Abstract
β-adrenergic stimulation of cardiac myocytes enhances intracellular calcium cycling, which frequently associates with pro-arrhythmic Ca waves. The threshold level of free calcium in the sarcoplasmic reticulum ([Ca]SR) where waves initiate is increased during β-adrenergic stimulation.1 Here, we measured [Ca]SR directly to monitor the [Ca]SR level at which spontaneous Ca waves terminated (termination threshold) during β-adrenergic stimulation. Compared with control conditions, application of the β-adrenergic receptor agonist isoproterenol (ISO; 1 μM) resulted in an increase in basal [Ca]SR and an increase in the [Ca]SR at which spontaneous Ca waves terminated. When [Ca]SR was experimentally matched, ISO stimulation resulted in a decrease in the depletion amplitude of the waves. Sensitization of ryanodine receptor SR Ca release channels to Ca with a low dose of caffeine in the presence of ISO was able to decrease the termination threshold compared with control conditions. Therefore, the Ca wave termination level may represent an important mode of altering Ca depletion from the SR and reducing the arrhythmogenic potential during β-adrenergic stimulation.
Keywords: beta-adrenergic stimulation, excitation-contraction coupling, Ca waves, ventricular myocytes, ryanodine receptor, intra-SR [Ca], arrhythmia
Introduction
During the cardiac cycle, Ca influx through L-type Ca channels triggers coordinated release of Ca via ryanodine receptor (RyR) Ca release channels of the sarcoplasmic reticulum (SR). This Ca-induced Ca release (CICR) leads to the whole-cell cytosolic Ca ([Ca]i) transient that initiates contraction of the heart. RyR activity is critically dependent on both [Ca]i and the intra-SR Ca content ([Ca]SR), which gives rise to complex dynamic RyR gating behavior.2-4 Increases in both [Ca]i and [Ca]SR cause positive inotropic effects during systole, yet also lead to spontaneous openings of the RyR during diastole resulting in cytosolic Ca waves. Ca waves are a highly arrhythmogenic form of CICR, and it has been shown that they arise when [Ca]SR reaches a certain overload threshold level. This level has been termed the “Ca wave threshold” and represents the intra-SR Ca content at which spontaneous Ca release activates.5,6 Experimental evidence suggests that the wave threshold may be set by the state of the RyR, as alteration of the wave threshold has been observed with agents that modulate RyR activity6-8 or in disease conditions associated with RyR dysfunction including heart failure9,10 and catecholaminergic polymorphic ventricular tachycardia.11-13
Due to the inherently regenerative nature of CICR, distinct mechanisms must be in place to ensure termination of the Ca release process and allow for sequestration of Ca back into the SR. It has been shown by our laboratory and others that the termination of Ca release is governed by a luminal [Ca]SR-dependent mechanism and dependent upon SR Ca depletion.14-17 The [Ca]SR at which release terminates is known as the termination threshold. Furthermore, we have shown that the termination threshold of local spontaneous Ca release is related to the luminal Ca sensitivity of the RyR as, like the wave threshold, it can be modified by agents that sensitize the RyR to activation by Ca.17,18 Thus, it has become evident that both the wave threshold and the termination threshold are relevant parameters that can be tuned to meet the demands imposed on the working heart.
In our recent study1 we utilized the low-affinity fluorescent Ca indicator fluo-5N to directly monitor the intra-SR Ca wave threshold during β-adrenergic stimulation. We found that the [Ca]SR level where waves initiated was increased during β-adrenergic stimulation, and the primary cause of increased Ca wave activity during β-adrenergic stimulation was the large increase in [Ca]SR above this higher threshold level. In the work presented here, we examined the effect of β-adrenergic stimulation on the termination threshold of spontaneous Ca waves. We find that in the presence of the β-adrenergic agonist isoproterenol the [Ca]SR at which spontaneous Ca waves terminated was increased compared with control conditions. At matched initial [Ca]SR levels this resulted in a decrease of the SR Ca depletion amplitude and a decrease of the amount of Ca released from the SR during spontaneous Ca waves. Taken together, these results indicate that the elevation of the Ca wave termination threshold, synergistically with the increase of Ca wave threshold,1 represents a protective mechanism against arrhythmogenic events during β-adrenergic stimulation.
Results and Discussion
In this study we directly monitored the [Ca]SR levels where Ca release during spontaneous Ca waves terminated under control conditions and during β-adrenergic stimulation. The SR of rabbit ventricular myocytes was loaded with the low-affinity fluorescent Ca indicator dye fluo-5N to monitor [Ca]SR. Cells were field-stimulated in elevated extracellular Ca ([Ca]o = 7 mM) at increasing frequencies (between 0.8–2.0 Hz) to overload the SR with Ca and induce spontaneous Ca waves during a rest period from stimulation. The Ca wave termination threshold was defined as the [Ca]SR at the nadir of the fluo-5N fluorescence signal during the Ca wave (dashed lines in Fig. 1). Using this approach, the spontaneous Ca wave termination threshold could be measured in the same cell under control conditions (Fig. 1A), during β-adrenergic stimulation in the absence (Fig. 1B) and presence of sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) inhibitors (Fig. 1C), and after RyR sensitization with caffeine (Fig. 1D). An example trace of a cell under control conditions exhibiting a spontaneous Ca wave is shown in Figure 1A. The dashed line (1) marks the termination threshold for control conditions. Application of the β-adrenergic receptor agonist isoproterenol (ISO; 1 μM; [Ca]o = 2 mM) resulted in an increase in the diastolic [Ca]SR (Fig. 1B) due to the stimulatory effects of ISO on SERCA. In the presence of ISO the [Ca]SR at which spontaneous Ca waves terminated was increased compared with control conditions (dashed line 2). To decrease [Ca]SR to the same level as in control conditions but in the maintained presence of ISO, extracellular Ca was reduced ([Ca]o = 1 mM) and SERCA was partially inhibited with cyclopiazonic acid (CPA; 3 μM). Under these conditions (Fig. 1C), the [Ca]SR at which Ca waves terminated was still increased compared with control (dashed line 3). In the continued presence of ISO+CPA a low dose (250 μM) of caffeine was applied to sensitize the RyR to activation by Ca (Fig. 1D). Caffeine significantly decreased the wave termination threshold compared with control conditions (dashed line 4) showing that sensitization of the RyR with a low dose of caffeine can overcome the effects of β-adrenergic stimulation. These results are consistent with our earlier observation that acute application of 250 μM caffeine resulted in a decrease in the termination threshold of spontaneous Ca release.17,18
Figure 1. Direct measurements of termination threshold of spontaneous Ca waves. [Ca]SR traces, measured with fluo-5N entrapped in the SR, show an electrically induced SR Ca depletion transient (arrow) followed by a period of rest to observe spontaneous Ca waves (wave denoted by star). This protocol was applied under (A) control conditions, and repeated in the presence of (B) isoproterenol (ISO; 1 μM), (C) ISO plus cyclopiazonic acid (CPA; 3 μM), and (D) during addition of caffeine (Caff; 250 μM). The dashed lines indicate Ca wave termination thresholds in control (1), ISO (2), ISO+CPA (3) and ISO+CPA+Caff (4).
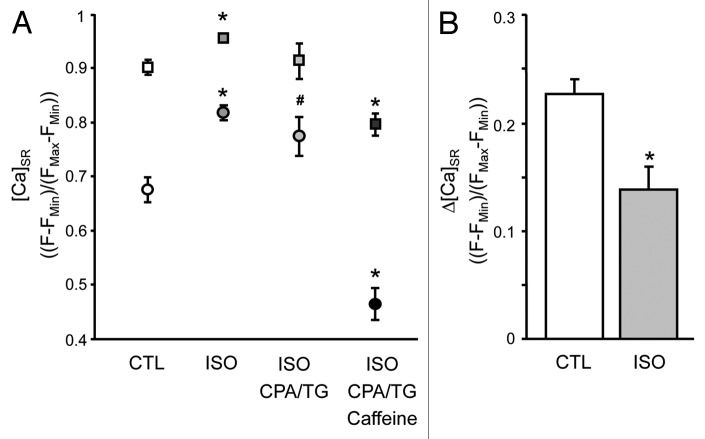
Figure 2A shows summary results for average [Ca]SR levels immediately preceding a spontaneous Ca wave (pre-wave [Ca]SR; squares) and at the nadir (circles) of the wave under control conditions, in the presence of ISO, ISO+CPA/TG and ISO+CPA/TG+Caff. (In some experiments SERCA was partially inhibited with thapsigargin (TG; 1 μM) yielding identical results. Therefore, for summary purposes CPA and TG results were pooled together). Under control conditions (CTL), pre-wave [Ca]SR [expressed as (F-FMin)/(FMax-FMin)] was 0.90 ± 0.01 (n = 23), and increased significantly (p = 0.0015, n = 22) to 0.96 ± 0.01 after addition of ISO. Notably, the termination threshold was also significantly increased under β-adrenergic stimulation (0.82 ± 0.01 ISO vs. 0.68 ± 0.02 CTL; p < 0.0001). After addition of a SERCA blocker (CPA or TG) in the maintained presence of ISO pre-wave [Ca]SR could be lowered experimentally to a level essentially identical to that observed under control conditions ([Ca]SR = 0.91 ± 0.03, n = 8; not significantly different from control), however the termination threshold remained significantly elevated compared with control (0.78 ± 0.04; p < 0.05 vs CTL). This indicates that at the same pre-wave [Ca]SR levels, the Ca wave termination threshold was indeed increased during β-adrenergic stimulation. Prolonged exposure to ISO+CPA/TG, together with Ca wave activity, was accompanied with a steady decline of [Ca]SR. Subsequent addition of caffeine (250 μM) at a decreased pre-wave [Ca]SR of 0.80 ± 0.01 (n = 8; p = 0.001 vs. CTL) triggered Ca waves immediately that terminated at a significantly lower [Ca]SR (0.47 ± 0.02; n = 8; p = 0.0001 vs. CTL).
Figure 2. Summary: intra-SR termination threshold and depletion amplitude of spontaneous Ca waves. (A) pre-wave [Ca]SR (squares) and [Ca]SR at the wave nadir (termination threshold; circles) in control (CTL; n = 23), ISO (n = 22), ISO+CPA/TG (n = 8), and ISO+CPA/TG+Caff (n = 8). (B) Ca wave depletion amplitudes at matched average pre-wave [Ca]SR in the absence (CTL) and presence of ISO [ISO+CPA/TG data from (A)]. *p < 0.01 vs. CTL, #p < 0.05 vs. CTL.
Figure 2B compares depletion amplitudes in the presence and absence of ISO when pre-wave [Ca]SR was matched (compare CTL and ISO+CPA/TG in Fig. 2A). The depletion amplitude was calculated as the difference between pre-wave [Ca]SR and [Ca]SR at the termination threshold. The graph shows that, when pre-wave [Ca]SR was identical, ISO significantly decreased the depletion amplitude.
Figure 3 illustrates correlations between wave termination threshold (wave nadir) and pre-wave [Ca]SR for two sets of data. Open symbols represent control data, and filled symbols represent wave threshold data in the presence of ISO. For this analysis ISO data in the absence and presence of CPA or TG were pooled together as the experimental focus was on the effect of β-adrenergic stimulation on wave parameters, and CPA and TG solely served as tools to manipulate pre-wave [Ca]SR independent of ISO and to achieve sufficient overlap of pre-wave [Ca]SR among the two conditions. Linear regression analysis of the two data sets yielded correlation coefficients of R = 0.82 (control) and 0.72 (ISO). Several interesting features are apparent from the regression analysis of Figure 3. In contrast to Ca sparks and Ca transients which terminate at a set [Ca]SR independent of initial [Ca]SR,14,17 Ca wave termination exhibited a degree of positive correlation with pre-wave [Ca]SR for both control and ISO data. In the presence of ISO pre-wave [Ca]SR showed a tendency toward higher values (right shift of the data along the abscissa), which is consistent with ISO increasing [Ca]SR compared with control conditions. Furthermore, in the presence of ISO there was a shift in the regression line toward higher termination [Ca]SR levels. As indicated by the vertical arrows that are positioned at the average pre-wave [Ca]SR in control and ISO, a particular pre-wave [Ca]SR level results in a higher termination threshold in the presence of ISO compared with control (illustrated by the vertical difference between the two regression lines). The vertical shift in the regression line in the presence of ISO suggests that release generally terminates at a higher [Ca]SR, likely due to a decrease in sensitivity to luminal Ca. This would be in agreement with the effects of ISO on the [Ca]SR threshold for wave initiation.1 Interestingly, the positive slope of both regression lines may indicate that wave termination is more complex than termination of elementary Ca release events (Ca sparks) or Ca transients during excitation-contraction coupling14,17,18 where Ca release ceases at the same [Ca]SR independent of initial [Ca]SR. However, because the slopes of the regression lines are similar between the two conditions this suggests that the overall termination mechanisms may be conserved but with an altered sensitivity to luminal Ca. The shift of Ca wave termination toward higher [Ca]SR could occur through one of the various post-translational modifications initiated by β-adrenergic receptor activation.
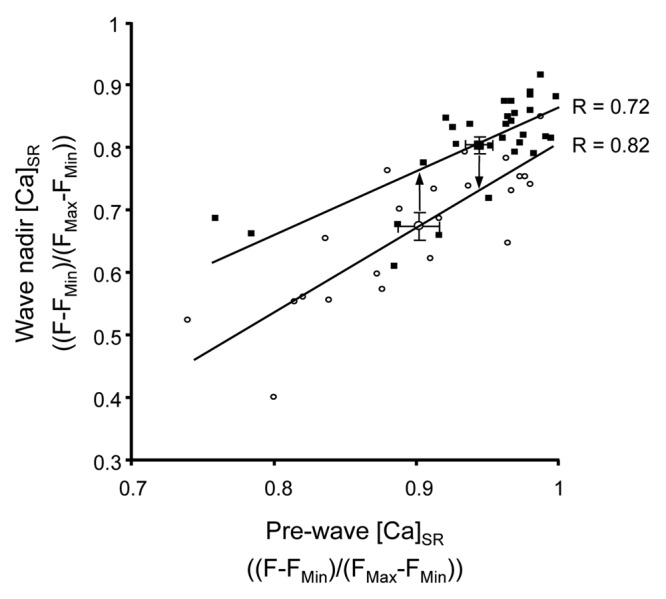
Figure 3. Correlation between pre-wave [Ca]SR and Ca wave termination threshold. Scatter plot with average values (± SEM) and linear regression lines of pre-wave [Ca]SR and [Ca]SR at the nadir of Ca depletion (termination threshold) during spontaneous Ca waves under control conditions (CTL; open symbols; n = 23) and in the presence of ISO (filled symbols; n = 30).
In summary, our data show that in the presence of β-adrenergic stimulation (ISO) the level at which spontaneous Ca waves initiate and terminate are both increased compared with control. The increase of Ca wave frequency typically observed in the presence of β-adrenergic stimulation is due to the strong stimulatory effect on SERCA that increases SR Ca load well above the increased threshold for wave initiation. Thus, the wave initiation threshold1 and wave termination threshold are key points of regulation of spontaneous pro-arrhythmic Ca release in cardiac myocytes. The observed elevations of the Ca wave threshold and of the Ca wave termination threshold during acute β-adrenergic stimulation potentially represent protective mechanisms against arrhythmogenic Ca release.
Materials and Methods
Solutions, chemicals and cell isolation
All reagents were purchased from Sigma-Aldrich unless otherwise noted. Control Tyrode solution with elevated Ca contained (in mM): 130 NaCl, 4 KCl, 7 CaCl2, 1 MgCl2, 10 D-glucose, 10 Hepes, pH 7.4 with NaOH. Tyrode solutions with different [Ca]o were prepared with iso-osmotic substitution of CaCl2 and NaCl. Isoproterenol (ISO) was prepared daily as a 100 mM stock solution (in H2O, maintained at 4°C), diluted in Tyrode solution immediately prior to experimental procedures, and used within 30 min. Cyclopiazonic acid (CPA) and thapsigargin (TG) were dissolved in dimethyl sulfoxide (DMSO) and diluted to working concentrations in Tyrode solution. All experiments were conducted at room temperature (22–24°C). Left-ventricular myocytes were isolated from male New Zealand White rabbits (2.5 kg, Myrtle’s Rabbitry) using retrograde Langendorff perfusion with Liberase Blendzyme TH (Roche Applied Science) as previously described.1
Intra-SR Ca measurements and experimental procedures
Ventricular myocytes were incubated with 10 μM of membrane permeant fluo-5N/AM (Molecular Probes-Life Technologies) for 2.5 h, washed for 30 min at 37°C to promote accumulation of dye within the SR, and plated on laminin-coated coverslips. Laser-scanning fluorescence confocal microscopy was performed using the resonant scan head of the Nikon A1R system (Nikon Instruments Inc.) in frame scan mode (60 fps at 256 × 512 pixels, 400 nm/pixel). Fluo-5N was excited using the 488 nm line of the argon ion laser and emission was recorded at 500–530 nm. Changes in [Ca]SR are presented as (F-FMin)/(FMax-FMin). FMin is the quench-corrected (15%) fluorescence value following complete emptying of the SR with 10 mM caffeine, and FMax is taken as the maximal diastolic fluo-5N fluorescence observed during electrical pacing in the presence of 1 μM ISO. Rabbit ventricular myocytes underwent several consecutive experimental trials consisting of electrical field stimulation of cells for a period of 30–60 sec, followed by 8 sec of rest. The stimulation-rest protocol was repeated at incrementally increasing pacing frequencies (0.05 to 0.2 Hz increments) to increase [Ca]SR until Ca waves were observed during the rest period. The nadir of the [Ca]SR depletion signal during a Ca wave was defined as the termination threshold (compare Fig. 1).
Statistics and data analysis
Data are presented as individual observations from single myocytes or as mean ± standard error of the mean (SEM). Statistical comparisons were performed using Student’s t-test for unpaired data, with significance set at p < 0.05. “n” refers to the number of individual experimental trials.
Acknowledgments
This work was supported by National Institutes of Health grants HL62231, HL80101 and HL101235 (to L.A.B.) and F32HL090211 and 1K01AG041208 (to T.L.D.) and the Leducq Foundation (to L.A.B). The authors would also like to thank Dr. Elisa Bovo, Stephen Shonts and Demetrio Santiago for assistance with myocyte isolation.
Glossary
Abbreviations:
- [Ca]i
cytosolic calcium concentration
- [Ca]SR
intra-sarcoplasmic reticulum free calcium concentration
- CICR
calcium-induced calcium release
- CPA
cyclopiazonic acid
- DMSO
dimethyl sulfoxide
- ISO
isoproterenol
- RyR
ryanodine receptor
- SERCA
sarcoplasmic/endoplasmic reticulum calcium ATPase
- SR
sarcoplasmic reticulum
- TG
thapsigargin
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/24173
References
- 1.Domeier TL, Maxwell JT, Blatter LA. β-Adrenergic stimulation increases the intra-sarcoplasmic reticulum Ca2+ threshold for Ca2+ wave generation. J Physiol. 2012;590:6093–108. doi: 10.1113/jphysiol.2012.236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82:893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 3.Györke I, Györke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–10. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Györke S, Györke I, Lukyanenko V, Terentyev D, Viatchenko-Karpinski S, Wiesner TF. Regulation of sarcoplasmic reticulum calcium release by luminal calcium in cardiac muscle. Front Biosci. 2002;7:d1454–63. doi: 10.2741/gyorke. [DOI] [PubMed] [Google Scholar]
- 5.Díaz ME, Trafford AW, O’Neill SC, Eisner DA. Measurement of sarcoplasmic reticulum Ca2+ content and sarcolemmal Ca2+ fluxes in isolated rat ventricular myocytes during spontaneous Ca2+ release. J Physiol. 1997;501:3–16. doi: 10.1111/j.1469-7793.1997.003bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ Res. 2007;100:105–11. doi: 10.1161/01.RES.0000252828.17939.00. [DOI] [PubMed] [Google Scholar]
- 7.Kong H, Jones PP, Koop A, Zhang L, Duff HJ, Chen SR. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem J. 2008;414:441–52. doi: 10.1042/BJ20080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overend CL, Eisner DA, O’Neill SC. The effect of tetracaine on spontaneous Ca2+ release and sarcoplasmic reticulum calcium content in rat ventricular myocytes. J Physiol. 1997;502:471–9. doi: 10.1111/j.1469-7793.1997.471bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belevych AE, Terentyev D, Terentyeva R, Ho HT, Gyorke I, Bonilla IM, et al. Shortened Ca2+ signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death. Circ Res. 2012;110:569–77. doi: 10.1161/CIRCRESAHA.111.260455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maxwell JT, Domeier TL, Blatter LA. Dantrolene prevents arrhythmogenic Ca2+ release in heart failure. Am J Physiol Heart Circ Physiol. 2012;302:H953–63. doi: 10.1152/ajpheart.00936.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashimura T, Briston SJ, Trafford AW, Napolitano C, Priori SG, Eisner DA, et al. In the RyR2(R4496C) mouse model of CPVT, β-adrenergic stimulation induces Ca waves by increasing SR Ca content and not by decreasing the threshold for Ca waves. Circ Res. 2010;107:1483–9. doi: 10.1161/CIRCRESAHA.110.227744. [DOI] [PubMed] [Google Scholar]
- 12.Sedej S, Heinzel FR, Walther S, Dybkova N, Wakula P, Groborz J, et al. Na+-dependent SR Ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human CPVT mutation. Cardiovasc Res. 2010;87:50–9. doi: 10.1093/cvr/cvq007. [DOI] [PubMed] [Google Scholar]
- 13.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–83. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zima AV, Picht E, Bers DM, Blatter LA. Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ Res. 2008;103:e105–15. doi: 10.1161/CIRCRESAHA.107.183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zima AV, Picht E, Bers DM, Blatter LA. Partial inhibition of sarcoplasmic reticulum ca release evokes long-lasting ca release events in ventricular myocytes: role of luminal ca in termination of ca release. Biophys J. 2008;94:1867–79. doi: 10.1529/biophysj.107.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Györke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ Res. 2002;91:414–20. doi: 10.1161/01.RES.0000032490.04207.BD. [DOI] [PubMed] [Google Scholar]
- 17.Domeier TL, Blatter LA, Zima AV. Alteration of sarcoplasmic reticulum Ca2+ release termination by ryanodine receptor sensitization and in heart failure. J Physiol. 2009;587:5197–209. doi: 10.1113/jphysiol.2009.177576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domeier TL, Blatter LA, Zima AV. Changes in intra-luminal calcium during spontaneous calcium waves following sensitization of ryanodine receptor channels. Channels (Austin) 2010;4:87–92. doi: 10.4161/chan.4.2.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]