Abstract
The bacterial mechanosensitive channel MscS provides an excellent model system for the study of mechanosensitivity and for investigations into the cellular response to hypoosmotic shock. Numerous studies have elucidated the structure, function and gating mechanism of Escherichia coli MscS, providing a wealth of information for the comparative analysis of MscS family members in bacteria, archaea, fungi and plants. We recently reported the electrophysiological characterization of MscS-Like (MSL)10, a MscS homolog from the model flowering plant Arabidopsis thaliana. Here we summarize our results and briefly compare MSL10 to previously described members of the MscS family. Finally, we comment on how this and other recently published studies illuminate the possible mechanisms by which ion selectivity is accomplished in this fascinating family of channels.
Keywords: MscS, MscS-Like, Xenopus oocyte, arabidopsis, ion channel, mechanosensitive
Introduction
Mechanosensitive channels sense and respond to membrane tension
All organisms must sense and respond to mechanical force for proper growth and development, and one way in which this may be accomplished is through the activation of mechanosensitive (MS) channels.1,2 MS channels are implicated in the perception of sound, touch and osmotic pressure. Mechanical force applied to the membrane is converted into a change in channel conformation and thus into increased open state probability. Once open, a MS channel allows ions (and in some cases other solutes as well as water) to pass across a membrane; the resulting ion flux may relieve osmotic stress and/or provide a biochemical signal for cellular response.
MscS as a paradigm of MS channel structure and function
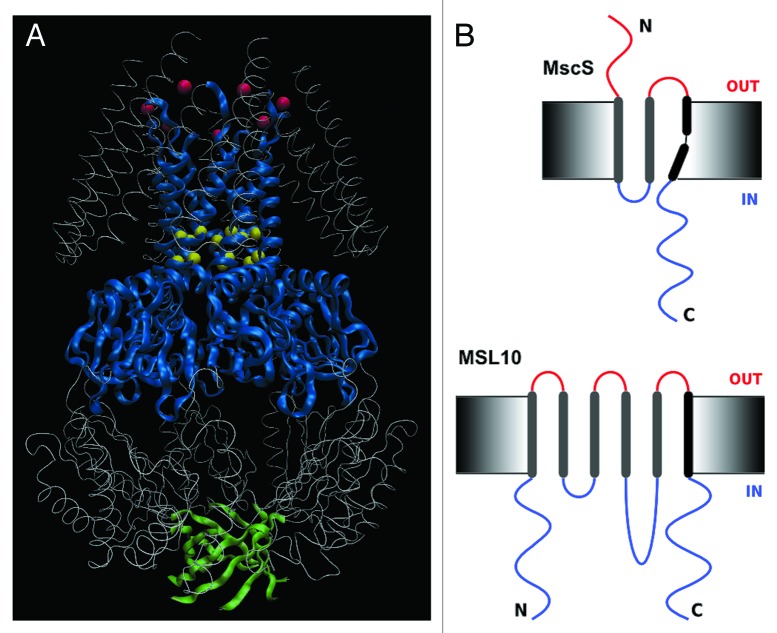
A particularly productive model system for the study of MS channel structure and function has been the Mechanosensitive channel of Small conductance (MscS), one of several channels discovered in the inner membrane of Escherichia coli giant spheroplasts.3 MscS is a weakly anion-selective4-7 ion channel, gated directly through tension in the membrane. When bacterial cells are subjected to hypoosmotic stress, cellular swelling increases membrane tension, which gates MscS and a second MS channel called MscL (Large conductance). Once open, these channels allow solutes to exit the cell, thereby preventing lysis and cell death; for this reason, MscS and MscL are often referred to as “osmotic safety valves.” Crystal structures of E. coli MscS (EcMscS) in two distinct conformational states have revealed a homoheptameric channel with three transmembrane (TM) helices per subunit and a large, C-terminal cytoplasmic domain (Fig. 1A). The interior vestibule formed by the soluble domain connects to the cytoplasm through seven side portals and a β-barrel at the C-terminal end.8-10 These structures provide a firm foundation for interpretation of the functional effects of mutagenic perturbations, for molecular dynamic simulations of gating and inactivation events and for comparative analyses with potential EcMscS homologs from other species.11
Figure 1. Structure and topology of MscS and MSL10. (A) Diagram of the homoheptameric structure of MscS from the revised crystal structure PDB 20AU generated with VMD 9.1 software. Blue ribbons mark TM3 and the middle β-domain; green ribbons indicate the β-barrel at the extreme C-terminus; yellow spheres mark L105 and L109; red spheres mark R88. (B) Topology of MscS (top) and predicted topology of MSL10 (bottom). Black rods represent transmembrane helices, extracellular domains are shown in red, and intracellular domains are blue.
MscS family
MscS family members are found throughout bacterial and archaeal species, occasionally in fungi and in all land plants examined.12,13 Multiple family members are often present in a single genome. MscS homologs have not been identified in animal systems, suggesting they may serve as therapeutic targets for pathogenic bacteria, fungi and protozoa. The domain conserved between EcMscS and its homologs, indicated in blue ribbon in Figure 1A, comprises the pore-lining TM helix TM3 and a portion of the soluble C-terminus called the middle β-domain. Outside of this ~90 amino acid region, MscS family members are highly variable in size, predicted topology and sequence. Diversity in topology is seen both between and within species; the E. coli genome encodes five MscS-related proteins in addition to MscS itself that include different numbers of TM helices (3 to 11 per subunit) and elaborations to periplasmic and cytoplasmic domains. We and others have proposed that the additional protein domains found in MscS homologs may impose novel kinds of regulatory control on a conserved mechanosensitive channel pore through the binding of ions, small molecules or other proteins.11,14
Several MscS homologs have been characterized by single-channel electrophysiology including MscMJ and MscMJLR from Methanococcus jannaschii,15 MscK and MscM from E. coli,16-18 TtMscS from Thermoanaerobacter tengcongensis,19 MscCG from Corynebacterium glutamicum20 and MSC1, a chloroplast-targeted homolog from the green alga Chlamydomonas reinhardtii.21 In addition, a family of bacterial cyclic nucleotide gated channels with homology to MscS has been reported.14 Msy1 and Msy2, two MscS homologs from Schizosaccharomyces pombe, have been functionally characterized but have not been analyzed at the single-channel level.22
MscS-like proteins in land plants
We have focused our investigations on the ten MscS-Like (MSL) proteins encoded in the genome of the flowering plant Arabidopsis thaliana, an excellent model system for molecular, genetic and cell biological analysis. Their diverse topologies, expression patterns and subcellular localizations, as well as reverse genetic analyses indicate that the ten MSL proteins have multiple and distinct functions in plant physiology.13 MSL2 and MSL3, two MscS homologs targeted to the envelope of plastids, serve to relieve hypoosmotic stress sensed within the organelle, suggesting that they function similarly to MscS.23 However, little is known about the physiological function of the other eight MSL proteins, and given the low sequence identity and differing topologies between EcMscS and plant MSLs (see Fig. 1B for a comparison), it was unclear whether these distant MscS homologs were capable of directly forming MS channels.
Results
Electrophysiological characterization of Arabidopsis MSL10
We previously demonstrated that EcMscS can be expressed in Xenopus oocytes and that it exhibits the same conductance and voltage dependence as the channel in its native environment.24 EcMscS is now one of several prokaryotic channels to be successfully expressed in Xenopus ooctyes (a heterologous system with the advantages of large patch size, control of expression levels and the potential for use in automated systems) and can serve as a standard reference to calibrate the gating threshold of other channels expressed in the same oocyte. Recently, we used a similar approach to characterize the gating characteristics of the Arabidopsis MscS homolog MSL10 by single-channel patch-clamp electrophysiology.25 Like EcMscS, expression of MSL10 in Xenopus oocytes produces a mechanically gated ion channel activity, and the latter closely resembles a MSL10-dependent MS channel activity previously characterized in Arabidopsis root protoplast membranes.26 These results provide strong evidence that MscS homologs from land plants are bona fide MS channels and that mechanosensitive gating can be achieved in the MscS family without conservation of many of the specific residues previously shown to be critical for bacterial EcMscS function.
MscS and MSL10 share several channel characteristics in addition to their mechanosensitivity.3,24,25 They share a slight conductance asymmetry under opposite membrane potentials, both exhibiting larger conductances under negative membrane potentials. Like EcMscS, MSL10 activation does not depend on the sign of membrane curvature and is easily observed both under positive and negative membrane potentials. In addition, both MscS and MSL10 are reversibly inhibited by relatively high concentrations of Gd3+ ions when the inner monolayer of the cellular membrane, which is enriched in negatively charged lipids, is exposed to the Gd3+. This behavior was shown to result from increased membrane stiffness caused by the coordinated binding of Gd3+ ions to the negatively charged headgroups of membrane lipids.27
However, MSL10 differs from MscS in several key aspects.3,24,25 Though both channels are mechanosensitive, their tension-dependent gating results in different single-channel conductances: approximately 300 pS for EcMscS and approximately 100 pS for MSL10 when measured in excised oocyte membrane patches in 100 mM symmetric salt. This smaller conductance of MSL10 correlates with a smaller pore; the large tetraethylammonium (TEA+) ion does not pass through (nor does it block) MSL10, though it is easily transported by EcMscS. Unlike EcMscS, which displays hysteresis only at very short pressure ramps, MSL10 closes more slowly and always at lower tension than at which it opened, regardless of the length of pressure ramp, transmembrane potential or sign of membrane curvature. Interestingly, the closing pressure threshold for MSL10 is close to zero, and often a fraction of the channels stay open for seconds after membrane pressure is released. Similar behavior has been reported for the algal MscS homolog MSC1,21 and it is possible that this gating behavior is restricted to the eukaryotic lineage. Thus, MSL10 shares many basic features with EcMscS but differs in a number of details that are likely to reflect MSL10s distinctive functions in a multicellular eukaryote.
MscS homologs show different ion channel selectivities
Ion selectivity is one of the key features that distinguish different MscS family members. MSL10 demonstrates a preference for anions, with PCl: PNa = 5.9. This is similar to the ion selectivity of MSC1 from Chlamydomonas (PCl: PK = 7)21 and that of TtMscS (PCl: PK = 8.7).19 Other MscS homologs either show a slight preference for anions (PCl: PK = 1.2 − 3 for EcMscS)4-7 or demonstrate a preference for cations (MscMJ and MscMJLR have PCl: PK = 0.17 and 0.2 respectively,15 MscM has PCl: PK = 0.417,18 and MscCG has PCl: PK = 0.320). These preferences are moderate; for comparison, the bacterial potassium channel KcsA prefers K+ over Na+ by a factor of over 1000.28
Like that of KscA, the selectivity filter of MscS family members could be located in the permeation pathway and differences in ion selectivity or preference observed between MscS family members attributed to changes in the pore size, electrostatic interactions, or both. In EcMscS, two “hydrophobic seal” residues, L105 and L109, generate the narrowest constriction of the pore. Figure 2 shows an alignment of the protein sequence corresponding to MscS TM2, TM3 and the upper middle β-domain from the best-studied MscS homologs. This alignment shows that the bulky hydrophobic residue at the L109 position is conserved among all the MscS family members (boxes). The presence of charged or polar residues within the permeation pathway may naively be expected to impact the efficiency with which a particular ion passes through the channel pore, but there is no clear correlation for any of the channels presented in Figure 2 except for MscCG, indicating that simply adding up the charged residues present in the pore-lining helix is an insufficient approach to predicting selectivity. MscCG has demonstrated a preference for cations, and its pore-lining TM helix harbors six negatively charged/polar residues (indicated in blue) and only 1 positively charged/polar residue (indicated in red). Even in this case, the contribution of charged residues in the pore-lining helix to ion selectivity is not straightforward. For example, MscCG D117 is unlikely to face the channel pore by analogy to the corresponding residue in MscS, which is suggested to instead interact with G168 in the cytoplasmic cage, thus stabilizing MscS’s inactivated state.29 The original EcMscS crystal structure revealed a ring of basic residues at the periplasmic end of TM2 corresponding to R88, an arrangement that was proposed to contribute to the anionic preference of EcMscS.8,9 However, R88 (indicated with a star) is not conserved among the other homologs, even those that show a relatively strong preference for anions. TtMscS and MscCG have nearby residues that are positively charged or polar (K86 and Q89, respectively), but these channels show opposite ionic preferences so these residues are unlikely to contribute substantially to ion selection.

Figure 2. Alignment of relevant amino acid sequences from selected MscS homologs. Sequence from archaeal (M. jannaschii), bacterial (E. coli, T. tengcongensis, C. glutamicum), algal (C. reinhardtii) and plant (A. thaliana) MscS homologs corresponding to TM2, TM3 and a portion of the middle-β domain were aligned with UGENE software (ugene.unipro.ru/). Bars in the bottom indicate the relevant domains from the EcMscS structure. Charged residues located at the end of TM2 and possibly affecting channel selectivity are in green, while the green star marks EcMscS R88. In the sequences that align with EcMscS TM3, hydrophobic residues are labeled gray, positively charged residues are labeled red and negatively charged residues are blue. The conservation of the pore constriction-forming residues from EcMscS, L105 and L109, is indicated with boxes.
It is now becoming clear that structural elements other than the permeation pore contribute to the variation in ion selectivity observed in MscS family members. Recent evidence indicates that at least one way in which ion selectivity is modulated is through the structure of the cytoplasmic vestibule. Recent molecular dynamics simulations support a model wherein the cytoplasmic domain of EcMscS serves as a pre-filter, ensuring that the loss of cations and anions upon channel gating are relatively closely balanced.30 In addition, the recently reported structure of TtMscS, the anion-selective MscS homolog from Thermoanaerobacter tengcongensis, differs from the structure of EcMscS both in the size of the side portals in the cytoplasmic vestibule and in the structure and sequence of a β-barrel at the extreme C-terminus (indicated in green in Fig. 1).19 Analysis of EcMscS/TtMscS chimeras provides evidence that the difference in ion selectivities can be attributed in part, to this β-barrel sequence (which is distinct from the middle β-domain, indicated in blue). Elaborations to this C-terminal β-barrel sequence may in fact underlie the ability to transport specialized solutes such as glutamate. Each monomer of the MscCG channel has a 247 amino acid C-terminal extension, and this unique domain may confer the ability to efficiently transport glutamate onto EcMscS.31 The relevance of these observations to the ion selectivity filter of MSL10 is not yet clear, as we were unable to identify any residues in the cytoplasmic domain (beyond the middle β-domain) that are conserved between EcMscS and MSL10.
Physiological implications of ion selectivity
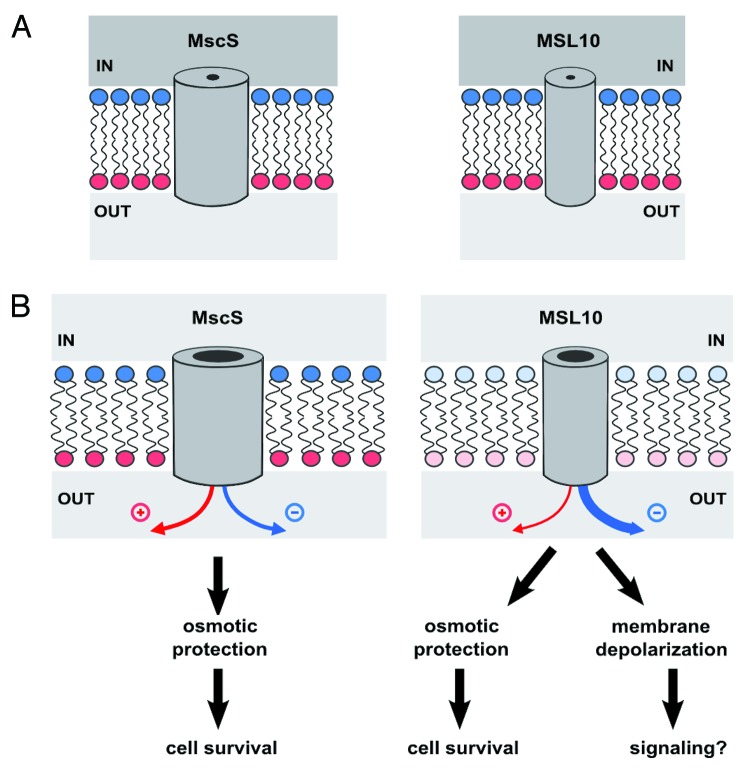
Two aspects of MSL10 behavior have important implications for its potential roles in plant physiology: its moderate preference for anions and its slow closing kinetics. Depolarization of the plant membrane (from a typical resting potential of around −150 mV)32 occurs in response to plant hormone signal transduction, low ambient temperature, pathogen infection and other signals and is in many cases mediated by Cl− efflux through anion channels.33 Membrane depolarization leads to the activation of depolarization-activated Ca2+ channels, Shaker-type K+ channels and possibly to the propagation of signals from cell to cell.33 A classic example is the activation of the Slow Anion Channel-Associated 1 (SLAC1) channel by perceived dehydration stress, which leads to the closing of stomata (pores in the epidermis of the leaf) via membrane depolarization and a well-studied cascade of ion and osmolyte efflux. We thus speculate that MSL10, once gated by high membrane tension (perhaps generated by osmotic stress, cell wall damage, organ bending or pathogenic invasion), preferentially releases anions from the cell, continuing to depolarize the cellular membrane until tension returns to baseline. MSL10 gating may therefore have two distinct consequences, as illustrated in Figure 3: (1) the release of osmolytes and the subsequent relief from hypoosmotic stress and (2) the generation of a cellular signal through membrane depolarization, which in turn would trigger the activation of depolarization-activated ion channels, leading to (as yet unknown) downstream cellular responses. It is important to note that EcMscS appears to accomplish the opposite of what we propose above for MSL10, carefully balancing the passage of positively and negatively charged osmolytes in order to prevent membrane depolarization.30
Figure 3. Model for the function of MSL10, as compared with MscS. (A) MscS and MSL10 channels in cells under severe hypoosmotic stress, before the channels open. Channels are drawn as simple tubes piercing the lipid bilayer. Positively and negatively charged monolayers are indicated with red and blue lipid headgroups, respectively. Hyperosmotic medium inside the cell is dark gray, while hypoosmotic medium in the extracellular space in light gray. (B) After opening of the channels. Both MscS and MSL10 release osmolytes, providing protection from lysis and allowing cell survival. However, the increased preference for anions of MSL10 results in the depolarization of the plasma membrane upon gating (shown as decreased intensity of red and blue). Therefore, MSL10 functions are likely not restricted to relief of hypoosmotic stress but also may include signaling through downstream depolarization-activated ion channels.
In summary, recently reported characterizations of additional members of the MscS family of MS channels—including MSL10 from a multicellular land plant and TtMscS from a thermophilic anaerobe—bring new information to bear on the structure and function of this intriguing family of proteins. The wealth of information regarding the structure and function of EcMscS provides a solid basis for the interpretation of these data. The observed diversity in ion selectivity in the MscS family may be attributable to differences in the structure of the soluble C-terminus and/or to differences in the constriction and electrostatic interactions of each channel’s permeation pathway. Ion selectivity has important implications for the potential biological functions of a channel; for MSL10 its predicted function expands to include signal transduction as well as osmoregulation. Future investigations in our and other laboratories should soon test these predictions.
Acknowledgments
We are grateful to Kira Veley, Margaret Wilson and Elizabeth Frick for helpful discussions and comments on the manuscript. This project was supported in part by American Recovery and Reinvestment Act (ARRA) funds through grant R01GM084211-01 from the National Institutes of Health to Doug Rees, Rob Phillips and E.S.H.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/channels/article/24505
References
- 1.Arnadóttir J, Chalfie M. Eukaryotic mechanosensitive channels. Annu Rev Biophys. 2010;39:111–37. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- 2.Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annu Rev Microbiol. 2010;64:313–29. doi: 10.1146/annurev.micro.112408.134106. [DOI] [PubMed] [Google Scholar]
- 3.Booth IR, Rasmussen T, Edwards MD, Black S, Rasmussen A, Bartlett W, et al. Sensing bilayer tension: bacterial mechanosensitive channels and their gating mechanisms. Biochem Soc Trans. 2011;39:733–40. doi: 10.1042/BST0390733. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, Moe PC, Chandrasekaran S, Booth IR, Blount P. Ionic regulation of MscK, a mechanosensitive channel from Escherichia coli. EMBO J. 2002;21:5323–30. doi: 10.1093/emboj/cdf537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sukharev S. Purification of the small mechanosensitive channel of Escherichia coli (MscS): the subunit structure, conduction, and gating characteristics in liposomes. Biophys J. 2002;83:290–8. doi: 10.1016/S0006-3495(02)75169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sotomayor M, Vásquez V, Perozo E, Schulten K. Ion conduction through MscS as determined by electrophysiology and simulation. Biophys J. 2007;92:886–902. doi: 10.1529/biophysj.106.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards MD, Bartlett W, Booth IR. Pore mutations of the Escherichia coli MscS channel affect desensitization but not ionic preference. Biophys J. 2008;94:3003–13. doi: 10.1529/biophysj.107.123448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bass RB, Strop P, Barclay M, Rees DC. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298:1582–7. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- 9.Steinbacher S, Bass RB, Strop P, Rees DC. Structures of the prokaryotic mechanosensitive channels MscL and MscS. In: Hamill OP, ed. Mechanosensitive Ion Channels, Part A. Amsterdam: Academic Press, 2007:1-24. [Google Scholar]
- 10.Wang W, Black SS, Edwards MD, Miller S, Morrison EL, Bartlett W, et al. The structure of an open form of an E. coli mechanosensitive channel at 3.45 A resolution. Science. 2008;321:1179–83. doi: 10.1126/science.1159262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haswell ES, Phillips R, Rees DC. Mechanosensitive channels: what can they do and how do they do it? Structure. 2011;19:1356–69. doi: 10.1016/j.str.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pivetti CD, Yen MR, Miller S, Busch W, Tseng YH, Booth IR, et al. Two families of mechanosensitive channel proteins. Microbiol Mol Biol Rev. 2003;67:66–85. doi: 10.1128/MMBR.67.1.66-85.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haswell ES. MscS-like proteins in plants. In: Hamill OP, ed. Mechanosensitive Ion Channels, Part A. Amsterdam: Academic Press, 2007:329-59. [Google Scholar]
- 14.Malcolm HR, Maurer JA. The mechanosensitive channel of small conductance (MscS) superfamily: not just mechanosensitive channels anymore. Chembiochem. 2012;13:2037–43. doi: 10.1002/cbic.201200410. [DOI] [PubMed] [Google Scholar]
- 15.Kloda A, Martinac B. Mechanosensitive channels in archaea. Cell Biochem Biophys. 2001;34:349–81. doi: 10.1385/CBB:34:3:349. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Edwards MD, Jeong H, Roth J, Booth IR. Identification of mutations that alter the gating of the Escherichia coli mechanosensitive channel protein, MscK. Mol Microbiol. 2007;64:560–74. doi: 10.1111/j.1365-2958.2007.05672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berrier C, Besnard M, Ajouz B, Coulombe A, Ghazi A. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J Membr Biol. 1996;151:175–87. doi: 10.1007/s002329900068. [DOI] [PubMed] [Google Scholar]
- 18.Schumann U, Edwards MD, Rasmussen T, Bartlett W, van West P, Booth IR. YbdG in Escherichia coli is a threshold-setting mechanosensitive channel with MscM activity. Proc Natl Acad Sci U S A. 2010;107:12664–9. doi: 10.1073/pnas.1001405107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Wang J, Feng Y, Ge J, Li W, Sun W, et al. Structure and molecular mechanism of an anion-selective mechanosensitive channel of small conductance. Proc Natl Acad Sci U S A. 2012;109:18180–5. doi: 10.1073/pnas.1207977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Börngen K, Battle AR, Möker N, Morbach S, Marin K, Martinac B, et al. The properties and contribution of the Corynebacterium glutamicum MscS variant to fine-tuning of osmotic adaptation. Biochim Biophys Acta. 2010;1798:2141–9. doi: 10.1016/j.bbamem.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama Y, Fujiu K, Sokabe M, Yoshimura K. Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc Natl Acad Sci U S A. 2007;104:5883–8. doi: 10.1073/pnas.0609996104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama Y, Yoshimura K, Iida H. Organellar mechanosensitive channels in fission yeast regulate the hypo-osmotic shock response. Nat Commun. 2012;3:1020. doi: 10.1038/ncomms2014. [DOI] [PubMed] [Google Scholar]
- 23.Veley KM, Marshburn S, Clure CE, Haswell ES. Mechanosensitive channels protect plastids from hypoosmotic stress during normal plant growth. Curr Biol. 2012;22:408–13. doi: 10.1016/j.cub.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maksaev G, Haswell ES. Expression and characterization of the bacterial mechanosensitive channel MscS in Xenopus laevis oocytes. J Gen Physiol. 2011;138:641–9. doi: 10.1085/jgp.201110723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maksaev G, Haswell ES. MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc Natl Acad Sci U S A. 2012;109:19015–20. doi: 10.1073/pnas.1213931109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol. 2008;18:730–4. doi: 10.1016/j.cub.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 27.Ermakov YA, Kamaraju K, Sengupta K, Sukharev S. Gadolinium ions block mechanosensitive channels by altering the packing and lateral pressure of anionic lipids. Biophys J. 2010;98:1018–27. doi: 10.1016/j.bpj.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noskov SY, Roux B. Ion selectivity in potassium channels. Biophys Chem. 2006;124:279–91. doi: 10.1016/j.bpc.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 29.Koprowski P, Grajkowski W, Isacoff EY, Kubalski A. Genetic screen for potassium leaky small mechanosensitive channels (MscS) in Escherichia coli: recognition of cytoplasmic β domain as a new gating element. J Biol Chem. 2011;286:877–88. doi: 10.1074/jbc.M110.176131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gamini R, Sotomayor M, Chipot C, Schulten K. Cytoplasmic domain filter function in the mechanosensitive channel of small conductance. Biophys J. 2011;101:80–9. doi: 10.1016/j.bpj.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Becker M, Börngen K, Nomura T, Battle AR, Marin K, Martinac B, et al. Glutamate efflux mediated by Corynebacterium glutamicum MscCG, Escherichia coli MscS, and their derivatives. Biochim Biophys Acta. 2013;1828:1230–40. doi: 10.1016/j.bbamem.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Hedrich R. Ion channels in plants. Physiol Rev. 2012;92:1777–811. doi: 10.1152/physrev.00038.2011. [DOI] [PubMed] [Google Scholar]
- 33.Ward JM, Mäser P, Schroeder JI. Plant ion channels: gene families, physiology, and functional genomics analyses. Annu Rev Physiol. 2009;71:59–82. doi: 10.1146/annurev.physiol.010908.163204. [DOI] [PMC free article] [PubMed] [Google Scholar]