Abstract
The axon/dendrite specification collapsin response mediator protein 2 (CRMP2) bidirectionally modulates N-type voltage-gated Ca2+ channels (CaV2.2). Here we demonstrate that small ubiquitin-like modifier (SUMO) protein modifies CRMP2 via the SUMO E2-conjugating enzyme Ubc9 in vivo. Removal of a SUMO conjugation site KMD in CRMP2 (K374A/M375A/D376A; CRMP2AAA) resulted in loss of SUMOylated CRMP2 without compromising neurite branching, a canonical hallmark of CRMP2 function. Increasing SUMOylation levels correlated inversely with calcium influx in sensory neurons. CRMP2 deSUMOylation by SUMO proteases SENP1 and SENP2 normalized calcium influx to those in the CRMP2AAA mutant. Thus, our results identify a novel role for SUMO modification in CRMP2/CaV2.2 signaling pathway.
Keywords: SUMO, CRMP2, Ubc9, CaV2.2, calcium influx, sensory neurons
Introduction
The multiple phosphorylated collapsin response mediator protein 2 (CRMP2) specifies axon/dendrite fate and axonal growth of neurons.1 Recent mapping of the CRMP2 interactome has, with great alacrity, revealed novel functions including regulation of microtubule dynamics, protein endocytosis and vesicle recycling, as well as synaptic assembly (reviewed in ref. 2). While extensive post-translational modifications of CRMP2, including glycosylation, oxidation, proteolysis, and phosphorylation have been reported, it is unclear as to exactly how these post-translational modifications affect CRMP2’s interactions and functional regulation of its burgeoning proteome.
Our work has identified CRMP2 as a modulator of neurotransmitter release via interactions with the presynaptic N-type voltage-gated Ca2+ channel (CaV2.2).3-5 A CRMP2/CaV2.2 complex was observed in sensory neurons4; the interaction was enhanced in an activity-dependent fashion implying dynamic regulation.3 Facile phosphorylation of CRMP2 increased its association with CaV2.26 further supporting the importance of a “tunable” interaction, especially in light of findings that disruption of the CRMP2/CaV2.2 complex in vivo reduces pain behavior in rodent models of neuropathic pain; anti-retroviral drug treatment,7-10 focal nerve demyelination,11 and persistent peripheral neuropathic pain.8,10,12 Further studies have demonstrated that interference between the CRMP2/N-Methyl-d-Aspartate receptor interaction is neuroprotective in vitro and reduces neuronal death in a moderate controlled cortical impact model of traumatic brain injury13 or against focal cerebral ischemic damage in the middle cerebral artery occlusion model.14
Here we provide evidence for further regulation of the CRMP2/CaV2.2 interaction by a hitherto unreported post-translational modification of CRMP2 by Small Ubiquitin-Like Modifier (SUMO). SUMOylation involves the covalent attachment of an ~12 kilodalton SUMO group to a lysine residue embedded within a SUMO-interaction motif (SIM) typified by the presence of large hydrophobic residue (ψ) preceding the modification site lysine and a negatively charged amino acid two residues downstream (ψ-K-X-E/D).15,16 Ubc9, the E2 SUMO-conjugating enzyme, may add one or more of the three vertebrate SUMO proteins to substrates.17 SUMOylation is a reversible process, and SUMO/sentrin-specific peptidases (SENP) 1 or SENP2 free the SUMO moiety.18 SUMOylation has been linked to alteration in the subcellular localization, activity and stability of its targeted proteins19 and is emerging as a novel regulator of neuronal function especially in control of ion channels (e.g., voltage-gated potassium channel Kv1.5,20 Kv2.1,21 the K+ leak channel K2P122,23) and receptors (e.g., the kainate receptor subunit GluR6,24 and the ionotropic glutamate receptor subunits GluR7a/b16).
We are focusing on protein-protein interactions that regulate CaV2.2—channels that are essential mediators of the neurotransmitter release pathway in nerve terminals,25,26 including those involved in pain networks.27,28 Our approach has been to identify modulators of CaV2.2 and then block these interactions to circumvent problems associated with drugs that directly block this channel. Here, we identify a putative SIM in CRMP2, removal of which has functional consequences on CRMP2’s regulation of calcium influx via CaV2.2. Thus, SUMOylation by regulating CRMP2 may ultimately “tune” CaV2.2 function in physiology and pathophysiology, especially in light of the well-established role for these channels in chronic pain.27
Results
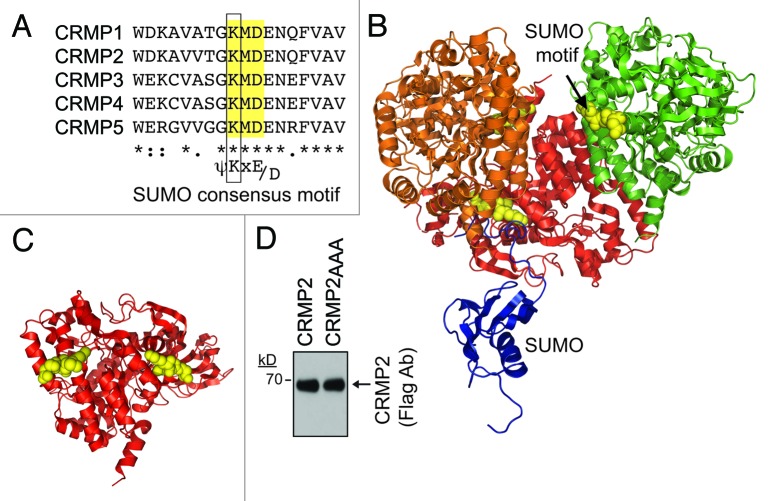
Scanning the CRMP2 sequence identified a putative SUMOylation motif conforming to the canonical ψ-K-X-E/D (where ψ is a hydrophobic residue) pattern reminiscent in some SUMO substrates.16,29 The motif, centered on Lys-374, lies on an accessible surface of each of the CRMP2 monomers with likely unhindered access to SUMO (Fig. 1A and B). Sequence comparison across vertebrate species revealed that the motif is fully conserved across human, rodent, bovine and fish species (not shown) as well as across other CRMP family members (Fig. 1C).
Figure 1. CRMP2 contains a conserved SUMOylation motif. (A) Aligned rodent CRMPs 1 to 5 sequences centered on the K374 residue (boxed) with the flanking residues Met/Asp (yellow box) conforming to the predicted consensus SUMOylation motif (shown below alignment). (B) Structural representation of CRMP2 (PDB code: 2GSE49) with the core SUMOylation motif identified in yellow spheres (arrow). For clarity, only three monomers of the CRMP2 tetramer are shown with their putative SUMOylation motifs represented in yellow spheres. The structure of SUMO-1 (PDB code: 1A5R50; blue) is also illustrated to highlight relative proximity to putative SUMOylation sites in CRMP2. (C) SUMOylation motifs of CRMP2 monomer and that from an adjoining monomer (not shown) are shown in yellow spheres. (D) Immunoblot with anti-Flag antibody from CAD lysates transfected with wild type (CRMP2) or the putative SUMOylation conjugation site mutant (CRMP2AAA) showing comparable levels of expression of the proteins.
To evaluate whether CRMP2 is SUMOylated in vivo in cells, we first created a CRMP2 construct in which the putative SUMOylation consensus motif (KMD) was replaced with alanine residues. Mutating the SUMOylated lysine to either arginine or alanine has been demonstrated to destroy the SUMOylation site for SUMO addition by Ubc930-34; thus, here we chose to mutate the K374 to alanine. We also mutated the downstream residues in the consensus motif as these can also influence SUMOylation. The generated CRMP2, designated CRMP2AAA, expressed robustly in heterologous CAD cells at levels equivalent to wild type CRMP2 (Fig. 1D). Notably, the triple alanine CRMP2 mutant did not compromise the structure/function of CRMP2 as it was able to increase neurite branching in transfected neurons compared with EGFP-transfected neurons (Fig. 2A–D) consistent with the previously reported increase in neurite/axon growth facilitated by CRMP2.1,35 Next, CAD cells were co-transfected with plasmids encoding Flag-tagged CRMP2 or CRMP2AAA in the presence of HA-tagged SUMOs 1 to 3 with or without the SUMO E2 conjugating enzyme Ubc9, then cell lysates were subjected to immunoprecipitation using antibodies against HA (to pull down SUMOylated proteins) followed by immunoblotting with Flag antibody (to detect CRMP2). The Flag antibody detected an ~67 kilodalton (kD) CRMP2 band in immunoprecipitates from cells expressing HA-tagged SUMOs 1 to 3 either alone or in the presence of Ubc9 (Fig. 3A), suggesting the presence of a tonic level of SUMOylation machinery in CAD cells. The detected SUMOylated CRMP2 was only about < 1% of the total CRMP2. In contrast, no SUMOylated CRMP2 bands were present in CAD cells transfected with CRMP2AAA in any of the transfected conditions (Fig. 3A), despite robust expression of CRMP2-Flag (Fig. 3B, lysate blot). These results are supportive of the conclusions that (1) the CRMP2 band in the presence of SUMOs and Ubc9 represented SUMOylated CRMP2 and (2) the K374 residue (along with residues 375 and 376) in CRMP2 contributed to the SUMOylation of CRMP2.
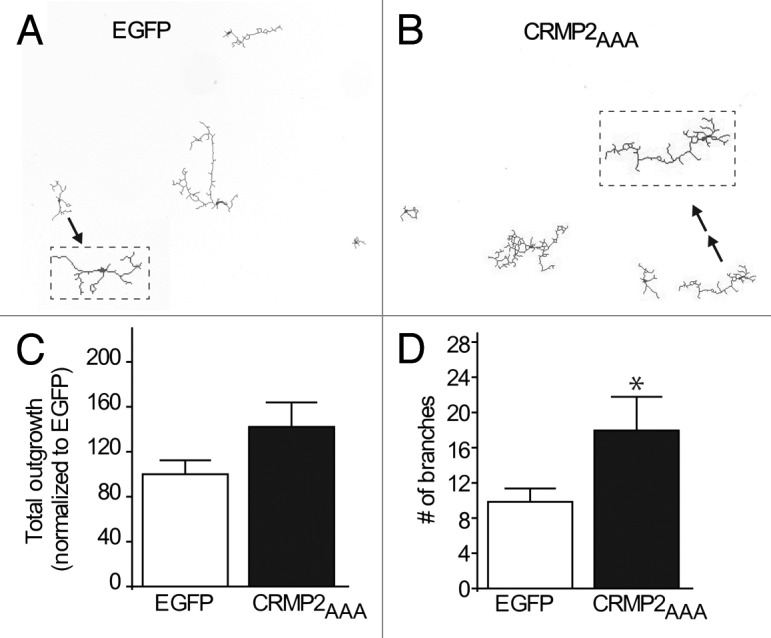
Figure 2. CRMP2AAA SUMOylation mutant increases neuronal outgrowth. Representative tracings of cortical neurons expressing EGFP (A) or CRMP2AAA + 10% EGFP (B) at 4× magnification. Tracing indicated by arrows have been expanded for better observation. (C) Total outgrowth of cells expressing either EGFP or CRMP2AAA + 10% EGFP, n = 23–39 cells. (D) Total number of branches in cells expressing EGFP or CRMP2 AAA + 10% EGFP. n = 23–39 cells. *p < 0.05, Student’s t-test vs. the EGFP condition.
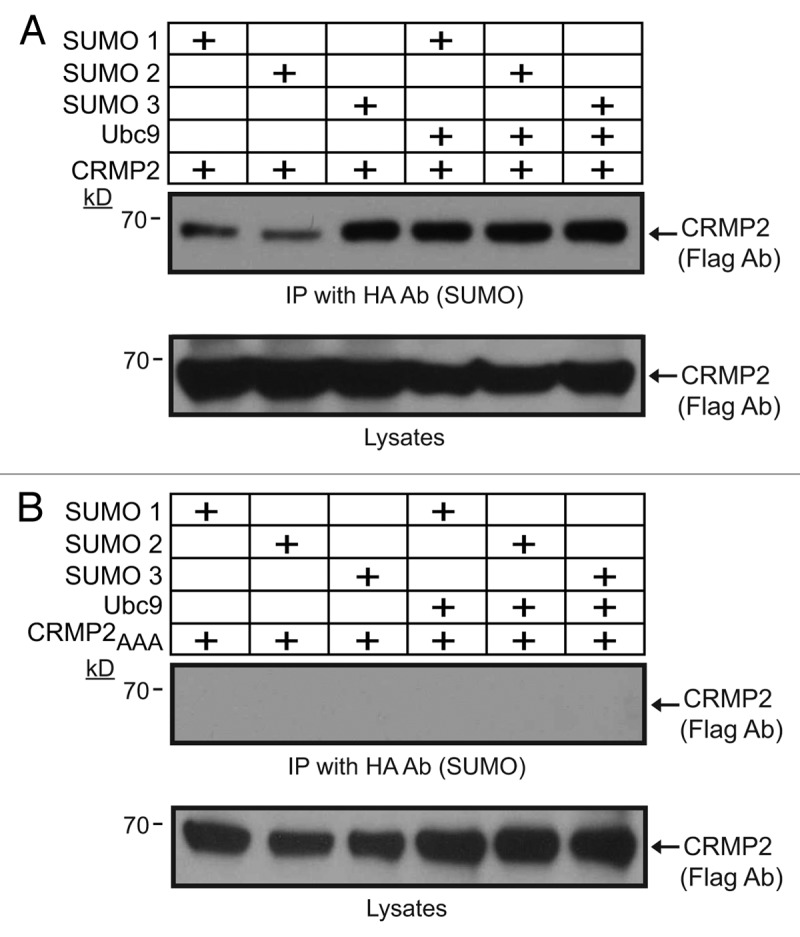
Figure 3. CRMP2 is minimally SUMOylated by multiple SUMO isoforms. CAD cells were transfected with Flag-CRMP2 (A) or putative SUMOylation conjugation site mutant Flag-CRMP2AAA (B) and 2 μg pCDNA3 Ubc9 alone or in the presence of pCDNA3 HA-SUMO1, pCDNA HA-SUMO2, or pCDNA HA-SUMO3. HA-tagged (top blots) proteins were purified and immunoblotted with an anti-Flag antibody. Lysates, representing < 1% of the input used for the immunoprecipitations, were immunoblotted with an anti-Flag antibody to reveal CRMP2. SUMOylated CRMP2 was detectable in the presence or absence of Ubc9; this represents less than < 1% of the total cellular CRMP2. Representative blots from three independent experiments are shown. Following incubation with enhanced ECL, the top blot in (A) was exposed for over an hour to film to resolve the really low signal.
We have previously shown that CRMP2 levels and phosphorylation state affect its biochemical interaction with CaV2.23,6 and regulate calcium influx in neurons.3,4,6 Thus, to investigate the role of SUMOylation in the regulation of CRMP2 functions, the effect of overexpression of SUMOs 1 to 3 and Ubc9 on calcium influx in sensory neurons was determined. Adult dorsal root ganglion (DRG) neurons were transfected with Ubc9 and each of the SUMOs plus wildtype CRMP2 or mutant CRMP2AAA and depolarization-evoked Ca2+-influx was monitored 48 h later with the Ca2+-sensitive dye Fura-2AM. Transfected cells were visualized using the red fluorescent protein DsRed (arrow, Fig. 4A). Stimulation with 90 mM KCl led to a rapid increase in the Fura-2AM ratio (Fig. 4A, 90 mM K+ panel) which is indicative of an increase in intracellular calcium concentration ([Ca2+]i). Overexpression of SUMOs/Ubc9 led to a decrease in the peak fluorescence ratios in neurons co-expressing wildtype CRMP2 and an increase in ratios co-expressing CRMP2AAA compared with dsRed-transfected cells (Fig. 4B), suggesting that (1) increasing SUMOylation decreases calcium influx and (2) decreasing CRMP2’s SUMOylation, as we earlier demonstrated in the CRMP2AAA SUMO conjugation site mutant (Fig. 3), increases calcium influx. No suppression of calcium influx was observed in the absence of Ubc9 (relative fluorescence ratio of 0.70 ± 0.04 (n = 8) for dsRed+CRMP2+SUMO 1+ Ubc9-transfected neurons vs. 0.67 ± 0.04 (n =) for dsRed+CRMP2+SUMO 1-transfected neurons). Collectively, this data supports the hypothesis for an inverse relationship between SUMOylation levels and calcium influx.
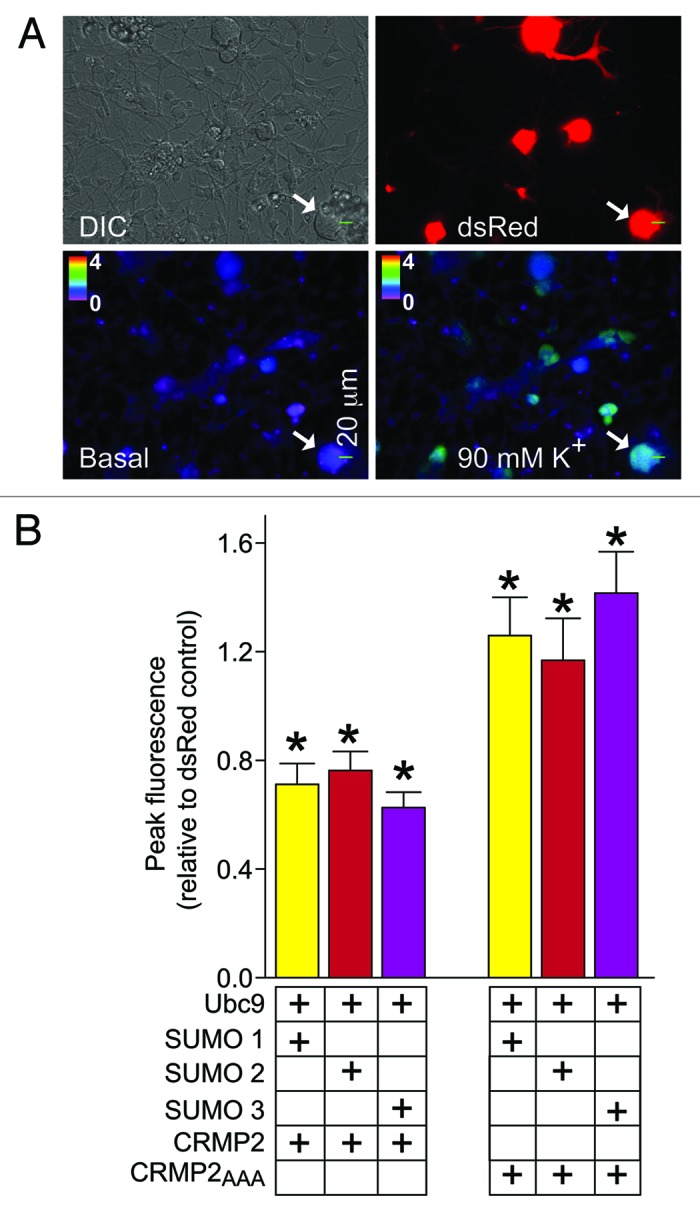
Figure 4. DeSUMOylation of CRMP2 affects depolarization-evoked Ca2+ influx in dorsal root ganglion (DRG) neurons. (A) Differential interference contrast (DIC) and pseudocolored fluorescent images of a field of DRG neurons visualized for Fura-2 a.m., before (Basal) and after stimulation with KCl (90 mM K+). Arrowhead shows a typical responding sensory neuron. Transfected cells are identified with dsRed fluorescence (arrow). (B) Ca2+ imaging was performed on adult mouse DRG neurons using the ratiometric Ca2+-sensitive dye Fura-2 a.m. Following a 1 min baseline measurement, neurons were stimulated with 90 mM KCl for 10 sec to induce Ca2+ influx. Bar graphs show the peak fluorescence response ± s.e.m. (adjusted for background and normalized to dsRed-transfection) of DRGs. Imaging was performed 48 h after transfection. Values represent the average ± SEM from four separate imaging experiments from 30 to 44 cells per condition (from at least four rats per condition). Asterisks indicate statistical significance compared with dsRed-transfected cells (p < 0.05, one-way ANOVA with Dunnett’s post-hoc test).
We further tested this hypothesis by boosting deSUMOylation in CAD cells by overexpression of the SUMO/sentrin-specific peptidases (SENP) 1 or 2 and monitoring calcium influx. DeSUMOylation increased peak calcium influx in CAD cells co-expressing wildytpe CRMP2 to levels statistically similar to cells expressing CRMP2AAA (data not shown). Calcium influx in CRMP2AAA-transfected cells was similar irrespective of presumptive increase or decrease in SUMOylation. Collectively, these findings support the notion that SUMOylation of CRMP2 at Lys-374 (as well as Met-375 and Asp-376) is an important determinant of CRMP-2’s modulation of CaV2.2 function.
Discussion
The “biochemical” CRMP2 interactome now encompasses motor proteins, kinases, channels, receptors, enzymes, and endocytosis-exocytosis related proteins supporting the notion that CRMP2 may serve as adaptors/scaffold molecules and as traffic “cops” (see review in ref. 2). We are focusing on delineating the ‘functional’ CRMP2 interactome and have demonstrated CRMP2’s modulation of CaV2.2 activity and channel trafficking as the molecular basis for its regulation of synaptic transmission.3,5-7 Our recent findings demonstrated that the cyclin dependent kinase 5 (Cdk5) phosphorylation of CRMP2 enhanced the CRMP2/CaV2.2 interaction and increased calcium influx,6 leading us to propose the concept that post-translational modifications of CRMP2 may act as “molecular switches” for CRMP2 to regulate its interactions with putative protein partners as well as controls its function to enforce select signaling cascades.
Here, we show for the first time, that SUMOylation modifies CRMP2, and that this post-translational modification alters calcium influx. Our results are entirely consistent with a recent report, which demonstrated that promoting synaptic protein SUMOylation reduces Ca2+ influx.36 Mutation of the CRMP2 SUMO conjugation site Lys-374 (as well as Met-375 and Asp-376) to an alanine leads to an elimination of SUMOylation. Importantly, our data demonstrated that calcium influx via voltage-gated calcium channels in sensory neurons, which we have shown previously to be augmented by CRMP2,3,4,6,12 is also affected by the SUMOylation state of CRMP2. Enabling enhanced deSUMOylation by introduction of SENPs or favoring CRMP2 deSUMOylation (as in the CRMP2AAA mutant lacking the SUMOylation site K374) correlates with increased calcium influx, implicating an inverse relationship between between SUMOylation of CRMP2 and calcium flux into cells. It is also possible that CaV2.2 itself is the target of SUMOylation and that channel, and not CRMP2, modification accounts for the altered calcium response. However, the first cytoplasmic loop of CaV2.2, despite the presence of three predicted SUMO motifs, does not appear to be SUMOylated.16 While the possibility that other intracellular regions of the channel may be SUMOylated remains to be tested, it is known that CaV2.2 is a target for another related modification, ubiquitination,37 which tags the channels for degradation culminating in a decrease in calcium influx. As both modifications target lysine residues, an intriguing possibility is that SUMOylation and ubiquitination may compete for the same site, adding yet another tier of regulation to this interaction. Precedence for this scenario exists as SUMOylation of the neuronal protein tau is decreased in the face of proteasome inhibition, which decreases ubiquitination.38
Very little is known about SUMO and chronic pain, although one study reported that the antidiabetic drug rosiglitazone (Avandia®), which SUMOylates the peroxisome proliferator-activated receptor leading to control of macrophages, can suppress the development of neuropathic pain activation by regulating macrophage infiltration and production of proinflammatory molecules following injury.39 Protein SUMOylation has been reported to modulate neurotransmitter release36 perhaps via N-type calcium channels that, in turn, are modulated by CRMP2. In summary, SUMO regulation of CRMP2/CaV2.2 signaling may have important implications for chronic pain because CaV2.2 channels are genetically40,41 and clinically28,42,43 validated targets for pain management. Future work will also investigate whether SUMOylation of CRMP2 affects its other functions, as well as how it regulates its other interactions.
Materials and Methods
Plasmids and antibodies
The following plasmids were from Addgene: HA-SUMO-1, HA-SUMO-2, HA-SUMO-3, HA-Ubc9, FLAG-SENP1, and FLAG-SENP2. Mutations in mouse CRMP2 cDNA (Mus musculus accession # NM_009955.3) were introduced by Quikchange II XL (Agilent Technologies)6 and cloned into Flag-epitope containing pCDNA3.1 plasmid. The introduced alanine mutations were verified by DNA sequencing. While typically arginine mutations have been used to investigate putative SUMOylation status of proteins, this is not always the case as illustrated by a study wherein the Lys to Arg mutation in the potassium leak channel K2P1 failed to increase potassium currents.44 For this reason and additional ones described in the Results section, we chose to mutate the lysine residue to an alanine.
Antibodies were purchased from the following: polyclonal CRMP2, polyclonal Flag and monoclonal HA-epitope, and control mouse and rabbit isotype-specific control IgG (Sigma).
Primary dorsal root ganglion (DRG) neuronal cultures
Sensory DRG neurons from 150–200 g Sprague Dawley rats (Harlan Labs) were isolated as described.12 All animals were housed with free access to food and water in the Indiana University Laboratory Animal Research Center and used in procedures approved by the Animal Use and Care Committee of the Indiana University School of Medicine.
Primary cortical neuron cultures, transfection and Neurite outgrowth analyses
Embryonic day 19 cortical neurons were prepared exactly as described previously.13 Briefly, cortices were dissected and cells suspensions were plated onto poly-d-lysine-coated 96-well plates. Cells were grown in Neurobasal medium containing 2% NuSerum, 5% NS21, supplemented with penicillin/streptomycin (100 U/ml; 50 µg/ml), 0.1 mM L-glutamine and 0.4 mM L-glutamax (Invitrogen). 48h after plating, cells were transfected with either EGFP or CRMP2AAA + 10% EGFP via Lipofectamine 2000 (Invitrogen). Transfections were allowed to proceed ~3 h, at which point, cells were fed with media containing 5-Fluoro-2’-deoxyuridine (1.5 µg/ml) (Sigma) to reduce the number of non-neuronal cells. At 48 h post transfection, cells were fixed with 4% paraformaldehyde (Sigma) and imaged using the ImageXpress Micro Widefield High Content Screening System (Molecular Devices). Multiple parameters involved in neurite outgrowth were examined via the neurite outgrowth application module within the MetaXpress Software. This analysis combines the following measurements: number of primary neurites, number of branches, mean process length, and maximum process length to determine a summary of total outgrowth per cell.
Culturing Catecholamine A Differentiated (CAD) cells
The neuronally derived CAD cells were grown at 37°C and in 5% CO2 as previously described.45-47 CAD cells were transfected with 1 μg/μl of polyethylenimine (PEI; Sigma-Aldrich)10 and plasmids as indicated below for the DRGs. Efficiency of CAD cell transfection was > 90% with this method.
DRG transfection
Isolated rat DRG neurons (~1.5 X 106) were transfected with 2 μg of CRMP2, CRMP2AAA, SUMOs1–3, Ubc9 or SENP1/2 cDNAs plus dsRed plasmid (0.2 μg) using the rat neuron Nucleofector™ solution (program O-003; Amaxa Biosystems) and plated on 12 mm BD BioCoat™ Poly-D-Lysine/Laminin coated glass coverslips (BD Biosciences) and maintained at 37°C and 3% CO2 in F-12 media supplemented with nerve growth factor (30 ng/ml).4,48 Under these conditions, transfection efficiencies of ~20–25% were routinely observed along with ~10–15% cell death.
Fura-2AM Ca2+ imaging of transfected DRG neurons
Fura-2AM (Invitrogen) Ca2+ imaging was performed on DRG neurons 48 h following transfection.12 A standard bath solution containing 139 mM NaCl, 3 mM KCl, 0.8 mM MgCl2, 1.8 mM CaCl2, 10 mM NaHEPES, pH 7.4, 5 mM glucose was used. Depolarization was evoked with a 10 sec pulse of 90 mM potassium chloride delivered via a Valvelink 8.1 controlled gravity-driven perfusion system (Automate Scientific). Fluorescence imaging was performed with an inverted microscope, Nikon Eclipse TE2000-U, using objective Nikon Super Fluor 20 × 0.75 numerical aperture and a Photometrics cooled CCD camera CoolSNAPHQ (Roper Scientific) controlled by MetaFluor 6.3 software (Molecular Devices).
Co-immunoprecipitation and western blotting
CAD cell lysates were generated by homogenization and gentle sonication in a detergent free modified RIPA buffer (50 mM TRIS-HCl, pH 8, 150 mM NaCl, and 1 mM EDTA with protease inhibitors plus 10 mM NEM). Immunoprecipitations were performed as before3,6,7,13 with antibodies overnight at 4°C with gentle rotation, followed by recovery of precipitates with Protein A/G-agarose for 2 h at 4°C before immunoblotting with antibodies as indicated in the Figures.
Data analysis
Data are presented as the means ± SEM. Statistical differences between calcium influx in control cells and those obtained under various transfected conditions were determined by using ANOVA with a Dunnett’s post-hoc test. Values of p < 0.05 were judged to be statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Erik Dustrude, Dr May Khanna for helpful discussions and Dr Fletcher A. White for generous use of his calcium imaging microscope. This work was supported, in part, by grants from the Indiana Clinical and Translational Sciences Institute funded, in part by a Project Development Team Grant Number (RR025761) from the National Institutes of Health (NIH), National Center for Research Resources, Clinical and Translational Sciences Award, the Indiana State Department of Health – Spinal Cord and Brain Injury Fund (A70-9-079138 to R.K.), a National Scientist Development grant from the American Heart Association (SDG5280023 to R.K.), a Neurofibromatosis New Investigator Award from the Department of Defense Congressionally Directed Military Medical Research and Development Program (NF1000099 to R.K.), and a Ralph and Grace Showalter Trust Foundation award (to R.K.). S.M.W. was partially supported by a Stark Medical Neuroscience Fellowship.
Glossary
Abbreviations:
- CaV2.2
N-type voltage-gated Ca2+ channel
- CRMP2
collapsin response mediator protein 2
- DRG
dorsal root ganglion
- DsRed
Discosoma sp. red fluorescent protein
- NEM
N-ethylmaleimide
- SIM
SUMO interaction motif
- SENP
SUMO/sentrin-specific peptidases
- SUMO
small ubiquitin-like modifier
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/24224
References
- 1.Inagaki N, Chihara K, Arimura N, Ménager C, Kawano Y, Matsuo N, et al. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4:781–2. doi: 10.1038/90476. [DOI] [PubMed] [Google Scholar]
- 2.Khanna R, Wilson SM, Brittain JM, Weimer JM, Sultana R, Butterfield AD, et al. Opening Pandors' jar: a primer on the putative roles of CRMP2 in a panoply of neurodegenerative, sensory and motor neurons, and central disorders. Future Neurol. 2012;5:749–71. doi: 10.2217/fnl.12.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brittain JM, Piekarz AD, Wang Y, Kondo T, Cummins TR, Khanna R. An atypical role for collapsin response mediator protein 2 (CRMP-2) in neurotransmitter release via interaction with presynaptic voltage-gated calcium channels. J Biol Chem. 2009;284:31375–90. doi: 10.1074/jbc.M109.009951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi XX, Schmutzler BS, Brittain JM, Wang Y, Hingtgen CM, Nicol GD, et al. Regulation of N-type voltage-gated calcium channels (Cav2.2) and transmitter release by collapsin response mediator protein-2 (CRMP-2) in sensory neurons. J Cell Sci. 2009;122:4351–62. doi: 10.1242/jcs.053280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Brittain JM, Wilson SM, Khanna R. Emerging roles of collapsin response mediator proteins (CRMPs) as regulators of voltage-gated calcium channels and synaptic transmission. Commun Integr Biol. 2010;3:1–4. doi: 10.4161/cib.3.2.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brittain JM, Wang Y, Eruvwetere O, Khanna R. Cdk5-mediated phosphorylation of CRMP-2 enhances its interaction with CaV2.2. FEBS Lett. 2012;586:3813–8. doi: 10.1016/j.febslet.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Brittain JM, Duarte DB, Wilson SM, Zhu W, Ballard C, Johnson PL, et al. Suppression of inflammatory and neuropathic pain by uncoupling CRMP-2 from the presynaptic Ca²⁺ channel complex. Nat Med. 2011;17:822–9. doi: 10.1038/nm.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piekarz AD, Due MR, Khanna M, Wang B, Ripsch MS, Wang R, et al. CRMP-2 peptide mediated decrease of high and low voltage-activated calcium channels, attenuation of nociceptor excitability, and anti-nociception in a model of AIDS therapy-induced painful peripheral neuropathy. Mol Pain. 2012;8:54. doi: 10.1186/1744-8069-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ripsch MS, Ballard CJ, Khanna M, Hurley JH, White FA, Khanna R. A PEPTIDE UNCOUPLING CRMP-2 FROM THE PRESYNAPTIC Ca(2+) CHANNEL COMPLEX DEMONSTRATES EFFICACY IN ANIMAL MODELS OF MIGRAINE AND AIDS THERAPY-INDUCED NEUROPATHY. Transl Neurosci. 2012;3:1–8. doi: 10.2478/s13380-012-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson SM, Schmutzler BS, Brittain JM, Dustrude ET, Ripsch MS, Pellman JJ, et al. Inhibition of transmitter release and attenuation of anti-retroviral-associated and tibial nerve injury-related painful peripheral neuropathy by novel synthetic Ca2+ channel peptides. J Biol Chem. 2012;287:35065–77. doi: 10.1074/jbc.M112.378695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson SM, Brittain JM, Piekarz AD, Ballard CJ, Ripsch MS, Cummins TR, et al. Further insights into the antinociceptive potential of a peptide disrupting the N-type calcium channel-CRMP-2 signaling complex. Channels (Austin) 2011;5:449–56. doi: 10.4161/chan.5.5.17363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju W, Li Q, Allette YM, Ripsch MS, White FA, Khanna R. Suppression of pain-related behavior in two distinct rodent models of peripheral neuropathy by a homopolyarginine-conjugated CRMP2 peptide. J Neurochem. 2012; 124:869–79. doi: 10.1111/jnc.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brittain JM, Chen L, Wilson SM, Brustovetsky T, Gao X, Ashpole NM, et al. Neuroprotection against traumatic brain injury by a peptide derived from the collapsin response mediator protein 2 (CRMP2) J Biol Chem. 2011;286:37778–92. doi: 10.1074/jbc.M111.255455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brittain JM, Pan R, You H, Brustovetsky T, Brustovetsky N, Zamponi GW, et al. Disruption of NMDAR-CRMP-2 signaling protects against focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Channels (Austin) 2012;6:52–9. doi: 10.4161/chan.18919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anckar J, Sistonen L. SUMO: getting it on. Biochem Soc Trans. 2007;35:1409–13. doi: 10.1042/BST0351409. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson KA, Nishimune A, Henley JM. Analysis of SUMO-1 modification of neuronal proteins containing consensus SUMOylation motifs. Neurosci Lett. 2008;436:239–44. doi: 10.1016/j.neulet.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkinson KA, Henley JM. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J. 2010;428:133–45. doi: 10.1042/BJ20100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhopadhyay D, Dasso M. Modification in reverse: the SUMO proteases. Trends Biochem Sci. 2007;32:286–95. doi: 10.1016/j.tibs.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Müller S, Hoege C, Pyrowolakis G, Jentsch S. SUMO, ubiquitin’s mysterious cousin. Nat Rev Mol Cell Biol. 2001;2:202–10. doi: 10.1038/35056591. [DOI] [PubMed] [Google Scholar]
- 20.Benson MD, Li QJ, Kieckhafer K, Dudek D, Whorton MR, Sunahara RK, et al. SUMO modification regulates inactivation of the voltage-gated potassium channel Kv1.5. Proc Natl Acad Sci U S A. 2007;104:1805–10. doi: 10.1073/pnas.0606702104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plant LD, Dowdell EJ, Dementieva IS, Marks JD, Goldstein SA. SUMO modification of cell surface Kv2.1 potassium channels regulates the activity of rat hippocampal neurons. J Gen Physiol. 2011;137:441–54. doi: 10.1085/jgp.201110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plant LD, Dementieva IS, Kollewe A, Olikara S, Marks JD, Goldstein SA. One SUMO is sufficient to silence the dimeric potassium channel K2P1. Proc Natl Acad Sci U S A. 2010;107:10743–8. doi: 10.1073/pnas.1004712107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA. Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell. 2005;121:37–47. doi: 10.1016/j.cell.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Konopacki FA, Jaafari N, Rocca DL, Wilkinson KA, Chamberlain S, Rubin P, et al. Agonist-induced PKC phosphorylation regulates GluK2 SUMOylation and kainate receptor endocytosis. Proc Natl Acad Sci U S A. 2011;108:19772–7. doi: 10.1073/pnas.1111575108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci. 1998;18:6319–30. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atlas D. The Voltage-Gated Calcium Channel Functions as the Molecular Switch of Synaptic Transmission. Annu Rev Biochem. 2013 doi: 10.1146/annurev-biochem-080411-121438. [DOI] [PubMed] [Google Scholar]
- 27.Zamponi GW, Lewis RJ, Todorovic SM, Arneric SP, Snutch TP. Role of voltage-gated calcium channels in ascending pain pathways. Brain Res Rev. 2009;60:84–9. doi: 10.1016/j.brainresrev.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J, Luo ZD. Calcium channel functions in pain processing. Channels (Austin) 2010;4:510–7. doi: 10.4161/chan.4.6.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson KA, Nakamura Y, Henley JM. Targets and consequences of protein SUMOylation in neurons. Brain Res Rev. 2010;64:195–212. doi: 10.1016/j.brainresrev.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merrill JC, Melhuish TA, Kagey MH, Yang SH, Sharrocks AD, Wotton D. A role for non-covalent SUMO interaction motifs in Pc2/CBX4 E3 activity. PLoS One. 2010;5:e8794. doi: 10.1371/journal.pone.0008794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duprez E, Saurin AJ, Desterro JM, Lallemand-Breitenbach V, Howe K, Boddy MN, et al. SUMO-1 modification of the acute promyelocytic leukaemia protein PML: implications for nuclear localisation. J Cell Sci. 1999;112:381–93. doi: 10.1242/jcs.112.3.381. [DOI] [PubMed] [Google Scholar]
- 32.Zhu J, Zhu S, Guzzo CM, Ellis NA, Sung KS, Choi CY, et al. Small ubiquitin-related modifier (SUMO) binding determines substrate recognition and paralog-selective SUMO modification. J Biol Chem. 2008;283:29405–15. doi: 10.1074/jbc.M803632200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perdomo J, Verger A, Turner J, Crossley M. Role for SUMO modification in facilitating transcriptional repression by BKLF. Mol Cell Biol. 2005;25:1549–59. doi: 10.1128/MCB.25.4.1549-1559.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampson DA, Wang M, Matunis MJ. The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J Biol Chem. 2001;276:21664–9. doi: 10.1074/jbc.M100006200. [DOI] [PubMed] [Google Scholar]
- 35.Wilson SM, Xiong W, Wang Y, Ping X, Head JD, Brittain JM, et al. Prevention of posttraumatic axon sprouting by blocking collapsin response mediator protein 2-mediated neurite outgrowth and tubulin polymerization. Neuroscience. 2012;210:451–66. doi: 10.1016/j.neuroscience.2012.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feligioni M, Nishimune A, Henley JM. Protein SUMOylation modulates calcium influx and glutamate release from presynaptic terminals. Eur J Neurosci. 2009;29:1348–56. doi: 10.1111/j.1460-9568.2009.06692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marangoudakis S, Andrade A, Helton TD, Denome S, Castiglioni AJ, Lipscombe D. Differential ubiquitination and proteasome regulation of Ca(V)2.2 N-type channel splice isoforms. J Neurosci. 2012;32:10365–9. doi: 10.1523/JNEUROSCI.0851-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorval V, Fraser PE. Small ubiquitin-like modifier (SUMO) modification of natively unfolded proteins tau and alpha-synuclein. J Biol Chem. 2006;281:9919–24. doi: 10.1074/jbc.M510127200. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi Y, Hasegawa-Moriyama M, Sakurai T, Inada E. The macrophage-mediated effects of the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone attenuate tactile allodynia in the early phase of neuropathic pain development. Anesth Analg. 2011;113:398–404. doi: 10.1213/ANE.0b013e31821b220c. [DOI] [PubMed] [Google Scholar]
- 40.Kim C, Jun K, Lee T, Kim SS, McEnery MW, Chin H, et al. Altered nociceptive response in mice deficient in the alpha(1B) subunit of the voltage-dependent calcium channel. Mol Cell Neurosci. 2001;18:235–45. doi: 10.1006/mcne.2001.1013. [DOI] [PubMed] [Google Scholar]
- 41.Striessnig J, Koschak A. Exploring the function and pharmacotherapeutic potential of voltage-gated Ca2+ channels with gene knockout models. Channels (Austin) 2008;2:233–51. doi: 10.4161/chan.2.4.5847. [DOI] [PubMed] [Google Scholar]
- 42.McGivern JG, McDonough SI. Voltage-gated calcium channels as targets for the treatment of chronic pain. Curr Drug Targets CNS Neurol Disord. 2004;3:457–78. doi: 10.2174/1568007043336743. [DOI] [PubMed] [Google Scholar]
- 43.Cao YQ. Voltage-gated calcium channels and pain. Pain. 2006;126:5–9. doi: 10.1016/j.pain.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Feliciangeli S, Bendahhou S, Sandoz G, Gounon P, Reichold M, Warth R, et al. Does sumoylation control K2P1/TWIK1 background K+ channels? Cell. 2007;130:563–9. doi: 10.1016/j.cell.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Park KD, Salome C, Wilson SM, Stables JP, Liu R, et al. Development and characterization of novel derivatives of the antiepileptic drug lacosamide that exhibit far greater enhancement in slow inactivation of voltage-gated sodium channels. ACS Chem Neurosci. 2011;2:90–106. doi: 10.1021/cn100089b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Wilson SM, Brittain JM, Ripsch MS, Salomé C, Park KD, et al. Merging Structural Motifs of Functionalized Amino Acids and α-Aminoamides Results in Novel Anticonvulsant Compounds with Significant Effects on Slow and Fast Inactivation of Voltage-gated Sodium Channels and in the Treatment of Neuropathic Pain. ACS Chem Neurosci. 2011;2:317–22. doi: 10.1021/cn200024z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Brittain JM, Jarecki BW, Park KD, Wilson SM, Wang B, et al. In silico docking and electrophysiological characterization of lacosamide binding sites on collapsin response mediator protein-2 identifies a pocket important in modulating sodium channel slow inactivation. J Biol Chem. 2010;285:25296–307. doi: 10.1074/jbc.M110.128801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leclere PG, Panjwani A, Docherty R, Berry M, Pizzey J, Tonge DA. Effective gene delivery to adult neurons by a modified form of electroporation. J Neurosci Methods. 2005;142:137–43. doi: 10.1016/j.jneumeth.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 49.Stenmark P, Ogg D, Flodin S, Flores A, Kotenyova T, Nyman T, et al. The structure of human collapsin response mediator protein 2, a regulator of axonal growth. J Neurochem. 2007;101:906–17. doi: 10.1111/j.1471-4159.2006.04401.x. [DOI] [PubMed] [Google Scholar]
- 50.Bayer P, Arndt A, Metzger S, Mahajan R, Melchior F, Jaenicke R, et al. Structure determination of the small ubiquitin-related modifier SUMO-1. J Mol Biol. 1998;280:275–86. doi: 10.1006/jmbi.1998.1839. [DOI] [PubMed] [Google Scholar]