Abstract
Recent findings indicate that a majority of action potentials originate from dendrites of GnRH neurons. This localization of the dendrite as the principle site of action potential initiation has sparked considerable interest in the nature of ionic channels throughout GnRH neurons. This paper will review the ionic conductances described within GnRH neurons and their implications for physiological output, such as sensitivity to steroids and diurnal state. To date, a majority of information regarding ionic conductances in GnRH neurons pertains to somata and the first 50–100 µm of dendrite length. Thus, unraveling the tapestry created by the nature and distribution of dendritic conductances in GnRH neurons lies at the forefront of understanding the control of reproductive hormone secretion.
Keywords: GnRH neurons, hypothalamus, conductance, estradiol, morphology, dendrites, modeling, puberty
Gonadotropin releasing-hormone (GnRH) is a small neuropeptide that drives pituitary release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), peripheral hormones critical in regulating gonadal function and fertility. The cell bodies of the GnRH-containing neurons are distributed diffusely throughout the rostral hypothalamus. Their axons project to the median eminence where they release the GnRH peptide into the hypophysiotropic portal system. In the portal capillaries, GnRH travels to the anterior pituitary gland to stimulate release of gonadotropins into the systemic circulation. GnRH release is not continuous, but rather is released in episodic pulses, approximately once/hour.1 It is well-established that the intermittent manner of GnRH release is essential for sexual reproduction and that changes in the frequency and amplitude of GnRH pulses govern transitions between reproductive states.2
The neuronal substrate that results in pulsatile GnRH release, and therefore comprises the “GnRH pulse generator,” is unknown. The intermittent stimulus for GnRH release may arise from input to the GnRH neurons and reflect synaptic interactions between GnRH neurons and a secondary network.3 Alternatively, pulsatile GnRH release may be a consequence of spontaneous activity of the GnRH neurons themselves, and therefore, is the result of properties that are intrinsic to this population of hypothalamic cells. These two hypotheses are not mutually exclusive; the GnRH pulse generator is likely derived from a combination of intrinsic properties and synaptic interactions.
With the development of transgenic rodent models in which the GnRH neurons express green fluorescent protein4-6 (GFP), it has become increasingly possible to study GnRH neurons using a variety of electrophysiological approaches.7 Thus, there is now a rapidly expanding literature focusing on the activity of GnRH neurons, their ionic conductances, and their physiological modulation by a variety of internal and external factors. Our purpose is to review what is known about the conductances described in both somatic and dendritic compartments of GnRH neurons, and their role within the mammalian reproductive axis.
This review will consider GnRH neurons as a single population. However, GnRH neurons display a physiological diversity that suggests they may not be one singular population of neurons. Before the broad-spread use of GnRH-GFP rodent models, Sim et al. (2000)8 provided a notable glimpse into the diversity that exists within the total population of GnRH neurons. Using post hoc identification of GnRH neurons, they demonstrated that sub-populations of GnRH neurons could be identified based on key electrophysiological properties such as repetitive firing and rectification. Since these behaviors reflect the expression of specific ion conductances, their study indicated that individual GnRH neurons likely differed in the type and densities of specific ion channels. Thus, while this review discusses GnRH neurons as a single population with the same, or largely the same ionic channels, it should be noted that even from the earliest junctures it has been clear that this is unlikely.
Ionic Channels in GnRH Neurons (see Table 1)
Table 1. Conductances and estrogen sensitivity .
| Current | E Sensitive | Diurnal | Citation |
|---|---|---|---|
| Ih |
No |
No |
Sim et al., 2001; Zhang et al., 2008; Chu et al., 2010 |
| Sodium | |||
|---|---|---|---|
| TTX-sensitive |
Yes |
No |
Spergel et al., 1999; Suter et al., 2000; Kuehl-Kovarik et al., 2005; Chu and Moenter, 2006; Liu and Herbison, 2008; Roberts et al., 2009 |
| Kisspeptin -induced inward |
No |
No |
Zhang et al., 2008 |
| Potassium | |||
|---|---|---|---|
| IA |
Yes |
Yes |
DeFazio and Moenter, 2002; Xu et al., 2008; Pielecka-Fortuna et al., 2011 |
| IK |
Yes |
Yes |
Herbison, 1998; Pielecka-Fortuna et al., 2011 |
| IM |
No |
No |
Xu et al., 2008 |
| SK |
Yes |
No |
Bosch et al., 2003; Kelly et al., 2002; Kelly et al., 2003; Kato et al., 2006; Liu and Herbison, 2008; Lee et al., 2010 |
| BK |
No |
No |
Hiraizumi et al., 2008 |
| KATP |
Yes |
No |
Zhang et al., 2007 |
| Calcium | |||
|---|---|---|---|
| L-type |
Yes |
Yes |
Nunemaker et al., 2003; Sun et al., 2010 |
| N-type |
Yes |
Yes |
Nunemaker et al., 2003; Sun et al., 2010 |
| P-type |
No |
No |
Nunemaker et al., 2003 |
| Q-type |
No |
No |
Nunemaker et al., 2003 |
| R-type |
Yes |
Yes |
Nunemaker et al., 2003; Sun et al., 2010 |
| T-type |
Yes |
No |
Kato et al., 2003; Nunemaker et al., 2003; Zhang et al., 2009 |
| Chloride | |||
|---|---|---|---|
| GABAA |
No |
No |
Weyler et al., 1999; Herbison and Moenter, 2011 |
Summary table of the ionic conductances described for GnRH neurons. Intrinsic GnRH conductances, estrogen sensitivity, and diurnal effects are listed per conductance type. Citations that describe conductances found in GnRH neurons are provided.
Table 1 describes some of the many ionic conductances that have been defined in GnRH neurons. The voltage-gated ion channels in GnRH neurons include sodium channels, potassium channels, and calcium channels. The combined impact of these channels largely determines the intrinsic properties of GnRH neurons. The relatively high input resistance of GnRH neurons suggests that at resting membrane potentials, most ionic conductances are inactive. However, even the earliest studies noted marked excursions and oscillations in resting membrane potential (Suter 2000) even in the absence of synaptic inputs (Keuhl 2003). Thus, ionic conductances in GnRH neurons likely underlie and modulate this critical electrophysiological behavior.
Sodium channels
There is general agreement that GnRH neurons express the classic TTX-sensitive voltage-gated sodium channels. Blocking these channels eliminates spontaneous action potentials in GnRH neurons as well as action potentials in response to current injection pulses. Thus, similar to other neurons, TTX-sensitive voltage-gated sodium channels underlie the rapid depolarization phase of the action potential in GnRH neurons4,6 It seems a substantial fraction of voltage-gated sodium channels (~93%) are inactive at resting membrane potentials. Spike threshold in GnRH neurons has been reported to range from -49 to -32 mV with the sodium current activating at about -40 mV4. Our own studies agree with the more depolarized range of spike threshold (~35 mV) which is consistent with the finding that the upstroke of the action potential is generated almost exclusively by voltage gated sodium channels.4
A TTX-sensitive sodium channel also contributes to slow after depolarizing potentials (so-called ADPs) in GnRH neurons. In other neurosecretory cells, ADPs have been implicated in repetitive firing, the mode of neuronal activity that facilitates hormone release.9 Both cultured GnRH somata and GnRH neurons in hypothalamic slices express ADPs.10-13 In female mice, ADPs recorded in GnRH neurons are modulated by estradiol, a key component of the positive and negative feedback system within the reproductive axis. High levels of estradiol, associated with positive feedback, increase the amplitude of ADPs and low levels of estradiol, associated with negative feedback, either have no effect14 or decrease ADP amplitude.15 The augmentation of ADP amplitude in response to estradiol has been suggested to increase the activity of GnRH neurons during times of maximum hormone release; for example, during the preovulatory surge of release in females.11 The latter finding is consistent with an additional study from the same laboratory indicating that low levels of estrogen decrease the conductance of both a persistent sodium current as well as the classical voltage-gated sodium channel.16 Similar studies have not been performed in males but estradiol is likely the negative feedback signal. Therefore, GnRH neurons from males may respond in a similar manner. The neuropeptide kisspeptin opens TRPC-like cation channels through which sodium influx makes a large contribution to kisspeptin’s depolarizing effect on GnRH neurons.17 Kisspeptin, a potent stimulator of GnRH release has been implicated in pubertal development, regulation of the female estrous cycle, and also as the effector in multiple signals that regulate reproduction.18
Potassium channels
Similar to other neurons, multiple potassium conductances are expressed in GnRH neurons. Most of these potassium conductances in neurons are activated by sub-threshold levels of membrane depolarization and can be distinguished by differing kinetic properties with respect to voltage and time. In GnRH neurons, the slowly inactivating potassium current (IK) and the fast inactivating A-type potassium current (IA) are both expressed.19 However, IA activates at more hyperpolarized voltages (~-50mV) than IK (~-40mV). IA inactivates rather quickly (~100 ms) and impacts the inter-action potential interval and therefore, the frequency of action potential firing. IK,, on the other hand, can remain activated for up to 5 sec, and generally acts to repolarize the membrane potential to remove the inactivation of sodium channels and enable repetitive firing. The so-called M-type potassium current (IM) is also expressed in GnRH neurons. M-type potassium currents are non-inactivating, sub-threshold currents that inhibit cell excitability. The presence of these currents on GnRH neurons may provide a mechanism in which GnRH autoregulates GnRH neurons via a self-feedback loop.20
Potassium channels in GnRH neurons create a rich substrate for modulation of activity. Kelly et al. (2002 and 2003)21,22 and Bosch et al. 200223 provided some of the earliest evidence for the regulation of reproductive hormone secretion via estrogen actions on potassium channels. Kelly et al.21,22 observed an estrogen-mediated reduction of inwardly rectifying potassium currents activated by µ-opioids in POMC neurons. Moreover, neurons throughout the preoptic area and the arcuate nuclei were hyperpolarized by steroids with a particularly marked response in GnRH neurons. In recent studies, estradiol has been shown to alter both IK and IA, two voltage-gated potassium conductances that are largely responsible for the overall excitability and discharge activity in GnRH neurons.19 Since the effects of estradiol are relatively rapid, it most likely operates through a mechanism other than genomic signaling.24 Estradiol shifts (in the depolarizing direction) the voltage of both the half activation and half inactivation of IA in female mice. In addition, action potential latency was reduced in animals treated with estradiol following ovariectomy when compared with females without estrogen replacement following ovariectomy.19 These effects of estradiol appear to be modulated by time of day, a finding that has the potential to explain how estrogen shifts between the positive and negative modes of feedback regulation on GnRH neurons.25 The GnRH peptide itself also has been reported to activate IM to hyperpolarize GnRH neurons and thus form a potential autoregulatory mechanism of negative feedback to GnRH neurons.20
Perhaps the most interesting potassium current in GnRH neurons is the ATP-sensitive KATP. This inwardly rectifying conductance is expressed in about 50% of GnRH neurons from both male and female mice, which approximates the proportion of GnRH neurons that are also glucose sensitive. The current carried by KATP channels (when input resistance is about 1.0 Ω) ranges from about 35 pA in males and varies in females based on estradiol levels (~27 pA in estrogen treated females vs. ~14 pA in oil treated females).26 In a variety of species, the release of GnRH is influenced by metabolic status.27 Thus, it is possible that the strong hyperpolarization imposed by KATP (~-8.0 mV) in either GnRH somata or dendrites is partly responsible for the link between nutritional status and fertility.
Repetitive action potentials result in the accumulation of calcium in neurons. The elevated levels of intracellular calcium activate calcium-dependent potassium currents. One of these currents has a relatively small conductance. Thus, it is called a small conductance potassium channel or SK. A second calcium-sensitive potassium conductance exists in most neurons. Because it has a larger conductance than SK channels, it is called the large-conductance calcium activated potassium channel or BK (for big potassium conductance). There is evidence for both SK and BK conductance in GnRH neurons. The SK conductance is reported to be present in all GnRH neurons derived from mice12 and rats.28 Tonic activity of SK currents in GnRH neurons accounts for a relatively large hyperpolarization (~7 mV). Thus, multiple parameters shift in the presence of apamin, a specific blocker of SK channels. For example, the action potential frequency increases. However, the changes in the underlying pattern of action potentials account for the higher frequency of discharge. More repetitive (as opposed to basal) action potentials are exhibited and the duration of episodes of repetitive action potentials is longer.12 Broad calcium transients have also been found to control slow calcium-dependent potassium conductances, including an apamin-sensitive conductance, which regulates activity within and between bursts of activity.29 Like SK, the BK conductance has also been reported to control activity patterns in GnRH neurons from rats.30
Calcium
Voltage-gated calcium channels can be broadly characterized as high voltage-gated channels (HVGCCs) and low voltage-activated channels (LVA). HVGCC are activated by strong depolarization and further distinguished as L-, N-, R- and Q/P subtypes. Each of these channels requires depolarization to activate them but they differ in terms of the required magnitude and/or duration of depolarization for activation. They also differ in the duration of the active conductance. Low voltage activated calcium channels are T-type conductances. Voltage-gated calcium channels can also be distinguished based on their region of expression through the body and by their physiological function.31
Multiple calcium conductances are present in GnRH neurons. GnRH somata in mice express high voltage activated calcium currents, with the L-type calcium channel dominating over N-, P, Q- and R-type. Low voltage-activated calcium channels have not been detected in mouse GnRH neurons.32 In GnRH somata derived from rats, HVGCC have been detected, and a small component of a T-type conductance is reported to emerge around the time of puberty.33 However, in mice, Spergel34 did not report a developmental change in GnRH neuron calcium currents. Nonetheless, the observation of an increase in T-type currents in rats is interesting in that puberty requires a shift in activity of GnRH neurons to support increased GnRH release. Consistent with the findings of Kato et al.,33 T-type calcium conductances are frequently present in cells with rhythmic bursting or pacemaker activity.31 The potential role of T-type calcium channels during times of elevated GnRH secretion is underscored by the recent findings of Zhang et al. (2009),35 indicating that T-type channels are positively modulated by estrogen and may be particularly important in the large release of GnRH that results in ovulation.
Chloride channels: A ligand-gated ion channel
Until recently, chloride ions were assumed to be passively distributed across neuronal membranes. Thus, the emergence of chloride channels in neurons has attracted intense interest.36 In GnRH neurons, the GABAA receptor is the major chloride ion channel identified thus far. As both receptor and channel, GABAA is involved in synaptic transmission to GnRH neurons to modulate their firing patterns. However, whether its activation results in excitation or inhibition of GnRH neurons remains a topic of controversy and discussion.37 In most adult neurons, GABAA receptor activation causes hyperpolarization through chloride entry into the cell. This hyperpolarization is dependent upon low intracellular chloride concentration driving Cl- movement into the cell down its concentration gradient. If intracellular chloride concentration is elevated, the reversal potential for chloride will be depolarized and chloride ions will move out of the cell causing depolarization of the membrane. It is now well established that GABAA activation is depolarizing and excitatory in most immature neurons, and switches to become hyperpolarizing and inhibitory in most mature neurons. However, there is evidence that Cl- conductance may remain predominantly depolarizing in peripubertal38 and even adult GnRH neurons.39 Despite the interest surrounding the distribution of chloride in GnRH neurons, GnRH neuron-specific knockout of GABAA channels does not ultimately disrupt normal fertility.40
Ion Channels and Information Processing in GnRH Neuron Dendrites
It is clear that the dendrites in many, if not most, populations of neurons express active ionic conductances that endow them with active properties. The expression of active conductances in dendrites compensates for the capacitance-induced distortion of the temporal aspects of synaptic potentials.41 These active conductances can underlie plateau potential generation and create bistability leading to repetitive action potentials. Finally, the effectiveness of distal synaptic inputs often relies on active conductances in the intervening segments of the dendrite to boost signals that would, in the absence of active properties, decay before reaching somata.42 Therefore, the expression of active conductances in dendrites not only defines, in part, neuronal excitability; these conductances often fundamentally alter how neurons integrate information.43-46
The full complement, density and distribution of ion channels with their respective conductances in dendrites of any population of neurons is not completely known, but the following parameters described here are generally characteristic within a population of neurons. Conductances can vary from uniform along the entire length of a dendrite, or as gradients that either increase or decrease along the dendrite length (see ref. 47 for detailed discussion on the issue of distribution of active conductances within dendrites). The most common active conductances in dendrites are the voltage-gated sodium channels, voltage-dependent potassium channels, hyperpolarization-activated cation ion current (Ih), voltage-dependent calcium channels and the NMDA receptor channels. In some dendrites, Ih reduces the time course of subthreshold events that would otherwise be distorted by the increased capacitive load of larger diameter segments of dendrites.48 The opening of the Ih channel reduces membrane resistance. This slows the time course of events by offsetting the increased capacitance. Multiple forms of potassium conductances are also expressed in dendrites, most notably fast potassium channels (A-type) or slower D-type potassium channels. Many dendrites also express voltage-gated calcium channels of several possible varieties (L, N, P/Q, R and T). Depending on the population of neurons, there can either be preferential expression of the high or low-voltage activated calcium channels in dendrites, or expression of the entire complement of voltage-activated calcium channels. The high voltage activated channels appear to be important for amplifying synaptic input, inducing synaptic plasticity, generating dendritic spikes and underlying calcium-dependent neurotransmitter release from dendrites.31 The T-type calcium channels in dendrites are implicated in burst firing patterns. Perhaps the most important and most commonly expressed active conductance in dendrites is the voltage-gated sodium channel. This voltage-gated sodium channel is the same tetrodotoxin (TTX)-sensitive voltage-gated sodium channel that underlies both the generation of the action potential at the axon hillock and the propagation of action potential in axons. These voltage gated sodium channels serve the same function in dendrites.
Most of the electrophysiological studies in GnRH neurons to date have relied on recordings from isolated GnRH somata or somata of GnRH neurons in acute brain slice preparations. Thus, many of the conductances noted above may actually exist in GnRH neuron dendrites but, due to the location of recordings, cannot be definitively attributed to any more than the proximal portions of dendrites. The persistence of ADPs detected in isolated GnRH somata indicates that they are most likely generated in the proximal regions of the GnRH neuron. This could be somata or within the first 30 µm of the dendrite that is usually present in cultured somata.49 Therefore the TTX-sensitive sodium-based current that has been implicated in the generation of ADPs may reside in the proximal dendrite.
Until recently, very little was understood about the GnRH neuron dendrite beyond the most proximal portions. It is now clear that the dendrites of GnRH neurons are morphologically and functionally more complex than was previously appreciated. Structurally, dendrites of GnRH neurons are now recognized as being exceptionally long processes that50,51 bundle with one another52 and exhibit extensive branching in areas outside the blood-brain barrier.53 Based on work from our laboratory54 and those of the Herbison group,55 dendrites are also the major site of action potential generation in GnRH neurons in brain slices. Both slow basal firing54 and repetitive action potentials45,55 primarily originate in GnRH dendrites. Thus, the fast sodium conductance is present in GnRH dendrites. Moreover, somatically-induced action potentials faithfully back-propagate in GnRH dendrites.54 As shown in Figure 1, action potentials occur in both somata and dendrites following applied current injection pulses at somata. However, this signal shows consistent back-propagation into dendrites whereby each induced action potential in somata also results in a corresponding action potential in dendrites. The physiological significance of back-propagation of action potentials into dendrites may include activation of voltage-gated calcium channels or removing the voltage-dependent magnesium block on NMDA receptors.56
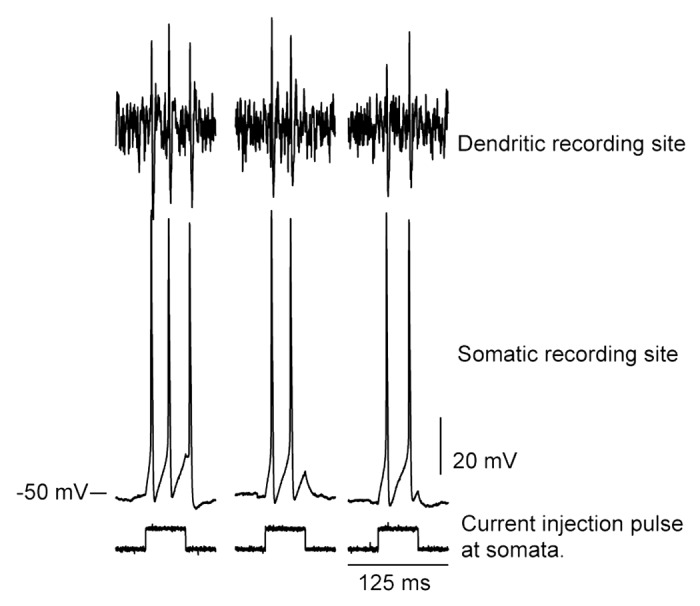
Figure 1. Back propagation of high frequency action potentials. Dual-recording at GnRH neuron soma and dendrites illustrates faithful back propagation of somatically generated high frequency action potentials. Expression of TTX-sensitive voltage-gated sodium channels and fast potassium conductances results in the active properties of GNRH neuron dendrites. Used with permission from the Endocrine Society for reference 46.
In any regard, the ability of the GnRH neuron dendrite to back-propagate relatively high-frequency action potentials supports the notion that these dendrites also express voltage-gated potassium channels in appropriate densities. In the absence of a rapid repolarization of dendritic membrane potential, repetitive action potentials could not be generated due to inactivation of the voltage-gated sodium channels. In contrast to the strong back-propagation of action potentials into GnRH neuron dendrites, CA1 pyramidal neurons exhibit a marked attenuation of high frequency action potentials during back-propagation into their dendrites.57 The observation of back-propagation failure during high frequency discharges presumably reflects persistent inactivation of voltage-gated sodium channels due to lack of the membrane repolarization generally provided by voltage-gated potassium channels. In the case of pyramidal neurons, the density of IA current channels decreases as the dendrite extends from the soma.57 Thus, the failure of high-fidelity back-propagation of somatically-generated action potentials is specifically due to weak dendritic expression of potassium channels which underlie membrane repolarization.58,59 In contrast, the robust back-propagation of somatically induced action potentials suggests the presence of sufficient densities of potassium conductances, mostly likely the fast-activating IA in GnRH dendrites. Moreover, if GnRH dendrites have enriched IA expression, the modulations of estradiol on IA channels (see above) may well be exerted on GnRH dendrites and somata.
The Ih current is a nonspecific cation current that utilizes sodium and potassium ions as current carriers. It is activated by hyperpolarization and inactivated by depolarization. It can serve as an important component of pacemaking in some neurons with primarily fast oscillations on the millisecond time scale (like cortical neurons).60 At resting membrane potentials, Ih can reduce/offset local elevations in membrane resistance (Rm) due to its opening and closing. These local changes in Rm would have substantial implications for activity in the thin distal dendrites of GnRH neurons. Moreover, because GnRH neurons have been reported in some cases to exhibit periodic activity,3,49,61-63 one might reasonably assume that Ih would be a dominant current. In the early study of Sim et al. (2000)7 only about 5% of GnRH neurons exhibited the inward rectification consistent with an Ih current. In contrast, later studies17,34,64 suggested that as many as half of GnRH neurons express Ih. An evaluation of the expression of Ih in GnRH dendrites may reconcile these differences.
Interaction of Ionic Channels and Synaptic Inputs
The GnRH neurons express a host of post-synaptic receptors that allow them to integrate multiple signals about internal homeostasis and the external environment.57 In response to these signals, activation of intrinsic conductances can shape the overall response of the GnRH cell. One of the most intriguing issues is how dendrites might process multiple and sometimes conflicting signals. In other neurons with elaborate dendritic arbors, individual branches within the same neuron function as the smallest unit of dendritic integration.58,59 Because segments of branches have unique ionic channel expression, even the same synaptic signal can be processed quite differently depending on the location of the synapse on the dendrite and the filtering properties of that dendritic segment. Segments of the exceedingly long GnRH dendrite have different passive filtering properties due to their morphology.12,60 However, the majority of GnRH neuron dendrites examined to date express only modest branching; those that project into the oranum vasculosum of the lamina terminalis (OVLT) being an exception.53 Thus, some of the tactics of most neurons would not be well-suited for the long, relatively unbranched dendrite of GnRH neurons.
The most common of experimental approaches used in other neurons to characterize interactions between incoming synaptic signals and intrinsic conductances (i.e., whole-cell recordings) are largely intractable in the very thin GnRH neuron dendrites. To provide insight into dendritic integration in GnRH dendrites, multi-compartmental models of GnRH neurons have been useful.46,60,61
Glutamate and GABA are key neurotransmitters throughout the brain and the neuroendocrine hypothalamus is no exception. Each bind to specific ligand-gated channels in GnRH neurons. As described above, GABA causes the opening of the GABAA chloride channel in the GnRH neurons. Glutamate can bind to the ionotropic glutamate receptors, NMDA and AMPA, which are non-specific cation channels. Multiple studies in a variety of species have provided compelling evidence that glutamate provides a key excitatory drive to GnRH neurons.62 However, GABA can be excitatory or inhibitory in GnRH neurons.36 The latter circumstance adds an additional layer of complexity for processing in GnRH neurons. Both glutamate and GABA have linear current-voltage relationships, thus making them quite amenable for computational modeling approaches.
Using compartmental models of GnRH neurons, one can examine the filtering properties of GnRH dendrites60 (Fig. 2). Part of the power of this approach is that one can distribute synaptic inputs along the simulated dendrite. When simulated synaptic inputs are applied along the length of the model GnRH neurons’ dendrite, the induced action potential frequency is reduced as synapses are placed in increasingly distal positions. This occurs in the dendrite shaft (in black line in Fig. 2) of a GnRH neuron with a branched dendrite (dark gray and dark, dashed gray lines) and along the dendrite of the classic bipolar GnRH neuron with a non-branching dendrite (light gray trace; Fig. 2). Since these model neurons do not contain simulated voltage-gated sodium channels in their dendrites, the response represents the impact of passive filtering by the plasma membrane.
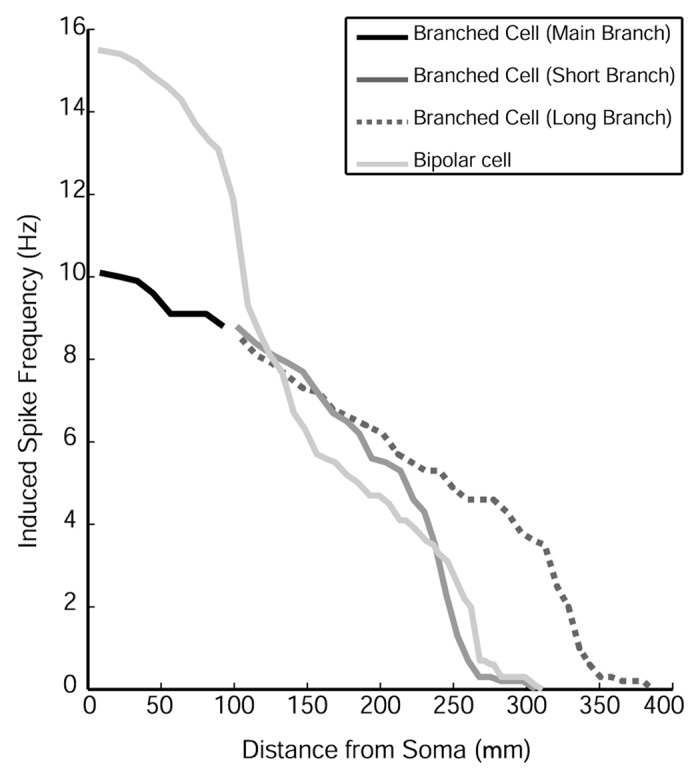
Figure 2. Spike frequency and dendritic location. The application of simulated AMPA conductances results in robust action potentials until about 150 µm of dendrite length. Beyond this most proximal portion of the GnRH dendrite, the passive filtering of synaptic input prevents generation of somatic action potentials. Used with permission from the Endocrine Society for reference 60.
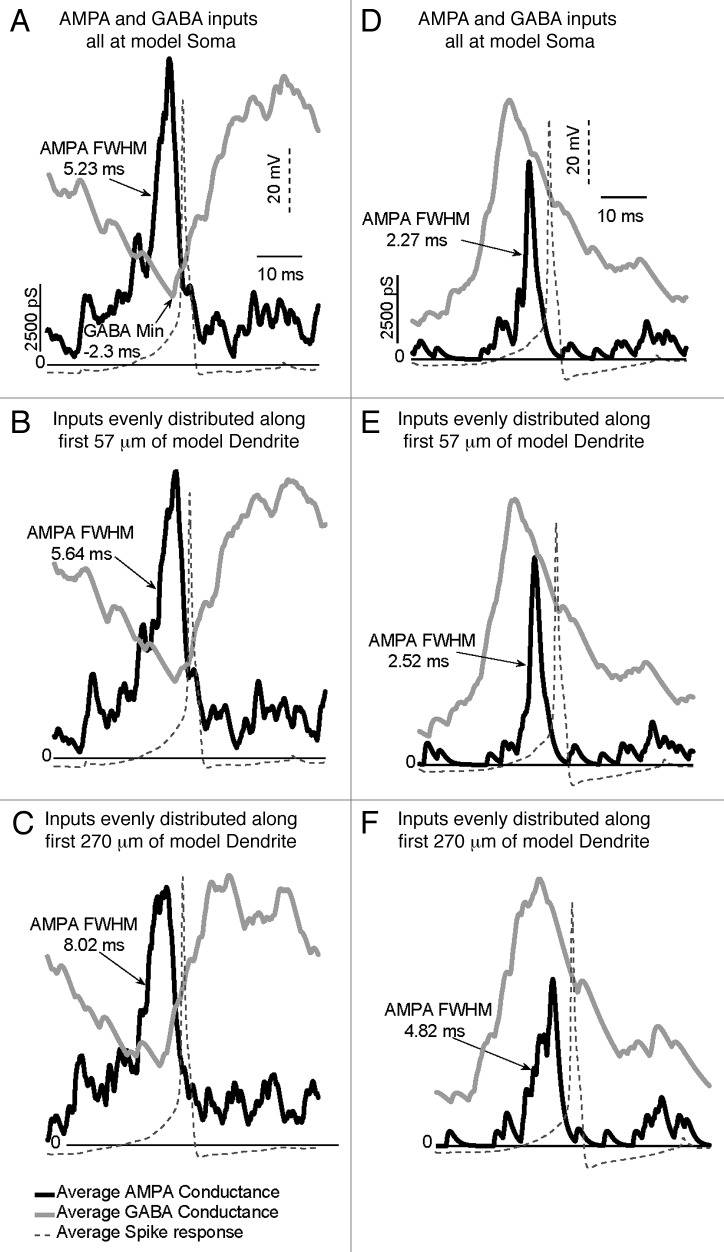
Using the above approach, one can also determine the impact of GABA in both the excitatory and inhibitory mode in the same GnRH neuron (albeit a simulated one) using a measure called spike-triggered averaging on conductance. This analysis allows one to directly relate action potentials to a particular temporal pattern and/or spatial arrangements of simulated synaptic inputs.61 As shown in Figure 3, distributing inputs along the model dendrite alters temporal aspects of synaptic integration of glutamate and GABA. Independent of whether GABA is in the inhibitory mode (panels A–C) or in the excitatory mode (panels D–F), action potentials are most effectively generated at all locations when the GABA conductance is at a nadir or declining relative to the peak AMPA conductance. As simulated synaptic inputs are distributed through increasing dendrite lengths (panels B–F), propagation delays (from site of simulated input to somata) are introduced. This can be assessed by measuring a conductance’s full width at half maximum (FWHM). Distribution of inputs along the dendrite more strongly impacts on AMPA inputs than GABA input due to the longer time course of GABA conductances. Thus, the effect of distributing inputs is to increase the time domain over which glutamatergic and GABAergic inputs can be integrated.
Figure 3. Model schematic of GnRH input and firing. Distributed inputs on model GnRH dendrites widen the temporal window for integration of AMPA-type inputs with GABA-A type inputs in GnRH somata (panels (A) and (D), proximal dendrite (panels (B) and (E) and in distal dendrite (panels (C) and (F). Panels A-C show the integration of AMPA and GABA inputs when GABA is excitatory. Panels D-F show integration of these inputs when GABA is inhibitory. The dashed wave form in all panels indicates aligned action potentials in response to the respective simulations. Distributing inputs along dendrites increases the temporal window for integration by introducing propagation delays. Used with permission from reference 61.
To study the issue of synaptic integration from the perspective of endogenous receptor activation, we have turned to photolysis of caged compounds, often termed “uncaging.”64 At the core of uncaging, is a molecule that contains a neurotransmitter “encased” in a photo-labile moiety. When the caged moiety is briefly exposed to light, its conformation changes and the functional portion of the caged compound is either released from the cage or at least exposed. It can then interact as a ligand with its receptors. Responses to a particular uncaged moiety indicate the presence of its receptor and how the neuron responds when that receptor is activated. Thus, we use can use photolysis of caged compounds to probe GnRH dendrites for their processing capabilities.
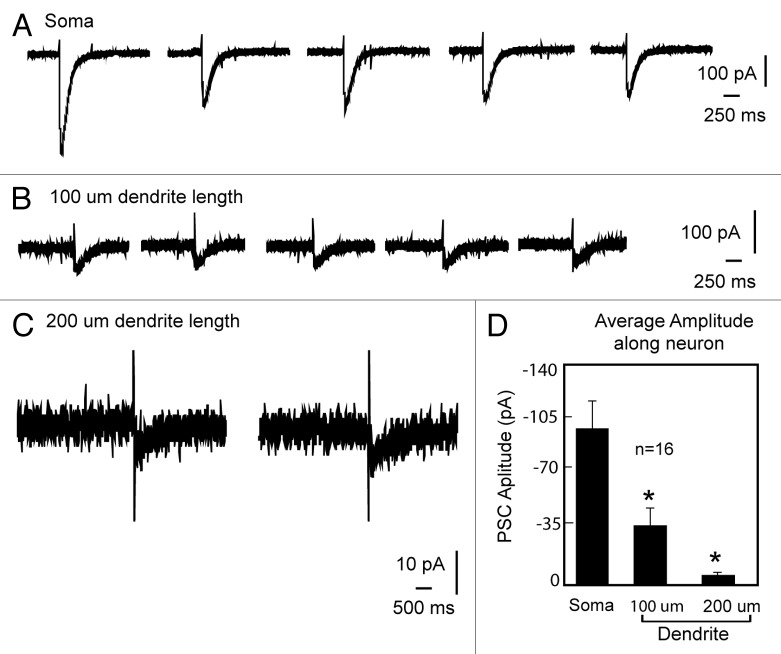
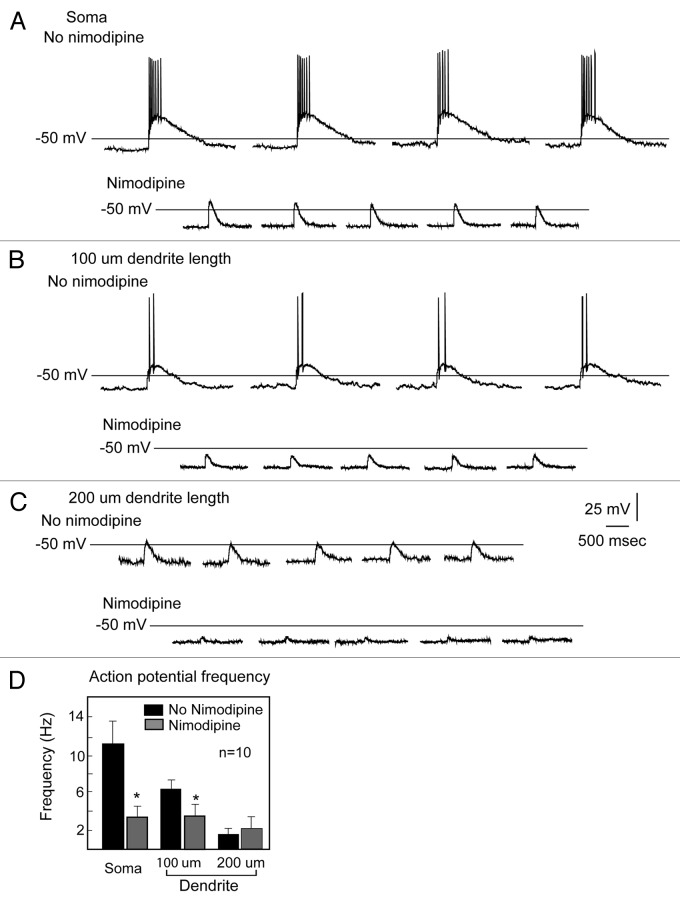
Using uncaging along the length of the GnRH dendrite, we have identified a functional microdomain composed of GABAergic receptors and L-type calcium channels.63 Previously, Constantin et al. (2010)65 demonstrated entry of calcium through L-type calcium channels when GABAA type receptors were activated. We used the release of caged GABA to activate GABAA receptors in GnRH somata and dendrites (Fig. 4). We rendered GABA excitatory by using high chloride in the pipette solution such that the reversal potential for chloride was -36.5 mV.36 Consistent with earlier observations using anatomical approaches,66 there is a decreasing response to GABA as the GnRH dendrite extends. Moreover, blocking L-type calcium channels eliminates the response to uncaging GABA (Fig. 5). Note that the post-synaptic potential in response to uncaging GABA in the soma approximates that at 200 µm of dendrite length. The increase in the GABA induced post-synaptic potential between 100 and 200 µm of dendrite length reflects a higher density of the L-type calcium channels in the distal portions of the dendrite. Thus, for their action on GnRH neurons, GABAergic inputs must engage L-type calcium channels, and the density of the intrinsic L-type calcium channels creates areas of variable sensitivity.
Figure 4. Attenuation of GABAergic PSPs and fendritic location. The GABAergic post synaptic current evoked by uncaging GABA decreases along the GnRH dendrite. Multiple trials at soma, proximal and distal dendritic locations are shown. Used with permission from 63.
Figure 5. L-type calcium channels and GABA excitation. Blocking L-type calcium channels in GnRH neurons eliminates the response to excitatory GABA. Thus, interaction with an intrinsic conductance long the length of the GnRH dendrite is required for excitatory GABA to generate action potentials. Used with permission from reference 63.
One of the hallmarks of the GnRH neuron population is the extensive range of internal and external cues that influence their activity. The active properties and unique morphology of GnRH neuron dendrites render them a multi-functional substrate on which internal and external cues can exert their signaling. Thus, understanding the physiology, morphology and behavior of GnRH neuron dendrites has moved to the forefront of the GnRH field.
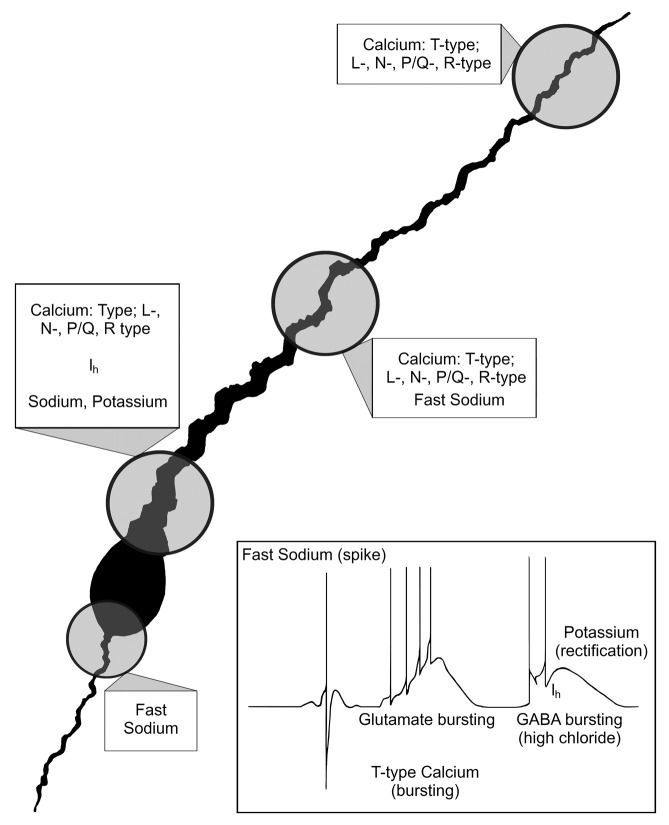
Figure 6. Morphology and corresponding physiology of GnRH neurons. Illustration summarizing the morphology and physiology of GnRH neurons. Known conductances are shown within their respective regions of the GnRH neuron. Inset summarizes the conductances observed in physiological recordings.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Supported by the Health Research Council of New Zealand and HD-045436 from The Eunice Kennedy Shriver National Institute of Child Health and Human Development. RC and KJS acknowledge the collective effort of individuals in their respective laboratories. We also acknowledge the contributions of the relatively small cadre of laboratories which study GnRH neurons using electrophysiological approaches. We thank attendees of the 2011 Gordon Conference: Dendrites: Molecules, Structure and Function for insightful discussions.
Glossary
Abbreviations:
- GnRH
gonadatropin releasing-hormone
- LH
luteinizing hormone
- FSH
follicle-stimulating hormone
- GFP
green fluorescent protein
- TTX
tetrodotoxin
- ADP
after depolarization
- ATP
adenosine triphosphate, GABA, gamma-aminobutyric acid
- Cl-
chloride
- NMDA
N-methyl-D-aspartic acid
- AMPA
2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl)-propanoic acid
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/24228
References
- 1.Goodman RL, Parfitt DB, Evans NP, Dahl GE, Karsch FJ. Endogenous opioid peptides control the amplitude and shape of gonadotropin-releasing hormone pulses in the ewe. Endocrinology. 1995;136:2412–20. doi: 10.1210/en.136.6.2412. [DOI] [PubMed] [Google Scholar]
- 2.Herbison A. Physiology of the GnRH neuronal network. In: Neill JD, ed. Knobil and Neill's Physiology of Reproduction. San Deigo, CA: Academic Press, 2006:1415-82. [Google Scholar]
- 3.Kusano K, Fueshko S, Gainer H, Wray S. Electrical and synaptic properties of embryonic luteinizing hormone-releasing hormone neurons in explant cultures. Proc Natl Acad Sci U S A. 1995;92:3918–22. doi: 10.1073/pnas.92.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spergel DJ, Krüth U, Hanley DF, Sprengel R, Seeburg PH. GABA- and glutamate-activated channels in green fluorescent protein-tagged gonadotropin-releasing hormone neurons in transgenic mice. J Neurosci. 1999;19:2037–50. doi: 10.1523/JNEUROSCI.19-06-02037.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, et al. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–9. doi: 10.1210/en.141.1.412. [DOI] [PubMed] [Google Scholar]
- 6.Suter KJ, Wuarin JP, Smith BN, Dudek FE, Moenter SM. Whole-cell recordings from preoptic/hypothalamic slices reveal burst firing in gonadotropin-releasing hormone neurons identified with green fluorescent protein in transgenic mice. Endocrinology. 2000;141:3731–6. doi: 10.1210/en.141.10.3731. [DOI] [PubMed] [Google Scholar]
- 7.Moenter SM. Identified GnRH neuron electrophysiology: a decade of study. Brain Res. 2010;1364:10–24. doi: 10.1016/j.brainres.2010.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sim JA, Skynner MJ, Herbison AE. Heterogeneity in the basic membrane properties of postnatal gonadotropin-releasing hormone neurons in the mouse. J Neurosci. 2001;21:1067–75. doi: 10.1523/JNEUROSCI.21-03-01067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutton A, Dyball RE. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979;290:433–40. doi: 10.1113/jphysiol.1979.sp012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuehl-Kovarik MC, Partin KM, Handa RJ, Dudek FE. Spike-dependent depolarizing afterpotentials contribute to endogenous bursting in gonadotropin releasing hormone neurons. Neuroscience. 2005;134:295–300. doi: 10.1016/j.neuroscience.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 11.Chu Z, Moenter SM. Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility. J Neurosci. 2006;26:11961–73. doi: 10.1523/JNEUROSCI.3171-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Herbison AE. Small-conductance calcium-activated potassium channels control excitability and firing dynamics in gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2008;149:3598–604. doi: 10.1210/en.2007-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts CB, O’Boyle MP, Suter KJ. Dendrites determine the contribution of after depolarization potentials (ADPs) to generation of repetitive action potentials in hypothalamic gonadotropin releasing-hormone (GnRH) neurons. J Comput Neurosci. 2009;26:39–53. doi: 10.1007/s10827-008-0095-5. [DOI] [PubMed] [Google Scholar]
- 14.Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci. 2009;29:5616–27. doi: 10.1523/JNEUROSCI.0352-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Kuehl-Kovarik MC. Estradiol directly attenuates sodium currents and depolarizing afterpotentials in isolated gonadotropin-releasing hormone neurons. Brain Res. 2012;1436:81–91. doi: 10.1016/j.brainres.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Garro M, Kuehl-Kovarik MC. Estradiol attenuates multiple tetrodotoxin-sensitive sodium currents in isolated gonadotropin-releasing hormone neurons. Brain Res. 2010;1345:137–45. doi: 10.1016/j.brainres.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28:4423–34. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30:713–43. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeFazio RA, Moenter SM. Estradiol feedback alters potassium currents and firing properties of gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16:2255–65. doi: 10.1210/me.2002-0155. [DOI] [PubMed] [Google Scholar]
- 20.Xu C, Roepke TA, Zhang C, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone (GnRH) activates the m-current in GnRH neurons: an autoregulatory negative feedback mechanism? Endocrinology. 2008;149:2459–66. doi: 10.1210/en.2007-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly MJ, Rønnekleiv OK, Ibrahim N, Lagrange AH, Wagner EJ. Estrogen modulation of K(+) channel activity in hypothalamic neurons involved in the control of the reproductive axis. Steroids. 2002;67:447–56. doi: 10.1016/S0039-128X(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 22.Kelly MJ, Qiu J, Rønnekleiv OK. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann N Y Acad Sci. 2003;1007:6–16. doi: 10.1196/annals.1286.001. [DOI] [PubMed] [Google Scholar]
- 23.Bosch MA, Kelly MJ, Rønnekleiv OK. Distribution, neuronal colocalization, and 17beta-E2 modulation of small conductance calcium-activated K(+) channel (SK3) mRNA in the guinea pig brain. Endocrinology. 2002;143:1097–107. doi: 10.1210/en.143.3.1097. [DOI] [PubMed] [Google Scholar]
- 24.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–30. doi: 10.1210/er.19.3.302. [DOI] [PubMed] [Google Scholar]
- 25.Pielecka-Fortuna J, DeFazio RA, Moenter SM. Voltage-gated potassium currents are targets of diurnal changes in estradiol feedback regulation and kisspeptin action on gonadotropin-releasing hormone neurons in mice. Biol Reprod. 2011;85:987–95. doi: 10.1095/biolreprod.111.093492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci. 2007;27:10153–64. doi: 10.1523/JNEUROSCI.1657-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wade GN, Jones JE. Neuroendocrinology of nutritional infertility. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1277–96. doi: 10.1152/ajpregu.00475.2004. [DOI] [PubMed] [Google Scholar]
- 28.Kato M, Tanaka N, Usui S, Sakuma Y. The SK channel blocker apamin inhibits slow afterhyperpolarization currents in rat gonadotropin-releasing hormone neurones. J Physiol. 2006;574:431–42. doi: 10.1113/jphysiol.2006.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee K, Duan W, Sneyd J, Herbison AE. Two slow calcium-activated afterhyperpolarization currents control burst firing dynamics in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30:6214–24. doi: 10.1523/JNEUROSCI.6156-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiraizumi Y, Nishimura I, Ishii H, Tanaka N, Takeshita T, Sakuma Y, et al. Rat GnRH neurons exhibit large conductance voltage- and Ca2+-Activated K+ (BK) currents and express BK channel mRNAs. J Physiol Sci. 2008;58:21–9. doi: 10.2170/physiolsci.RP013207. [DOI] [PubMed] [Google Scholar]
- 31.Catterall WA. Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunemaker CS, DeFazio RA, Moenter SM. Calcium current subtypes in GnRH neurons. Biol Reprod. 2003;69:1914–22. doi: 10.1095/biolreprod.103.019265. [DOI] [PubMed] [Google Scholar]
- 33.Kato M, Ui-Tei K, Watanabe M, Sakuma Y. Characterization of voltage-gated calcium currents in gonadotropin-releasing hormone neurons tagged with green fluorescent protein in rats. Endocrinology. 2003;144:5118–25. doi: 10.1210/en.2003-0213. [DOI] [PubMed] [Google Scholar]
- 34.Spergel DJ. Calcium and small-conductance calcium-activated potassium channels in gonadotropin-releasing hormone neurons before, during, and after puberty. Endocrinology. 2007;148:2383–90. doi: 10.1210/en.2006-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 17Beta-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2009;29:10552–62. doi: 10.1523/JNEUROSCI.2962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–68. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 37.Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011;23:557–69. doi: 10.1111/j.1365-2826.2011.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han SK, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology. 2002;143:1459–66. doi: 10.1210/en.143.4.1459. [DOI] [PubMed] [Google Scholar]
- 39.DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16:2872–91. doi: 10.1210/me.2002-0163. [DOI] [PubMed] [Google Scholar]
- 40.Lee K, Porteous R, Campbell RE, Lüscher B, Herbison AE. Knockdown of GABA(A) receptor signaling in GnRH neurons has minimal effects upon fertility. Endocrinology. 2010;151:4428–36. doi: 10.1210/en.2010-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook EP, Johnston D. Voltage-dependent properties of dendrites that eliminate location-dependent variability of synaptic input. J Neurophysiol. 1999;81:535–43. doi: 10.1152/jn.1999.81.2.535. [DOI] [PubMed] [Google Scholar]
- 42.Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature. 2000;404:285–9. doi: 10.1038/35005094. [DOI] [PubMed] [Google Scholar]
- 43.Williams SR, Stuart GJ. Voltage- and site-dependent control of the somatic impact of dendritic IPSPs. J Neurosci. 2003;23:7358–67. doi: 10.1523/JNEUROSCI.23-19-07358.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Häusser M, Mel B. Dendrites: bug or feature? Curr Opin Neurobiol. 2003;13:372–83. doi: 10.1016/S0959-4388(03)00075-8. [DOI] [PubMed] [Google Scholar]
- 45.Spruston N, Kath WL. Dendritic arithmetic. Nat Neurosci. 2004;7:567–9. doi: 10.1038/nn0604-567. [DOI] [PubMed] [Google Scholar]
- 46.Gulledge AT, Kampa BM, Stuart GJ. Synaptic integration in dendritic trees. J Neurobiol. 2005;64:75–90. doi: 10.1002/neu.20144. [DOI] [PubMed] [Google Scholar]
- 47.Migliore M, Shepherd GM. Emerging rules for the distributions of active dendritic conductances. Nat Rev Neurosci. 2002;3:362–70. doi: 10.1038/nrn810. [DOI] [PubMed] [Google Scholar]
- 48.Lüthi A, McCormick DA. H-current: properties of a neuronal and network pacemaker. Neuron. 1998;21:9–12. doi: 10.1016/S0896-6273(00)80509-7. [DOI] [PubMed] [Google Scholar]
- 49.Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM. Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J Neurosci. 2002;22:2313–22. doi: 10.1523/JNEUROSCI.22-06-02313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell RE, Han SK, Herbison AE. Biocytin filling of adult gonadotropin-releasing hormone neurons in situ reveals extensive, spiny, dendritic processes. Endocrinology. 2005;146:1163–9. doi: 10.1210/en.2004-1369. [DOI] [PubMed] [Google Scholar]
- 51.Roberts CB, Best JA, Suter KJ. Dendritic processing of excitatory synaptic input in hypothalamic gonadotropin releasing-hormone neurons. Endocrinology. 2006;147:1545–55. doi: 10.1210/en.2005-1350. [DOI] [PubMed] [Google Scholar]
- 52.Campbell RE, Gaidamaka G, Han SK, Herbison AE. Dendro-dendritic bundling and shared synapses between gonadotropin-releasing hormone neurons. Proc Natl Acad Sci U S A. 2009;106:10835–40. doi: 10.1073/pnas.0903463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herde MK, Geist K, Campbell RE, Herbison AE. Gonadotropin-releasing hormone neurons extend complex highly branched dendritic trees outside the blood-brain barrier. Endocrinology. 2011;152:3832–41. doi: 10.1210/en.2011-1228. [DOI] [PubMed] [Google Scholar]
- 54.Roberts CB, Campbell RE, Herbison AE, Suter KJ. Dendritic action potential initiation in hypothalamic gonadotropin-releasing hormone neurons. Endocrinology. 2008;149:3355–60. doi: 10.1210/en.2008-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iremonger KJ, Herbison AE. Initiation and propagation of action potentials in gonadotropin-releasing hormone neuron dendrites. J Neurosci. 2012;32:151–8. doi: 10.1523/JNEUROSCI.3739-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Häusser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–44. doi: 10.1126/science.290.5492.739. [DOI] [PubMed] [Google Scholar]
- 57.Hoffman DA, Magee JC, Colbert CM, Johnston DK. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–75. doi: 10.1038/42571. [DOI] [PubMed] [Google Scholar]
- 58.Colbert CM, Magee JC, Hoffman DA, Johnston D. Slow recovery from inactivation of Na+ channels underlies the activity-dependent attenuation of dendritic action potentials in hippocampal CA1 pyramidal neurons. J Neurosci. 1997;17:6512–21. doi: 10.1523/JNEUROSCI.17-17-06512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jung HY, Mickus T, Spruston N. Prolonged sodium channel inactivation contributes to dendritic action potential attenuation in hippocampal pyramidal neurons. J Neurosci. 1997;17:6639–46. doi: 10.1523/JNEUROSCI.17-17-06639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dickson CT, Magistretti J, Shalinsky MH, Fransén E, Hasselmo ME, Alonso A. Properties and role of I(h) in the pacing of subthreshold oscillations in entorhinal cortex layer II neurons. J Neurophysiol. 2000;83:2562–79. doi: 10.1152/jn.2000.83.5.2562. [DOI] [PubMed] [Google Scholar]
- 61.Nunemaker CS, DeFazio RA, Moenter SM. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology. 2002;143:2284–92. doi: 10.1210/en.143.6.2284. [DOI] [PubMed] [Google Scholar]
- 62.Gay VL, Hemond PJ, Schmidt D, O’Boyle MP, Hemond Z, Best J, et al. Hormone secretion in transgenic rats and electrophysiological activity in their gonadotropin releasing-hormone neurons. Am J Physiol Endocrinol Metab. 2012;303:E243–52. doi: 10.1152/ajpendo.00157.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelly MJ, Wagner EJ. GnRH neurons and episodic bursting activity. Trends Endocrinol Metab. 2002;13:409–10. doi: 10.1016/S1043-2760(02)00698-7. [DOI] [PubMed] [Google Scholar]
- 64.Chu Z, Takagi H, Moenter SM. Hyperpolarization-activated currents in gonadotropin-releasing hormone (GnRH) neurons contribute to intrinsic excitability and are regulated by gonadal steroid feedback. J Neurosci. 2010;30:13373–83. doi: 10.1523/JNEUROSCI.1687-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Constantin S, Jasoni CL, Wadas B, Herbison AE. Gamma-aminobutyric acid and glutamate differentially regulate intracellular calcium concentrations in mouse gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:262–70. doi: 10.1210/en.2009-0817. [DOI] [PubMed] [Google Scholar]
- 66.Cottrell EC, Campbell RE, Han SK, Herbison AE. Postnatal remodeling of dendritic structure and spine density in gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:3652–61. doi: 10.1210/en.2006-0296. [DOI] [PubMed] [Google Scholar]