Abstract
GATA-4 is an important transcription factor involved in several developmental processes of the heart, such as cardiac myocyte proliferation, differentiation and survival. The precise mechanisms underlying the regulation of GATA-4 remain unclear, this is especially true for the mechanisms that mediate the post-transcriptional regulation of GATA-4. Here, we demonstrate that miR-200b, a member of the miR-200 family, is a critical regulator of GATA-4. Overexpression of miR-200b leads to the downregulation of GATA-4 mRNA and a decrease in GATA-4 protein levels. Moreover, miR-200b not only inhibits cell growth and differentiation but also reverses the growth response mediated by GATA-4, whereas depletion of miR-200b leads to a slight reversal of the anti-growth response achieved by knocking down endogenous GATA-4. More importantly, the cell cycle-associated gene cyclin D1, which is a downstream target of GATA-4, is also regulated by miR-200b. Thus, miR-200b targets GATA-4 to downregulate the expression of cyclin D1 and myosin heavy chain (MHC), thereby regulating cell growth and differentiation.
Keywords: GATA-4, miR-200b, transcription regulation, cell growth, cell differentiation
Introduction
GATA-binding protein 4 (GATA-4), a zinc finger transcription factor, is a master regulator of developmental processes of the heart, such as cardiac myocyte proliferation, differentiation and survival.1-6 Recent studies indicate that it is also involved in a number of other processes such as female fertility and carcinogenesis.7-9 As a regulator of several target genes, GATA-4 plays many important roles.4,9-12 However, the precise mechanisms by which GATA-4 itself is regulated are not yet fully understood. The expression of GATA-4 could be regulated at the post-translational or post-transcriptional level. Mechanisms of post-translational regulation include protein phosphorylation, acetylation, sumoylation and methylation, whereas post-transcriptional modification mechanisms include the stabilization of mRNA prior to protein synthesis. Although it has been established that the activity of GATA-4 can be modulated through post-translational modifications, including protein phosphorylation, acetylation, sumoylation and methylation,13,14 the mechanisms underlying the post-transcriptional regulation of GATA-4 remain unclear.
MicroRNAs (miRNAs) are short, highly conserved noncoding RNA molecules that play a role in post-transcriptional regulation by targeting the 3′ untranslated region (3′-UTR) of target gene mRNAs, leading to mRNA degradation and translational repression. Recent studies have shown that miR-26b binds the GATA-4 3′-UTR to repress its translation.15 Interestingly, bioinformatic analysis predicted that the 3′- UTR of GATA-4 also contains a miR-200b target site, raising the possibility that miR-200b targets GATA-4.
The miR-200 family consists of five members, miR-200a, miR-200b, miR-200c, miR-429 and miR141, which regulate the transcription factors Zeb1 and Ets-1 as well as Suz12, a subunit of the polycomb repressor complexes.16-18 Previous studies have shown that miR-200b is involved in epithelial to mesenchymal transition, formation and maintenance of cancer stem cells, invasion of prostate cancer cells and gastric carcinoma.16-24 Recently, miR-200b was found to be involved in the angiogenic response of endothelial cells.18 miR-200b exerts these effects through targeting specific genes, such as ZEB1 and SIP1, Suz12 and Ets-1.16-18 However, it remains unclear whether miR-200b targets the transcription factor GATA-4. Bioinformatics analyses suggest that the mouse GATA-4 3′-UTR contains binding sites for miR-26ab/1297/4465, miR-200bc/429/548a, miR-122/122a/1352 and miR-208ab. Among these miRNAs, miR-26b has been demonstrated to target GATA-4 during cardiac hypertrophy,15 so it would be interesting to determine whether miR-200b targets GATA-4, which contributes to the establishment of the post-transcriptional mechanisms in regulating GATA-4.
In this study, we have identified GATA-4 as a novel direct target of miR-200b. We demonstrate for the first time that miR-200b-mediated downregulation of GATA-4 leads to subsequent downregulation of cyclin D1 and myosin heavy chain (MHC) expression, resulting in inhibition of cell growth and differentiation.
Results
miR-200b inhibits cell proliferation by inducing cell cycle arrest and apoptosis
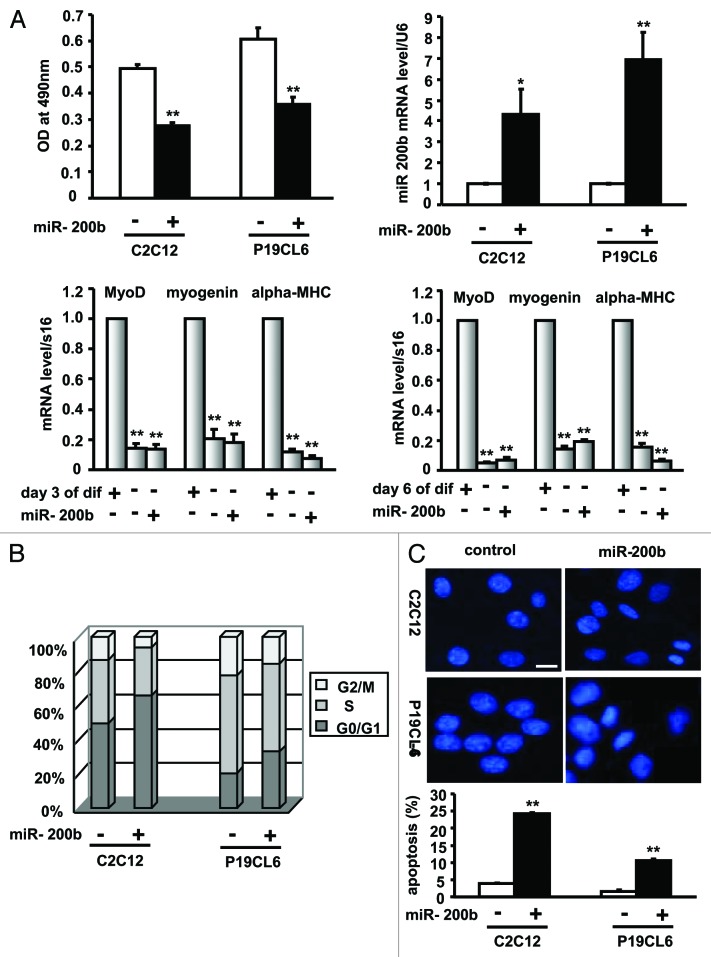
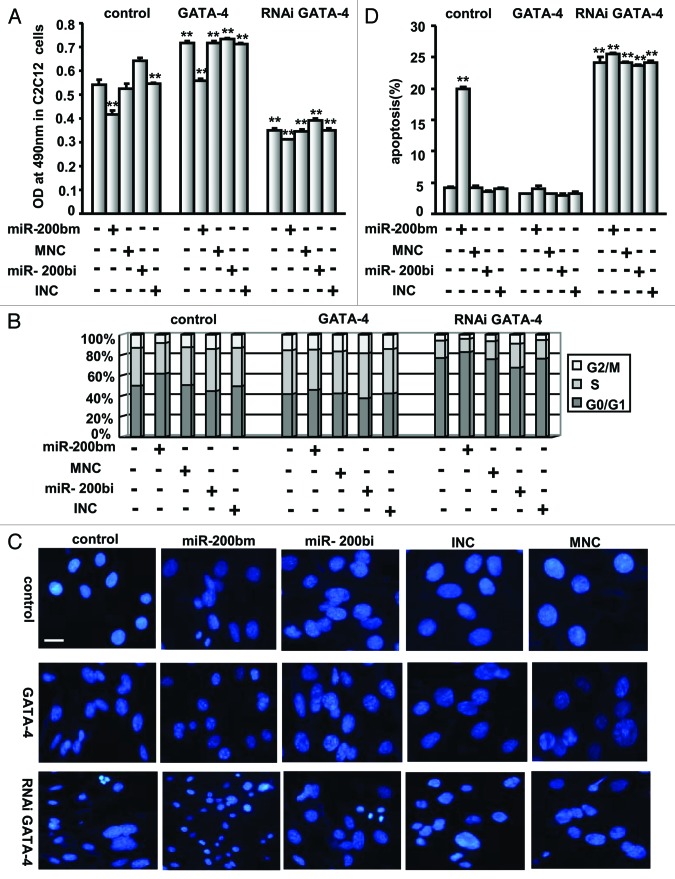
To elucidate the specific role of miR-200b in cell growth, C2C12 and P19CL6 cells were stably transfected with pri-miR-200b to upregulate endogenous miR-200b and subsequently plated in 96-well plates to measure cell viability. The miR-200b level in each stable cell line was determined by quantitative real-time PCR (qPCR) (Fig. 1A, upper right panel), and cell viability was measured by the MTT assay (Fig. 1A, upper left panel). Interestingly, C2C12 and P19CL6 cells stably expressing miR-200b demonstrated a 44% and 41% reduction in cell number and a 4.3- and 6.9-fold increase in miR-200b levels, respectively (Fig. 1). These data suggested that miR-200b has an anti-proliferative effect on C2C12 and P19CL6 cells. To further determine whether C2C12 cells stably transfected with pri-miR-200b were reserved in an undifferentiated state, the expression of myogenin, MyoD and α-MHC, three muscle-specific genes, was analyzed by real-time PCR. As shown in Figure 1A (lower), when compared with C2C12 cells on differentiation day 3 and day 6, myogenin, MyoD and α-MHC mRNA levels were significantly decreased, suggesting that miR-200b maintains C2C12 cells in an undifferentiated state.
Figure 1. miR-200b inhibits cell growth. (A) The effect of miR-200b on cell proliferation. C2C12 and P19CL6 cells stably transfected with pri-miR-200b or with pcDNA3.1(+) empty vector were seeded in 96-well plates and grown for 24 h. Cell viability was determined by MTT assay as described in the Materials and Methods section (upper left). miR-200b expression levels were analyzed by qPCR (upper right). The expression of myogenin, MyoD and α-MHC, three muscle-specific genes was analyzed by real-time PCR in C2C12 cells treated as described above and differentiated for 3 d (lower left) and 6 d (lower right). dif, differentiation. The data shown are the mean ± SEM of three independent experiments. * indicates p < 0.05 vs. control, and ** indicates p < 0.01 vs. control. (B) The effect of miR-200b on cell cycle progression. miR-200b-expressing C2C12 and P19CL6 cells and control cells were subjected to FACS analysis. (C) The effect of miR-200b on apoptosis. C2C12 and P19CL6 cells were treated as described in Figure 1A and subjected to DAPI staining to monitor chromatin fragmentation as an indicator of apoptosis (upper), or subjected to annexin V-FITC- propidium iodide (PI) double staining to quantify the percentage of apoptotic cells (lower). The results are the mean ± SE. M of three independent experiments. ** indicates p < 0.01 compared with control. Images were taken using a 20 × objective. Scale bar is 50 μm and applies to all images in Figure 1C.
To determine whether the anti-proliferative effect of miR-200b is due to cell cycle arrest, we performed FACS analysis following propidium iodide (PI) staining of C2C12 and P19CL6 cells stably transfected with pri-miR-200b. In comparison to control cells, the G0/G1 population among cells overexpressing miR-200b increased by 32% in C2C12 cells and 62% in P19CL6 cells, whereas the G2/M population decreased by 53% and 31%, respectively (Fig. 1B). These data suggested that miR-200b leads to cell cycle arrest in C2C12 and P19CL6 cells.
To determine whether the anti-proliferative effect of miR-200b results from apoptosis, we examined the effect of miR-200b on apoptosis in C2C12 and P19CL6 cells by evaluating chromatin condensation. Cells overexpressing miR-200b had higher levels of condensed chromatin than the control cells (Fig. 1C, upper). Consistent with these observations, FACS analysis using annexin V-FITC-propidium iodide (PI) double staining showed a 6.2- and 6.6-fold increase in the percentage of apoptotic C2C12 and P19CL6 cells, respectively (Fig. 1C, lower), suggesting that miR-200b induces apoptosis in C2C12 and P19CL6 cells.
Taken together, these results indicated that miR-200b inhibits the growth of C2C12 and P19CL6 cells by arresting cell cycle and inducing apoptosis.
miR-200b blocks the differentiation of P19CL6 and C2C12 cells
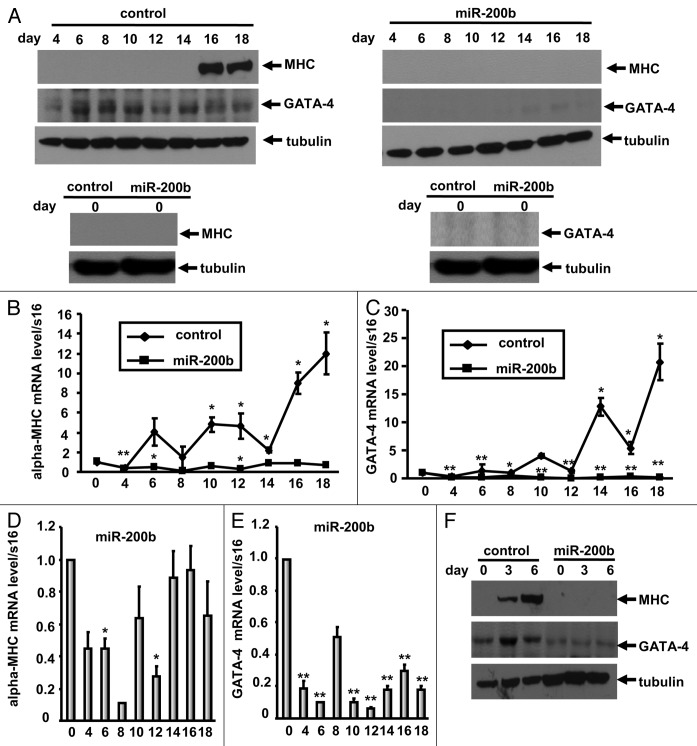
P19CL6 embryonal carcinoma cells have been established as an effective model for studying cardiomyogenesis because they can be efficiently differentiated into beating cardiomyocytes in the presence of 1% DMSO, this finding has been confirmed by immunoblotting using the anti-sarcomeric MHC antibody MF20.19 To elucidate the specific role of miR-200b in cardiomyocyte differentiation, we established a stable cell line constitutively overexpressing miR-200b and cultured these cells in medium containing 1% DMSO. Immunoblot analysis of 50-μg cell lysate samples using the anti-sarcomeric MHC antibody MF20 suggested that in contrast to P19CL6 cells, which differentiate into cardiomyocytes with the expresssion of the cardiac markers α-MHC (Fig. 2A and B), the cells overexpressing miR-200b did not differentiate into cardiomyocytes with absent expression of α-MHC until day 18 (Fig. 2A). Consist with these results, qPCR analysis revealed that during differentiation, mRNA levels of the cardiac transcription factor GATA-4 and sarcomeric α-MHC were significantly decreased in cells overexpressing miR-200b compared with pcDNA 3.1(+) empty vector-transfected cells (Fig. 2B and C). Furthermore, when compared with the cells on differentiation day 0, GATA-4 and α-MHC mRNA levels decreased in miR-200b-overexpressing cells during the differentiation time course, resulting in levels of GATA-4 and α-MHC that were insufficient for efficient differentiation into cardiomyocytes (Fig. 2D and E). These results suggest that miR-200b inhibits the expression of cardiac-specific genes and blocks cardiomyocyte differentiation of P19CL6 cells.
Figure 2. miR-200b inhibits cell differentiation. (A) The effect of miR-200b on cardiomyocyte differentiation was assessed using MF20 immunoblotting in pCDNA3.1(+) empty vector- (upper left) and pri-miR-200b-transfected (upper right) P19CL6 cell lysates. The cells were cultured for 18 d in 1% DMSO. At the indicated time points, the cell lysates were subjected to immunoblotting analysis with the antibodies indicated in the figure. The cell lysates were also subjected to immunoblotting analysis on day 0 (before treatment with DMSO) (lower left and lower right). Western blot analysis shows inhibition of GATA-4 and sarcomeric MHC during the differentiation of P19CL6 cells stably expressing miR-200b when compared with control cells. (B‒E) The effect of miR-200b on cardiac marker gene expression was assessed by qPCR. On the indicated days, P19CL6 cells transfected with pCDNA3.1(+) empty vector (control) (B and C) or pri-miR-200b (B‒E) were evaluated for α-MHC and GATA-4 mRNA levels. Notably, expression levels of α-MHC and GATA-4 were decreased in P19CL6 cells stably expressing miR-200b. (F) The effect of miR-200b on myogenic differentiation was assessed using MF20 immunoblotting in pCDNA3.1(+) empty vector- and pri-miR-200b-transfected C2C12 cell lysates. The cells were cultured for 6 d in differentiation medium. At the indicated time points, the cell lysates were subjected to immunoblotting analysis with the antibodies indicated in the Fig. Western blot analysis shows inhibition of GATA-4 and sarcomeric MHC during the differentiation of C2C12 cells stably expressing miR-200b when compared with control cells.
To elucidate the specific role of miR-200b in myogenic differentiation of C2C12 myoblasts, we established a stable cell line constitutively overexpressing miR-200b and cultured these cells in medium containing 2% horse serum. Immunoblot analysis of 50-μg cell lysate samples using the anti-sarcomeric MHC antibody MF20 suggested that in contrast to C2C12 cells, which differentiate into contractile myotubes with the expresssion of the muscle-specific gene α-MHC (Fig. 2F), the cells overexpressing miR-200b did not differentiate into myotubes with absent expression of α-MHC until day 6 (Fig. 2F). Furthermore, when compared with C2C12 cells, GATA-4 protein levels decreased in miR-200b-overexpressing cells during the differentiation time course, suggesting that miR-200b inhibits the expression of muscle-specific genes and blocks myogenic differentiation of C2C12 cells.
GATA-4 is a novel direct target of miR-200b
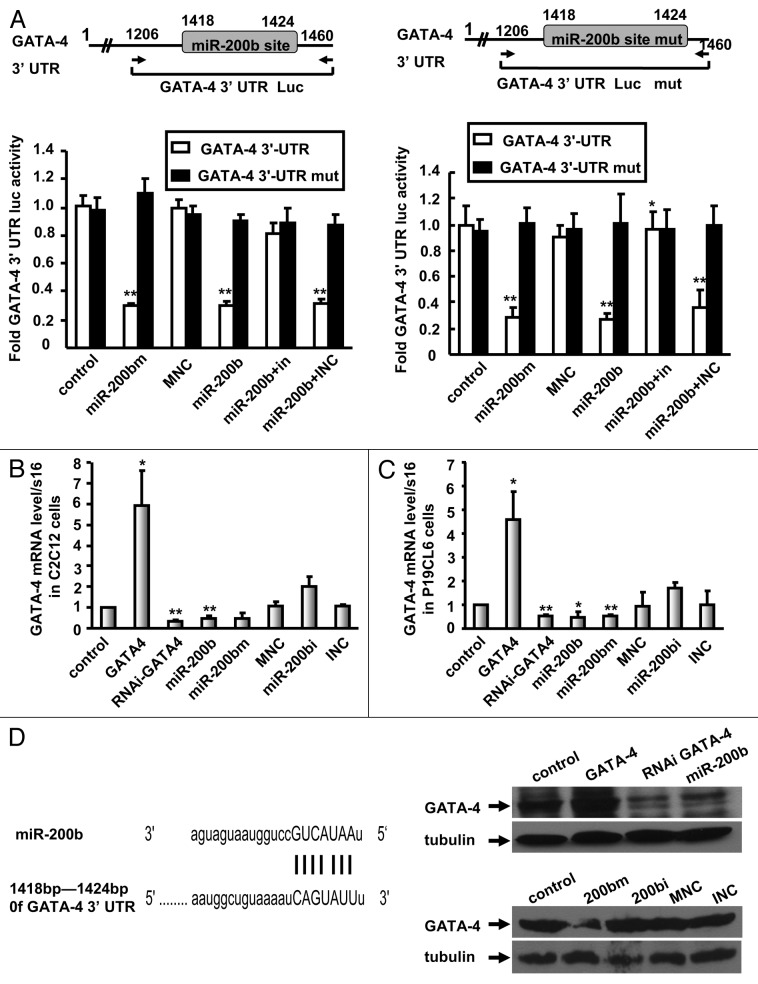
Because our results showed that miR-200b blocks cardiomyocyte differentiation of P19CL6 cells and myogenic differentiation of C2C12 myoblasts by inhibiting GATA-4 expression, we tested whether miR-200b directly targets GATA-4. Bioinformatic analysis predicted perfect complementarity between the miR-200b seed sequence and the GATA-4 3′-UTR sequence (1,418–1,424 bp). To validate the direct binding of the GATA-4 3′-UTR to miR-200b, we performed a miR target luciferase reporter assay using a GATA-4 3′-UTR-Luc plasmid in P19CL6 and C2C12 cells. In contrast to mock-transfected cells, luciferase activity was reduced in miR-200b mimic delivered cells by approximately 70% (C2C12) and 72% (P19CL6) (Fig. 3A). Similar results were observed in miR-200b-overexpressing cells. Furthermore, a miR-200b inhibitor reversed the reduction of luciferase activity induced by miR-200b (Fig. 3A). Importantly, mutation of the miR-200b target site in the GATA-4 3′-UTR abolished miR-200b-mediated repression luciferase expression (Fig. 3A). These results demonstrate that miR-200b binds the GATA-4 3′-UTR sequence.
Figure 3. Direct targeting of GATA-4 by miR-200b. (A) miR target reporter luciferase activity mediated by reporter constructs harboring the wild-type and mutated 3′-UTR of GATA-4 in C2C12 (left) or P19CL6 (right) cells containing miR-200b mimic (miR-200bm), miR-200b mimic negative control (MNC), miR-200b inhibitor negative control (INC), miR-200b and/or miR-200b inhibitor (in or miR-200bi). Schematic representation of the GATA-4 3′ -UTR showing the GATA-4 wild-type and mutant 3′-UTR-Luc constructs harboring the miR-200b target site. The data shown are the mean ± SEM of three independent experiments. ** indicates p < 0.01 vs. the control. (B and C) GATA-4 mRNA levels in C2C12 (B) or P19CL6 cells (C) transfected with control vector, GATA-4, RNAi GATA-4, miR-200b, miR-200b mimic, miR-200b inhibitor or control miRNAs. The results are the mean ± SE. M of three independent experiments. * indicates p < 0.05, and ** indicates p < 0.01 compared with control. (D) GATA-4 and α-tubulin protein levels after transfection with control vector, GATA-4, RNAi GATA-4, miR-200b, miR-200b mimic (200 bm), miR-200b inhibitor (200 bi), miR-200b mimic negative control (MNC), and miR-200b inhibitor negative control (INC) in C2C12 cells. Schematic of the predicted miR-200b-binding sites in the GATA-4 3′-UTR.
To determine whether miR-200b regulates GATA-4 expression, we established stable cell lines expressing GATA-4 or miR-200b or GATA-4-silenced cell lines (RNAi GATA-4) in C2C12 and P19CL6 cells. The GATA-4 mRNA level was reduced by 69% in RNAi GATA-4 cells and by 51% in miR-200b-overexpressing C2C12 cells (Fig. 3B), and was upregulated 5.9-fold in GATA-4-overexpressing C2C12 cells (Fig. 3B). Moreover, upon delivery of the miR-200b mimic in C2C12 cells, the GATA-4 mRNA level was reduced by 54%, whereas the miR-200b inhibitor caused a 2.0-fold increase in the GATA-4 mRNA level (Fig. 3B). Similar results were observed in P19CL6 cells (Fig. 3C). Consistent with the observed effects on mRNA levels, the GATA-4 protein level increased in cells overexpressing GATA-4 and decreased in miR-200b-overexpressing cells and in RNAi GATA-4 cells (Fig. 3D, upper right). Moreover, miR-200b mimic inhibits the protein levels of GATA-4 and miR-200b inhibitor induces the expression of GATA-4 protein (Fig. 3D, lower right). These data established that miR-200b binds the GATA-4 3′-UTR region, resulting in mRNA degradation and translation repression.
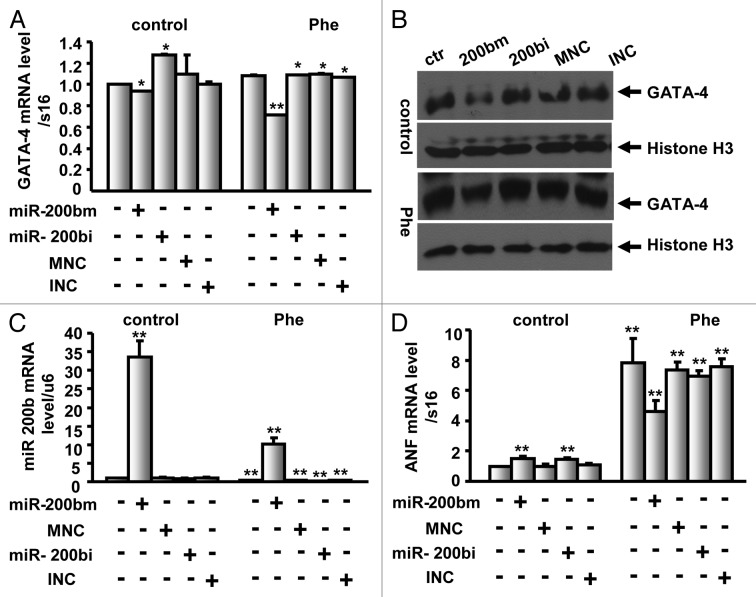
To further determine whether miR-200b targets GATA-4 in normal and hypertrophic neonatal rat ventricular myocytes, cardiomyocytes were treated with the α adrenoceptor agonist Phenylephrine (100 μM) and/or transfected with miR-200b mimic, miR-200b inhibitor or control miRNAs. Upon delivery of the miR-200b mimic, the GATA-4 mRNA level was reduced by 6% and 29% in untreated and Phenylephrine-treated cardiomyocytes, respectively (Fig. 4A), whereas the miR-200b inhibitor caused a 28% increase and no change in the GATA-4 mRNA level in untreated and Phenylephrine-treated cardiomyocytes, respectively (Fig. 4A). However, the GATA-4 protein level was inhibited in untreated and Phenylephrine-treated cardiomyocytes in response to miR-200b mimic (Fig. 4B), suggesting that miR-200b targets GATA-4 in normal and hypertrophic neonatal rat ventricular myocytes, whereas the miR-200b inhibitor caused no significant change in the GATA-4 protein level in untreated and Phenylephrine-treated cardiomyocytes (Fig. 4B). Moreover, the miR-200b level was increased by 32.5-fold in miR-200b mimic delivered cells, and the upregulation was reduced to 10.2-fold upon Phenylephrine treatment (Fig. 4C), indicating that Phenylephrine inhibits the expression of miR-200b. The mRNA levels of ANF, one of the hypertrophy marker genes, were significantly increased upon Phenylephrine treatment (Fig. 4D), indicating that Phenylephrine induces cardiac hypertrophy. However, upon delivery of the miR-200b mimic in untreated and Phenylephrine-treated cardiomyocytes, the expression of ANF exhibited a reverse trend when compared with their respective control cells (Fig. 4D), suggesting that miR-200b may contribute to the reduced hypertrophic response to Phenylephrine.
Figure 4. miR-200b targets GATA-4 in neonatal rat ventricular cardiomyocytes with or without 100 μM phenylephrine (Phe). (A) Neonatal rat ventricular cardiomyocytes were treated with 100 μM Phe and/or transfected with miR-200b mimic, miR-200b inhibitor or control miRNAs, and the mRNA levels of GATA-4 were determined by qPCR using Taqman methods. (B) Nuclear extracts were prepared from neonatal rat ventricular cardiomyocytes treated as described in Figure 4A, and the protein levels of GATA-4 were determined by western blot analysis. Histone H3 was used as a loading control. ctr, control; 200 bm, miR-200b mimic; 200 bi, miR-200b inhibitor; MNC, miR-200b mimic negative control; INC, miR-200b inhibitor negative control. (C and D) miR-200b (C) and ANF (D) expression levels were analyzed by qPCR in cardiomyocytes treated as described in Figure 4A. The results are the mean ± SE. M of three independent experiments. ** indicates p < 0.01 compared with control.
GATA-4 overexpression reverses miR-200b-mediated anti-growth effects in C2C12 cells
To determine the roles of GATA-4 in cell growth, the miR-200b mimic, miR-200b inhibitor or control miRNAs were delivered into the GATA-4-expressing stable cell line. The cells were analyzed for cellular growth by the MTT assay. GATA-4 protein expression was detected in the stable C2C12 cells (Fig. 3D). GATA-4 overexpression was found not only to induce cell growth but also to reverse miR-200b-associated inhibition of cell growth (Fig. 5A).
Figure 5. GATA-4 overexpression reverses miR-200b mimic-mediated anti-growth effects, and GATA-4 downregulation simulates the effects of miR-200b mimic on cell growth. (A) MTT assay for cellular growth after delivery of miR-200b mimic (miR-200bm), miR-200b inhibitor (miR-200bi), miR-200b mimic negative control (MNC) or miR-200b inhibitor negative control (INC) into C2C12 cells in the presence or absence of the GATA-4 expression plasmid. The data shown are the mean ± SEM of three independent experiments. ** indicates p < 0.01 vs. control. (B) FACS analysis of cell cycle progression in C2C12 cells treated as described in (A). (C) DAPI staining to monitor chromatin fragmentation in C2C12 cells treated as described in (A). Images were taken using a 20 × objective. Scale bar is 50 μm and applies to all images in Figure 4C. (D) Annexin V-FITC- propidium iodide (PI) double staining to quantify the percentage of apoptotic cells in C2C12 cells treated as described in (A). The results are the mean ± SE. M of three independent experiments. ** indicates p < 0.01 compared with control.
Consistent with these results, GATA-4 was found to accelerate the cell cycle in C2C12 cells and reverse the miR-200b-mediated cell cycle arrest (Fig. 5B). DAPI staining and FACS analysis using annexin V-FITC-propidium iodide (PI) double staining showed that overexpression of GATA-4 abolishes miR-200b-induced apoptosis in C2C12 cells (Fig. 5C and D). These results suggest that GATA-4 overexpression reverses miR-200b-mediated anti-growth effects by accelerating cell cycle progression and blocking apoptosis.
Downregulation of GATA-4 simulates the effects of miR-200b on cell growth
To further determine the roles of GATA-4 in cell growth, the miR-200b mimic, miR-200b inhibitor or control miRNAs were delivered into the cell line in which GATA-4 was stably knocked down (RNAi GATA-4), and cell growth was evaluated by the MTT assay. GATA-4 protein expression was downregulated in the GATA-4-silenced C2C12 cells (Fig. 3D). Silencing GATA-4 not only blocked cell growth but also simulated the effects of miR-200b on cell growth, whereas miR-200b inhibitor slightly reversed the inhibition of cell growth mediated by RNAi GATA-4 (Fig. 5A).
Consistent with these observations, silencing GATA-4 was found to arrest the cell cycle in C2C12 cells and to simulate miR-200b-mediated cell cycle arrest (Fig. 5B). DAPI staining and FACS analysis using annexin V-FITC- propidium iodide (PI) double staining showed that in addition to inducing apoptosis, silencing GATA-4 simulates miR-200b-mediated apoptosis in C2C12 cells (Fig. 5C and D). These results suggest that GATA-4 silencing simulates miR-200b-mediated anti-growth effects by arresting the cell cycle and inducing apoptosis.
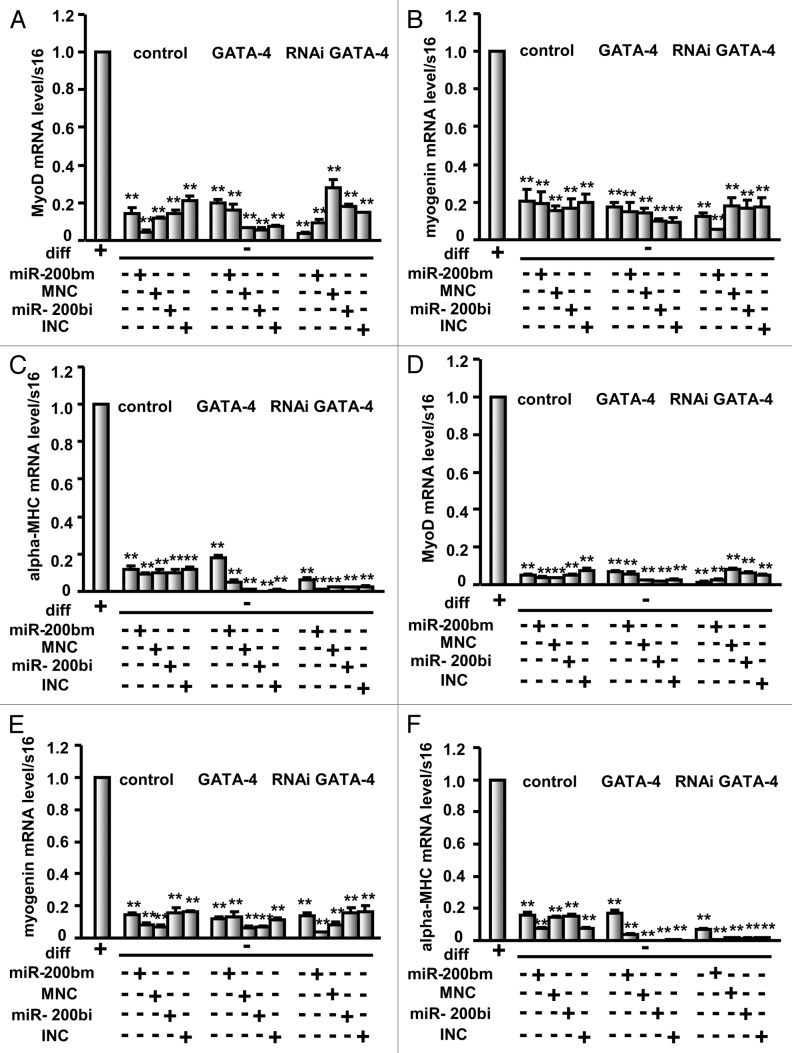
To further determine whether C2C12 cells treated as described above were reserved in an undifferentiated state, the expression of myogenin, MyoD and α-MHC was analyzed by real-time PCR. As shown in Figure 6, when compared with C2C12 cells on differentiation day 3, myogenin, MyoD and α-MHC mRNA levels were significantly decreased in cells transfected with GATA-4 or GATA-4 RNAi expression vector and/or the miR-200b mimic, miR-200b inhibitor or control miRNAs (Fig. 6A‒C). Similar results were observed when compared with C2C12 cells on differentiation day 6 (Fig. 6D‒F), suggesting that these cells treated as described above were in an undifferentiated state.
Figure 6. miR-200b maintains C2C12 cells in an undifferentiated state. The expression of myogenin, MyoD and α-MHC, three muscle-specific genes was analyzed by real-time PCR in C2C12 cells treated as described in Figure 5 and differentiated for 3 d (A‒C) and 6 d (D‒F). diff, differentiation. The results are the mean ± SE. M of three independent experiments. ** indicates p < 0.01 compared with control.
GATA-4 overexpression reverses miR-200b-mediated anti-growth effects in P19CL6 cells
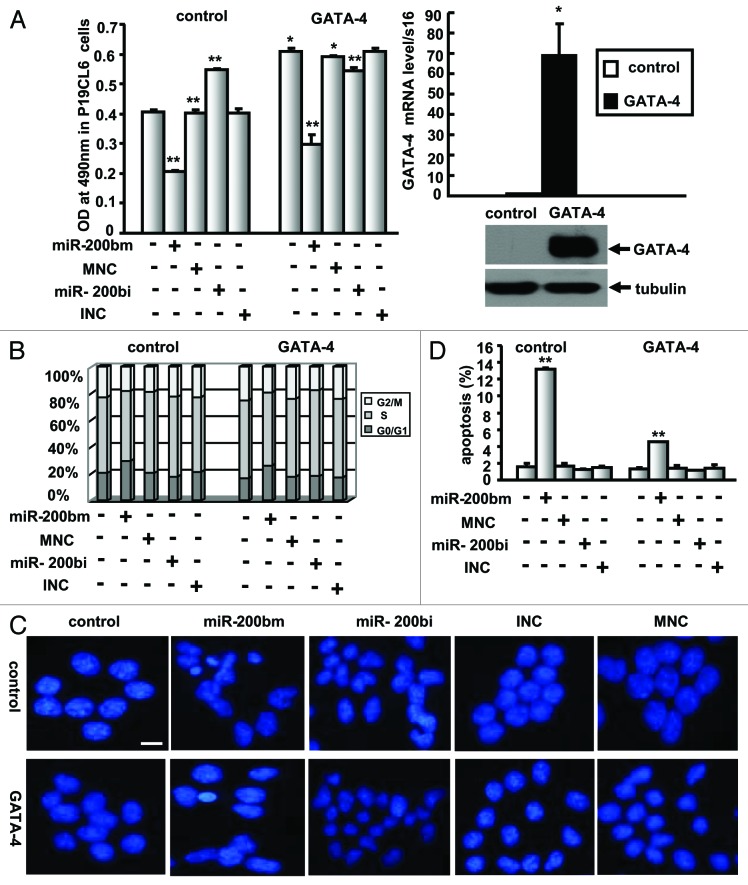
To further determine the role of GATA-4 in cell growth, the stable cell lines expressing GATA-4 were treated with miR-200b mimic, miR-200b inhibitor or control miRNAs followed by MTT assay for cellular growth. The mRNA and protein levels of GATA-4 were increased in the stable P19CL6 cells (Fig. 7A, right panel). Overexpression of GATA-4 not only induced cell growth but also reversed the miR-200b-associated inhibition of cell growth (Fig. 7A).
Figure 7. GATA-4 overexpression reverses miR-200b mimic-mediated anti-growth effects in P19CL6 cells. (A) MTT assay for cellular growth after the delivery of miR-200b mimic (miR-200bm), miR-200b inhibitor (miR-200bi), miR-200b mimic negative control (MNC) or miR-200b inhibitor negative control (INC) into P19CL6 cells in the presence of the GATA-4 expression vector (left). GATA-4 mRNA and protein expression levels were analyzed by qPCR and western blot (right). The data shown are the mean ± SEM of three independent experiments. * indicates p < 0.05 vs. control, and ** indicates p < 0.01 vs. control. (B) FACS analysis of cell cycle progression in P19CL6 cells treated as described in (A). (C) DAPI staining to monitor chromatin fragmentation in P19CL6 cells treated as described in (A). Images were taken using a 20 × objective. Scale bar is 50 μm and applies to all images in Figure 5C. (D) Annexin V-FITC- propidium iodide (PI) double staining to quantify the percentage of apoptotic cells in P19CL6 cells treated as described in (A).The results are the mean ± S. E. M of three independent experiments. ** indicates p < 0.01 compared with control.
Consistent with these results, GATA-4 overexpression was found to accelerate cell cycle progression in P19CL6 cells and to reverse miR-200b-mediated cell cycle arrest (Fig. 7B). Moreover, DAPI staining and FACS analysis using annexin V-FITC-propidium iodide (PI) double staining showed that overexpression of GATA-4 inhibits miR-200b-induced apoptosis in P19CL6 cells (Fig. 7C and D). These results suggest that GATA-4 overexpression leads to the reversal of miR-200b-mediated anti-growth effects by accelerating cell cycle progression and inhibiting apoptosis.
Cyclin D1, a downstream target of GATA-4, is regulated by miR-200b
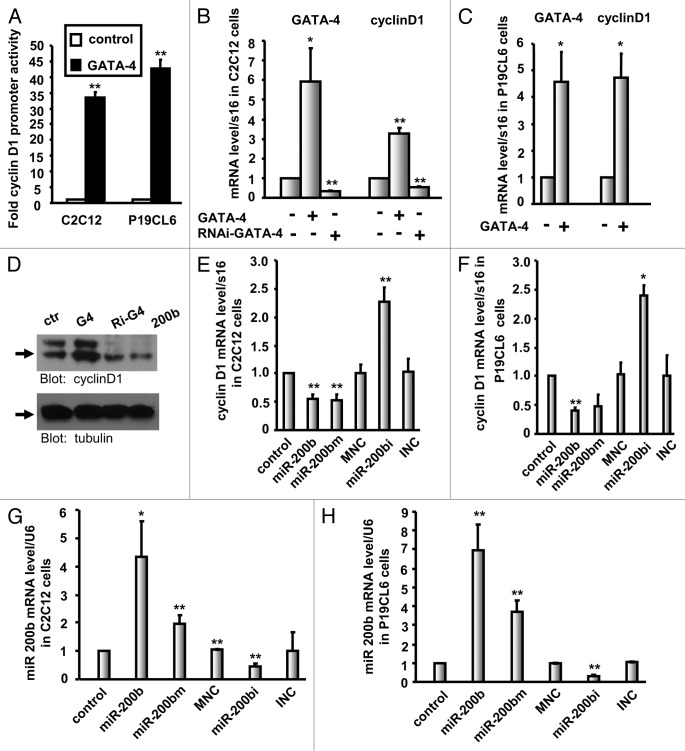
We hypothesized that the effects of miR-200b on GATA-4 regulation and its inhibition of cell cycle and cell growth in C2C12 and P19CL6 cells may depend on cyclin D1 inactivation. To verify this, we first tested whether GATA-4 regulates the transcription and expression of cyclin D1, which is an activator of cell cycle progression. C2C12 and P19CL6 cells were transfected with either the control vector or the GATA-4 expression plasmid along with a reporter plasmid containing the firefly luciferase gene driven by a 1,000-bp fragment of the human cyclin D1 promoter, and luciferase activity was measured 36 h post-transfection. Luciferase activity was found to be higher in GATA-4-transfected C2C12 and P19CL6 cells (up to 33.6- and 42.6-fold increases, respectively) compared with control cells (Fig. 8A). At the same time, we observed an increase in cyclin D1 mRNA levels and protein levels in GATA-4-overexpressing C2C12 or P19CL6 cells (Fig. 8B‒D). Furthermore, knocking down endogenous GATA-4 resulted in reduced expression of cyclin D1 in C2C12 cells (Fig. 8B and D). Taken together, these results suggest that cyclin D1 is a downstream target of GATA-4.
Figure 8. Cyclin D1 is regulated by GATA-4 and is downregulated in miR-200b-transfected cells. (A) Luciferase activity of reporter constructs harboring the 1,000-bp human cyclin D1 promoter sequence in C2C12 or P19CL6 cells in the presence of the GATA-4 expression vector. The data shown are the mean ± SEM of three independent experiments. ** indicates p < 0.01 vs. control. (B and C) Real-time PCR analysis of GATA-4 and cyclin D1 expression in C2C12 (B) or P19CL6 (C) cells in the presence or absence of the GATA-4 expression plasmid. The results are the mean ± SEM * indicates p < 0.05 vs. control, and ** indicates p < 0.01 vs. control. (D) Cyclin D1 and α-tubulin protein levels after transfection of C2C12 cells with the control vector (ctr), GATA-4 (G4), RNAi GATA-4 (Ri-G4) and miR-200b (200b). (E and F) Real-time PCR analysis of cyclin D1 expression in C2C12 (E) or P19CL6 (F) containing miR-200b mimic (miR-200bm), miR-200b inhibitor (miR-200bi), miR-200b mimic negative control (MNC), miR-200b inhibitor negative control (INC) or stably expressing miR-200b. (G and H) miR-200b expression levels were analyzed by real-time PCR. The results shown are the mean ± SEM * indicates p < 0.05 vs. control, and ** indicates p < 0.01 vs. control.
To further confirm whether the expression of cyclin D1 was regulated by miR-200b, C2C12 and P19CL6 cells were transfected with the miR-200b mimic, miR-200b inhibitor or control miRNAs. Measurement of cyclin D1 mRNA levels after 24 h confirmed the miR-200b-mediated inhibition of cyclin D1 transcription (Fig. 8E and F). Similar results were obtained in the stable cell line overexpressing miR-200b (Fig. 8E and F); in these cells, cyclin D1 inhibition was completely abolished by inhibiting miR-200b (Fig. 8E and F). Consistent with these results, the expression of cyclin D1 was decreased in miR-200b-expressing stable cell lines (Fig. 8D). The level of miR-200b in each stable or transfected cell line was determined by qPCR (Fig. 8G and H). Thus, our results suggest that miR-200b inhibits cyclin D1 expression.
In conclusion, we have demonstrated that cyclin D1 is a downstream target of GATA-4 and is inhibited by miR-200b, clearly establishing the presence of a regulatory cascade involving miR-200b, GATA-4 and cyclin D1 in C2C12 and P19CL6 cells.
Discussion
Our results show that miR-200b inhibits cell proliferation and differentiation, and induces cell cycle arrest and apoptosis in C2C12 and P19CL6 cells by targeting GATA-4 and reducing cyclin D1 expression. To the best of our knowledge, we are the first to describe an anti-growth and anti-differentiation function of miR-200b in C2C12 or P19CL6 cells and to establish the regulatory cascade of miR-200b to cyclin D1 via GATA-4, which plays an important role in cardiac biology. Recently, Chan and colleague have shown that miR-200b directly targets GATA-2 to inhibit endothelial cell proliferation and angiogenesis,20 suggesting that miR-200b may regulate all members of the GATA family via the post-transcriptional regulation mechanism.
Three lines of evidence indicate that GATA-4 plays a critical role in cell growth and differentiation. First, the RNAi GATA-4 experiments indicate that GATA-4 is critical for C2C12 cell growth. Second, overexpression of GATA-4 induces cell growth. Third, GATA-4 is downregulated in the miR-200b-expressing stable cell lines, leading to the downregulation of the cell cycle activator cyclin D1 and cardiac differentiation marker gene α-MHC, which are responsible for the repression of growth and differentiation, indicating that GATA-4 is required for growth and differentiation. Our findings are consistent with previous studies describing an essential role for GATA-4 in the cardiac differentiation of mesenchymal stem cells, P19 cells, embryonic stem (ES) cells, mesenchymal stromal cells and P19CL6 cells.1,2,21,22,23 Other studies also describe a direct correlation between GATA-4 and cardiac proliferation showing that GATA-4 regulates cardiac myocyte proliferation in mice through cooperation with GATA-5 or activation of cyclin D2 and Cdk4.4,5 Here, we demonstrate that GATA-4 is required for C2C12 and P19CL6 cell growth, suggesting that GATA-4 stimulates proliferation in various cell types.
The regulation of cyclin D1 expression by miR-200b is a critical step in cell growth. As mentioned above, the loss of miR-200b induces cell growth, and this function of miR-200b depends on cyclin D1 activation. Furthermore, overexpression of miR-200b inhibits cell growth by inhibiting cyclin D1 expression. Thus, cyclin D1 is critical for miR-200b-mediated anti-growth effects. Xia and colleague have shown that miR-200b directly targets RND3 to upregulate cyclin D1 expression and increases S-phase entry.24 The discrepancy between our study and theirs may be due to three factors. First, we observed a downregulation of cyclin D1 in C2C12 and P19CL6 cells, whereas as no downregulation of cyclin D1 was observed in the HeLa cells used by Xia et al. Therefore, miR-200b-dependent regulation of cyclin D1 may be cell type-dependent. Second, we observed a downregulation of cyclin D1 in cells stably overexpressing miR-200b; in contrast, Xia et al. studied cells transiently transfected with miR-200b. Third, we demonstrated that miR-200b targets GATA-4 to downregulate the expression of cyclin D1, whereas Xia et al. showed that miR-200b directly targets RND3 to upregulate the expression of cyclin D1. It is plausible that miR-200b targets genes differentially in different cell types.
In addition to showing that miR-200b regulates cyclin D1, we have also found that GATA-4 activates the transcription and expression of cyclin D1 to induce cell growth. The relevance of the GATA-4 cyclin D1 pathway in the proliferation and differentiation of embryonic cardiomyocytes has also been demonstrated by Nakajima et al., who showed that overexpression of cyclin D1 results in cardiomyocyte hyperproliferation, and cyclin D1 inhibits the differentiation of cardiomyocytes through the degradation of GATA-4.25 In conclusion, a regulatory cascade involving miR-200b, GATA-4 and cyclin D1 maintains cell growth and cell cycle progression.
Because GATA-4 is a critical transcription factor involved in heart development, it is important to understand the mechanisms regulating its mRNA stability and protein translation. In this study, we investigated the roles of miR-200b in regulating the expression of GATA-4 during cell growth and differentiation. We demonstrated that: (1) miR-200b inhibits cell proliferation through inducing cell cycle arrest and apoptosis (Fig. 1), (2) miR-200b blocks the differentiation of C2C12 and P19CL6 cells (Fig. 2), (3) overexpression of miR-200b significantly reduces levels of GATA-4 mRNA protein and GATA-4 transcriptional activity (Fig. 3), (4) GATA-4 overexpression reverses the miR-200b-mediated anti-growth effects in C2C12 and P19CL6 cells, whereas downregulation of GATA-4 simulates the effects of miR-200b on cell growth (Figs. 5 and 7) and (5) miR-200b regulates cyclin D1, a downstream target of GATA-4 (Fig. 8). Taken together, these results provide strong evidence that miR-200b regulates cell growth and differentiation by targeting GATA-4 to downregulate the expression of cyclin D1 and α-MHC.
Except for miR-200b, miR-26b has been demonstrated to target GATA-4 during cardiac hypertrophy.15 However, the interaction of other miRNAs with the GATA-4 3′-UTR and the contributions of these interactions to cell growth and differentiation remain unclear. Interestingly, GATA-4 lies upstream of miR-144/451 in the transcriptional regulatory cascade,26 suggesting that a negative feedback loop between GATA-4 and miRNAs functions in transcriptional regulation.
In conclusion, the anti-growth and anti-differentiation effects of miR-200b are mediated through the targeting of GATA-4 and the suppression of cyclin D1 and α-MHC expression in C2C12 and P19CL6 cells. These findings represent a novel mechanism of GATA-4 post-transcriptional regulation that could be potentially applied in cell growth and differentiation.
Materials and Methods
Plasmids
GATA-4 full-length cDNA was cloned into the EcoRI/KpnI sites of the pcDNA 3.1(+) vector (Invitrogen). The RNAi GATA-4 constructs were designed according to the pSilencer neo™ Instruction Manual (Ambion) as described previously.27 A 404-bp rat genomic fragment encompassing the miR-200b coding region was amplified by PCR and ligated into HindIII/EcoRI sites of the pcDNA 3.1(+) vector. Murine cDNA fragments corresponding to the GATA-4 3′-UTR encompassing the miR-200b binding site were PCR-amplified and ligated into the firefly luciferase reporter construct (pGL3-control, Promega). For the mutant construct, the bases GTAT in the seed sequence of miR-200b were changed to TAGC. All constructs were confirmed by DNA sequencing. The sequences of the primers are listed in Table 1.
Table 1. Primer sequences.
| Forward primer | Reverse primer | ||
|---|---|---|---|
| cloning |
GATA-4 |
GATCGGTACCATGTACCAAAGCCTGGC |
GATCGAATTCTTACGCGGTGATTATGTC |
| Pri-miR-200b |
GATCAAGCTTGGGAGCTCT CCCTAA ATTCC |
GATCGAATTCTTTGGAGATGACAGGTGC TG |
|
| GATA-4 3′ UTR Luc |
ATCGTCTAGACCCACAGAGCTGTAGCCAACT |
TAATGGCCGGCCCGGAGGAAACAGGAATACAGAT |
|
| GATA-4 3′ UTR Luc mut |
ATCGTCTAGACCCACAGAGCTGTAGCCAACT |
TAATGGCCGGCCCGGAGGAAACAGGAATACAGATAAATTTATTAGTTAAGCTATGATTTTACAGCCATTTC |
|
| RNAi GATA-4 |
GATCCGTCTCGATATGTTTGATGACTTCAAGAGA GTCATCAAACATATCGAGATTTTTTGGAAA |
AGCTTTTCCAAAAAATCTCGATATGTTTGATGACTCTCTTGAAGTCATCAAACATATCGAGACG |
|
| q-PCR | GATA-4 |
CAGCAGCAGTGAAGAGATGC |
ATGTCCCCATGACTGTCAGC |
| cyclin D1 |
ATGAGAACAAGCAGACCATCCGCA |
GCTTGACTCCAGAAGGGCTTCAAT |
|
| α-MHC |
GACGCCCAGATGGCTGACTT |
GGTCAGCATGGCCATGTCCT |
|
| 40S ribosomal protein S16 |
TCTGGGCAAGGAGAGATTTG |
CCGCCAAACTTCTTGGATTC |
|
| U6 |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
|
| MiR-200b |
GCGGCTAATACTGCCTGGT |
GTGCAGGGTCCGAGGT |
|
| GATA-4 probe |
FAM-c agg acc agg ctg ttc caa gag-TAMRA |
|
|
| S16 probe | FAM-ctggtgtggatatccggg tccg-TAMRA |
Cell culture, transfection and differentiation
C2C12 (ATCC) and P19CL6 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM, GIBCO) and minimum essential medium alpha medium (α-MEM, GIBCO) supplemented with 10% fetal bovine serum (FBS), respectively. Neonatal rat ventricular cardiomyocytes culture was performed as described previously.28 Transfections were performed 24 h after plating cells using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. Briefly, the cells expressing the cyclin D1 promoter and wild-type or mutant GATA-4 3′-UTR-luc reporter constructs were transiently transfected with GATA-4, miR-200b mimic, miR-200b inhibitor or control miRNAs. The miR-200b mimic, the mimic negative control (mimic-NC), miR-200b inhibitor and the inhibitor negative control (inhibitor-NC) were purchased from GenePharma Co. Ltd. The cells were transfected with 50 nM of the mimic, the inhibitor, the mimic-NC or the inhibitor-NC. Thirty-six hours post-transfection, the cells were harvested, and luciferase activity was measured with a Berthold LB960 luminometer. The amount of reporter was maintained at 1 μg per well of a 12-well plate, and the amount of DNA was kept constant using the empty expression vector.
To construct cells stably expressing GATA-4 and miR-200b, C2C12 and P19CL6 cells were transfected with the empty pcDNA3.1(+) vector (G418-resistant), pcDNA3-GATA-4 or miR-200b using Lipofectamine (Invitrogen). GATA-4-silenced cell lines were generated by transfecting C2C12 cells with RNAi GATA-4 using Lipofectamine (Invitrogen). All cells were transfected for 24 h and treated with 400 μg/ml G418 (Amersham Pharmacia Biotech). G418-resistant colonies were selected after 2 wk.
To induce differentiation, P19CL6 cells were plated at a density of 1.8 × 105 cells in a 6-well plate with a-MEM containing 1% dimethyl sulfoxide (DMSO) for 18 d. The medium was changed every 2 d. For myogenic differentiation, C2C12 cells were cultured until at approximately 90% confluence and induced in differentiation medium consisting of DMEM with 2% horse serum.
Quantitative real-time PCR
Stem-loop qRT-PCR for mature miR-200b was performed as previously described.29 RNA was extracted from C2C12, P19CL6 cells and neonatal rat ventricular cardiomyocytes using the TRIzol method (Invitrogen). First-strand cDNAs were synthesized and used as a template in subsequent real-time PCR reactions as described previously.28 A comparative quantification method was used, and the levels of mRNA and miR-200b were normalized to those of the ribosomal protein S16 and U6, respectively. The mRNA levels of GATA-4 in neonatal rat ventricular myocytes were determined using Premix Ex Taq reagent (Takara) according to the manufacturer’s instructions. Taqman probes were purchased from Applied Biosystems. The sequences of the primers and probes are listed in Table 1.
Western blot analysis
Cell lysates were prepared from C2C12 cells stably expressing GATA-4, miR-200b or RNAi GATA-4, or from C2C12 cells transfected with miR-200b mimic, miR-200b inhibitor or control miRNAs as previously described.28 Nuclear extracts were prepared from neonatal rat ventricular myocytes treated with 100 μM Phe and/or transfected with miR-200b mimic, miR-200b inhibitor or control miRNAs. Western blotting was performed using cell lysates according to standard protocols as previously described.28 Briefly, proteins were separated by SDS-PAGE, transferred to PVDF membranes and subjected to immunoblotting using antibodies specific for the sarcomeric MHC (MF-20, Developmental Studies Hybridoma Bank), GATA-4, cyclin D1, Histone H3, or tubulin and visualized using the standard ECL protocol (Pierce).
Cell proliferation assay
C2C12 and P19CL6 cells, GATA-4-silenced cells or cells stably expressing GATA-4 or miR-200b were transiently transfected with miR-200b mimic, miR-200b inhibitor or control miRNAs. After 48 h, the cells were seeded at 10,000 cells per well in a 96-well plate. After 24 h, an MTT assay was performed to monitor the growth rate of these cells using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma). Briefly, 20 µl of a 5 mg/ml MTT solution was added to each well and incubated for 4 h at 37°C until a purple precipitate was visible. The MTT medium was then removed, and 150 µl of DMSO was added to dissolve the formazan. The absorbance was read at 490 nm using a Bio-Rad 550 Microplate Reader. All experiments were plated in sextuplicate and were performed three times.
Flow cytometric analysis
Flow cytometry was performed as previously described.30 In brief, C2C12 and P19CL6 cells, GATA-4-silenced cells or cells stably expressing GATA-4 or miR-200b were seeded at 5 × 105 cells per well in a 6-well plate the day before transfection. The cells were then transfected with the miR-200b mimic, miR-200b inhibitor or control miRNAs. After 48 h, the cells were harvested and fixed with 70% ice-cold ethanol overnight at -20°C. After centrifugation and washing with ice-cold PBS, the cells were stained with propidium iodide (PI, Sigma) solution for 30 min at room temperature in the dark. Flow cytometric analysis was performed using a BD FACScan flow cytometer (Becton Dickinson Immunocytometry System), and cell cycle and apoptosis were analyzed using Cell-Quest software (BD Biosciences). The experiments were performed three times in duplicate.
DAPI staining
C2C12 and P19CL6 cells, GATA-4-silenced cells or cells stably expressing GATA-4 or miR-200b were allowed to adhere to glass coverslips (22 × 22 mm) in 6-well plates. After 24 h, the cells were transfected with the miR-200b mimic, miR-200b inhibitor or control miRNAs. Forty-eight hours post-transfection, the cells on the glass coverslips were rinsed with PBS and fixed with 95% ethanol for 30 min at room temperature. The cells were DNA stained with 0.5 µg/ml DAPI (Sigma) solution for 15 min at room temperature in the dark. The coverslips were mounted and imaged with a Nikon Eclipse E800 fluorescent microscope.
Annexin V-FITC/PI apoptosis assay
Apoptosis was quantified with Annexin V-FITC/ PI apoptosis detection kit (KeyGen BioTECH) according to the manufacturer's recommendations. In brief, C2C12 and P19CL6 cells, GATA-4-silenced cells or cells stably expressing GATA-4 or miR-200b were seeded at 5 × 105 cells per well in a 6-well plate the day before transfection. The cells were then transfected with the miR-200b mimic, miR-200b inhibitor or control miRNAs. After 48 h, the cells were harvested and stained with annexin V-FITC and PI to detect the percentage of apoptotic cells by flow cytometric analysis using a BD FACScan flow cytometer (Becton Dickinson Immunocytometry System), and cell apoptosis were analyzed using Cell-Quest software (BD Biosciences). The experiments were performed three times in duplicate.
Statistics
The data are reported as the mean ± SEM. Student’s unpaired t-tests were used to compare two groups. In all cases, differences were considered to be statistically significant when p < 0.05.
Acknowledgments
We thank Dr Issei Komuro and Dr Chunyan Zhou for providing the P19CL6 mouse embryonic carcinoma cells. This study was supported by grants from the Ministry of Science and Technology of China (863 Program: 2007AA02Z122 and 2012AA022501 to M.-X.Z.), the National Natural Science Foundation of China (no. 81141044 to M.-X.Z and no.31071206 to L-X.X), the International Cooperation and Exchange Foundation of Henan Scientific Committee (082102310024 and 104300510016 to M.-X.Z), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, the State Education Ministry (SRF for ROCS, SEM) and the Ministry of Personnel and the Henan University Key Teacher Foundation.
Glossary
Abbreviations:
- MHC
myosin heavy chain
- 3′-UTR
3′ untranslated region
- DNEM
Dulbecco’s modified Eagle’s medium
- α-MEM
minimum essential medium alpha medium
- FBS
fetal bovine serum
- DMSO
dimethyl sulfoxide
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- qPCR
quantitative real-time PCR
- ES
embryonic stem
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/rnabiology/article/24370
References
- 1.Armiñán A, Gandía C, Bartual M, García-Verdugo JM, Lledó E, Mirabet V, et al. Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev. 2009;18:907–18. doi: 10.1089/scd.2008.0292. [DOI] [PubMed] [Google Scholar]
- 2.Hu DL, Chen FK, Liu YQ, Sheng YH, Yang R, Kong XQ, et al. GATA-4 promotes the differentiation of P19 cells into cardiac myocytes. Int J Mol Med. 2010;26:365–72. [PubMed] [Google Scholar]
- 3.Kobayashi S, Volden P, Timm D, Mao K, Xu X, Liang Q. Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. J Biol Chem. 2010;285:793–804. doi: 10.1074/jbc.M109.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojas A, Kong SW, Agarwal P, Gilliss B, Pu WT, Black BL. GATA4 is a direct transcriptional activator of cyclin D2 and Cdk4 and is required for cardiomyocyte proliferation in anterior heart field-derived myocardium. Mol Cell Biol. 2008;28:5420–31. doi: 10.1128/MCB.00717-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh MK, Li Y, Li S, Cobb RM, Zhou D, Lu MM, et al. Gata4 and Gata5 cooperatively regulate cardiac myocyte proliferation in mice. J Biol Chem. 2010;285:1765–72. doi: 10.1074/jbc.M109.038539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki YJ. Cell signaling pathways for the regulation of GATA4 transcription factor: Implications for cell growth and apoptosis. Cell Signal. 2011;23:1094–9. doi: 10.1016/j.cellsig.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng R, Blobel GA. GATA Transcription Factors and Cancer. Genes Cancer. 2010;1:1178–88. doi: 10.1177/1947601911404223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu B, Guo M, Wang S, Campagna D, Luo M, Herman JG, et al. Evaluation of GATA-4 and GATA-5 methylation profiles in human pancreatic cancers indicate promoter methylation patterns distinct from other human tumor types. Cancer Biol Ther. 2007;6:1546–52. doi: 10.4161/cbt.6.10.4708. [DOI] [PubMed] [Google Scholar]
- 9.Kyrönlahti A, Rämö M, Tamminen M, Unkila-Kallio L, Butzow R, Leminen A, et al. GATA-4 regulates Bcl-2 expression in ovarian granulosa cell tumors. Endocrinology. 2008;149:5635–42. doi: 10.1210/en.2008-0148. [DOI] [PubMed] [Google Scholar]
- 10.Brewer AC, Alexandrovich A, Mjaatvedt CH, Shah AM, Patient RK, Pizzey JA. GATA factors lie upstream of Nkx 2.5 in the transcriptional regulatory cascade that effects cardiogenesis. Stem Cells Dev. 2005;14:425–39. doi: 10.1089/scd.2005.14.425. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi S, Lackey T, Huang Y, Bisping E, Pu WT, Boxer LM, et al. Transcription factor gata4 regulates cardiac BCL2 gene expression in vitro and in vivo. FASEB J. 2006;20:800–2. doi: 10.1096/fj.05-5426fje. [DOI] [PubMed] [Google Scholar]
- 12.Ounzain S, Kobayashi S, Peterson RE, He A, Motterle A, Samani NJ, et al. Cardiac expression of ms1/STARS, a novel gene involved in cardiac development and disease, is regulated by GATA4. Mol Cell Biol. 2012;32:1830–43. doi: 10.1128/MCB.06374-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitta K, Day RM, Kim Y, Torregroza I, Evans T, Suzuki YJ. Hepatocyte growth factor induces GATA-4 phosphorylation and cell survival in cardiac muscle cells. J Biol Chem. 2003;278:4705–12. doi: 10.1074/jbc.M211616200. [DOI] [PubMed] [Google Scholar]
- 14.He A, Shen X, Ma Q, Cao J, von Gise A, Zhou P, et al. PRC2 directly methylates GATA4 and represses its transcriptional activity. Genes Dev. 2012;26:37–42. doi: 10.1101/gad.173930.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han M, Yang Z, Sayed D, He M, Gao S, Lin L, et al. GATA4 expression is primarily regulated via a miR-26b-dependent post-transcriptional mechanism during cardiac hypertrophy. Cardiovasc Res. 2012;93:645–54. doi: 10.1093/cvr/cvs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 17.Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39:761–72. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan YC, Khanna S, Roy S, Sen CK. miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J Biol Chem. 2011;286:2047–56. doi: 10.1074/jbc.M110.158790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habara-Ohkubo A. Differentiation of beating cardiac muscle cells from a derivative of P19 embryonal carcinoma cells. Cell Struct Funct. 1996;21:101–10. doi: 10.1247/csf.21.101. [DOI] [PubMed] [Google Scholar]
- 20.Chan YC, Roy S, Khanna S, Sen CK. Downregulation of endothelial microRNA-200b supports cutaneous wound angiogenesis by desilencing GATA binding protein 2 and vascular endothelial growth factor receptor 2. Arterioscler Thromb Vasc Biol. 2012;32:1372–82. doi: 10.1161/ATVBAHA.112.248583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura T, Ono K, Morimoto T, Wada H, Hirai M, Hidaka K, et al. Acetylation of GATA-4 is involved in the differentiation of embryonic stem cells into cardiac myocytes. J Biol Chem. 2005;280:19682–8. doi: 10.1074/jbc.M412428200. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Zuo S, Pasha Z, Yu B, He Z, Wang Y, et al. GATA-4 promotes myocardial transdifferentiation of mesenchymal stromal cells via up-regulating IGFBP-4. Cytotherapy. 2011;13:1057–65. doi: 10.3109/14653249.2011.597380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snyder M, Huang XY, Zhang JJ. Stat3 directly controls the expression of Tbx5, Nkx2.5, and GATA4 and is essential for cardiomyocyte differentiation of P19CL6 cells. J Biol Chem. 2010;285:23639–46. doi: 10.1074/jbc.M110.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia W, Li J, Chen L, Huang B, Li S, Yang G, et al. MicroRNA-200b regulates cyclin D1 expression and promotes S-phase entry by targeting RND3 in HeLa cells. Mol Cell Biochem. 2010;344:261–6. doi: 10.1007/s11010-010-0550-2. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima K, Inagawa M, Uchida C, Okada K, Tane S, Kojima M, et al. Coordinated regulation of differentiation and proliferation of embryonic cardiomyocytes by a jumonji (Jarid2)-cyclin D1 pathway. Development. 2011;138:1771–82. doi: 10.1242/dev.059295. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Wang X, Zhu H, Zhu C, Wang Y, Pu WT, et al. Synergistic effects of the GATA-4-mediated miR-144/451 cluster in protection against simulated ischemia/reperfusion-induced cardiomyocyte death. J Mol Cell Cardiol. 2010;49:841–50. doi: 10.1016/j.yjmcc.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zang MX, Li Y, Wang H, Wang JB, Jia HT. Cooperative interaction between the basic helix-loop-helix transcription factor dHAND and myocyte enhancer factor 2C regulates myocardial gene expression. J Biol Chem. 2004;279:54258–63. doi: 10.1074/jbc.M408502200. [DOI] [PubMed] [Google Scholar]
- 28.Yao CX, Xiong CJ, Wang WP, Yang F, Zhang SF, Wang TQ, et al. Transcription factor GATA-6 recruits PPARα to cooperatively activate Glut4 gene expression. J Mol Biol. 2012;415:143–58. doi: 10.1016/j.jmb.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 29.Belaguli NS, Sepulveda JL, Nigam V, Charron F, Nemer M, Schwartz RJ. Cardiac tissue enriched factors serum response factor and GATA-4 are mutual coregulators. Mol Cell Biol. 2000;20:7550–8. doi: 10.1128/MCB.20.20.7550-7558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim EJ, Kho JH, Kang MR, Um SJ. Active regulator of SIRT1 cooperates with SIRT1 and facilitates suppression of p53 activity. Mol Cell. 2007;28:277–90. doi: 10.1016/j.molcel.2007.08.030. [DOI] [PubMed] [Google Scholar]