Downstream signaling is then triggered through phosphorylation of STATs on specific tyrosine sites, inducing activation, homo- or hetero-dimerization and translocation into the nucleus.2 In the nucleus STAT molecules bind as dimers or more complex oligomers to specific enhancer sequences (ISRE/GAS elements) in target genes, thus inducing transcriptional activation2 (Fig. 1). Inhibitory effects upon JAK-STAT signaling are mediated primarily by suppressor of cytokine signaling proteins.7
Figure 1. Principles in JAK-STAT signaling. Following receptor binding of a relevant cytokine or growth factor, the receptor undergoes homo- or hetero-dimerization and binds cytosolic JAKs (JAK1, 2, 3 or Tyk2) for receptor auto-phosphorylation and transactivation. This event allows recruitment of transcription factors belonging to the STAT family (STAT 1, 2, 3, 4, 5A, 5B or 6) that bind the cytoplasmic domain of the receptor through their SH2 domain. Phosphorylated STAT proteins subsequently undergo homo- or hetero-dimerization and translocate to the nucleus, where they induce transcriptional activation of target genes by binding to ISRE/GAS elements.
The STAT3 Molecule and STAT3 Signaling Pathways
The STAT3 molecule was discovered in 1994, when Akira and coworkers purified and cloned STAT3 from mouse liver extracts and found that it binds to the interleukin (IL)-6 responsive element within the acute phase response promoter.8 Based on sequence similarities to STAT1, Zhong et al. simultaneously described STAT3 as a DNA-binding protein downstream of epidermal growth factor receptor.9 STAT3 is a 770 amino acid protein encoded on a gene located on chromosome 17q21. The molecule consists of a coiled coil-, a DNA binding- and an SH2 domain.9
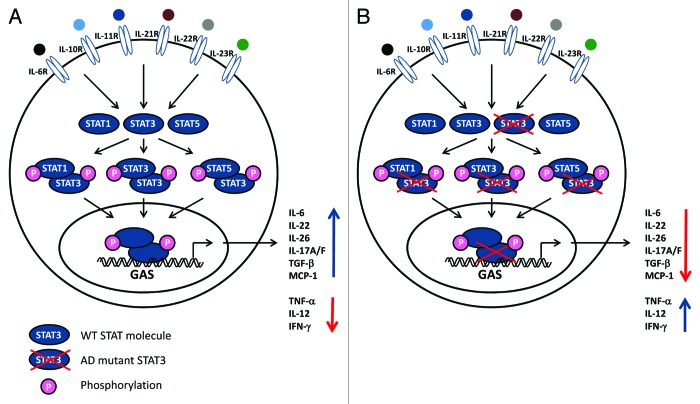
Pathways involving STAT3 activation are triggered by a number of cytokines and growth factors (Fig. 2). Receptor binding and activation leads to recruitment of intracellular JAK2 and Tyk2, resulting in specific STAT3 phosphorylation on Tyr 705, allowing dimerization, nuclear translocation and induction of target gene expression.2 Phosphorylated STAT3 primarily homodimerize but may also heterodimerize with STAT1 and STAT5, thus inducing another transcriptional program (Fig. 2). Moreover, STAT3 is further post-translationally modified by Tyr 727 phosphorylation, methylation and acetylation, which contribute to functional regulation of the molecule.10,11 An extraordinarily high diversity of genes is induced by STAT3, including IL-10, IL-17A/F, IL-22, IL-26, transforming growth factor (TGF)β, IL-6 and monocyte chemotactic protein 1. On the other hand, pro-inflammatory mediators, including tumor necrosis factor (TNF)α, IL-12 and IFNγ, are downregulated through STAT3 signaling2 (Fig. 2). In normal cells, activation of STAT3 is a transient and tightly controlled process, after which nuclear dephosphorylated STAT3 shuttles back into the cytoplasm through nuclear pores.5
Figure 2. Impaired STAT3 function affects multiple pathways. (A) A wide range of cytokines and growth factors activate receptors utilizing the tyrosine kinases JAK2 and Tyk2 which trigger signaling pathways involving STAT3. Phosphorylated STAT3 can homo- or heterodimerize with other STAT3 molecules or STAT1 or STAT5, respectively. STAT complexes modulate transcription of various genes, including increased IL-6, IL-10, IL-17A/17F, IL-22, TGFβ, MCP1 production, as well as decreased TNFα, IL-12 and IFNγ synthesis. (B) Mutations in STAT3 molecules can lead to dominant negative effects of the molecules, hence reducing or abolishing STAT3-dependent activities.
Hyper-IgE Syndrome (HIES)
HIES, or Job syndrome, was first described in 1966 by Davis et al. and defined by eosinophilia, eczema and recurrent skin and pulmonary infections.12 Subsequently, Buckley et al. added to the description the characteristically elevated IgE levels, which gave rise to the name HIES or Buckley disease.12 This primary immunodeficiency is characterized by a number of immunologic and infectious disease manifestations as well as a pleiotropic range of non-immunological features, including craniofacial, dental, musculoskeletal, neurological and vascular abnormalities.13 These immunological and somatic phenotypes and the underlying pathogenesis are described in more detail below and schematically presented in Table 1.
Table 1. Immunological and somatic phenotypes and associated pathogenesis in HIES.
| Clinical phenotype | Immunological abnormality |
|---|---|
| Staphylococcal cold abcesses (skin and lungs) |
IL-6 ↓, IL-17 ↓, IL-22 ↓, β-defensin ↓ |
| Reduced neutrophil chemotaxis and function | |
| Chronic mucocutaneous candidiasis |
Th17 responses (IL-17A/F, IL21, IL-22) ↓ |
| Impaired antifungal immunity | |
| Elevated serum-IgE |
IL-21 signaling ↓ |
| Atopy |
IL-10 responses ↓, T regulatory cells ↓ |
| B cell lymphoma |
IL-21 signaling ↓ |
| Disturbed B cell differentiation | |
| Craniofacial abnormalities (craniosynostosis, childhood dentition, high-arched palate) |
IL-11 signaling ↓ |
| Pneumatocele |
Increased matrix metalloprotease activity |
| Osteoporosis, scoliosis, fractures |
Enhanced osteoclastogenesis and osteopenia |
| Vascular abnormalities (aneurysms, turtousity) |
TGFβ signaling ↓, TNFα and RANTES production |
| Parenchymal brain lesions | Increased inflammation, demyelinization and astrocytosis following nerve injury |
Immunological phenotype of HIES
As described in the original report of Job syndrome, “cold” abscesses caused mainly by Staphylococcus aureus is a typical feature of the disease.12 Other characteristics are eczematoid rashes frequently present already during the neonatal period, skin abscesses, recurrent sinopulmonary infections, chronic mucocutaneous candidiasis (CMC) and eosinophilia together with markedly elevated serum IgE > 2,000 IU/mL.13 The infectious etiology in recurrent sinopulmonary infections is Staphylococcus aureus in particular, and less frequently Streptococcus pneumonia and Haemophilus species.14 These infections contribute to the formation of bronchiectasias, parenchymal lung damage and the formation of pneumatoceles, which may eventually constitute a site for chronic infection with Pseudomonas aeruginosa, atypical mycobacteria and Aspergillus.13 Another prominent infectious complication is CMC and to a lesser extent Pneumocystis jirovecii pneumonia. Moreover, other fungal pathogens including Histoplasma, Coccidioides and Cryptococcus have been reported to cause gastrointestinal infections as well as meningitis in patients with HIES.13 Recently, varicella zoster virus reactivation has been described in a relatively large proportion of patients with classical autosomal dominant (AD)-HIES.15
Somatic phenotype of HIES
The multisystem nature of AD-HIES is clearly demonstrated by the extensive list of non-immunological manifestations reported. Abnormal craniofacial features are characteristic facies, craniosynostosis, retained childhood dentition and high-arched palate.15,16 Within the musculoskeletal system hyperextensibility, scoliosis, osteoporosis and minimal trauma fractures are prominent.17 Several vascular abnormalities have also been described, including coronary artery tourtousity/dilatation, aneurysms and hypertension.18 Parenchymal brain lesions of unknown etiology have also been reported.19 Finally, AD-HIES patients are at an increased risk of developing malignancies, in particular non-Hodgkin lymphoma.20
Genetic origin of HIES
The identification of specific genetic mutations has allowed a much better understanding of the pathogenesis underlying the immunological abnormalities and infectious disease spectrum as well as the complex somatic features observed in HIES. A major progress was made in 2007 with the identification of mutations in STAT3 as a cause of AD-HIES.21 This study, which included 50 HIES patients, 48 family members and relevant controls, revealed increased innate immune responses and impaired IL-6 signaling, and identified familial and sporadic mutations on chromosome 17 in the STAT3 locus.21 These mutations were either missense mutations or in-frame deletions.21,22 Additionally, “hot spots” with independent mutations affecting the same codons were identified. STAT3 mutations appear to be located primarily in the SH2- and the DNA-binding domains of the molecule and act in a dominant negative manner, thus inhibiting the function of the wild-type molecule. However, expression, phosphorylation and nuclear translocation of STAT3 appeared to be largely identical in leukocytes from patients with STAT3 mutations and controls.22 Therefore, despite several mutations being identified, the specific mechanism, by which STAT3 mutations abolish the functions of the molecule are not fully understood. Several additional mutations in STAT3 resulting in classical AD-HIES have been reported.23 Moreover, an international collaboration directed by Grimbacher and associates in the setting of the European Society for Immunodeficiencies (ESID) has been initiated in a combined effort to further genetically characterize AD-HIES.
Recently, mutations in alternative molecules have been associated with syndromes that share many features with classical AD-HIES, although they are inherited by an autosomal recessive (AR) trait and tend to have a milder clinical picture. Mutations in dedicator of cytokinesis (DOCK)8 cause a phenotype similar to AD-HIES but with less prominent pulmonary features and instead with multiple specific allergies and an increased susceptibility to viral diseases, including herpes zoster, disseminated varicella and molluscum contagiosum.24,25 An even more heterogeneous picture is observed in patients with Tyk2 deficiency, which differs from AD-HIES by the more modest IgE-elevation and absence of the somatic phenotypes, but instead is dominated by pulmonary mycobacterial infections.26
Pathogenesis of the Immunological and Infectious Phenotype of HIES
A characteristic feature of HIES is aberrant inflammation, which involves on the one hand insufficient inflammation leading to “cold” skin abscesses, and on the other hand exaggerated pathological inflammatory responses in the lung resulting in formation of parenchymal lung damage in the form of pneumatoceles.21 As previously described and illustrated in Figure 2, the STAT3 molecule plays a central role in signal transduction induced by multiple cytokines, including IL-6, IL-10, IL-11, IL-17, IL-21 and IL-22. Therefore STAT3 deficiency leads to upregulation of many Th1 cytokines, such as IFNγ and TNFα, and downregulation of pro-inflammatory and anti-inflammatory responses regulated by IL-6 and IL-10, respectively.21 However, the precise mechanisms that allow STAT3 to promote inflammation in some instances and to inhibit inflammation at other times have not been fully resolved.2 An important explanation for this may be the fact that STAT3 knock-down in mice is embryonically lethal thus precluding murine studies in vivo, at least not without cellular modifications, such as cell type-specific STAT3 knockout in restricted tissues.27
A central role is played by STAT3 in signaling downstream of IL-6 and induction of the acute phase response,2 and therefore impaired IL-6 functions are believed to account for a substantial part of the immunodeficiency present in HIES. In addition, IL-22, acting through STAT3, has been ascribed a role in regulating epithelial cell barrier function,28 which is also disturbed in HIES. Moreover, STAT3 has been demonstrated to negatively regulate IFN responses and Toll-like receptor signaling occurring either through induction of anti-inflammatory molecules, or alternatively, by suppression of nuclear factor κB.29
A markedly decreased Th17 response is a hallmark of HIES, and indeed STAT3 mutations have been demonstrated to result in a failure of Th17 CD4 cell differentiation.30 Defective Th17 responses play a central role in several immunodeficiencies with CMC characterized by increased susceptibility to Candida infection.31 Furthermore, IL-17 signaling is involved in neutrophil proliferation and chemotaxis. Therefore, impaired neutrophil responses and recruitment to lung and skin may, at least partly, account for the recurrent staphylococcal infections observed at these particular sites.32-34 IL-17 and IL-22 also play a role in upregulation of antimicrobial peptides such as β-defensins, which have been implicated in both staphylococcal-driven atopic dermatitis and CMC.35,36 Specifically, respiratory epithelial cells and keratinocytes have been demonstrated to be tightly dependent on IL-17 for induction of antimicrobial molecules, including those responsible for antifungal responses.37
HIES patients may display a decrease in B cell numbers and variable antibody responses, although hypogammaglobulinemia is not a typical finding.38,39 Within the T cell compartment, T cell function has previously been regarded as largely normal.34 More recently however, a report has described impaired control of varicella zoster virus and Epstein-Barr virus in the presence of normal control of cytomegalovirus and herpes simplex virus, which could be associated to defects in generation of T memory cells in patients with HIES.40
The highly elevated IgE levels characteristic of the disorder have been suggested to reflect a role for STAT3 in mediating IL-21 receptor signaling based on the observation that mice deficient in IL-21R have elevated IgE levels.21,41 In addition, HIES patients have low numbers of antigen specific memory B cells, and since B cell immaturity has been linked to the preferential production of IgE in mice, this may account for the elevated IgE in patients with HIES.42 Regarding atopy, which may be observed in HIES patients already within the very first days of life, this feature may be linked to the impaired development of regulatory T cells and abolished responses to IL-10.43 Finally, hypereosinophilia, which is a relatively consistent feature of HIES and also included in the initial description of this immunodeficiency, has also been observed in mice with myeloid-specific STAT3 deficiency.44
Pathogenesis of the Somatic Phenotype of HIES
One of the most prominent non-immunological features of HIES is the presence of craniofacial abnormalities, including craniosynostosis, delayed tooth eruption and high-arched palate. This phenotype was recently suggested to be due to impaired IL-11 signaling based on a study involving Pakistani families with these specific features (but not HIES) caused by missense mutations in the gp130-associated IL-11RA impairing downstream STAT3-mediated signal transduction.45
Another characteristic finding in HIES is the exaggerated parenchymal damage and formation of pulmonary cysts (pneumatoceles) following pneumonia. Studies in mice have suggested that pulmonary cellular defects in migration, orientation and ciliation after injury are prominent in the absence of STAT3.46 In addition, STAT3 is involved in regulating the activity of a number of matrix metalloproteases, which is supported by a clinical study involving 37 HIES patients demonstrating altered levels of some of these proteases compared with controls.47 Such alterations may result in greater enzymatic activity against collagen as well as enhanced fibrinolysis and angiogenesis, resulting in increased tissue injury in the lung and possibly in arterial remodeling abnormalities.13 Dysregulated TGFβ as well as exaggerated TNFα-induced production of RANTES has also been suggested to play a role in the pathogenesis of cardiovascular abnormalities, such as coronary artery turtousity and aneurism formation in HIES patients.18,48,49
Regarding abnormalities in the skeletal system, such as scoliosis, osteoporosis and minimal trauma fractures, hemopoiesis-specific STAT3 deletion is associated with increased osteoclastogenesis and osteopenia in mice.50 Finally, parenchymal brain lesions observed in the majority of HIES patients and increasing in frequency with age may results from increased inflammation, demyelinization and astrocytosis in response to nerve injury, which has been demonstrated in mice in the absence of STAT3.51
HIES patients have an increased risk of malignancy, which is the result of STAT3 mediating the signaling downstream of several growth hormone receptors. Overexpression or persistent activation of STAT3 has been reported in most human hematological malignancies and solid tumors.7 This critical role for STAT3 in malignant transformation and tumorigenesis can be explained by the multifaceted roles played by STAT3 homodimers inducing a wide range of molecules involved in cell survival and apoptosis (Bcl-2, Bcl-xl, Cyclin D, c-Myc, survivin and others). Furthermore, STAT3/c-Jun mediated downregulation of Fas/CD95 transcription and Fas surface expression also mediates anti-apoptotic effects7 which are diminished in STAT3 deficiency. In particular, HIES patients have a much higher risk of developing aggressive B cell lymphomas, which may be linked to abnormalities in STAT3/IL-21 dependent differentiation of B cells into plasma cells with the possible involvement of T follicular helper cells.1,39
Diagnosis and Differential Diagnoses
HIES is a rare disease affecting an estimated 1:100,000 with an equal distribution between males and females. To help diagnose classical AD-HIES a scoring system has been proposed.17 In the presence of significantly elevated IgE combined with staphylococcal pulmonary or skin infections and/or CMC, it may be relevant to perform genetic analysis for STAT3 mutations. In certain cases AR-HIES (with absence of somatic phenotype and viral or mycobacterial infections), examining for DOCK8 or Tyk2 mutations may be considered.25-27 However, a number of differential diagnoses may also be relevant to take into consideration. Alternative immunodeficiencies associated with elevated IgE levels are Wiscott-Aldrich syndrome, Omenn syndrome, atypical complete DiGeorge syndrome, Netherton syndrome and immune dysregulation, polyendocrinopathy and enteropathy X-linked (IPEX) syndrome.52 High levels of IgE may also be caused by other conditions, such as allergy, parasitic disease or malignant hematological disorders, most frequently combined with hypereosinophilia. Finally, increased susceptibility to staphylococcal infections may also be a feature of patients with chronic granulomatous disease, in whom generation of neutrophil/macrophage oxidative burst is impaired, or may be associated with diabetes mellitus.53 Finally, a rapidly growing list of conditions associated with CMC with diverse underlying immunological basis exists.31
Treatment and Prophylaxis
One of the cornerstones in HIES management is to prevent skin and pulmonary infections with prophylactic antibacterial and antifungal treatment. For prophylaxis antibiotics against Staphylococcus aureus, such as sulfamethoxazole/trimethoprim may be used.4 In cases with pulmonary Aspergillus infection, colonization or pneumatocele, itraconazole is valuable. Prophylactic antifungal therapy may also be required to prevent CMC and onychomycosis in some patients. In order to control eczema and skin abscesses anti-staphylococcal oral therapy combined with bleach baths and chlorhexidine washes are recommended.13 Immunoglobulin substitution therapy may be indicated in select HIES patients with hypogammaglobulinemia but is not a general recommendation. Finally, bone marrow transplantation has been tried in a few HIES patients but with mixed results, hence leaving the role of bone marrow transplantation in HIES unclear.13
Other Immunodeficiencies Involving Mutations in JAK-STAT Molecules
Defects in JAK-STAT molecules may result in quite different immunodeficiencies and clinical pictures. To illustrate this point, other known immunodeficiencies involving JAKs and STATs will be briefly summarized below.
First, JAK3 mutations represent one of the genetic origins of SCID, giving rise to an autosomally recessive (AR) inherited T− B+ NK− SCID variant.54 SCID is characterized by the absence of autologous T cells resulting in excessive susceptibility to a broad range of pathogens. In SCID originating from JAK3 mutations the lack of T cells and NK cells can be attributed to defects in IL-7- and IL-15 signaling, respectively.55,56 Although the generation of circulating B cells is not disturbed, B cells in this form of SCID display impaired class-switch recombination and defective antibody production.57 Moreover, hypomorphic mutations in JAK3 give rise to less severe phenotypes, including combined immunodeficiencies, lymphoproliferation and autoimmunity.1
A homozygous missense mutation in STAT5B has been described in a patient with growth hormone insensitivity syndrome (GHIS) and short stature, facial dysmorphism and severe infections.58 Moreover patients with bi-allelic STAT5B mutations with GHIS, autoimmunity and eczema have been described.59 The STAT5B gene encodes a central signaling molecule in the IL-2R signaling pathway. Intriguingly, STAT5B immunodeficiency bears some similarity to HIES caused by STAT3 mutations, thus suggesting some degree of overlapping cellular functions of STAT5B and STAT3.1
In the case of STAT1, mutations may give rise to either loss-of-function or gain-of-function, resulting in distinct immunological and clinical phenotypes. The first mutations identified in STAT1 are associated with the clinical picture of MSMD,60 consisting of susceptibility to atypical mycobacteria or Bacille Calmette-Guerin (BCG) vaccines, which in these patients lead to clinical disseminated disease. In addition, patients with STAT1 mutations may acquire salmonellosis and infections with other intracellular bacteria, fungi and parasites.61 The identification of mutations in IL-12 and IL-12R molecules also resulting in MSMD, underscores the close interplay between IL-12 and IFNγ in the response against pathogens targeting macrophages, particularly mycobacteria.62 Additional AR forms of MSMD have been identified, in which patients are susceptible not only to mycobacteria but also to viral infections, including herpes encephalitis.63 These patients therefore harbor defects in both type I (IFNα and IFNβ) and type II (IFNγ) immunity.64 Finally, STAT1 gain-of-function mutations have been described in patients with CMC, in whom the pathogenesis may be explained by a negative regulatory role of STAT1 upon Th17 responses.65
Novel Findings and Areas of Uncertainty
Many fundamental aspects of STAT3 biology remain unanswered. The paradoxical pro- and anti-inflammatory functions of STAT3 and the underlying molecular mechanisms are challenging questions that are directly linked to our understanding of the immunological and somatic features of HIES. Moreover, it will be interesting to learn in the future if specific mutations in STAT3, DOCK8 and Tyk2 can be further correlated with the immunological and somatic phenotypes of this intriguing disease, and furthermore to broaden our understanding of the differences between AD-HIES caused by STAT3 mutations and AR-HIES originating from mutations in DOCK8, Tyk2 or in as yet unidentified molecules. Theoretically, mutations altering the function of any receptors, signaling pathways, and cytokines related to STAT3 may cause a spectrum of diseases more or less similar to classical AD-HIES.
Insight into JAK-STAT signaling has grown enormously during the past decade. From a therapeutic clinical perspective, the most promising results have been obtained in the field of cancer treatment by the use of small molecule inhibitors of STAT3 function.7 Indeed, there may be a great potential for developing STAT3 inhibitors for therapy of cancer and other conditions with chronic inflammation, such as psoriasis and renal/pulmonary fibrosis, and several STAT3 inhibitors are in early clinical trials.7 Whether the multiple functions of STAT3 may be substituted in HIES patients by providing the missing cytokines, for example IL-17, may be a very challenging task and remain unanswered. Based on the pleiotropic range of cellular functions exerted by STAT3-induced mediators however, it appears safe to state that a total reversal of the HIES phenotype would be impossible.
Conclusion
The study of primary immunodeficiencies combining genetic and molecular immunological analyses in vitro with the observed clinical infectious phenotype has previously proven very fruitful.1 Combining a basic scientific and clinical approach appears to be a prerequisite for gaining novel insights into fundamental immunological mechanisms. Moreover, this may also provide information and understanding of the immune system that can be translated into clinical diagnosis, treatment and management of these rare hereditary immunodeficiencies. In the case of STAT3 and HIES, the intriguing immunological and somatic phenotype has taught scientists and clinicians important aspects of basic immunology, such as mechanisms of anti-staphylococcal immunity in skin and lungs, and also contributed to the relatively recent appreciation of the importance of Th17 responses in the control of Candida infection. In addition, the study of somatic features of HIES has yielded insights into the biology of skeletal and vascular remodeling and angiogenesis. In the future, it will be interesting to further dissect the molecular mechanisms underlying STAT3-mediated control of immunity, cell survival and malignant transformation.
Acknowledgments
The author and ICID were supported by a grant from the Central Denmark Region. The author wishes to thank Dr Carsten Schade Larsen for critical reading of the manuscript.
Glossary
Abbreviations:
- AD
autosomal dominant
- AR
autosomal recessive
- CMC
chronic mucocutaneous candidiasis
- DOCK
dedicator of cytokinesis
- HIES
Hyper-IgE syndrome
- IFN
interferon
- Ig
immunoglobulin
- IL
interleukin
- JAK
Janus kinase
- MSMD
Mendelian susceptibility to mycobacterial disease
- SCID
severe combined immunodeficiency
- STAT
signal transducers and activators of transcription
- TGF
transforming growth factor
- TNF
tumor necrosis factor
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/jak-stat/article/23435
References
- 1.Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36:515–28. doi: 10.1016/j.immuni.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–50. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison DA. The Jak/STAT pathway. Cold Spring Harb Perspect Biol. 2012;4:a011205. doi: 10.1101/cshperspect.a011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–87. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnell JE, Jr., Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–21. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 6.Stark GR, Darnell JE., Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36:503–14. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debnath B, Xu S, Neamati N. Small molecule inhibitors of signal transducer and activator of transcription 3 (Stat3) protein. J Med Chem. 2012;55:6645–68. doi: 10.1021/jm300207s. [DOI] [PubMed] [Google Scholar]
- 8.Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, et al. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 9.Zhong Z, Wen Z, Darnell JE., Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–8. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Huang J, Dasgupta M, Sears N, Miyagi M, Wang B, et al. Reversible methylation of promoter-bound STAT3 by histone-modifying enzymes. Proc Natl Acad Sci U S A. 2010;107:21499–504. doi: 10.1073/pnas.1016147107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan ZL, Guan YJ, Chatterjee D, Chin YE. Stat3 dimerization regulated by reversible acetylation of a single lysine residue. Science. 2005;307:269–73. doi: 10.1126/science.1105166. [DOI] [PubMed] [Google Scholar]
- 12.Davis SD, Schaller J, Wedgwood RJ. Job’s Syndrome. Recurrent, “cold”, staphylococcal abscesses. Lancet. 1966;1:1013–5. doi: 10.1016/S0140-6736(66)90119-X. [DOI] [PubMed] [Google Scholar]
- 13.Sowerwine KJ, Holland SM, Freeman AF. Hyper-IgE syndrome update. Ann N Y Acad Sci. 2012;1250:25–32. doi: 10.1111/j.1749-6632.2011.06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley RH, Wray BB, Belmaker EZ. Extreme hyperimmunoglobulinemia E and undue susceptibility to infection. Pediatrics. 1972;49:59–70. [PubMed] [Google Scholar]
- 15.Smithwick EM, Finelt M, Pahwa S, Good RA, Naspitz CK, Mendes NF, et al. Cranial synostosis in Job’s syndrome. Lancet. 1978;1:826. doi: 10.1016/S0140-6736(78)93028-3. [DOI] [PubMed] [Google Scholar]
- 16.O’Connell AC, Puck JM, Grimbacher B, Facchetti F, Majorana A, Gallin JI, et al. Delayed eruption of permanent teeth in hyperimmunoglobulinemia E recurrent infection syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89:177–85. doi: 10.1067/moe.2000.103129. [DOI] [PubMed] [Google Scholar]
- 17.Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, et al. Hyper-IgE syndrome with recurrent infections--an autosomal dominant multisystem disorder. N Engl J Med. 1999;340:692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- 18.Freeman AF, Avila EM, Shaw PA, Davis J, Hsu AP, Welch P, et al. Coronary artery abnormalities in Hyper-IgE syndrome. J Clin Immunol. 2011;31:338–45. doi: 10.1007/s10875-011-9515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman AF, Collura-Burke CJ, Patronas NJ, Ilcus LS, Darnell D, Davis J, et al. Brain abnormalities in patients with hyperimmunoglobulin E syndrome. Pediatrics. 2007;119:e1121–5. doi: 10.1542/peds.2006-2649. [DOI] [PubMed] [Google Scholar]
- 20.Leonard GD, Posadas E, Herrmann PC, Anderson VL, Jaffe ES, Holland SM, et al. Non-Hodgkin’s lymphoma in Job’s syndrome: a case report and literature review. Leuk Lymphoma. 2004;45:2521–5. doi: 10.1080/10428190400004463. [DOI] [PubMed] [Google Scholar]
- 21.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 22.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 23.Mogensen TH, Jakobsen MA, Larsen CS. Identification of a novel STAT3 mutation in a patient with hyper-IgE syndrome. Scand J Infect Dis. 2012 doi: 10.3109/00365548.2012.715750. In press. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046–55. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol. 2012;13:612–20. doi: 10.1038/ni.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25:745–55. doi: 10.1016/j.immuni.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 27.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci U S A. 1997;94:3801–4. doi: 10.1073/pnas.94.8.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12:383–90. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 29.Wang WB, Levy DE, Lee CK. STAT3 negatively regulates type I IFN-mediated antiviral response. J Immunol. 2011;187:2578–85. doi: 10.4049/jimmunol.1004128. [DOI] [PubMed] [Google Scholar]
- 30.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–6. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puel A, Cypowyj S, Maródi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. 2012;12:616–22. doi: 10.1097/ACI.0b013e328358cc0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laan M, Cui ZH, Hoshino H, Lötvall J, Sjöstrand M, Gruenert DC, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–52. [PubMed] [Google Scholar]
- 33.Hill HR, Ochs HD, Quie PG, Clark RA, Pabst HF, Klebanoff SJ, et al. Defect in neutrophil granulocyte chemotaxis in Job’s syndrome of recurrent “cold” staphylococcal abscesses. Lancet. 1974;2:617–9. doi: 10.1016/S0140-6736(74)91942-4. [DOI] [PubMed] [Google Scholar]
- 34.Buckley RH, Becker WG. Abnormalities in the regulation of human IgE synthesis. Immunol Rev. 1978;41:288–314. doi: 10.1111/j.1600-065X.1978.tb01469.x. [DOI] [PubMed] [Google Scholar]
- 35.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 36.Conti HR, Baker O, Freeman AF, Jang WS, Holland SM, Li RA, et al. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol. 2011;4:448–55. doi: 10.1038/mi.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med. 2009;206:1291–301. doi: 10.1084/jem.20082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speckmann C, Enders A, Woellner C, Thiel D, Rensing-Ehl A, Schlesier M, et al. Reduced memory B cells in patients with hyper IgE syndrome. Clin Immunol. 2008;129:448–54. doi: 10.1016/j.clim.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Avery DT, Deenick EK, Ma CS, Suryani S, Simpson N, Chew GY, et al. B cell-intrinsic signaling through IL-21 receptor and STAT3 is required for establishing long-lived antibody responses in humans. J Exp Med. 2010;207:155–71. doi: 10.1084/jem.20091706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siegel AM, Heimall J, Freeman AF, Hsu AP, Brittain E, Brenchley JM, et al. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35:806–18. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ozaki K, Spolski R, Feng CG, Qi CF, Cheng J, Sher A, et al. A critical role for IL-21 in regulating immunoglobulin production. Science. 2002;298:1630–4. doi: 10.1126/science.1077002. [DOI] [PubMed] [Google Scholar]
- 42.Wesemann DR, Magee JM, Boboila C, Calado DP, Gallagher MP, Portuguese AJ, et al. Immature B cells preferentially switch to IgE with increased direct Sμ to Sε recombination. J Exp Med. 2011;208:2733–46. doi: 10.1084/jem.20111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, Agematsu K, et al. Defective IL-10 signaling in hyper-IgE syndrome results in impaired generation of tolerogenic dendritic cells and induced regulatory T cells. J Exp Med. 2011;208:235–49. doi: 10.1084/jem.20100799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panopoulos AD, Zhang L, Snow JW, Jones DM, Smith AM, El Kasmi KC, et al. STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood. 2006;108:3682–90. doi: 10.1182/blood-2006-02-003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieminen P, Morgan NV, Fenwick AL, Parmanen S, Veistinen L, Mikkola ML, et al. Inactivation of IL11 signaling causes craniosynostosis, delayed tooth eruption, and supernumerary teeth. Am J Hum Genet. 2011;89:67–81. doi: 10.1016/j.ajhg.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kida H, Mucenski ML, Thitoff AR, Le Cras TD, Park KS, Ikegami M, et al. GP130-STAT3 regulates epithelial cell migration and is required for repair of the bronchiolar epithelium. Am J Pathol. 2008;172:1542–54. doi: 10.2353/ajpath.2008.071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sekhsaria V, Dodd LE, Hsu AP, Heimall JR, Freeman AF, Ding L, et al. Plasma metalloproteinase levels are dysregulated in signal transducer and activator of transcription 3 mutated hyper-IgE syndrome. J Allergy Clin Immunol. 2011;128:1124–7. doi: 10.1016/j.jaci.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–98. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 49.Kovacic JC, Gupta R, Lee AC, Ma M, Fang F, Tolbert CN, et al. Stat3-dependent acute Rantes production in vascular smooth muscle cells modulates inflammation following arterial injury in mice. J Clin Invest. 2010;120:303–14. doi: 10.1172/JCI40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Z, Welte T, Troiano N, Maher SE, Fu XY, Bothwell AL. Osteoporosis with increased osteoclastogenesis in hematopoietic cell-specific STAT3-deficient mice. Biochem Biophys Res Commun. 2005;328:800–7. doi: 10.1016/j.bbrc.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 51.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–34. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 52.Ozcan E, Notarangelo LD, Geha RS. Primary immune deficiencies with aberrant IgE production. J Allergy Clin Immunol. 2008;122:1054–62, quiz 1063-4. doi: 10.1016/j.jaci.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 53.Song E, Jaishankar GB, Saleh H, Jithpratuck W, Sahni R, Krishnaswamy G. Chronic granulomatous disease: a review of the infectious and inflammatory complications. Clin Mol Allergy. 2011;9:10. doi: 10.1186/1476-7961-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macchi P, Villa A, Giliani S, Sacco MG, Frattini A, Porta F, et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID) Nature. 1995;377:65–8. doi: 10.1038/377065a0. [DOI] [PubMed] [Google Scholar]
- 55.Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T(-)B(+)NK(+) severe combined immunodeficiency. Nat Genet. 1998;20:394–7. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buckley RH. B-cell function in severe combined immunodeficiency after stem cell or gene therapy: a review. J Allergy Clin Immunol. 2010;125:790–7. doi: 10.1016/j.jaci.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, et al. Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med. 2003;349:1139–47. doi: 10.1056/NEJMoa022926. [DOI] [PubMed] [Google Scholar]
- 59.Nadeau K, Hwa V, Rosenfeld RG. STAT5b deficiency: an unsuspected cause of growth failure, immunodeficiency, and severe pulmonary disease. J Pediatr. 2011;158:701–8. doi: 10.1016/j.jpeds.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 60.Dupuis S, Dargemont C, Fieschi C, Thomassin N, Rosenzweig S, Harris J, et al. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science. 2001;293:300–3. doi: 10.1126/science.1061154. [DOI] [PubMed] [Google Scholar]
- 61.Haverkamp MH, van Dissel JT, Holland SM. Human host genetic factors in nontuberculous mycobacterial infection: lessons from single gene disorders affecting innate and adaptive immunity and lessons from molecular defects in interferon-gamma-dependent signaling. Microbes Infect. 2006;8:1157–66. doi: 10.1016/j.micinf.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 62.Altare F, Durandy A, Lammas D, Emile JF, Lamhamedi S, Le Deist F, et al. Impairment of mycobacterial immunity in human interleukin-12 receptor deficiency. Science. 1998;280:1432–5. doi: 10.1126/science.280.5368.1432. [DOI] [PubMed] [Google Scholar]
- 63.Sancho-Shimizu V, Zhang SY, Abel L, Tardieu M, Rozenberg F, Jouanguy E, et al. Genetic susceptibility to herpes simplex virus 1 encephalitis in mice and humans. Curr Opin Allergy Clin Immunol. 2007;7:495–505. doi: 10.1097/ACI.0b013e3282f151d2. [DOI] [PubMed] [Google Scholar]
- 64.Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, et al. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- 65.Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–48. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]