Abstract
Epilepsy is a brain disorder characterized by seizures and convulsions. The basis of epilepsy is an increase in neuronal excitability that, in some cases, may be caused by functional defects in neuronal voltage gated sodium channels, Nav1.1 and Nav1.2. The effects of antiepileptic drugs (AEDs) as effective therapies for epilepsy have been characterized by extensive research. Most of the classic AEDs targeting Nav share a common mechanism of action by stabilizing the channel’s fast-inactivated state. In contrast, novel AEDs, such as lacosamide, stabilize the slow-inactivated state in neuronal Nav1.1 and Nav1.7 isoforms. This paper reviews the different mechanisms by which this stabilization occurs to determine new methods for treatment.
Keywords: VGSC, epilepsy, anticonvulsants, AEDs, hyperexcitability, steady-state slow inactivation, steady-state fast inactivation
Introduction
There has been much research into the effects of anticonvulsants as effective therapies for epilepsy, a brain disorder characterized by epileptic seizures. This disorder is often linked to voltage-gated sodium channel (VGSC) channelopathies, with approximately 200 mutations in patients with epilepsy.1-3 Research into VGSCs as a therapeutic target is necessary due to the channel’s importance in the initiation and propagation of electrical signals in excitable cells. The α subunit is the core protein of the channel. It can exist in several different isoforms that localize in different tissues. The main neuronal VGSC subtypes are Nav1.1, Nav1.2 and Nav1.6.3-5 Epilepsy is characterized by an increase in neuronal excitability that may be caused by changes in the voltage-dependent properties of the VGSC. A variety of anticonvulsants are used for the treatment of epilepsy caused by malfunctioning VGSCs. Experimentally, these drugs have been shown to have similar mechanism of action: They tend to stabilize the fast-inactivated state of the channel.4,6-8 However, novel anticonvulsants, such as lacosamide, effectively stabilize the slow-inactivation state.4,9,10 Despite numerous studies, questions still remain about the mechanisms of action of novel anticonvulsants and their relationship with the variety of epileptic syndromes.
Epilepsy: A Neurological Disorder Related to VGSC
Epilepsy is a broad term encompassing a variety of conditions and syndromes originating from different causes. This review will specifically focus on the role of mutations in the VGSC and how those underlie certain forms of epilepsy. It is necessary to understand the mechanisms of alteration in the biophysical properties to determine how anticonvulsants are used to treat voltage-gated sodium channelopathies.
Genetics and clinical phenotypes
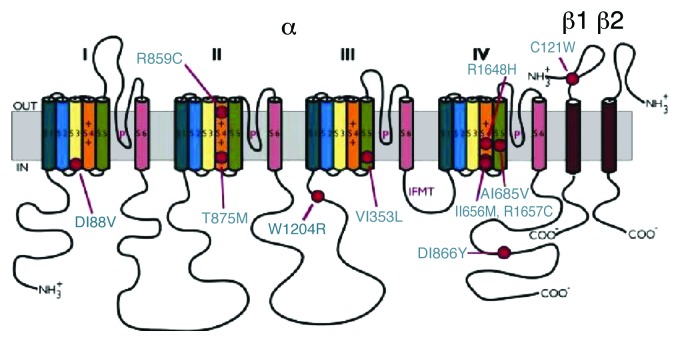
Genetic mutations related to the development of epilepsy were discovered mostly in the SCN1A gene encoding the Nav1.1 core protein.2,3,11,12 More than 500 mutations were found in the SCN1A relating to a variety of epileptic syndromes.12 Several mutations in SCN2A gene encoding the Nav1.2 isoform have also been reported.2 A few mutations exist in other VGSC forming genes suggesting that epileptic syndromes have multiple origins.2,5,13 Epilepsy patients are usually heterozygous for the mutant channel.2,11,13 Some of the mutations of SCN1A gene are shown in Figure 1. Many of these mutations are missense where there is a single amino acid substitution. This includes T875M and R1648H,2,11 which give rise to the epileptic syndrome known as generalized epilepsy with febrile seizures plus (GEFS+). GEFS+ is one of the mildest forms of epilepsy wherein seizures occur at elevated body temperature.1,3,5,13,14 Generalized epilepsy is a result of electrical excitation in both hemispheres of the brain as opposed to partial seizures, which originate from discrete areas across the cerebral cortex.15
Figure 1. Mutations in voltage-gated sodium channels are associated with epilepsy. More than 100 mutations alone appear to be involved with VGSC channelopathies and give rise to the GEFS+ syndrome. This figure gives reference to a few of the mutations causing Generalized Epilepsy Febrile Seizures + (GEFS+) indicated by red circles. Auxillary subunits, which contain IgG domains (transmembrane), form associations with the main core protein of the channel. Mutations leading GEFS+ can also be found in subunits like β1 subunits shown in the figure (for a review see ref. 11). Image from reference 11.
The same phenotype (GEFS+) may also originate from a mutation in the auxiliary sodium channel β1 subunit. β1 subunits are associated with cell adhesion and membrane trafficking, they modulate biophysical properties of VGSC and may also have a thermoprotective role.5,14,16
The core α subunit of the Nav channel is usually associated with one or more of the auxiliary subunits β1, β2, β3 and β4 by covalent or non-covalent bonds. The β2 subunit is covalently linked to the α subunit via a disulfide bridge. β1 associates with the α subunit via non-covalent interactions.7 β1 and β2 enhance the kinetics of fast-inactivation when co-expressed with the α core protein.17 Studies conducted by Tammaro et al. (2002) showed that co-expression of wild-type β1 enhances onset kinetics of fast-inactivation as opposed to the mutant C121W β1 subunit. Due to the enhanced kinetics, the fast-inactivation during an action potential is more complete resulting in decreased persistent sodium current. Since more channels enter into fast-inactivation with the co-expression of the β1 subunit, there is a notable hyperpolarized shift in the steady-state voltage dependence of fast-inactivation.7,17 The β1 subunit mutation C121W disrupts the normal disulfide bridge formed between residues 121 and 21. Coexpression with the β1 C121W alters the biophysical properties of the channel protein complex.5,13,14,16 Egri et al. (2012) recently demonstrated temperature-dependent effects of the C121W mutation that may contribute to the pathophysiology of GEFS+.
Another type of epileptic syndrome genetically based in VGSC is severe myoclonic epilepsy of infancy (SMEI). The SMEI mutations are often nonsense or frameshifts in the SCN1A gene resulting in non-functional channels.2,18
Non VGSC related mechanisms that contribute to the increase in neuronal excitability include enhancements of the actions of glutamate, a common CNS neurotransmitter, which is secreted from pre-synaptic neurons into the synaptic clefts at larger amounts in some syndromes of epilepsy.19 The increased concentration causes more neurotransmitters to bind to their glutamatergic receptors in the post synaptic neurons. As a result, Ca2+ ions diffuse down their electrochemical gradients into the neurons causing an ever greater depolarization in the membrane. In comparison, mutations may cause a defect in the actions of GABA, a common brain inhibitory neurotransmitter, which normally brings the membrane potential to hyperpolarized potentials.19 In these types of epilepsy, GABA is ineffective, thus causing post-synaptic neurons to be depolarized. These two counteracting mechanisms are referred to as gain and loss of function, in which excitatory pathways are stimulated and inhibitory pathways are inhibited, respectively.2
Mechanisms of hyper-excitability
Many mutations in the VGSC alter the biophysical properties of the channel in a similar manner. As with all epileptic seizures, these mutations result in neuronal hyper-excitability.2,13,20
The VGSC is composed of four homologous domains that contain six transmembrane segments. Transmembrane segments S1-S4 helices are known as the voltage sensing domain. Its function is to elicit conformational alterations during depolarization and repolarization phases. The S4 helices can translate upward or downward depending on the polarity of the electric field thus causing channel activation or deactivation.1,18,21 This voltage sensing property is due to conserved positively charged amino acid residues in each third position in the S4 segments.1,3,4,7,18,20,22-24 The conformational change generated by translation of these segments is transmitted to the activation gate within the S5-S6 pore segment.7,23 The S6 segment is then displaced laterally, opening the central pore for the conduction of Na current.23
Voltage-gated sodium channels activate and start to conduct Na current in a voltage and time dependent manner. The counteracting process where Na entry ceases is known as inactivation and occurs in a time-dependent manner.25 The intracellular loop linking Domains III and IV contains the IFMT (isoleucine-phenylalanine-methionine-threonine) motif, which is vital for the fast-inactivation process. Ragsdale et al. (1998) showed that adding or cleaving residues to/from this motif can contribute to the inability of the channel to enter the fast-inactivated state. As the Na current rises rapidly during depolarization, the fast-inactivation process takes place in which the IFMT motif structure flips to occlude the pore.1,7,18,20,24,26 In the brain, VGSC can cycle through this process in a few milliseconds, thus being able to sustain high frequency trains of action potentials.1 Another mechanism of inactivation is known as slow-inactivation in which channels inactivate at slow time constant extending to a few seconds. However, the conformational mechanism by which this inactivation occurs still remains obscure.17,24,27
In epilepsy, one of the common mechanisms of neuronal hyper-excitability is the inability to inactivate quickly. A portion of the Na channels is left in a conducting state resulting in the late sodium currents.3,7,20 Consequently, the threshold for depolarization is lowered and more easily attained so a subsequent depolarizing stimulus is more likely to activate sodium channels.
Another mechanism underlying neuronal hyper-excitability, as seen in the D188V and R1648H mutations in the SCN1A gene, is a rapid recovery from fast-inactivation.2,5 With increased recovery, more channels are available to be activated upon subsequent depolarization, thus contributing to an increase in neuronal excitability.
Contribution of the mutant β1C121W to hyper-excitability
The C121W mutation in the β1 subunit causes GEFS+ syndrome through similar mechanisms of excitability.5,14 Recently, Egri et al. (2012) found that upon co-expression with β1 C121W, the voltage-dependence of Nav1.2 activation is significantly altered at high temperatures (34°C) as observed by hyperpolarized shifts in the conductance curve compared with room temperature (22°C). β1C121W also acts to destabilize the fast-inactivated state as seen by a depolarized shift in the fast-inactivation curve relative to the wild-type β subunit. Nevertheless, the kinetics of recovery from fast-inactivation is enhanced with both mutated and wild-type forms at elevated temperature. The destabilization of fast-inactivation is also observable in the use-dependence protocol as there is a significant reduction in the use-dependence at elevated temperatures. The mean UDI asymptote is greater than 0.6 (more than 60% of channels are available for activation) at 34°C compared with only 30% channel availability at 22°C. This result suggests that a significantly greater proportion of channels are available to be activated at higher temperatures, supporting the hyperexcitability of neuronal tissues. Additionally, Egri et al. (2012) observed a higher proportion of β1C121W-coexpressed channels remaining in a non-inactivated state at elevated temperatures; thus an increase in the persistent sodium current. These and other results support the notion of neuronal hyperexcitability accompanying epileptic seizures in patients with β1C121W mutation.
Anti-Epileptic Drugs
Voltage-gated sodium channels have been the target of many antiepileptic drugs. A variety of toxins and pharmacological modulators exert their effects by binding to different biophysical states of the VGSC. Certain states of VGSC are stabilized or destabilized depending on the effects of modulatory agents, resulting in biophysical alterations of the channel.1 The modulated receptor hypothesis formulates a link between the functional states of VGSC and the activity of drug molecules. This hypothesis postulates that anticonvulsants and related compounds, such as local anesthetics, preferentially bind to the activated or inactivated states of the channel over the closed/deactivated state.7
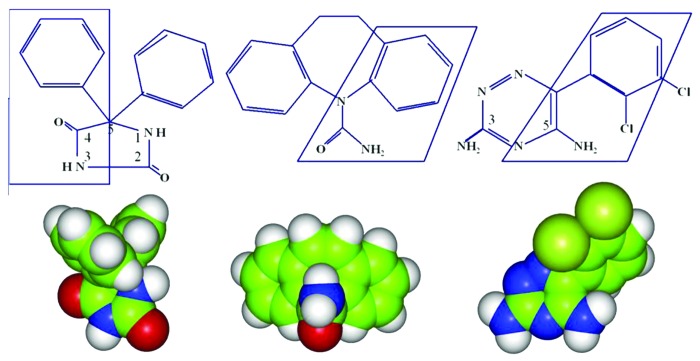
Antiepileptic drugs (anticonvulsants) are most often tricyclic compounds that share a basic structure with an amide group in the middle making the molecule both polar and rigid.28 Phenytoin, carbamazepine, lamotrigine and a variety of others play a role in VGSC modulation by blocking them from the cytoplasmic side (Fig. 2). However, there are novel anticonvulsants such as lacosamide that do not share these structural characteristics,4 suggesting different mechanisms of binding.
Figure 2. Anticonvulsants like phenytoin (left), carbamazepine (middle) and Lamotrigine (right) share common molecular structures. They are tricyclic compounds that form interactions with the inner pore of the channel, specifically in Domain IV and the S6 helix. The two biding sites that these compounds share with local anesthetics in DIV-S6 are Phe-1764 and Tyr-1771. The interactions include aromatic-aromatic interactions and aromatic-polar interactions. However there are additional interactions formed elsewhere that helps block the pore (for a review see ref. 28). Image from reference 28.
The Food and Drug Administration in the U.S. has approved 20 different anticonvulsants for treatment of epilepsy. About half of these drugs have similar mechanism of action, inhibiting VGSC by stabilization of fast inactivation: Fosphenytoin, oxcarbazepine, primidone, zonisamide, valproic acid and valproate semisodium all share this mode of action. In this review, phenytoin will be examined greatly and minor reference will be given to other anticonvulsants with similar properties. A few drugs with different modes of action, such as lacosamide and ranolazine, an antiarrhythmic, will also be considered in this review.
Phenytoin and comparable anticonvulsants
Voltage-dependent effects
Phenytoin was the first anticonvulsant used to successfully treat epileptic syndromes without having negative side effects, such as brain sedation. Ragsdale et al. (1998) have shown that phenytoin inhibits high frequency neuronal action potentials. Electrophysiological studies and site-directed mutagenesis have proven that phenytoin blocks the VGSC from the inner vestibule of the pore.3 Phenytoin binding is voltage- and frequency-dependent. At hyperpolarized membrane potentials where the channel resides in the closed/deactivated states, the binding affinity of phenytoin is low. According to Montegazza et al. (2010) at reduced membrane potentials the dissociation constant is > > 100 μM. Over a range of depolarized voltages from −80 to −30 mV the affinity of the channel to the drug rises linearly. Phenytoin preferentially binds to the activated and inactivated states as shown by a thermodynamic stabilization in fast-inactivation steady-state curves. This reduces the channel availability for the next intermittent depolarizing train.7,8 As epileptic seizures are characterized by an increased frequency of action potentials, the likelihood of finding channels available for activation in the next successive AP is highly reduced with phenytoin application.7 In addition, there are suggestions that phenytoin plays a role in inhibiting late sodium current through non-inactivated channels, which sustain depolarisations during epilepsy.
During repolarization, the drugs affinity for the channel diminishes. As channels return to the resting state, the drug starts dissociating from the channel. The drug dissociates at a rate slower than the recovery from inactivation. Thus, the likelihood of eliciting a response during the next depolarizing pulse is relatively low.7
Carbamazepine is another anticonvulsant similar to phenytoin, exhibiting a voltage- dependent and frequency-dependent block of channels.29,30 Carbamazepine binds to VGSC with 3-fold lower affinity but almost five times faster kinetics than phenytoin.3,7 As the concentration of carbamazepine increases, there is a significant reduction in the amplitude of peak current. In line with the modulated receptor hypothesis, carbamazepine binds preferentially to channels in the depolarized state.29,30
Binding sites
Local anesthetics and anticonvulsants such as phenytoin share common binding site in the VGSC. This receptor site includes residues F1764 and Y1771 in the S6 segment of Domain IV.6,28 The location of these residues in the inner vestibule of the pore indicates that these drugs bind intracellularly to the VGSC. In the open-state conformation, the C-termini of S6 in Domains I to IV form a wide opening, and the two receptor site residues face the pore. In this high affinity conformational state, phenytoin forms interactions with the two aromatic rings of the two receptor residues and thus effectively blocks the pore.28 For phenytoin, one of its aromatic rings forms a non-polar aromatic-aromatic interaction with the Y1771 residue. The other amide containing branch of phenytoin (hydantoin ring) forms a polar bond between the electron-donating π electron of the F1764 aromatic residue. An interaction also occurs between phenytoin and the residues of the IFM inactivation gate motif, contributing to the stabilization of the fast-inactivated state. Earlier Ragsdale et al. (1998) suggested that the phenylalanine residue (F1764) and the tyrosine residue (Y1771) situated in the inner vestibule of the pore form thermodynamically favorable interactions with the inactivation IFMT motif and thus stabilize the fast inactivation state.31 Site-directed mutagenesis of the receptor site residues, F1764 and Y1771, were performed by replacing these residues with alanine. These experiments show an alteration in the voltage-dependence of fast-inactivation exhibiting a destabilization in fast-inactivation.
The structure of carbamazepine is similar to phenytoin signifying the same pharmacological activity.28 Both are tricyclic compounds. Carbamazepine contains two nitrogen atoms. The same interactions that occur between the aromatic rings of phenytoin and the receptor site in sodium channel apply to carbamazepine as well: One aromatic–aromatic non-polar bond and another N-aromatic polar hydrogen bond are formed.28
Lidocaine, a Class I antiarrhythmic and a common local anesthetic, has effects similar to phenytoin by binding to the same receptor site as shown by site-directed mutagenesis at the S6 transmembrane segment to the F1764 and Y1771 residues in Domain IV.6,7,32 After the deletion of aromatic side chains, there is a decrease in the affinity of VGSC for lidocaine and a reduction in the voltage-dependent and frequency-dependent block.7 Study of Yang et al. (2010) demonstrated that lidocaine has optimal binding affinity to the activated/inactivated states of the neuronal sodium channel. This in effect proves that most antiarrhythmic and local anesthetics function to stabilize the fast-inactivated states just as most anticonvulsants do.32
Lacosamide
Lacosamide is a novel anticonvulsant that effectively treats partial seizures.9 Lacosamide stabilizes the slow-inactivated state in contrast to other anticonvulsants that exhibit their effects primarily on the fast-inactivation state.10 In a prolonged train of depolarizing stimuli, lacosamide is more effective at reducing the amplitudes and frequency of sustained repetitive firing spikes when the stimulus was prolonged to tens of seconds as opposed to less than 1 sec. This effect was even more significant as the concentration of the drug was increased from 32–100 μM.9 By contrast, older anticonvulsants, such as phenytoin, carbamazepine, and lamotrigine, exert their action over substantially shorter time scale. Another effect of lacosamide is blocking neuronal channels without any shifts in the voltage-dependent curves of activation. Additionally, lacosamide seems to have a preferential affinity to the slow inactivated state of other neuronal VGSCs, Nav 1.3 and Nav 1.7.10 Experiments conducted by Errington et al. (2008) indicate no notable change in the kinetics of fast-inactivation with the perfusion of 100 μM lacosamide. The only significant change detected in experiments of Errington et al. (2008) as well as Sheets et al. (2008) is on slow-inactivation, as lacosamide acts to stabilize this state causing a reduction in channel availability on a time scale of seconds to tens of seconds.9,10 We tested the effects of lacosamide on the mutated C121W β1 auxiliary subunit associated with GEFS+ (unpublished results). The mutant β1 subunit was transfected into a stable Nav1.2 cell line. The channels coexpressed with the wild-type and mutant β1 subunits were both compared at elevated and normal room temperature. The results obtained support the earlier synopsis that lacosamide selectively stabilizes the slow-inactivated state. We observed more significant stabilization of steady-state slow-inactivation by lacosamide at elevated temperatures (34°C) compared with normal temperature (22°C, unpublished results). Interestingly, the stabilization effect was greater for the wild-type as opposed to the mutant C121W β1 subunit. This supports the thermoprotective role of the wild-type β1 subunit in stabilizing the slow-inactivated state and indicates the importance of the β1 subunit in regulating sodium channel slow inactivation. The thermoprotective role is lost in C121W β1mutant leading to hyperexcitability associated with GEFS+ but also results in a decrease in the efficacy of the anticonvulsant, lacosamide. These results confirm the importance of auxiliary subunits β1 subunit in the interaction of the drug with the channel. Study of Uebachs et al. (2010) have shown that the absence β1 subunits associated with neuronal VGSC cause altered neuronal sensitivity to the anticonvulsant carbamazepine.33 Additionally, more effective stabilization of slow-inactivation steady-state at elevated temperatures as opposed to normal temperatures suggests that lacosamide is more potent in the physiological range of temperatures than at room temperature, which is commonly used in electrophysiological studies in heterologous expression systems.
Ranolazine
Ranolazine is an antiarrhythmic or antianginal drug used in the treatment of angina pectoris, cardiac instability, arrhythmias and reduced contractility due to an improper sodium balance within myocytes.34-36 This imbalance is the result of an increase in persistent currents in Nav 1.5 channels. Persistent currents may lead to an increase in the amplification of synaptic potentials, generation of subthreshold oscillations and facilitation of repetitive firing.3
Ranolazine has been shown to inhibit persistent late sodium currents, which may elongate the duration of action potentials, by stabilizing the inactivated state.36 Kahlig et al. (2010) tested the effects of ranolazine on epilepsy-associated Nav1.1 mutations that result in an increase in persistent current. Control data was obtained for the wild-type form of Nav1.1 where perfusion of 30 μM of ranolazine reduced persistent current by one third while causing no significant tonic block of peak current. The effect of ranolazine on R1648H, a mutation that increases persistent current leading to GEFS+, was even more pronounced (50% reduction in persistent current with 30 μM of ranolazine). Ranolazine thus leads to a decrease in sodium and calcium overload in cardiac myocytes, which contributes to a reduction in myocardial contractility during periods of ischemia.35 Ranolazine significantly acts to reduce persistent current as opposed to peak current in cardiac and neuronal sodium channels.34,36-38
There are other mutations that have been shown to contribute to an increase in the amplitude of persistent Na current in various sodium channel isoforms. Huang et al. (2011) demonstrated that Y1767C mutation in Nav1.5 cardiac channel, associated with long QT syndrome, increases persistent Na current.39 Interestingly, the tyrosine residue in this position contributes to the binding site of Class I antiarrhythmics.6,28,32 Concordantly, Huang et al. (2011) show that when this tyrosine is changed to cysteine Class I antiarrhythmics quinidine, mexiletine and flecainide have no inhibitory effect on persistent current. However, 50 μM ranolazine effectively blocks persistent current in Nav1.5 Y1767C channel. Moreover, use-dependent inactivation protocol shows enhanced efficiency of ranolazine for the Y1767C mutant compared with the wild-type Nav1.5 channel. These results suggest that there are important distinctions between binding sites of ranolazine and Class I antiarrhythmics. Ranolazine is structurally different and bulkier than many Class I antiarrhythmics. Replacement of the large aromatic group at the inner pore entrance with smaller cysteine side chain may reduce the steric hindrance experienced by ranolazine while accessing its binding site within the pore.39
Recently, Peters et al. (2013) tested the effects of ranolazine on Nav1.2.40 They observed significant stabilization of steady-state slow inactivation, faster onset of slow inactivation and delayed recovery from it in the presence of 10 or 100 μM ranolazine. Thermodynamic stabilization of the slow-inactivated state might be the most important effect. The −8mV shift in the slow inactivation curve with the addition of 10 μM (therapeutic range) suggests that there will be a significant decrease in the number of available channels over time. This conclusion is corroborated in use-dependent inactivation experiments where channel availability after 500 depolarizations decreases from 0.9 (Control) to 0.6 with perfusion of 10 μM ranolazine.40 The decrease in the number of available of channels over a long time scale suggests that ranolazine might be similar to lacosamide in its ability to stabilize the slow-inactivated state.
Summary and Future Studies
Voltage-gated sodium channels play a vital role in excitable cells, like cardiomyocytes and neurons, to generate and propagate action potentials. Functional deficits in the VGSC may alter their biophysical properties and thus alter the physiological behaviors, as in the neurological disorder of epilepsy resulting in seizures. Anticonvulsants play an important role in compensating for these channel deficits and treating epileptic conditions. Lacosamide, the novel anticonvulsant, could be tested with other mutated forms of VGSC that contribute to epileptic disorders. In addition, mechanisms of action of lacosamide should be determined in order to fully understand why they are different than the other classes of anticonvulsants. Ranolazine, although not recognized as an anti-epileptic drug, could be tested using epileptogenic mutant VGSCs. Further studies are necessary to determine whether the actions of ranolazine are similar to lacosamide or the older class of anticonvulsants and which biophysical state it alters. There also could be studies on the effects of local anesthetics with molecular structures similar to primary anticonvulsants, such as phenytoin, to determine whether they could be potential anti-epileptics.
Acknowledgments
Thanks to Dr Peter Ruben for his revisions and comments. Thanks to Colin Peters for his input.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/24380
References
- 1.Clare JJ, Tate SN, Nobbs M, Romanos MA. Voltage-gated sodium channels as therapeutic targets. Drug Discov Today. 2000;5:506–20. doi: 10.1016/S1359-6446(00)01570-1. [DOI] [PubMed] [Google Scholar]
- 2.Meisler MH, Kearney JA. Sodium channel mutations in epilepsy and other neurological disorders. J Clin Invest. 2005;115:2010–7. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010;9:413–24. doi: 10.1016/S1474-4422(10)70059-4. [DOI] [PubMed] [Google Scholar]
- 4.Lees G, Shipton E. Voltage-gated sodium channels in nociception and their potential as targets for new drugs in treatment of chronic neuropathic pain. Curr Anaesth Crit Care. 2009;20:204–8. doi: 10.1016/j.cacc.2009.06.002. [DOI] [Google Scholar]
- 5.Egri C, Vilin YY, Ruben PC. A thermoprotective role of the sodium channel β1 subunit is lost with the β1 (C121W) mutation. Epilepsia. 2012;53:494–505. doi: 10.1111/j.1528-1167.2011.03389.x. [DOI] [PubMed] [Google Scholar]
- 6.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci U S A. 1996;93:9270–5. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ragsdale DS, Avoli M. Sodium channels as molecular targets for antiepileptic drugs. Brain Res Brain Res Rev. 1998;26:16–28. doi: 10.1016/S0165-0173(97)00054-4. [DOI] [PubMed] [Google Scholar]
- 8.Karoly R, Lenkey N, Juhasz AO, Vizi ES, Mike A. Fast- or slow-inactivated state preference of Na+ channel inhibitors: a simulation and experimental study. PLoS Comput Biol. 2010;6:e1000818. doi: 10.1371/journal.pcbi.1000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Errington AC, Stöhr T, Heers C, Lees G. The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol Pharmacol. 2008;73:157–69. doi: 10.1124/mol.107.039867. [DOI] [PubMed] [Google Scholar]
- 10.Sheets PL, Heers C, Stoehr T, Cummins TR. Differential block of sensory neuronal voltage-gated sodium channels by lacosamide [(2R)-2-(acetylamino)-N-benzyl-3-methoxypropanamide], lidocaine, and carbamazepine. J Pharmacol Exp Ther. 2008;326:89–99. doi: 10.1124/jpet.107.133413. [DOI] [PubMed] [Google Scholar]
- 11.Escayg A, Goldin AL. Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia. 2010;51:1650–8. doi: 10.1111/j.1528-1167.2010.02640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thompson CH, Kahlig KM, George AL., Jr. SCN1A splice variants exhibit divergent sensitivity to commonly used antiepileptic drugs. Epilepsia. 2011;52:1000–9. doi: 10.1111/j.1528-1167.2011.03040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wimmer VC, Reid CA, Mitchell S, Richards KL, Scaf BB, Leaw BT, et al. Axon initial segment dysfunction in a mouse model of genetic epilepsy with febrile seizures plus. J Clin Invest. 2010;120:2661–71. doi: 10.1172/JCI42219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meadows LS, Malhotra J, Loukas A, Thyagarajan V, Kazen-Gillespie KA, Koopman MC, et al. Functional and biochemical analysis of a sodium channel β1 subunit mutation responsible for generalized epilepsy with febrile seizures plus type 1. J Neurosci. 2002;22:10699–709. doi: 10.1523/JNEUROSCI.22-24-10699.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krumholz A, Wiebe S, Gronseth G, Shinnar S, Levisohn P, Ting T, et al. Quality Standards Subcommittee of the American Academy of Neurology. American Epilepsy Society Practice Parameter: evaluating an apparent unprovoked first seizure in adults (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2007;69:1996–2007. doi: 10.1212/01.wnl.0000285084.93652.43. [DOI] [PubMed] [Google Scholar]
- 16.Tammaro P, Conti F, Moran O. Modulation of sodium current in mammalian cells by an epilepsy-correlated beta 1-subunit mutation. Biochem Biophys Res Commun. 2002;291:1095–101. doi: 10.1006/bbrc.2002.6570. [DOI] [PubMed] [Google Scholar]
- 17.Shcherbatko A, Ono F, Mandel G, Brehm P. Voltage-dependent sodium channel function is regulated through membrane mechanics. Biophys J. 1999;77:1945–59. doi: 10.1016/S0006-3495(99)77036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev. 2005;57:387–95. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- 19.Czapiński P, Blaszczyk B, Czuczwar SJ. Mechanisms of action of antiepileptic drugs. Curr Top Med Chem. 2005;5:3–14. doi: 10.2174/1568026053386962. [DOI] [PubMed] [Google Scholar]
- 20.Denac H, Mevissen M, Scholtysik G. Structure, function and pharmacology of voltage-gated sodium channels. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:453–79. doi: 10.1007/s002100000319. [DOI] [PubMed] [Google Scholar]
- 21.Payandeh J, Gamal El-Din TM, Scheuer T, Zheng N, Catterall WA. Crystal structure of a voltage-gated sodium channel in two potentially inactivated states. Nature. 2012;486:135–9. doi: 10.1038/nature11077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong CM, Hille B. Voltage-gated ion channels and electrical excitability. Neuron. 1998;20:371–80. doi: 10.1016/S0896-6273(00)80981-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Ren W, DeCaen P, Yan C, Tao X, Tang L, et al. Crystal structure of an orthologue of the NaChBac voltage-gated sodium channel. Nature. 2012;486:130–4. doi: 10.1038/nature11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulbricht W. Sodium channel inactivation: molecular determinants and modulation. Physiol Rev. 2005;85:1271–301. doi: 10.1152/physrev.00024.2004. [DOI] [PubMed] [Google Scholar]
- 25.ten Tusscher KHWJ, Noble D, Noble PJ, Panfilov AV. A model for human ventricular tissue. Am J Physiol Heart Circ Physiol. 2004;286:H1573–89. doi: 10.1152/ajpheart.00794.2003. [DOI] [PubMed] [Google Scholar]
- 26.Catterall WA, Raman IM, Robinson HPC, Sejnowski TJ, Paulsen O. The Hodgkin-Huxley heritage: from channels to circuits. J Neurosci. 2012;32:14064–73. doi: 10.1523/JNEUROSCI.3403-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quandt FN. Burst kinetics of sodium channels which lack fast inactivation in mouse neuroblastoma cells. J Physiol. 1987;392:563–85. doi: 10.1113/jphysiol.1987.sp016797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipkind GM, Fozzard HA. Molecular model of anticonvulsant drug binding to the voltage-gated sodium channel inner pore. Mol Pharmacol. 2010;78:631–8. doi: 10.1124/mol.110.064683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh JN, Jain G, Ramarao P, Sharma SS. Inhibition of sodium current by carbamazepine in dorsal root ganglion neurons in vitro. Indian J Physiol Pharmacol. 2009;53:147–54. [PubMed] [Google Scholar]
- 30.Willow M, Gonoi T, Catteral W. Voltage clamp analysis of the inhibitory actions of diphenyihydantoin and carbamazepine on voltage-sensitive sodium channels in neuroblastoma cells. J Mol Pharmacol. 1985;27:549–58. [PubMed] [Google Scholar]
- 31.Molnár P, Erdö SL. Vinpocetine is as potent as phenytoin to block voltage-gated Na+ channels in rat cortical neurons. Eur J Pharmacol. 1995;273:303–6. doi: 10.1016/0014-2999(94)00755-V. [DOI] [PubMed] [Google Scholar]
- 32.Yang YC, Huang CS, Kuo CC. Lidocaine, carbamazepine, and imipramine have partially overlapping binding sites and additive inhibitory effect on neuronal Na+ channels. Anesthesiology. 2010;113:160–74. doi: 10.1097/ALN.0b013e3181dc1dd6. [DOI] [PubMed] [Google Scholar]
- 33.Uebachs M, Opitz T, Royeck M, Dickhof G, Horstmann MT, Isom LL, et al. Efficacy loss of the anticonvulsant carbamazepine in mice lacking sodium channel beta subunits via paradoxical effects on persistent sodium currents. J Neurosci. 2010;30:8489–501. doi: 10.1523/JNEUROSCI.1534-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahlig KM, Lepist I, Leung K, Rajamani S, George AL. Ranolazine selectively blocks persistent current evoked by epilepsy-associated Naν1.1 mutations. Br J Pharmacol. 2010;161:1414–26. doi: 10.1111/j.1476-5381.2010.00976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belardinelli L, Shryock JC, Fraser H. Inhibition of late (sustained/persistent) sodium current: a potential drug target to reduce intracellular sodium-dependent calcium overload and its detrimental effects on cardiomyocyte function. Eur Heart J Suppl. 2004;6:I3–7. doi: 10.1016/S1520-765X(04)80002-6. [DOI] [Google Scholar]
- 36.Belardinelli L, Shryock JC, Fraser H. Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92(Suppl 4):iv6–14. doi: 10.1136/hrt.2005.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Undrovinas AI, Belardinelli L, Undrovinas NA, Sabbah HN. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S169–77. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belardinelli L, Shryock JC, Fraser H. The mechanism of ranolazine action to reduce ischemia-induced diastolic dysfunction. Eur Heart J Suppl. 2006;8:A10–3. doi: 10.1093/eurheartj/sui091. [DOI] [Google Scholar]
- 39.Huang H, Priori SG, Napolitano C, O’Leary ME, Chahine MY. Y1767C, a novel SCN5A mutation, induces a persistent Na+ current and potentiates ranolazine inhibition of Nav1.5 channels. Am J Physiol Heart Circ Physiol. 2011;300:H288–99. doi: 10.1152/ajpheart.00539.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters CH, Sokolov S, Rajamani S, Ruben PC. Effects of the antianginal drug, ranolazine, on the brain sodium channel NaV 1.2 and its modulation by extracellular protons. Br J Pharmacol. 2013 doi: 10.1111/bph.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]