Abstract
The differentiation of cardiac fibroblasts to myofibroblasts is one of the key events during cardiac remodeling, however, the molecular mechanism underlying this process is not well known. Calcium signaling plays an important role in the regulation of cardiac fibroblast function, but its role in the differentiation of fibroblasts is undefined. Recently four Transient Receptor Potential (TRP) channels TRPM7, TRPC3, TRPC6 and TRPV4 were shown to be crucial for the differentiation of cardiac fibroblasts to myofibroblasts. This addendum sums up the roles described for these four TRP channels in cardiac fibroblast differentiation, and discusses the possible molecular mechanisms underlying this process and its relevance for cardiac remodeling in disease.
Keywords: cardiac fibroblast, differentiation, mechanical signaling, myofibroblast, integrin, ECM stiffness, TRPV4, Rho/ROCK
Cardiac remodeling is critical for the function of the heart in response to injury or insult.1-3 Cardiac fibroblasts (CF) are the predominant cell types that secrete extracellular matrix (ECM) and help maintain the structural integrity of the heart.4 CFs proliferate, migrate and differentiate to myofibroblasts during cardiac remodeling in disease states such as myocardial infarction (MI), atrial fibrillation (AF), and pressure overload-induced hypertrophy.4-7 Myofibroblasts are hypersecretory, highly contractile and deposit excessive ECM proteins resulting in cardiac fibrosis that eventually form the scar. However, uncontrolled production of ECM proteins by prolonged survival of myofibroblasts can lead to pathological fibrosis. Therefore, most of the studies on cardiac fibrosis focus on understanding the molecular mechanism(s) controlling differentiation of cardiac fibroblasts to myofibroblasts. Fibroblast differentiation involves two major events including de novo expression of α-SMA and its incorporation into the stress fibers which are mediated by soluble and mechanical signaling.8-13 Most of the studies in the field have been focused on the profibrotic soluble factors such as transforming growth factor (TGFβ), angiotensin II (Ang II) and endothelin (ET-1) working through canonical SMAD and ERK1/2 pathways. Interestingly, intracellular calcium regulates a number of functions in fibroblasts such as contractile activity,14 however, it is not known whether calcium signaling plays a role in the differentiation of fibroblasts to myofibroblasts. Recently four different Transient Receptor Potential (TRP) channels, TRPM7, TRPC3, TRPC6 and TRPV4 have been shown to be critical for fibroblast differentiation to myofibroblasts.15-18
TRP channels are non-selective calcium entry channels19 and are widely expressed in a number of cells including fibroblasts. Du et al. (2010) demonstrated that atrial fibroblasts isolated from human cardiac tissue exhibit endogenous currents of TRPM7 and calcium entry is exclusively mediated through TRPM7 but not TRPC1, TRPC6, TRPV2 or TRPV4 which are also expressed in these cells.17 Importantly, they found that TRPM7 expression and activity is upregulated in fibroblasts from AF patients and that knockdown of TRPM7 significantly reduced basal differentiation of fibroblasts(from AF patients) to myofibroblasts. Further, TGFβ-mediated increased expression of TRPM7 was shown to be correlated with increased differentiation of human atrial fibroblasts to myofibroblasts in vitro. These results suggest an exclusive role for TRPM7 in fibroblast myofibroblast transition in the human atrium and that TRPM7 expression and activity is regulated by TGFβ. However, this study did not provide any evidence for the mechanisms by which TGFβ regulates TRPM7 expression/activity or differentiation. Two additional studies provided mechanistic insights. First, using animal models of atrial fibrillation, isolated fibroblasts and pharmacological inhibitors, Harada et al. (2012) demonstrated that TRPC3 channels are critical for atrial fibroblast differentiation.18 Further they described that NFAT-mediated downregulation of microRNA-26 increased TRPC3-dependent fibroblast proliferation via ERK1/2 pathway leading to increased fibroblast differentiation. Although this study provided a possible mechanism(s) for TGFβ -induced increased expression of TRPC3 and cardiac fibroblast proliferation, no mechanism(s) were given for the differentiation process. Davis et al. (2012) in the second study addressed this point albeit the candidate TRP channel here was TRPC6 not TRPC3. Using a genome-wide screen they identified TRPC6 as a calcium channel necessary and sufficient for fibroblast differentiation to myofibroblasts.16 Importantly, they demonstrated that the profibrotic mediators TGFβ and Ang II, independent of other, induced TRPC6 expression through a p38 MAPKinase/serum responsive factor (SRF)-dependent activation of TRPC6 promoter. They further demonstrated that TRPC6 activates calcineurin/NFAT pathway which induces α-SMA expression leading to fibroblast differentiation. Thus, this forms the first study to show a comprehensive mechanism for the role of a TRP channel in fibroblast differentiation to myofibroblasts, starting from the stimulation of growth factor to the expression of TRP channel which in turn contributed to the expression of α-SMA.
Although the three studies above identified a role for TRP channels in fibroblast differentiation, they are mostly focused on soluble factor signaling and did not attempt to distinguish between α-SMA expression and its incorporation in to stress fibers. However, the phenotypic differentiation of fibroblast to myofibroblasts is dependent on the mechanical forces that are generated by changes in the ECM stiffness and tensional forces generated by actin filaments. Indeed, the balance between the contractile forces generated by the cell and the resistance offered by ECM stiffness to these forces, both transferred by integrins, decides the phenotype of the cell. Moreover, α-SMA incorporation into stress fibers could also be dependent on integrin-dependent mechanical signaling.20,21 Integration of both soluble and mechanical signals are required for α-SMA expression and its incorporation in to stress fibers. However, neither the mechanism nor the molecule that integrate this signaling is known. Interestingly, all the TRP channels implicated in the cardiac fibroblast differentiation were shown to be involved in mechanotransduction. TRPM7 appears to exhibit direct mechanosensitivity in response to membrane stretch and osmotic swelling in heterologously expressed cells22 and regulate calcium flickers in fibroblasts.23 TRPC3 and TRPC6 were both demonstrated to play role in mechanotransduction during pressure overload-induced cardiac hypertrophy.24 However, TRPC6 mechanosensitivity requires the expression of Angiotensin II receptor (AT1R), importantly, even without the ligand binding to the receptor.25 Although these three TRP channels have mechanosensitive functions, there are no reports on their involvement in mechanotransduction in cardiac fibroblasts. In a recent study, we addressed this question and demonstrated that a mechanosensitive ion channel, TRPV4 mediates fibroblast differentiation via integration of soluble and mechanical signals.15 By using a TRPV4-specific antagonist AB159908 and siRNA knockdown of TRPV4, we conclusively demonstrated that TRPV4 is required for TGFβ1-induced differentiation as measured by incorporation of α-SMA into stress fibers. Furthermore, using bioengineered gels of varying stiffness we have shown that TGFβ1-induced fibroblast differentiation was dependent on ECM stiffness and was attenuated by TRPV4 blockade. Similar to TRPM7, TRPC3 and TRPC6, the expression of TRPV4 was also increased by TGFβ treatment. Our study proposed two roles for TRPV4. First, TRPV4 acts as a mechanosensor of ECM stiffness and induce fibroblast differentiation by increasing the expression and incorporation of α-SMA in to stress fibers13,15 may be via a Rho/ROCK dependent pathway.9,26 Second, increased cell contraction and tensional forces may activate additional integrins27,28leading to the activation of latent TGFβ29 forming a positive mechanical feedback loop that drives the differentiation of fibroblasts. Importantly, in contrast to the exclusive role of TRPM7 in atrial fibroblast differentiation shown by Du et al.,17 our study demonstrated that TRPM7 channels do not play a role in ventricular fibroblast differentiation.
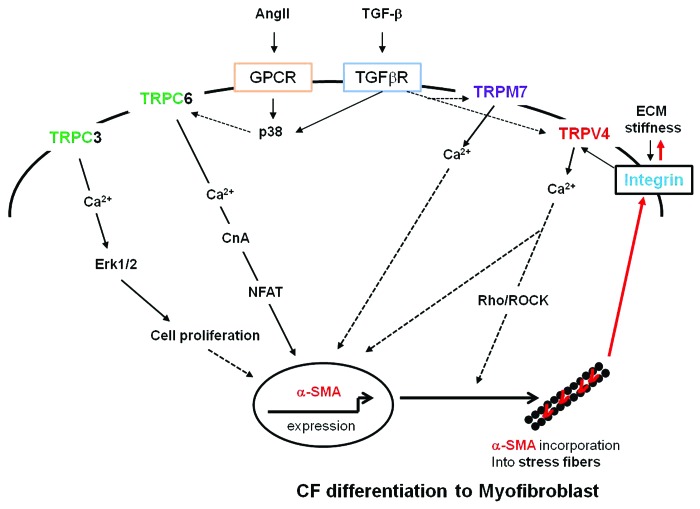
The findings from the above studies suggest that all four different TRP channels are critical for fibroblast differentiation to myofibroblasts in a cell-specific manner. This raises an important question, how and why would nature employ different TRP channels for the same function? The answer to this question comes partially from the studies discussed. TRPM7 and TRPC3 are exclusively involved in atrial fibroblast differentiation whereas TRPC6 and TRPV4 appear to participate in ventricular fibroblast differentiation. The fact that TRPM7 is exclusively required for atrial fibroblast differentiation and TRPV4 is critically utilized for ventricular differentiation, despite both being sufficiently expressed in atrial and ventricular fibroblasts, confirms the cell-specific roles for these TRP channels. This also could be due to the local mechanical environment, since ventricles are mechanically more active than atria, which may preferentially activate TRPV4. The local mechanical environment cannot explain the roles for TRPC3 and TRPC6 in fibroblast differentiation, however, the molecular signaling may shed light on this issue. Mechanistically, the unifying point of all these TRP channels is that their expression is regulated by soluble factors such as TGFβ irrespective of the origin of fibroblast. Furthermore, NFAT signaling is implicated in both TRPC3- and TRPC6-dependent fibroblast proliferation and α-SMA expression, respectively. Although these studies demonstrate that each of these TRP channels are critical for fibroblast differentiation, this is based on a single end point observation i.e., increased α-SMA expression. The role for mechanical forces which are required for α-SMA incorporation to stress fibers13 were only shown in the TRPV4 study.15 Based on these observations, we speculate that different TRP channels TRPM7, TRPC3, TRPC6 and TRPV4 channels may play distinct roles in fibroblast differentiation (Fig. 1). TRPC3 may regulate fibroblast proliferation through the Erk1/2 pathway, and TRPC6 induces α-SMA expression via activation of calcineurin/NFAT signaling. TRPV4 may integrate both of these signals with mechanical signals from integrins and facilitate the incorporation of α-SMA into stress fibers through the Rho/ROCK pathway. Alternatively, these channels may work together by forming hetero-tetramers,30,31 activating pathways implicated in fibroblast proliferation, α-SMA expression and stress fiber formation leading to the differentiation of fibroblasts to myofibroblasts. Unraveling specific roles for each TRP channel and their downstream signaling will further our understanding of myofibroblast differentiation and may provide the basis for the development of novel therapies to limit myocardial fibrosis and its complications.
Figure 1. A proposed model showing the mechanism(s) by which TRP channels regulate fibroblast differentiation to myofibroblasts during cardiac remodeling (adapted from ref. 16). The soluble profibrotic factors such as TGFβ15-17 and Ang II16 induce expression of TRP channels via p38/SRF16 or NFAT/miR-26,18 which in turn activate signaling cascades involved in fibroblast proliferation (TRPC3/ERK1/218) and α-SMA expression (TRPC6/CnA/NFAT16). These chemical signals then integrate with mechanical signals (ECM stiffness/integrin/TRPV4/Rho-ROCK15) leading to incorporation α-SMA into actin stress fibers. The increased cell contraction and tensional forces may activate additional integrins (red arrows) leading to the increased ECM stiffness and activation of latent TGFβ.TGFβ = transforming growth factor β; Ang II = angiotensin II; can, calcineurin; SRF, serum responsive factor; NFAT, Nuclear factor of activated T-cells; miR-26, microRNA26; α-SMA, α-smooth muscle actin; ECM, extracellular matrix; ROCK, Rho associated protein kinase.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work is supported by start-up funds from NEOMED (CKT) and, in part, by NIH-1R15HL106442–01 (JGM and CKT).
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/24328
References
- 1.Czubryt MP. Common threads in cardiac fibrosis, infarct scar formation, and wound healing. Fibrogenesis Tissue Repair. 2012;5:19. doi: 10.1186/1755-1536-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jourdan-Lesaux C, Zhang J, Lindsey ML. Extracellular matrix roles during cardiac repair. Life Sci. 2010;87:391–400. doi: 10.1016/j.lfs.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segura AM, Frazier OH, Buja LM. Fibrosis and heart failure. Heart Fail Rev. 2012 doi: 10.1007/s10741-012-9365-4. [DOI] [PubMed] [Google Scholar]
- 4.van Nieuwenhoven FA, Turner NA. The role of cardiac fibroblasts in the transition from inflammation to fibrosis following myocardial infarction. Vascul Pharmacol. 2012 doi: 10.1016/j.vph.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair. 2012;5:15. doi: 10.1186/1755-1536-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flack EC, Lindsey ML, Squires CE, Kaplan BS, Stroud RE, Clark LL, et al. Alterations in cultured myocardial fibroblast function following the development of left ventricular failure. J Mol Cell Cardiol. 2006;40:474–83. doi: 10.1016/j.yjmcc.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Ma Y, Halade GV, Lindsey ML. Extracellular matrix and fibroblast communication following myocardial infarction. J Cardiovasc Transl Res. 2012;5:848–57. doi: 10.1007/s12265-012-9398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–37. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 9.Hinz B. Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep. 2009;11:120–6. doi: 10.1007/s11926-009-0017-1. [DOI] [PubMed] [Google Scholar]
- 10.Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–55. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Hinz B, Gabbiani G. Mechanisms of force generation and transmission by myofibroblasts. Curr Opin Biotechnol. 2003;14:538–46. doi: 10.1016/j.copbio.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159:1009–20. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goffin JM, Pittet P, Csucs G, Lussi JW, Meister JJ, Hinz B. Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol. 2006;172:259–68. doi: 10.1083/jcb.200506179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Follonier Castella L, Gabbiani G, McCulloch CA, Hinz B. Regulation of myofibroblast activities: calcium pulls some strings behind the scene. Exp Cell Res. 2010;316:2390–401. doi: 10.1016/j.yexcr.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Adapala RK, Thoppil RJ, Luther DJ, Paruchuri S, Meszaros JG, Chilian WM, et al. TRPV4 channels mediate cardiac fibroblast differentiation by integrating mechanical and soluble signals. J Mol Cell Cardiol. 2013;54:45–52. doi: 10.1016/j.yjmcc.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JDA. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012;23:705–15. doi: 10.1016/j.devcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du J, Xie J, Zhang Z, Tsujikawa H, Fusco D, Silverman D, et al. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res. 2010;106:992–1003. doi: 10.1161/CIRCRESAHA.109.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harada M, Luo X, Qi XY, Tadevosyan A, Maguy A, Ordog B, et al. Transient receptor potential canonical-3 channel-dependent fibroblast regulation in atrial fibrillation. Circulation. 2012;126:2051–64. doi: 10.1161/CIRCULATIONAHA.112.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nilius B. TRP channels in disease. Biochim Biophys Acta. 2007;1772:805–12. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Ingber DE. Integrins, tensegrity, and mechanotransduction. Gravit Space Biol Bull. 1997;10:49–55. [PubMed] [Google Scholar]
- 21.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–27. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 22.Numata T, Shimizu T, Okada Y. Direct mechano-stress sensitivity of TRPM7 channel. Cell Physiol Biochem. 2007;19:1–8. doi: 10.1159/000099187. [DOI] [PubMed] [Google Scholar]
- 23.Wei C, Wang X, Chen M, Ouyang K, Song LS, Cheng H. Calcium flickers steer cell migration. Nature. 2009;457:901–5. doi: 10.1038/nature07577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel A, Sharif-Naeini R, Folgering JR, Bichet D, Duprat F, Honoré E. Canonical TRP channels and mechanotransduction: from physiology to disease states. Pflugers Arch. 2010;460:571–81. doi: 10.1007/s00424-010-0847-8. [DOI] [PubMed] [Google Scholar]
- 25.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomasek JJ, Vaughan MB, Kropp BP, Gabbiani G, Martin MD, Haaksma CJ, et al. Contraction of myofibroblasts in granulation tissue is dependent on Rho/Rho kinase/myosin light chain phosphatase activity. Wound Repair Regen. 2006;14:313–20. doi: 10.1111/j.1743-6109.2006.00126.x. [DOI] [PubMed] [Google Scholar]
- 27.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, et al. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 2009;104:1123–30. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24:4444–52. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, et al. The single-molecule mechanics of the latent TGFβ1 complex. Curr Biol. 2011;21:2046–54. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 30.Alessandri-Haber N, Dina OA, Chen X, Levine JD. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J Neurosci. 2009;29:6217–28. doi: 10.1523/JNEUROSCI.0893-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, et al. Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+ influx. Arterioscler Thromb Vasc Biol. 2010;30:851–8. doi: 10.1161/ATVBAHA.109.196584. [DOI] [PubMed] [Google Scholar]