Abstract
Certain bacterial pathogens are able to evade the host immune system and persist within the human host. The consequences of persistent bacterial infections potentially include increased morbidity and mortality from the infection itself as well as an increased risk of dissemination of disease. Eradication of persistent infections is difficult, often requiring prolonged or repeated courses of antibiotics. During persistent infections, a population or subpopulation of bacteria exists that is refractory to traditional antibiotics, possibly in a non-replicating or metabolically altered state. This review highlights the clinical significance of persistent infections and discusses different in vitro models used to investigate the altered physiology of bacteria during persistent infections. We specifically focus on recent work establishing increased protection against oxidative stress as a key element of the altered physiologic state across different in vitro models and pathogens.
Keywords: persistent bacterial infections, antibiotic tolerance, persisters, oxidative stress, small colony variants, biofilms
Introduction
For many bacterial pathogens, the host immune system successfully eliminates the invading bacteria and the infection resolves. In certain infections, however, bacteria evade the host immune system and persist within the host. In some cases these persistent infections are asymptomatic for long periods of time, but can undergo future reactivation into clinically significant disease, or can be associated with malignancy or subsequent disease dissemination. Alternatively, some persistent infections result in clinically apparent, chronic symptoms. In these cases, even standard treatment with antibiotics often fails to effectively sterilize persistent infections, and prolonged or repeated courses of antibiotics are required for successful eradication. At an extreme, lifelong chronic suppression with antibiotics can be required in the absence of eradication.
Many factors contribute to the ability of pathogens to establish persistent infections, including both host and bacterial factors. Certain pathogens appear uniquely adapted to evade the host immune system and persist in infected individuals for decades in the absence of symptoms, for example Mycobacterium tuberculosis or Salmonella Typhi.1,2 Other pathogens like Pseudomonas aeruginosa or Escherichia coli can cause both symptomatic acute and chronic infections, with specific changes in the host facilitating the establishment of a persistent infection. The first section of this review highlights the clinical significance of persistent infections and the wide range of strategies employed by bacteria to survive the host immune system response (see Table 1 for examples of bacteria associated with persistent infections). In the second section, we discuss different in vitro models used to investigate the physiology of bacteria involved in persistent infections. Despite differences, many models share a common theme: bacteria adapt to environmental stresses imposed by the host by entering a different physiologic state. A key element of this different physiologic state is a non-replicating or slowly replicating growth rate, which may have the additional benefit of contributing to a pathogen’s defense against antibiotic exposure. Walsh McDermott first suggested in the 1950s that the relative metabolic state of bacteria affects antibiotic efficacy, causing cells to become “indifferent” to antibiotics, thereby relating the physiologic state of bacteria to antibiotic efficacy.3
Table 1. Pathogens associated with persistent bacterial infections.
| Pathogen | Persistent disease | Biologic mechanisms | |
|---|---|---|---|
| Asymptomatic persistent infections |
Mycobacterium tuberculosis |
Latent tuberculosis |
Intracellular growth, persisters |
|
Helicobacter pylori |
Gastritis, gastric cancer |
Intracellular growth |
|
|
Salmonella Typhi |
Chronic carrier, gall bladder carcinoma |
Intracellular growth, biofilm formation |
|
|
Treponema pallidum |
Latent syphilis |
Intracellular growth |
|
| Symptomatic persistent infections |
Pseudomonas aeruginosa |
Bronchiectasis/pneumonia in CF patients |
Biofilms, small colony variants, persisters |
|
Escherichia coli |
Recurrent urinary tract infections |
Intracellular growth, biofilms |
|
|
Staphylococcus aureus |
Bronciectasis/pneumonia in CF patients; device-associated infections |
Biofilms, small colony variants |
|
|
Hemophilus influenza |
Recurrent otitis media |
Biofilms |
|
| Mycobacterium leprae | Leprosy | Intracellular growth |
One of the most significant environmental stresses encountered by bacteria is the host oxidative immune response. In addition, studies have suggested that treatment with bactericidal antibiotics may result in increased oxidative stress via the Fenton reaction, though this finding remains ontroversial with more recent studies questioning this mechanism of cell death.4-9 In this review we specifically focus on recent work demonstrating the role of increased defenses against oxidative stress in various in vitro models for persistent infections. Increased antioxidant capabilities may protect a bacterium from the host immune response as well as facilitate survival during antibiotic exposure, thereby enabling the establishment of a persistent infection.
Clinical Significance of Persistent Infections
Asymptomatic persistent infections
Several persistent infections are clinically asymptomatic yet still have significant consequences for their human host. In some cases these consequences represent an increased risk of developing clinically significant disease at a later time, exemplified by M. tuberculosis and Treponema pallidum, the causative agents of tuberculosis and syphilis, respectively. In 95% of patients infected with M. tuberculosis, the innate and adaptive immune response is able to successfully contain bacterial growth and a clinically silent or latent infection results. Macrophages, monocytes, and T cells are recruited to contain the infection in the lungs by forming well-organized structures called granulomas, containing a reservoir of live bacteria. The immune response is unable, however, to completely sterilize the lungs, resulting in the creation of a long-term reservoir of bacteria.1 An individual with latent M. tuberculosis infection can remain asymptomatic for decades, but has a 10% risk of developing symptomatic and infectious disease over the course of their lifetime. The risk of developing active disease increases if the immune system becomes compromised, for example, from HIV, cancer chemotherapy or treatment with a tumor necrosis factor (TNF) inhibitor. Similar to M. tuberculosis, T. pallidum, described as a stealth pathogen due to poorly antigenic surfaces and antigenic variation, represents another pathogen with a “latent” asymptomatic stage.10 Following an initial symptomatic infection period, the symptoms of syphilis normally resolve in the absence of antibiotic treatment. Despite an absence of symptoms, however, T. pallidum is able to persist within a diverse range of tissues.10 Ultimately, 1/3 of patients with latent T. pallidum infection can later progress to tertiary syphilis, the most dangerous stage of syphilis infection. Tertiary syphilis is characterized by the formation of chronic gummas, which are benign tumors commonly affecting skin or bones, neurologic disease affecting the central nervous system, and cardiovascular disease including syphilitic aortitis.11
In other asymptomatic persistent infections, the consequences of infection can include increased risk of malignancy and dissemination of disease. Helicobacter pylori is a gram-negative pathogen that can colonize the gastric epithelium, resulting in an asymptomatic, superficial chronic gastritis. H. pylori infection can persist for decades with the bacterium employing several strategies to evade the host immune system, including suppressing the adaptive immune response and adapting to an intracellular environment during part of its lifecycle.2 The consequences of H. pylori infection include increased risk of duodenal and peptic ulcers as well as an increased risk of gastric adenocarcinoma and gastric lymphoma.12,13 Finally, in the case of Salmonella Typhi, an asymptomatic, persistent infection in the gallbladder may occur following an acute episode of typhoid fever or even in the absence of a clinical history of typhoid fever.2 These asymptomatic carriers shed Salmonella Typhi bacteria in their stools and urine without any apparent signs of disease, exemplified by the historic case of Typhoid Mary, infecting other individuals and causing symptomatic disease. Thus, asymptomatic carriers represent a significant public health hazard. In addition, Salmonella Typhi infection in the gallbladder is associated with an increased risk of carcinoma of the gallbladder.14
Symptomatic persistent infections
In contrast to clinically asymptomatic persistent infections, some persistent infections are associated with clinically apparent symptoms at the time of or intermittently during infection. Many symptomatic persistent infections are associated with changes in the host that facilitate the establishment of a persistent infection, for example, the insertion of a foreign device. Device associated infections represent one of the most common forms of persistent infections. It is estimated that more than 50% of hospital-acquired infections are due to infected medical devices, including prosthetic joints, central venous catheters (CVCs), and prosthetic heart valves.15 Prosthetic joint infections are often very difficult to diagnose, as the patient may present with pain as the only complaint, and diagnostic aspiration of the joint may be unrevealing. The mortality rate for infected prosthetic hip and knee joints is reported to be as high as 2.5% and treatment often requires operative debridement of the joint, possibly including removal of the prosthesis, combined with long-term intravenous antibiotic treatment.16,17 The estimated cost for treating an infected prosthetic joint is $50 000, representing a significant burden on the healthcare system.18 In the case of CVCs, catheters are rapidly colonized with skin organisms after insertion into the body, complicating diagnosis of CVC-associated infections. While colonization does not uniformly result in disease, colonization increases the risk that bacteria may spread hematogenously, resulting in symptomatic blood stream infections or secondary infections like endocarditis.19 It is estimated that there are 80 000 episodes of catheter-related bloodstream infections annually in the US.20
Infection in patients with cystic fibrosis (CF) is another example of a persistent infection with devastating consequences. In CF patients, mutations in the cystic fibrosis transmembrane regulator (CFTR) lead to defects in chloride transport. As a result, mucus in the airways becomes thickened, which results in dilatation and cystic changes in the lungs. Together, these changes create a niche for pathogens such as P. aeruginosa, Staphylococcus aureus, Burkholderia cepacia, and Haemophilus influenza. These pathogens are extremely difficult to eradicate from CF patients, and ultimately 80–95% of patients with CF die of respiratory failure secondary to chronic bacterial infections, often either P. aeruginosa or S. aureus.21
In some cases, symptomatic persistent infections develop even in the absence of a clear change in the host environment. These pathogens are usually associated with acute infections, but for unclear reasons may establish a more persistent infection in some individuals. For example, recurrent or chronic cystitis is a common consequence of an acute urinary tract infection.22 In one study, 30% of women experienced at least one culture confirmed recurrence within six months of their initial infection.23 In some patients recurrent cystitis can become so problematic to necessitate prophylactic or suppressive therapy with antimicrobials.24 Other examples of symptomatic persistent infections without clearly identifiable host risk factors, aside from obvious anatomical factors, include native-valve endocarditis, osteomyelitis, chronic sinusitis, and otitis media.
Treatment of persistent infections
It is clear that there are indications to treat both asymptomatic and symptomatic persistent infections. Unfortunately, the treatment of persistent infections is generally more difficult than the treatment of acute infections. In the case of latent M. tuberculosis, the standard treatment regimen requires 9 mo of antibiotics, and treatment of latent syphilis is three times longer than treatment of acute syphilis infection.25 Chronic Salmonella Typhi infection is extremely difficult to eradicate, even with very aggressive antibiotic therapy, and can require removal of the gallbladder.26 Treatment of device-associated infections often requires removal of the infected device in addition to prolonged courses of antibiotics.27 Antibiotic therapy against P. aeruginosa and S. aureus infections in CF patients becomes less and less effective later in the course of disease with increasing resistance in the infecting pathogen, and many patients ultimately succumb to infection despite appropriate antibiotic therapy.21
Why are persistent infections so difficult to treat?
Numerous factors contribute to the difficulty in sterilizing persistent infections. One contributing factor may be that bacteria often establish persistent infections within a “protected niche” in the host. For example, there are specific regions within the host where physical structures may obstruct an effective immune response and prevent adequate penetration with antibiotics, notably the blood-brain barrier, joint spaces and the sinuses. In other cases, the host immune response may actually create a “protected niche” by attempting to sequester the bacteria, exemplified by granulomas. While granuloma formation during M. tuberculosis infection represents a mechanism of host-defense, granulomas are also believed to protect bacteria, though incompletely, from antibiotics. Finally, some pathogens may develop their own “protected niche,” either through the formation of bacterial communities known as biofilms or by adapting to intracellular growth. Biofilms are bacterial communities embedded within an extracellular matrix and adherent to a surface,28 for example a foreign device in the case of device-associated infections, or gallstones in the case of Salmonella Typhi.29 One important characteristic of biofilms is the intrinsic resistance of the bacterial community to the host immune system. This is due to multiple factors, including decreased efficacy of antibodies and antimicrobial peptides against bacteria within biofilms, decreased phagocytic uptake, and decreased sensitivity to polymorphonuclear leukocyte (PMN)-mediated killing.30-33 The extracellular matrix component of a biofilm is also believed to partly limit the diffusion of antibiotics. Finally, some persistent infections are associated with intracellular growth, including infection by M. tuberculosis, T. pallidum, H. pylori, and Salmonella Typhi.2,34 Similar to biofilms, the intracellular environment confers protection from host immune responses including antibodies and complement factors, and may limit the effective concentration of antibiotics presented to bacteria.
Evidence suggests, however, that the concept of a “protected niche” is not sufficient to fully explain persistent infections. For example, some pathogens establish persistent infections in regions of the host that are not considered protective niches. Furthermore, several studies have demonstrated that antibiotics can in fact diffuse through biofilms, and imaging studies with radiolabeled antibiotics demonstrate that antibiotics do successfully penetrate granulomas.35-38 Thus, the barrier argument is insufficient to explain the ability of bacteria in these niches to survive chemotherapy.
In addition to sequestration from the immune system and antibiotics, another factor contributing to persistent infection is the ability of bacteria to adopt an altered physiologic state against which current antibiotics that predominantly target replicating cells are less efficacious. Walsh McDermott first suggested the ability of bacteria to “play dead” or transform themselves by “adaptive plasticity” in 1958.3 He proposed that staphylococci and tubercle bacilli in mice adopt an alternative, reversible state that is “indifferent” to antibiotics.3 This hypothesis was supported by later work with M. tuberculosis in an in vivo mouse infection model. Mice infected with M. tuberculosis appear to clear infection after 12 weeks of treatment with multiple antibiotics, as cultures from the homogenized lungs and spleen of these mice are sterile. However, with continued observation, 2/3 of the mice relapse with drug-sensitive bacteria, either spontaneously or with immunosuppression.39 This observation suggests both that bacteria are present within the host in a non-replicating or slowly replicating state that cannot be easily cultured in vitro, and that antibiotics are relatively ineffective against this physiologic state.
Models Used to Study Persistent Infections In Vitro
As efforts have been made to understand the role of altered bacterial physiologic states, specifically non-replicating or slowly replicating states, in persistent infections, several different in vitro models have emerged. Here, we focus on five of these models: facultative intracellular growth, the small colony variant phenotype, a “persisters” subpopulation, environmentally induced antibiotic indifference, and biofilms. In each of these models, bacteria enter a different physiologic state during persistent infection that is associated with refractoriness to antibiotic-mediated killing. While there are clear differences between these models, this review focuses on recent work establishing increased protection against oxidative stress as a key element across models and pathogens. At the outset we acknowledge that the terminology used in the field to describe these phenomena is confusing and variable. The term antibiotic tolerance is often used in the scientific literature to describe the phenomena of non-heritable antibiotic resistance, but historically antibiotic tolerance has also been used to describe specific cases of heritable resistance.40 For clarity, in this review we define the term antibiotic tolerance as the reduced efficacy of antibiotics in the absence of genotypic resistance.
Intracellular pathogens
Many pathogens involved in persistent infections are facultative intracellular pathogens, including uropathogenic E. coli (UPEC), Salmonella Typhi and M. tuberculosis. For UPEC and Salmonella Typhimurium, the data suggests that adaptation to the intracellular environment causes the bacterium to enter a non-replicating state. Helaine et al. used a fluorescence dilution method to quantify bacterial replication of Salmonella Typhimurium within macrophages.41 They found that after entry into macrophages a subset of the initial population did not undergo any replication, suggesting the onset of a non-replicating state. Likewise, UPEC, which is primarily an extracellular pathogen, has been shown to survive intracellularly in a non-replicating state during the course of urinary tract infections and pyelonephritis. During acute infection, UPEC invades superficial epithelial cells. In some cases, the epithelial cells expel the bacteria via a TLR4-dependent pathway. However, some bacteria escape from endocytic vesicles into the cytoplasm and replicate to form intracellular bacterial communities (IBCs). These communities, which have been identified in vivo in women with acute cystitis,42 are protected from the host immune system, display antibiotic tolerance, and are required for infection.43,44 Ultimately the superficial epithelial cells undergo apoptosis and bacteria contained within IBCs are released.22 At this point, the acute infection may resolve, or UPEC may establish a persistent infection by invading exposed transitional cells and forming “quiescent intracellular reservoirs”.45 These bacteria, contained in membrane-bound compartments in bladder transitional cells, do not replicate, but are able to survive for months, even in the face of antibiotic therapy. These reservoirs of bacteria are then thought to represent a source for recurrent or chronic cystitis.45 Interestingly, the formation of quiescent intracellular reservoirs depends on the severity of the initial immune response during acute infection.46 It is unclear whether a more severe immune response induces phenotypic changes in bacteria that are more conducive to chronic infection, or whether a more severe immune response simply facilitates chronic infection through damage to the epithelium.
Similar to UPEC, M. tuberculosis can replicate intracellularly. However, while UPEC requires specific factors to escape from the endosome prior to active replication, M. tuberculosis is able to replicate within the phagosome, expressing specific factors to counteract the harsh conditions encountered in the lysosome. In non-activated macrophages mycobacteria are able to arrest phagosome maturation, preventing fusion with the lysosome. The mechanisms responsible for phagosome arrest are not completely understood, but likely involve multiple factors including mycobacterial cell wall lipids as well as effectors secreted via the ESX and SecA2 secretion systems.47,48 In IFN-γ-activated macrophages, phagolysosome fusion and maturation occurs; nevertheless, M. tuberculosis is able to survive in this harsh environment.49 In response to nitric oxide synthesized by the macrophage, the bacterium expresses multiple enzymes and antioxidants to counteract the reactive nitrogen species (RNS) and ROS encountered within the phagolysosome, including the KatG catalase-peroxidase, superoxide dismutases, low molecular weight thiols, and an NADH-dependent peroxidase.50,51 Mutants with defects in katG catalase-peroxidase or superoxide dismutases display decreased virulence in mouse infection models, demonstrating that the ability of M. tuberculosis to detoxify ROS and RNS is therefore crucial for its survival within the host.52,53
The multiplicity of pathways for ROS and RNI detoxification within M. tuberculosis may likely provide protection against bactericidal antibiotics as well, given data implicating ROS in death from bactericidal antibiotics.4-7 Evidence supporting this link between the oxidative stress response and antibiotic tolerance was recently described in the intracellular model for persistence using the pathogen Mycobacterium marinum. M. marinum displays antibiotic tolerance shortly after phagocytosis.54 This antibiotic-tolerant phenotype was found to depend on the expression of bacterial efflux pumps required for intracellular growth. Efflux pumps have recently been implicated in the oxidative stress response mechanism, perhaps functioning as a mechanism for the bacterium to efflux proteins damaged by ROS.55,56 While the upregulation of efflux pumps is classically associated with increased efflux of antibiotics resulting in lower intracellular concentrations, in intracellular mycobacteria, it could also serve both as a mechanism for the bacterium to adapt to intracellular growth as well as provide protection against hydroxyl radical mediated antibiotic killing.
Small colony variants
The small colony variant (SCV) phenotype represents another bacterial phenotype associated with persistent infections. First described in Salmonella Typhi in 1910, more recent work has associated the SCV phenotype with P. aeruginosa and S. aureus infection in CF patients.57-61 The phenotype, most well studied in S. aureus, is associated with smaller colony morphology when plated on agar plates, decreased expression of toxins, increased expression of adhesins, intracellular growth and resistance to antibiotics.62 The SCV phenotype is associated with a thick cell wall63 and clinical SCV isolates demonstrate upregulation of genes under the control of the alternative sigma factor SigB that plays a role in oxidative stress response, suggesting that the SCV phenotype may be a defense mechanism against environmental stress.64 SCV isolates obtained from clinical specimens rapidly revert to a wild-type phenotype when sub-cultured in vitro, complicating the study of this phenomenon.65 However, mutants in the electron transport chain, either hemB or menD mutants, mimic the SCV phenotype, and work with these mutants has suggested that the SCV phenotype may represent an adaptation to facilitate intracellular survival and growth.66 In support of this hypothesis, S. aureus hemB mutants have been reported to survive within the lysosome,67 and several studies have reported the proliferation and persistent survival of SCV S. aureus within endothelial cells.68-70 The survival of SCV within endothelial cells may also be a consequence of decreased α-toxin expression and consequently decreased apoptosis of infected cells.71 Tuchscherr et al. studied a virulent wild-type S. aureus clinical isolate in several in vitro and in vivo infection models and found that persistence in all models strongly favored the SCV phenotype.65
A mechanism for the rapid switching between SCV and wild-type phenotypes was suggested by Cui et al. after studying a S. aureus strain that simultaneously produces both SCVs and normal colony variants.72 Whole genome sequencing of the two variants revealed that a reversible, large-scale inversion of the chromosome occurs at high frequencies, accounting for the two observed phenotypes. Comparing the expression profiles of the two variants revealed that the SCV was associated with decreased expression of genes involved in thiamine metabolism (required for menadione synthesis, a component of the electron transport chain) as well as oxidative phosphorylation. These findings are consistent with prior observations associating SCV with defects in the respiratory chain.58 The SCV phenotype is associated with tolerance to antimicrobials, which previously has been attributed to the decreased growth rate and metabolism associated with this phenotype, and decreased antibiotic uptake due to a defect in the electron transport chain.73 While these may be contributing factors, the association of SCV with defects in the electron transport chain suggests that the SCV phenotype may also be a mechanism to protect the cell against oxidative stress. Repression of the electron transport chain will lead to decreased endogenous free radical production and decreased superoxide stress.74 The SVC phenotype may therefore protect the bacteria from superoxide stress encountered during intracellular persistence with the additional consequence of protection against bactericidal antimicrobials.
Persisters
The term “persisters” refers to a small population of bacteria that survives treatment with high doses of antibiotics in the absence of genetic resistance. The phenomenon was first described shortly after scientists began using penicillin to treat bacterial infections. In 1942, Hobby et al. noted that approximately 1% of hemolytic streptococci survived treatment with penicillin in vitro.75 Joseph Bigger, who similarly discovered that small numbers of S. aureus cells consistently survive antibiotic treatment in vitro without developing genetic resistance, then described these surviving bacteria as “persisters”.76 Since these initial observations, persister subpopulations have been observed in many different human pathogens, including E. coli, S. aureus, M. tuberculosis, and P. aeruginosa,76-79 as well as in vivo3 in several mouse infection models. Examination of persisters in E. coli was facilitated by the discovery of the high persistence hipA mutant.80 hipA encodes the toxin in a toxin-antitoxin module, with the antitoxin hipB neutralizing hipA activity. Expression of hipA in excess of hipB results in cessation of cell growth; the high persistence hipA7 mutant has two point mutations resulting in a gain of function and a high persistence phenotype.81 Balaban et al. used single cell microfluidic studies to characterize persisters in the hipA7 mutant as well as in a second toxin-antitoxin mutant, hipQ, and identified two persister types. Type I persisters are generated during stationary phase and are characterized by a prolonged lag time when transferred to fresh media.82 Type II persisters are continuously generated by a phenotype-switching mechanism independent of growth phase.
Stochastic processes within the cell are believed to result in the formation of the persisters observed by Balaban et al. Multiple pathways have been suggested for persister formation. One of the first classes of genes implicated in persister formation was toxin-antitoxin (TA) genes such as hipA7 and hipQ. Recent transcriptome profiling of persisters, isolated by collecting cells that failed to lyse after β-lactam or d-cycloserine exposure, has also revealed that several stress response regulons, including the SOS response, as well as several toxin-antitoxin (TA) genes, are upregulated in persister populations in E. coli77 and in M. tuberculosis.83 One mechanism by which TA loci may induce stochastic persistence in E. coli was recently described. Many toxin genes, activated upon degradation of the cognate antitoxin, encode mRNases that rapidly degrade mRNA, stopping translation and replication, thereby inducing antibiotic tolerance.84 The degradation of antitoxins is mediated by the Lon protease, and stochastic variation in the number of Lon molecules within a cell may affect antitoxin levels, toxin activity, and therefore persistence rates.84,85 More recently, an additional mechanism for stochastic persistence was described by Wakamoto et al. in Mycobacterium smegmatis using the antibiotic isoniazid (INH). Single cell microfluidic studies revealed that stochastic expression of KatG, the enzyme required for activation of INH, determined whether a cell survived antibiotic stress.86
Recently, several suggested pathways for persister formation implicate a role for oxidative stress in persister formation and survival. Kim et al. observed greater numbers of persisters in E. coli subpopulations with normal morphology and lower hydroxyl radical concentrations following antibiotic treatment, compared with filamentous populations with increased hydroxyl radical concentrations, suggesting a role for ROS in persister survival.87 Oxidative stress is one of several signals that results in induction of the SOS response, which has been shown in E. coli to induce both β-lactam and fluoroquinolone antibiotic tolerance.88-90 More recently, the small molecule indole, induced by oxidative stress, has been implicated in persister formation. Lee et al. found that exposure to antimicrobials and oxidative stress results in increased indole production through transcriptional upregulation of the tnaA gene responsible for indole synthesis.91 Indole is thus secreted by a small subpopulation of E. coli cells in response to antibiotic exposure. Indole then induces transcriptional changes in neighboring cells, most notably upregulation of efflux pumps and oxidative stress protective mechanisms, resulting in antibiotic tolerance in the greater population.91,92 In addition, Wu et al. demonstrated that treating an E. coli population with the pro-oxidant paraquot results in increased antibiotic tolerance within the population via upregulation of MDR efflux pumps.93 One possibility suggested by the authors is that upregulation of the MDR efflux pumps results in an effective decrease in fluoroquinolone concentration, allowing for the survival of a greater number of bacteria. An alternative explanation is that increased expression of MDR efflux pumps, implicated as an important component of the oxidative stress response, may provide increased protection against oxidative stress and facilitate bacterial survival even at the same intracellular concentration of antibiotic.55,56 The importance of the oxidative stress response in persister populations was further highlighted by recent work demonstrating that the persister subpopulation in M. smegmatis and M. tuberculosis is differentiated from the larger antibiotic susceptible population by differential sensitivities to ROS.94 When decreased concentrations of ROS are achieved, either through small (20%) reductions in oxygen tension or with the addition of thiourea, a free radical quencher, the antibiotic tolerant persister population survives antibiotic treatment. In contrast, when increased concentrations of ROS are achieved via redox cycling with the antibiotic clofazamine, the persister population is unable to survive antibiotic treatment.95 It is intriguing to conjecture based on the differential susceptibilities of persisters to ROS in M. smegmatis that the stochastic expression of KatG recently observed in M. smegmatis may affect bacterial survival not only from differential antibiotic activation, but also from enhanced protection against oxidative stress.86
Environmentally induced antibiotic indifference
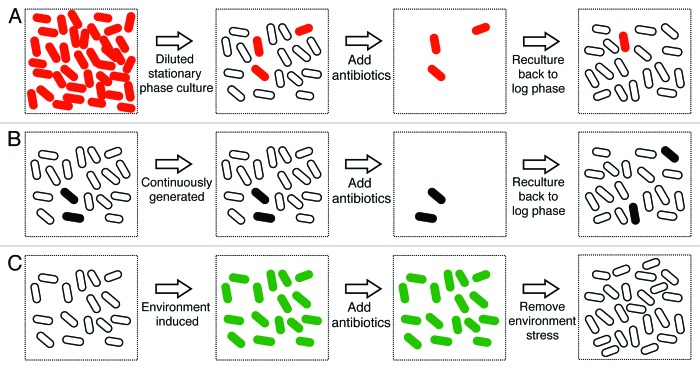
Environmental stress, for example the deprivation of nutrients, hypoxia or low pH, induces the phenotype of antibiotic indifference, characterized by arrested growth and population-wide antibiotic tolerance.96-102 Similarly, stationary phase bacteria, which encounter the depletion of nutrients and changes in the pH of the media, also exhibit antibiotic indifference. While stationary phase is associated with both “environmentally induced” antibiotic indifference and the generation of type I persisters described above, important differences exist between these two models for antibiotic tolerance. In the case of persisters, stationary phase cells are diluted into fresh media prior to antibiotic challenge and only a minority of the population survives. With antibiotic indifference, the bacteria are held in stationary phase at the time of antibiotic challenge and a majority of the population survives (Fig. 1). It is not known whether a common biological mechanism contributes to these two different antibiotic tolerant phenotypes.
Figure 1. Persistence vs. environmentally induced antibiotic indifference. (A) Type I persistence. Slowly replicating, antibiotic tolerant cells result from passage through stationary phase, and are characterized by an extended lag time after dilution in fresh media (type I persisters). The number of type I persisters observed is directly proportional to the size of the stationary phase inoculum. When the diluted population is exposed to antibiotics the majority of cells die, but the persisters survive. Persister cells are represented by red ovals, and rapidly growing, antibiotic-sensitive cells are represented by white ovals. (B) Type II persistence. Slowly replicating, antibiotic tolerant cells also may be continuously generated during exponential growth (type II persisters). When the population is exposed to antibiotics, the majority of cells die, but the persisters survive. When the surviving cells are regrown in fresh media, the bacteria regain antibiotic sensitivity as this is a phenotypic change, but new persister cells may be generated. Persister cells are represented by black ovals and rapidly growing, antibiotic-sensitive cells are represented by white ovals. (C) Environmentally induced antibiotic indifference. Slowly replicating or non-replicating, antibiotic-tolerant cells may also result from environmental stresses like hypoxia or carbon starvation, which induce population-wide antibiotic indifference. When the population is exposed to antibiotics, all the cells survive. When the environmental stress is removed, the surviving cells resume growth and regain antibiotic sensitivity as this is a phenotypic, not genetic, change. Environmental-induced antibiotic-tolerant cells are represented by green ovals and rapidly growing, antibiotic-sensitive cells are represented by white ovals. This figure depicts theoretical extremes for persistence and environmental-induced antibiotic indifference. In reality, all three types of antibiotic-tolerant cells can co-exist depending on the conditions of growth and the culture.
The antibiotic indifferent phenotype observed after nutrient deprivation is associated with a coordinated transcriptional response to carbon and amino acid starvation, called the stringent response. The stringent response is mediated by the production of hyperphosphorylated guanine nucleotides, (p)ppGpp, by the protein RelA.103 The (p)ppGpp molecule synthesized by RelA has multiple roles in the cell, including binding to RNA polymerase and changing transcription factor expression and activity as it coordinates the extensive bacterial response to stress. In addition, (p)ppGpp production is important for the antibiotic tolerance phenotype in vitro. In E. coli relA deletion mutants that are unable to synthesize (p)ppGpp, nutrient starvation does not elicit tolerance to penicillin.104 Conversely, when the relA gene was used to overexpress (p)ppGpp in E. coli, the bacteria exhibited tolerance to penicillin.105 In vivo, work in M. tuberculosis suggests RelA is also required for persistent infection, as bacteria with a relA deletion are significantly impaired in their ability to establish persistent infection in mice. The mechanism linking RelA and persistence has been further supported in recent work by Nguyen et al., who studied a relA deletion mutant in P. aeruginosa and found that the RelA mediated stringent response in P. aeruginosa directly affects antioxidant enzyme expression and decreases production of pro-oxidant molecules.106 Therefore, the coordinated stress response mediated by RelA results in increased protection against oxidative stress, potentially explaining the observed antibiotic tolerant phenotype.
Biofilms
Biofilms are bacterial communities embedded within an extracellular matrix and adherent to a variety of surfaces, including living tissue and indwelling medical devices.28 An extensive exopolysaccharide layer surrounds bacteria within biofilms. This physical barrier is believed to provide protection against environmental stresses like heat-shock and desiccation, as well as against the host immune system.107 Biofilm formation is induced by a variety of stresses, including nutrient limitation, iron limitation, and cell envelope stress. The general stress response, regulated by the alternative sigma subunit of RNA polymererase, RpoS, is important for biofilm formation as deletion of rpoS leads to decreased biofilm formation in E. coli.108 Once biofilms are established, the process of detachment, when planktonic bacteria periodically escape the biofilm, is also regulated.109 Recently, d-amino acids, produced by many bacteria, were shown to break down biofilms and signal for biofilm disassembly.110 The in vivo consequence of biofilm disassembly is bacterial dissemination and the establishment of a new nidus of infection, potentially explaining the natural history of chronic, relapsing infections.109 A caveat to this model, however, is that actual in vivo biofilms or bacterial communities may vary in their resemblance to in vitro biofilms, depending on the type of infection.
Similar to the other three models discussed, bacteria within biofilms display phenotypic antibiotic tolerance, and several links to oxidative stress within biofilms have recently been described. Boles et al. recently showed that bacteria within biofilms experience significant endogenous oxidative stress and consequently upregulate several genes involved in the oxidative stress response, including soxS, a regulator of the superoxide response.111,112 This induction suggests that increased antioxidant responses within biofilms could contribute to the antibiotic tolerant phenotype. A link between antibiotic tolerance and oxidative stress has also been illustrated in the yeast Candida albicans, using a strain with reduced antioxidant capabilities due to the deletion of two superoxide dismutases, Sod4 and 5. The C. albicans deletion strain exhibited significantly less antibiotic tolerance in biofilms compared with the wild-type strain.113 Finally, the work in P. aeruginosa demonstrating a RelA-dependent antioxidant response discussed in the context of antibiotic indifference is also relevant to biofilms. Bacteria within biofilms are relatively starved of nutrients due to reduced diffusion through the biofilm, resulting in induction of the RelA-mediated stringent response with subsequent increased protection from oxidative stress. In addition, RelA has been shown to be important for successful biofilm formation in Streptococcus mutans.114
In many ways, the biofilm model highlights how the different suggested mechanisms for antibiotic tolerance may be occurring simultaneously and may be interconnected. For example, some studies suggest that the antibiotic tolerance phenotype observed within biofilms results from antibiotic indifference induced in response to oxygen or nutrient deprivation.36,115 Other studies have suggested that the unique physiologic state of stochastic persisters is similar to the physiologic state of bacteria within biofilms, and pre-existing persisters in a population may even represent a mechanism for initiation of biofilm formation.37 Finally, bacteria with a small colony variant phenotype are associated with enhanced biofilm formation.59,116 Thus, antibiotic tolerance observed within biofilms is the result of the bacterial population exhibiting characteristics of several different in vitro models, including facultative intracellular growth, environmentally induced antibiotic indifference, stochastic persisters, and small colony variants. Biofilms are also believed to play a role in the intracellular bacterial communities in uropathogenic E. coli, as it has been suggested that the intracellular bacterial communities of UPEC are actually organized within biofilms on the surface of intracellular membrane surfaces.22 The complex relationships between the models for persistent infections can also be observed within the larger context of clinical infections, for example, pseudomonas infection in CF patients. P. aeruginosa forms biofilms within the cystic fibrosis lung,117 and P. aeruginosa and S. aureus infections in CF patients are both associated with small colony variant phenotypes. Finally, clinical isolates of P. aeruginosa cultured from CF patients contain increased numbers of “persisters” when tested in vitro.118
Conclusions
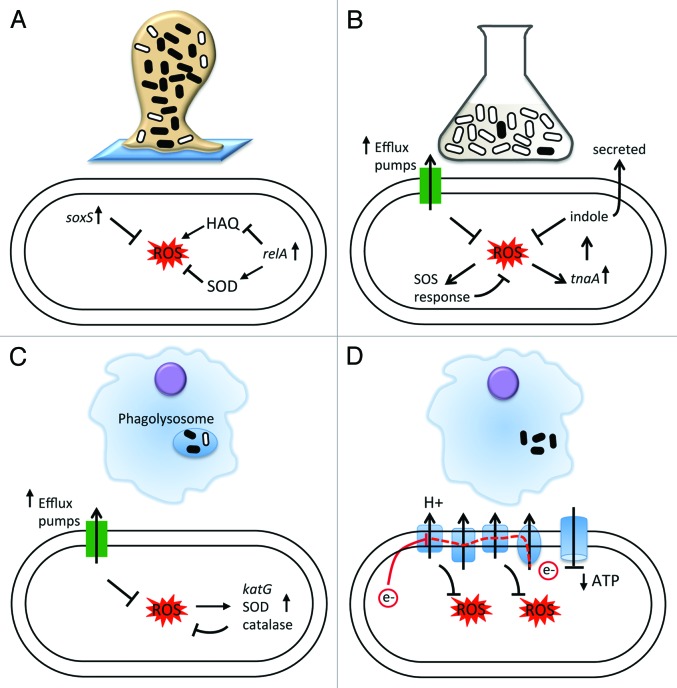
Many different models have been proposed to explain chronic infections and their associated antibiotic tolerance in the absence of genetic resistance, including facultative intracellular growth, small colony variants, persister populations, environmentally induced antibiotic indifference, and biofilm formation. In light of the wide range of experimentally supported models, it is likely that numerous mechanisms, rather than a single one, play a role in the establishment of a chronic infection and the associated difficulty in sterilizing these infections.119 In this review, we highlight that despite the heterogeneity of the mechanisms contributing to the antibiotic tolerant population, the cellular response to oxidative stress may be a shared theme among the diverse pathways to chronic infections and antibiotic tolerance (Fig. 2). Mounting evidence suggests that bacteria confronting host immunity within many different environments are trying to protect themselves from oxidative stress.74 Thus, it is tempting to hypothesize that the same bacterial adaptive responses to oxidative stresses in the host microenvironment may provide the additional benefit of facilitating bacterial survival during antibiotic exposure. This survival advantage may reflect the physiologic changes occurring in bacteria in response to oxidative stress, allowing survival at the same intracellular concentration of antibiotic. Alternatively, the upregulation of efflux pumps as part of the oxidative stress response may decrease the effective intracellular concentration of antibiotics, enabling bacteria survival. The link between the oxidative stress response and persistent infections suggests a new approach to managing persistent infections, regardless of the underlying mechanism. If small molecules can be identified that potentiate oxidative stress or subvert cellular mechanisms that protect against reactive oxygen species, these small molecules have the potential to more effectively sterilize infections that currently defy our antibiotic arsenal.95,119,120 By impairing protective mechanisms against oxidative stress, these “new therapeutics” may serve the dual function of acting on bacteria as primary antibiotics, as well as impairing the bacterial defense to host immunity.
Figure 2. The role of oxidative stress in different in vitro models for persistent infections. (A) Biofilms. Bacteria in biofilms are exposed to increased endogenous oxidative stress, resulting in upregulation of soxS, a regulator of the superoxide response. In addition, for bacteria in biofilms or exposed to starvation conditions, the RelA mediated stringent response results in increased expression of superoxide dismutases (SOD) and decreased expression of pro-oxidants (HAQ). As a result, bacteria exhibit higher tolerance to ROS. (B) Persisters. Persisters have been shown to exhibit differential sensitivity to ROS compared with rapidly growing, antibiotic susceptible bacteria. Proposed mechanisms include increased expression of efflux pumps, a component of the oxidative stress response, the secretion of indole, which may induce oxidative protective mechanisms in neighboring cells, and upregulation of the SOS response. (C) Intracellular infection. M. tuberculosis, an intracellular pathogen, expresses specific factors to counteract the ROS and RNS encountered in the phagosome, including expression of efflux pumps, superoxide dismutases and low molecular weight thiols. Antibiotic tolerance within the macrophages is mediated by the expression of efflux pumps in M. marinum. (D) Small colony variants. The small colony variant phenotype, which may represent an adaptation to facilitate intracellular survival and growth, is characterized by deficiencies in the electron transport chain. Repression of the electron transport chain results in the generation of fewer ROS within the cell as well as reduced ATP production and transmembrane potential, all of which may affect antibiotic efficacy. In all panels, antibiotic tolerant cells are represented by black ovals, and antibiotic sensitive bacteria are represented by white ovals.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/23987
References
- 1.Gomez JE, McKinney JD. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis (Edinb) 2004;84:29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Monack DM, Mueller A, Falkow S. Persistent bacterial infections: the interface of the pathogen and the host immune system. Nat Rev Microbiol. 2004;2:747–65. doi: 10.1038/nrmicro955. [DOI] [PubMed] [Google Scholar]
- 3.McDERMOTT W. Microbial persistence. Yale J Biol Med. 1958;30:257–91. [PMC free article] [PubMed] [Google Scholar]
- 4.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 5.Dwyer DJ, Kohanski MA, Hayete B, Collins JJ. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol Syst Biol. 2007;3:91. doi: 10.1038/msb4100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Zhao X. Contribution of oxidative damage to antimicrobial lethality. Antimicrob Agents Chemother. 2009;53:1395–402. doi: 10.1128/AAC.01087-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolodkin-Gal I, Sat B, Keshet A, Engelberg-Kulka H. The communication factor EDF and the toxin-antitoxin module mazEF determine the mode of action of antibiotics. PLoS Biol. 2008;6:e319. doi: 10.1371/journal.pbio.0060319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Imlay JA. Cell death from antibiotics without the involvement of reactive oxygen species. Science. 2013;339:1210–3. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science. 2013;339:1213–6. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- 10.Lafond RE, Lukehart SA. Biological basis for syphilis. Clin Microbiol Rev. 2006;19:29–49. doi: 10.1128/CMR.19.1.29-49.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kent ME, Romanelli F. Reexamining syphilis: an update on epidemiology, clinical manifestations, and management. Ann Pharmacother. 2008;42:226–36. doi: 10.1345/aph.1K086. [DOI] [PubMed] [Google Scholar]
- 12.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–6. doi: 10.1016/0140-6736(91)92035-Z. [DOI] [PubMed] [Google Scholar]
- 13.Nomura A, Stemmermann GN, Chyou PH, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and the risk for duodenal and gastric ulceration. Ann Intern Med. 1994;120:977–81. doi: 10.7326/0003-4819-120-12-199406150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Caygill CP, Hill MJ, Braddick M, Sharp JC. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet. 1994;343:83–4. doi: 10.1016/S0140-6736(94)90816-8. [DOI] [PubMed] [Google Scholar]
- 15.Bryers JD. Medical biofilms. Biotechnol Bioeng. 2008;100:1–18. doi: 10.1002/bit.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lentino JR. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis. 2003;36:1157–61. doi: 10.1086/374554. [DOI] [PubMed] [Google Scholar]
- 17.Shuman EK, Urquhart A, Malani PN. Management and prevention of prosthetic joint infection. Infect Dis Clin North Am. 2012;26:29–39. doi: 10.1016/j.idc.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Sculco TP. The economic impact of infected joint arthroplasty. Orthopedics. 1995;18:871–3. [PubMed] [Google Scholar]
- 19.Fux CA, Stoodley P, Hall-Stoodley L, Costerton JW. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Rev Anti Infect Ther. 2003;1:667–83. doi: 10.1586/14787210.1.4.667. [DOI] [PubMed] [Google Scholar]
- 20.Lane RK, Matthay MA. Central line infections. Curr Opin Crit Care. 2002;8:441–8. doi: 10.1097/00075198-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunstad DA, Justice SS. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu Rev Microbiol. 2010;64:203–21. doi: 10.1146/annurev.micro.112408.134258. [DOI] [PubMed] [Google Scholar]
- 23.Foxman B. Recurring urinary tract infection: incidence and risk factors. Am J Public Health. 1990;80:331–3. doi: 10.2105/AJPH.80.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooton TM. Recurrent urinary tract infection in women. Int J Antimicrob Agents. 2001;17:259–68. doi: 10.1016/S0924-8579(00)00350-2. [DOI] [PubMed] [Google Scholar]
- 25.Brown DL, Frank JE. Diagnosis and management of syphilis. Am Fam Physician. 2003;68:283–90. [PubMed] [Google Scholar]
- 26.Crawford RW, Rosales-Reyes R, Ramírez-Aguilar MdeL, Chapa-Azuela O, Alpuche-Aranda C, Gunn JS. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Natl Acad Sci U S A. 2010;107:4353–8. doi: 10.1073/pnas.1000862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Eiff C, Jansen B, Kohnen W, Becker K. Infections associated with medical devices: pathogenesis, management and prophylaxis. Drugs. 2005;65:179–214. doi: 10.2165/00003495-200565020-00003. [DOI] [PubMed] [Google Scholar]
- 28.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Escobedo G, Marshall JM, Gunn JS. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nat Rev Microbiol. 2011;9:9–14. doi: 10.1038/nrmicro2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otto M. Bacterial evasion of antimicrobial peptides by biofilm formation. Curr Top Microbiol Immunol. 2006;306:251–8. doi: 10.1007/3-540-29916-5_10. [DOI] [PubMed] [Google Scholar]
- 31.Ward KH, Olson ME, Lam K, Costerton JW. Mechanism of persistent infection associated with peritoneal implants. J Med Microbiol. 1992;36:406–13. doi: 10.1099/00222615-36-6-406. [DOI] [PubMed] [Google Scholar]
- 32.Shiau AL, Wu CL. The inhibitory effect of Staphylococcus epidermidis slime on the phagocytosis of murine peritoneal macrophages is interferon-independent. Microbiol Immunol. 1998;42:33–40. doi: 10.1111/j.1348-0421.1998.tb01966.x. [DOI] [PubMed] [Google Scholar]
- 33.Yasuda H, Ajiki Y, Aoyama J, Yokota T. Interaction between human polymorphonuclear leucocytes and bacteria released from in-vitro bacterial biofilm models. J Med Microbiol. 1994;41:359–67. doi: 10.1099/00222615-41-5-359. [DOI] [PubMed] [Google Scholar]
- 34.Nix RN, Altschuler SE, Henson PM, Detweiler CS. Hemophagocytic macrophages harbor Salmonella enterica during persistent infection. PLoS Pathog. 2007;3:e193. doi: 10.1371/journal.ppat.0030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderl JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2000;44:1818–24. doi: 10.1128/AAC.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walters MC, 3rd, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother. 2003;47:317–23. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson GG, O’Toole GA. Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol. 2008;322:85–105. doi: 10.1007/978-3-540-75418-3_5. [DOI] [PubMed] [Google Scholar]
- 38.Barclay WRER, Ebert RH, Le Roy GV, Manthei RW, Roth LJ. Distribution and excretion of radioactive isoniazid in tuberculous patients. J Am Med Assoc. 1953;151:1384–8. [PubMed] [Google Scholar]
- 39.McCune RM, Feldmann FM, McDermott W. Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J Exp Med. 1966;123:469–86. doi: 10.1084/jem.123.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomasz A, de Vegvar ML. Construction of a penicillin-tolerant laboratory mutant of Staphylococcus aureus. Eur J Clin Microbiol. 1986;5:710–3. doi: 10.1007/BF02013310. [DOI] [PubMed] [Google Scholar]
- 41.Helaine S, Thompson JA, Watson KG, Liu M, Boyle C, Holden DW. Dynamics of intracellular bacterial replication at the single cell level. Proc Natl Acad Sci U S A. 2010;107:3746–51. doi: 10.1073/pnas.1000041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Justice SS, Hung C, Theriot JA, Fletcher DA, Anderson GG, Footer MJ, et al. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc Natl Acad Sci U S A. 2004;101:1333–8. doi: 10.1073/pnas.0308125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blango MG, Mulvey MA. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob Agents Chemother. 2010;54:1855–63. doi: 10.1128/AAC.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mysorekar IU, Hultgren SJ. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc Natl Acad Sci U S A. 2006;103:14170–5. doi: 10.1073/pnas.0602136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 2010;6:e1001042. doi: 10.1371/journal.ppat.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell DG. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol Rev. 2011;240:252–68. doi: 10.1111/j.1600-065X.2010.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullivan JT, Young EF, McCann JR, Braunstein M. The Mycobacterium tuberculosis SecA2 system subverts phagosome maturation to promote growth in macrophages. Infect Immun. 2012;80:996–1006. doi: 10.1128/IAI.05987-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacMicking JD, Taylor GA, McKinney JD. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science. 2003;302:654–9. doi: 10.1126/science.1088063. [DOI] [PubMed] [Google Scholar]
- 50.Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, et al. Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J Exp Med. 2003;198:693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehrt S, Schnappinger D. Mycobacterial survival strategies in the phagosome: defence against host stresses. Cell Microbiol. 2009;11:1170–8. doi: 10.1111/j.1462-5822.2009.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edwards KM, Cynamon MH, Voladri RK, Hager CC, DeStefano MS, Tham KT, et al. Iron-cofactored superoxide dismutase inhibits host responses to Mycobacterium tuberculosis. Am J Respir Crit Care Med. 2001;164:2213–9. doi: 10.1164/ajrccm.164.12.2106093. [DOI] [PubMed] [Google Scholar]
- 53.Ng VH, Cox JS, Sousa AO, MacMicking JD, McKinney JD. Role of KatG catalase-peroxidase in mycobacterial pathogenesis: countering the phagocyte oxidative burst. Mol Microbiol. 2004;52:1291–302. doi: 10.1111/j.1365-2958.2004.04078.x. [DOI] [PubMed] [Google Scholar]
- 54.Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, et al. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell. 2011;145:39–53. doi: 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramón-García SMC, Martín C, Thompson CJ, Aínsa JA. Role of the Mycobacterium tuberculosis P55 efflux pump in intrinsic drug resistance, oxidative stress responses, and growth. Antimicrob Agents Chemother. 2009;53:3675–82. doi: 10.1128/AAC.00550-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poole K. Bacteria multidrug efflux pumps serve other functions. Microbes. 2008;3:179–85. [Google Scholar]
- 57.Tuchscherr L, Medina E, Hussain M, Völker W, Heitmann V, Niemann S, et al. Staphylococcus aureus phenotype switching: an effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol Med. 2011;3:129–41. doi: 10.1002/emmm.201000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, et al. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol. 2006;4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 59.Starkey M, Hickman JH, Ma L, Zhang N, De Long S, Hinz A, et al. Pseudomonas aeruginosa rugose small-colony variants have adaptations that likely promote persistence in the cystic fibrosis lung. J Bacteriol. 2009;191:3492–503. doi: 10.1128/JB.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kahl B, Herrmann M, Everding AS, Koch HG, Becker K, Harms E, et al. Persistent infection with small colony variant strains of Staphylococcus aureus in patients with cystic fibrosis. J Infect Dis. 1998;177:1023–9. doi: 10.1086/515238. [DOI] [PubMed] [Google Scholar]
- 61.Häussler S, Tümmler B, Weissbrodt H, Rohde M, Steinmetz I. Small-colony variants of Pseudomonas aeruginosa in cystic fibrosis. Clin Infect Dis. 1999;29:621–5. doi: 10.1086/598644. [DOI] [PubMed] [Google Scholar]
- 62.McNamara PJ, Proctor RA. Staphylococcus aureus small colony variants, electron transport and persistent infections. Int J Antimicrob Agents. 2000;14:117–22. doi: 10.1016/S0924-8579(99)00170-3. [DOI] [PubMed] [Google Scholar]
- 63.Bulger RJ, Bulger RE. Ultrastructure of small colony variants of a methicillin-resistant Staphylococcus aureus. J Bacteriol. 1967;94:1244–6. doi: 10.1128/jb.94.4.1244-1246.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moisan H, Brouillette E, Jacob CL, Langlois-Bégin P, Michaud S, Malouin F. Transcription of virulence factors in Staphylococcus aureus small-colony variants isolated from cystic fibrosis patients is influenced by SigB. J Bacteriol. 2006;188:64–76. doi: 10.1128/JB.188.1.64-76.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tuchscherr L, Heitmann V, Hussain M, Viemann D, Roth J, von Eiff C, et al. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J Infect Dis. 2010;202:1031–40. doi: 10.1086/656047. [DOI] [PubMed] [Google Scholar]
- 66.Balwit JM, van Langevelde P, Vann JM, Proctor RA. Gentamicin-resistant menadione and hemin auxotrophic Staphylococcus aureus persist within cultured endothelial cells. J Infect Dis. 1994;170:1033–7. doi: 10.1093/infdis/170.4.1033. [DOI] [PubMed] [Google Scholar]
- 67.Schröder A, Kland R, Peschel A, von Eiff C, Aepfelbacher M. Live cell imaging of phagosome maturation in Staphylococcus aureus infected human endothelial cells: small colony variants are able to survive in lysosomes. Med Microbiol Immunol. 2006;195:185–94. doi: 10.1007/s00430-006-0015-0. [DOI] [PubMed] [Google Scholar]
- 68.von Eiff C, Heilmann C, Proctor RA, Woltz C, Peters G, Götz F. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J Bacteriol. 1997;179:4706–12. doi: 10.1128/jb.179.15.4706-4712.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.von Eiff C, Becker K, Metze D, Lubritz G, Hockmann J, Schwarz T, et al. Intracellular persistence of Staphylococcus aureus small-colony variants within keratinocytes: a cause for antibiotic treatment failure in a patient with darier’s disease. Clin Infect Dis. 2001;32:1643–7. doi: 10.1086/320519. [DOI] [PubMed] [Google Scholar]
- 70.Sendi P, Proctor RA. Staphylococcus aureus as an intracellular pathogen: the role of small colony variants. Trends Microbiol. 2009;17:54–8. doi: 10.1016/j.tim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 71.Kahl BC, Belling G, Becker P, Chatterjee I, Wardecki K, Hilgert K, et al. Thymidine-dependent Staphylococcus aureus small-colony variants are associated with extensive alterations in regulator and virulence gene expression profiles. Infect Immun. 2005;73:4119–26. doi: 10.1128/IAI.73.7.4119-4126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui L, Neoh HM, Iwamoto A, Hiramatsu K. Coordinated phenotype switching with large-scale chromosome flip-flop inversion observed in bacteria. Proc Natl Acad Sci U S A. 2012;109:E1647–56. doi: 10.1073/pnas.1204307109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chuard C, Vaudaux PE, Proctor RA, Lew DP. Decreased susceptibility to antibiotic killing of a stable small colony variant of Staphylococcus aureus in fluid phase and on fibronectin-coated surfaces. J Antimicrob Chemother. 1997;39:603–8. doi: 10.1093/jac/39.5.603. [DOI] [PubMed] [Google Scholar]
- 74.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–76. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hobby GLMK, Chaffee E. Observations of the mechanism of action of penicillin. Proc Soc Exp Biol Med. 1942;50:281–5. [Google Scholar]
- 76.Bigger JW. Treatment of staphylococcal infections with penicillin. Lancet. 1944;244:497–500. doi: 10.1016/S0140-6736(00)74210-3. [DOI] [Google Scholar]
- 77.Keren I, Shah D, Spoering A, Kaldalu N, Lewis K. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J Bacteriol. 2004;186:8172–80. doi: 10.1128/JB.186.24.8172-8180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wood DN, Chaussee MA, Chaussee MS, Buttaro BA. Persistence of Streptococcus pyogenes in stationary-phase cultures. J Bacteriol. 2005;187:3319–28. doi: 10.1128/JB.187.10.3319-3328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 80.Moyed HS, Bertrand KP. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J Bacteriol. 1983;155:768–75. doi: 10.1128/jb.155.2.768-775.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schumacher MA, Piro KM, Xu W, Hansen S, Lewis K, Brennan RG. Molecular mechanisms of HipA-mediated multidrug tolerance and its neutralization by HipB. Science. 2009;323:396–401. doi: 10.1126/science.1163806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science. 2004;305:1622–5. doi: 10.1126/science.1099390. [DOI] [PubMed] [Google Scholar]
- 83.Keren I, Minami S, Rubin E, Lewis K. Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. MBio. 2011;2:e00100–11. doi: 10.1128/mBio.00100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Maisonneuve E, Shakespeare LJ, Jørgensen MG, Gerdes K. Bacterial persistence by RNA endonucleases. Proc Natl Acad Sci U S A. 2011;108:13206–11. doi: 10.1073/pnas.1100186108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Gerdes K, Maisonneuve E. Bacterial persistence and toxin-antitoxin loci. Annu Rev Microbiol. 2012;66:103–23. doi: 10.1146/annurev-micro-092611-150159. [DOI] [PubMed] [Google Scholar]
- 86.Wakamoto Y, Dhar N, Chait R, Schneider K, Signorino-Gelo F, Leibler S, et al. Dynamic persistence of antibiotic-stressed mycobacteria. Science. 2013;339:91–5. doi: 10.1126/science.1229858. [DOI] [PubMed] [Google Scholar]
- 87.Kim JS, Heo P, Yang TJ, Lee KS, Jin YS, Kim SK, et al. Bacterial persisters tolerate antibiotics by not producing hydroxyl radicals. Biochem Biophys Res Commun. 2011;413:105–10. doi: 10.1016/j.bbrc.2011.08.063. [DOI] [PubMed] [Google Scholar]
- 88.Miller C, Thomsen LE, Gaggero C, Mosseri R, Ingmer H, Cohen SN. SOS response induction by beta-lactams and bacterial defense against antibiotic lethality. Science. 2004;305:1629–31. doi: 10.1126/science.1101630. [DOI] [PubMed] [Google Scholar]
- 89.Dörr T, Vulić M, Lewis K. Ciprofloxacin causes persister formation by inducing the TisB toxin in Escherichia coli. PLoS Biol. 2010;8:e1000317. doi: 10.1371/journal.pbio.1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dörr T, Lewis K, Vulić M. SOS response induces persistence to fluoroquinolones in Escherichia coli. PLoS Genet. 2009;5:e1000760. doi: 10.1371/journal.pgen.1000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee HH, Molla MN, Cantor CR, Collins JJ. Bacterial charity work leads to population-wide resistance. Nature. 2010;467:82–5. doi: 10.1038/nature09354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vega NM, Allison KR, Khalil AS, Collins JJ. Signaling-mediated bacterial persister formation. Nat Chem Biol. 2012;8:431–3. doi: 10.1038/nchembio.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu Y, Vulić M, Keren I, Lewis K. Role of oxidative stress in persister tolerance. Antimicrob Agents Chemother. 2012;56:4922–6. doi: 10.1128/AAC.00921-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Grant SS, Kaufmann BB, Chand NS, Haseley N, Hung DT. Eradication of bacterial persisters with antibiotic-generated hydroxyl radicals. Proc Natl Acad Sci U S A. 2012;109:12147–52. doi: 10.1073/pnas.1203735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yano T, Kassovska-Bratinova S, Teh JS, Winkler J, Sullivan K, Isaacs A, et al. Reduction of clofazimine by mycobacterial type 2 NADH:quinone oxidoreductase: a pathway for the generation of bactericidal levels of reactive oxygen species. J Biol Chem. 2011;286:10276–87. doi: 10.1074/jbc.M110.200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Levin BRRD, Rozen DE. Non-inherited antibiotic resistance. Nat Rev Microbiol. 2006;4:556–62. doi: 10.1038/nrmicro1445. [DOI] [PubMed] [Google Scholar]
- 97.Eng RH, Padberg FT, Smith SM, Tan EN, Cherubin CE. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob Agents Chemother. 1991;35:1824–8. doi: 10.1128/AAC.35.9.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dahl JL, Kraus CN, Boshoff HI, Doan B, Foley K, Avarbock D, et al. The role of RelMtb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc Natl Acad Sci U S A. 2003;100:10026–31. doi: 10.1073/pnas.1631248100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wayne LG, Lin KY. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect Immun. 1982;37:1042–9. doi: 10.1128/iai.37.3.1042-1049.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43:717–31. doi: 10.1046/j.1365-2958.2002.02779.x. [DOI] [PubMed] [Google Scholar]
- 101.Xie Z, Siddiqi N, Rubin EJ. Differential antibiotic susceptibilities of starved Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother. 2005;49:4778–80. doi: 10.1128/AAC.49.11.4778-4780.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gold B, Pingle M, Brickner SJ, Shah N, Roberts J, Rundell M, et al. Nonsteroidal anti-inflammatory drug sensitizes Mycobacterium tuberculosis to endogenous and exogenous antimicrobials. Proc Natl Acad Sci U S A. 2012;109:16004–11. doi: 10.1073/pnas.1214188109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dalebroux ZD, Swanson MS. ppGpp: magic beyond RNA polymerase. Nat Rev Microbiol. 2012;10:203–12. doi: 10.1038/nrmicro2720. [DOI] [PubMed] [Google Scholar]
- 104.Kusser W, Ishiguro EE. Involvement of the relA gene in the autolysis of Escherichia coli induced by inhibitors of peptidoglycan biosynthesis. J Bacteriol. 1985;164:861–5. doi: 10.1128/jb.164.2.861-865.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodionov DG, Ishiguro EE. Direct correlation between overproduction of guanosine 3′,5′-bispyrophosphate (ppGpp) and penicillin tolerance in Escherichia coli. J Bacteriol. 1995;177:4224–9. doi: 10.1128/jb.177.15.4224-4229.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science. 2011;334:982–6. doi: 10.1126/science.1211037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Costerton JWS, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 108.Schembri MA, Kjaergaard K, Klemm P. Global gene expression in Escherichia coli biofilms. Mol Microbiol. 2003;48:253–67. doi: 10.1046/j.1365-2958.2003.03432.x. [DOI] [PubMed] [Google Scholar]
- 109.Otto M. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med. 2013;64:175–88. doi: 10.1146/annurev-med-042711-140023. [DOI] [PubMed] [Google Scholar]
- 110.Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science. 2010;328:627–9. doi: 10.1126/science.1188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boles BR, Singh PK. Endogenous oxidative stress produces diversity and adaptability in biofilm communities. Proc Natl Acad Sci U S A. 2008;105:12503–8. doi: 10.1073/pnas.0801499105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Landini P. Cross-talk mechanisms in biofilm formation and responses to environmental and physiological stress in Escherichia coli. Res Microbiol. 2009;160:259–66. doi: 10.1016/j.resmic.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 113.Bink A, Vandenbosch D, Coenye T, Nelis H, Cammue BP, Thevissen K. Superoxide dismutases are involved in Candida albicans biofilm persistence against miconazole. Antimicrob Agents Chemother. 2011;55:4033–7. doi: 10.1128/AAC.00280-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lemos JA, Brown TA, Jr., Burne RA. Effects of RelA on key virulence properties of planktonic and biofilm populations of Streptococcus mutans. Infect Immun. 2004;72:1431–40. doi: 10.1128/IAI.72.3.1431-1440.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anderl JN, Zahller J, Roe F, Stewart PS. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2003;47:1251–6. doi: 10.1128/AAC.47.4.1251-1256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Häussler S. Biofilm formation by the small colony variant phenotype of Pseudomonas aeruginosa. Environ Microbiol. 2004;6:546–51. doi: 10.1111/j.1462-2920.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- 117.Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–4. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 118.Mulcahy LR, Burns JL, Lory S, Lewis K. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J Bacteriol. 2010;192:6191–9. doi: 10.1128/JB.01651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Allison KR, Brynildsen MP, Collins JJ. Heterogeneous bacterial persisters and engineering approaches to eliminate them. Curr Opin Microbiol. 2011;14:593–8. doi: 10.1016/j.mib.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brynildsen MP, Winkler JA, Spina CS, Macdonald IC, Collins JJ. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat Biotechnol. 2013;31:160–5. doi: 10.1038/nbt.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]