Abstract
The hallmarks of the immune response to viral infections are the expansion of antigen-specific CD8+ cytotoxic T lymphocytes (CTLs) after they encounter antigen-presenting cells in the lymphoid tissues and their subsequent redistribution to nonlymphoid tissues to deal with the pathogen. Control mechanisms exist within CTL activation pathways to prevent inappropriate CTL responses against disseminating infections with a broad distribution of pathogen in host tissues. This is demonstrated during overwhelming infection with the noncytolytic murine lymphocytic choriomeningitis virus, in which clonal exhaustion (anergy and/or deletion) of CTLs prevents immune-mediated pathology but allows persistence of the virus. The mechanism by which the immune system determines whether or not to mount a full response to such infections is unknown. Here we present data showing that the initial encounter of specific CTLs with infected cells in lymphoid tissues is critical for this decision. Whether the course of the viral infection is acute or persistent for life primarily depends on the degree and kinetics of CTL exhaustion in infected lymphoid tissues. Virus-driven CTL expansion in lymphoid tissues resulted in the migration of large quantities of CTLs to nonlymphoid tissues, where they persisted at stable levels. Surprisingly, although virus-specific CTLs were rapidly clonally exhausted in lymphoid tissues under conditions of chronic infection, a substantial number of them migrated to nonlymphoid tissues, where they retained an effector phenotype for a long time. However, these cells were unable to control the infection and progressively lost their antiviral capacities (cytotoxicity and cytokine secretion) in a hierarchical manner before their eventual physical elimination. These results illustrate the differential tissue-specific regulation of antiviral T-cell responses during chronic infections and may help us to understand the dynamic relationship between antigen and T-cell populations in many persistent infections in humans.
A cardinal feature of the adaptive immune response to viruses is the activation of specific T cells in the lymphoid tissues after they encounter virally infected antigen-presenting cells (APCs), such as dendritic cells (DCs) (12, 17, 31). For most viral infections, CD8+ T cells form a crucial arm of the immune response through the actions of effector cytokines and cytolysis (20, 21, 27, 69). In addition, CD4+ T cells provide help for both CD8+ T-cell and B-cell responses (53). The activation of T cells proceeds to proliferative expansion and differentiation into effector T cells that are capable of promoting a rapid resolution of the infection. Because infections with most viruses are not initiated in or confined to lymphoid tissues, the initial antigen exposure and activation of specific T cells in lymphoid tissues are followed by their migration to sites of virus replication in nonlymphoid tissues. This migration facilitates a rapid protective response and is regulated by the expression of homing and adhesion molecules such as selectins, integrins, and chemokine receptors (9, 66, 71). After the initial proliferative burst, which produces large quantities of T cells with diverse subspecificities for viral peptides and clearance of the pathogen, the majority of antigen-specific T cells undergo apoptosis, and a stable, long-lived, but numerically reduced memory T-cell population is established. While the massive expansion of antigen-specific T cells at the onset of infection provides a mechanism for improved survival odds for the host by rapid control of the pathogen, an important limitation to this strategy is the potentially lethal tissue damage that the immune response can cause.
The current paradigm maintains that the immune system is remarkably flexible and capable of responding in qualitatively and quantitatively distinct ways to different infections, with tight regulatory mechanisms to ensure both protection and minimal associated pathological consequences (82, 83). This consideration is of particular importance during persistent viral infections, in which antigen-specific T cells (especially CD8+ T cells, which play a pivotal role in the control or eradication of persistent viruses such as Epstein-Barr virus, cytomegalovirus [CMV], hepatitis B virus [HBV], hepatitis C virus [HCV], and human immunodeficiency virus [HIV]) (10, 29, 45, 62, 75) fail to contain virus replication as a result of different mechanisms. Evasion mechanisms, utilized to variable degrees by different viruses, can counteract cytotoxic T-lymphocyte (CTL) immune responses, enabling a virus to survive and persist in the host. For example, the failure of CD8+ T cells to control infection at an early stage can lead to shifts in immune-mediated selective pressure and the emergence of T-cell escape variants with mutations in the presented peptides (8, 57). In addition, the expression of certain viral proteins can perturb antigen processing and peptide presentation, resulting in impaired T-cell recognition by infected cells (72). A combination of these factors leads to a state of relative deficiency in the antiviral activity of specific CD8+ T cells, which is a characteristic immunological feature in individuals suffering chronic viral infections. Increasing evidence suggests that such deficiencies may be a consequence of the functional dysregulation and/or physical deletion (clonal exhaustion) of virus-specific CD8+ T cells exposed to a high viral load or may be due to the selection of antigen-specific T cells with altered functions (3, 14, 16, 19, 36, 38, 47, 52, 60, 76, 79, 80). The fact that antigen-specific CD8+ T cells with a functional antiviral phenotype are often detectable in chronically infected individuals suggests that functional inactivation or physical deletion may occur at variable degrees to reduce or compromise the levels of functional virus-specific effector CD8+ T cells in different persistent infections. However, the impact of viral antigen persistence on the population dynamics and fate of virus-specific T cells with respect to their patterns of migration and abilities to control the infection within different tissues of the infected host remains poorly characterized. In particular, it is unknown whether the kinetics and characteristics of T-cell exhaustion (anergy and apoptotic deletion) determined for lymphoid tissues reflect those of the overall population of antigen-specific T cells in different host tissues. Moreover, the impact of persisting viral antigen on the functional characteristics of virus-specific CD8+ T-cell populations in lymphoid versus nonlymphoid tissues and its relationship to functionally active memory CD8+ T cells generated after an acute infection remain important but unresolved issues. An evaluation of these parameters with animal models is of particular interest because the study of chronic infections in humans is limited by the inability to recover virus-specific T cells from different tissues, forcing a reliance upon the analysis of antigen-specific T-cell populations recovered from peripheral blood (PBL).
To address these questions, we evaluated the functional properties of virus-specific CD8+ T cells in different tissues during acute versus persistent infections with lymphocytic choriomeningitis virus (LCMV). The murine LCMV system is an excellent model for the study of the dynamics of virus-host interactions during an acute or chronic infection (1, 50, 83). Infection of mice with a relatively low dose of LCMV-Docile leads to a strong and broadly directed virus-specific CD8+ T-cell response that is readily detectable in the spleen, resulting in the efficient clearance of virus within 2 weeks after infection. After resolution of the infection, the majority of expanded antigen-specific CD8+ T cells undergo an apoptotic death phase, and a stable population of memory T cells is established. However, the dynamics of this process change dramatically under conditions of infection with a relatively high dose of LCMV-Docile, which leads to a disseminating infection. High levels of antigen stimulation due to the increased viral load at the onset of infection result in a transient CD8+ T-cell response by which antigen-specific CD8+ T cells are induced, proliferate, and initially exhibit antiviral functions but progressively lose this ability. The loss of protective immunity results in viral persistence (47). Such functionally deficient T cells survive in the host for long periods but may eventually be physically eliminated (52, 80, 81). A detailed analysis of virus-specific CD8+ T-cell responses in lymphoid and nonlymphoid tissues under conditions of acute or persistent infection with LCMV is presented in this study, revealing a central role for the viral load in lymphoid tissue in the induction and maintenance of clonal exhaustion. The data strongly suggest that CD8+ T cells may be differentially regulated in the environments of lymphoid and nonlymphoid tissues, and the pattern of T-cell exhaustion observed with mice is likely a common feature of the immune response during chronic infections in humans.
MATERIALS AND METHODS
Animals and virus.
C57BL/6 and C57BL/6-CD4−/− mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Mice were infected intravenously with a relatively low dose (102 PFU) or a high dose (2 × 106 PFU) of LCMV-Docile to initiate an acute or persistent infection, respectively. LCMV-Docile (a variant isolated from an LCMV-UBC carrier mouse) was obtained from C. J. Pfau (Troy, N.Y.) as a plaque-purified second-passage virus (56). Virus titers were determined by use of an immunological focus assay (5).
Isolation of lymphocyte populations from nonlymphoid tissues.
Mice were perfused with phosphate-buffered saline (PBS) containing heparin (75 U/ml) prior to tissue removal. Lung tissues were minced and then treated at 37°C for 1 h with collagenase (150 U/ml; Gibco) in RPMI with 5% fetal calf serum (FCS). The resulting suspension was pelleted by centrifugation, resuspended in 44% Percoll (Pharmacia) in PBS with heparin (200 U/ml) layered on 67.5% Percoll, and centrifuged at 600 × g for 20 min at 20°C. Lymphocytes were harvested from the gradient interface and washed extensively before use. Liver or kidney tissues were mashed through a 70-μm-pore-size strainer in RPMI with 5% FCS. The resulting cell suspensions were centrifuged, and the pellet was resuspended in a 38% Percoll solution supplemented with heparin (200 U/ml) and centrifuged at 600 × g for 20 min at 20°C. Cells harvested from the pellet were treated with 0.83% ammonium chloride to remove red blood cells and were washed extensively in RPMI with 5% FCS before use.
Antibodies and viral peptides.
Antibodies were purchased from BD Pharmingen. Peptides were synthesized at the MCG Genomics Core Facility (Augusta, Ga.) in a Perkin-Elmer Applied Biosystems (Berkeley, Calif.) 433A peptide synthesizer. The peptides used for this study were the immunodominant H-2Db-restricted determinants for LCMV-specific CD8+ T-cell responses derived from the viral glycoprotein (GP1) GP133-41 (KAVYNFATC) or the viral nucleoprotein NP396-404 (FQPQNGQFI). Experiments measuring virus-specific T-cell functions were performed by using the optimized Db-binding peptide KAVYNFATM, which exhibits a high affinity for Db. The interaction of the T-cell receptor (TCR) with H-2Db/GP133-41 is not affected by the replacement of the cysteine at anchor position 9 in the natural peptide with methionine. Key functional analyses were confirmed by using the natural viral peptide (KAVYNFATC). Note that LCMV-Docile contains an amino acid change in peptide GP2276-286 (380N→S) derived from viral GP2, another immunodominant determinant for LCMV-specific CD8+ T-cell responses in C57BL/6 mice. This amino acid alteration substantially reduces the ability of the GP2276-286 peptide to bind H-2Db, and no GP2-specific CD8+ T cells were detectable in the infected mice.
Quantitative analysis of virus-specific CD8+ T cells.
Major histocompatibility complex (MHC)-peptide tetramers were prepared as previously described (2, 49, 52). Experiments utilized H-2Db tetramers complexed with the GP133-41 (KAVYNFATM) or NP396-404 (FQPQNGQFI) peptide. Single-cell suspensions prepared from the spleen or from lymphocytes isolated from different tissues were stained with tetramers along with an antibody specific for CD8 (clone 53-6.72) in fluorescence-activated cell sorting (FACS) buffer (PBS with 1% bovine serum albumin and 0.2% sodium azide). After being stained for 1 h at 4°C, cells were fixed in PBS containing 0.1% paraformaldehyde and were measured in a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, Calif.), and data were analyzed with CellQuest software.
Intracellular staining for cytokines.
Splenocytes or lymphocytes isolated from tissues were cultured in 96-well flat-bottomed plates at 106 cells/well in 200 μl of RPMI 1640 (Gibco) supplemented with 10% FCS in the presence or absence of the indicated peptide at a concentration of 1 μg/ml (49, 52). For the quantitation of total virus-specific CD8+ T-cell responses, virus-infected DC (DC2.4, kindly provided by K. Rock, Boston, Mass.) (68) stimulators were used in combination with intracellular cytokine staining. Effector cells (106) were incubated with 4 × 105 DC2.4 cells that were uninfected or had been infected 48 h previously with LCMV at a multiplicity of infection of 0.5. All stimulation reactions were performed for 6 h at 37°C in the presence of 10 U of murine interleukin-2 (IL-2)/well and 1 μg of brefeldin A (BD-Pharmingen)/well. After 6 h of culture, cells were harvested, washed once in FACS buffer, and surface stained with an antibody specific for mouse CD8α (clone 53-6-72). The cells were then fixed, permeabilized (Cytofix/Cytoperm kit; BD-Pharmingen), and stained for intracellular cytokines by use of a fluorescein isothiocyanate-conjugated antibody against murine gamma interferon (IFN-γ) (clone XMG1.2) or a phycoerythrin-conjugated antibody against murine tumor necrosis factor alpha (TNF-α) or IL-2 (clones MP6-XT22 and JES6-5H4, respectively). Cells were then analyzed by flow cytometry as described above.
Analysis of the cytotoxic T-cell response.
Direct ex vivo CTL activity was determined by a 5-h 51Cr release assay as described previously (48). Lymphocytes were incubated with peptide (GP133-41 or NP396-404)-loaded EL-4 cells or virus-infected MC57G cells at effector-to-target (E:T) ratios ranging from 50:1 to 200:1. E:T values shown were corrected for the number of GP133-41 or NP396-404 tetramer-positive cells in each epitope-specific CTL population or for the number of GP133-41 and NP396-404 tetramer-positive cells in the total virus-specific CTL population. CTL precursor activity was determined with a bulk culture system as described previously (48). Briefly, lymphocytes were prepared from LCMV-infected mice at the indicated time points. Cells were cultured in 24-well plates for 5 days at a density of 4 × 106 per well together with virus-infected peritoneal macrophages (1 × 105) in 2 ml of Iscove's modified Dulbecco's medium supplemented with 10% FCS and 10 U of murine IL-2/ml. Restimulated cells were resuspended in 1 ml of minimal essential medium with 5% FCS per culture well, and serial threefold dilutions of effector cells were tested in a 51Cr release assay, using MC57G cells infected with virus or pulsed with 10 μg of the indicated peptide/ml as target cells. The E:T cell ratios shown were corrected for the total number of tetramer-positive cells per culture well (prior to culture) by the equation E:T = (D × P × L) ÷ N, where P is the percentage of tetramer-positive lymphocytes, L is the total number of lymphocytes added to each well in the bulk culture, D is the dilution factor (10, as 1/10 of the bulk culture was used in the final 51Cr release assay), and N is the number of target cells per well (in this case, 104) in the 51Cr release assay.
TCR affinity measurement by dissociation assay.
The TCR affinities of GP133-41- and NP396-404-specific CD8+ T cells were measured by a tetramer dissociation assay as described previously (67). Briefly, lymphocytes isolated from the spleen or liver of infected mice were stained at room temperature with the Db/GP133-41 or Db/NP396-404 tetramer and CD8 antibody, as described above. Cells were washed twice with FACS buffer and incubated on ice in the presence of saturating amounts of a Db-specific monoclonal antibody (28-14-8s) to allow for tetramer dissociation and binding to the Db antibody. Dissociation was monitored for 0 to 120 min. Half-lives (t1/2) were determined by the equation (ln 2)/mean slope value and were expressed in minutes. The mean slope of each interval was equivalent to the variable ln(Fa/Fb)/t, where Fa is the normalized fluorescence at the start of the interval, Fb is the normalized total fluorescence at the end of the interval, and t is the length of the interval (67).
Depletion of CD8+ or CD4+ T-cell subsets in vivo.
Mice were depleted of CD8+ or CD4+ T-cell subsets on day 15 after infection by an intraperitoneal injection of a purified specific antibody (anti-CD8 antibody [YTS169] or a mixture of anti-CD4 antibodies [YTS191 plus YTA3.1]), as previously described (46). The antibody was administrated on days 15 and 17 after infection, and treatment was continued at weekly intervals throughout the experiment.
Biochemical and histological analysis of viral hepatitis.
Liver injury was monitored by immunohistochemical analysis and measurements of serum alanine aminotransferase (sALT) and serum aspartate aminotransferase (sAST) activity according to the manufacturer's protocol (Pointe Scientific Inc., Lincoln Park, Mich.). Liver tissues harvested from infected mice were embedded in OCT compound (Sakura Finetek, Torrance, Calif.), snap-frozen in a dry ice-2-methyl-butane bath, sectioned, air dried, and fixed in 10% acetone. Sections from each tissue specimen were stained with hematoxylin and eosin and were subjected to gross and microscopic pathological analyses.
RESULTS
Dynamics of virus-specific CD8+ T-cell response in lymphoid versus nonlymphoid tissues during an acute or persistent LCMV infection.
To examine the fate of virus-specific CD8+ T cells in relation to viral loads in lymphoid compared to nonlymphoid tissues during an acute or persistent infection, we measured the kinetics of viral titers and virus-specific CD8+ T-cell responses in different tissues of B6 mice infected with LCMV-Docile (102 or 2 × 106 PFU). Lymphocytes isolated from the spleen, peripheral (axillary, para-aortic, and mesenteric) lymph nodes (PLN), bone marrow (BM), liver, kidney, lung, and PBL were stained with the appropriate tetramer-peptide complex (Db/GP133-41 or Db/NP396-404) and an antibody specific to CD8α to identify antigen-specific CD8+ T cells within the entire lymphocyte population. The functioning of virus-specific T cells was measured by staining for IFN-γ secretion after peptide stimulation.
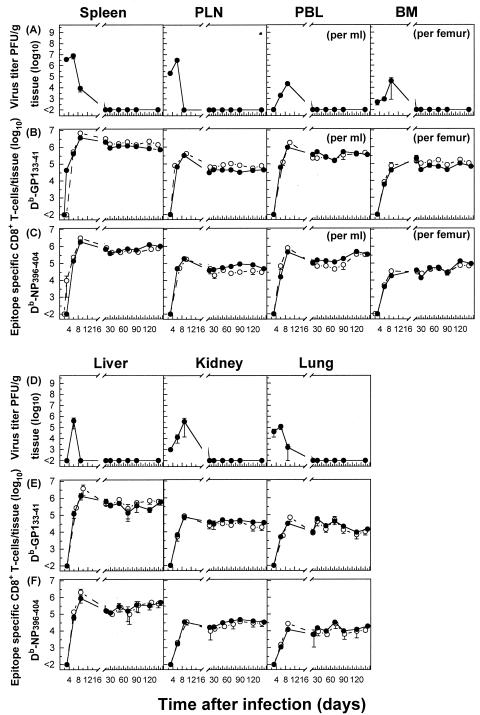
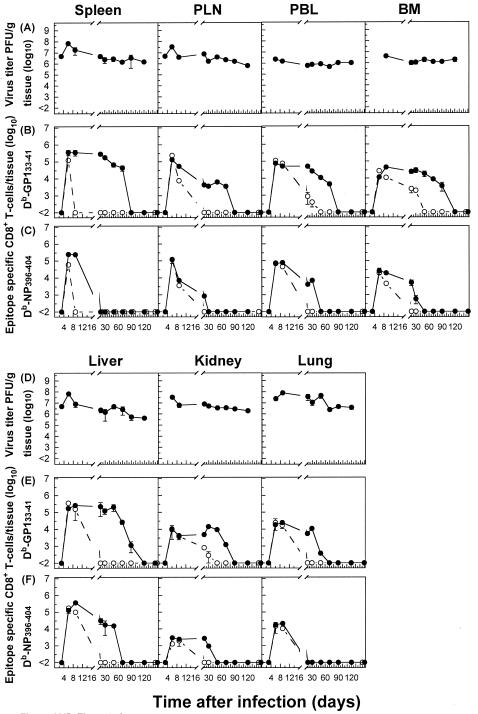
In agreement with earlier findings, the infection of mice with 102 PFU of LCMV-Docile causes an acute infection with initial viral replication in multiple tissues. Viral titers peaked between days 3 and 6, followed by a rapid decline below detectable levels by day 20 after infection for all tissues examined (Fig. 1A and D). The burst of virus-specific CD8+ T-cell responses in lymphoid tissues (spleen and PLN) focused toward the dominant GP133-41 or NP396-404 epitope accounted for almost (>80%) the entire expanded CD8+ T-cell population. The ensuing contraction phase, shown by a numerical reduction of antigen-specific CD8+ T cells, was followed by the memory phase, during which epitope-specific CD8+ T cells persisted at stable levels while retaining a functional phenotype (Fig. 1B, C, E, and F). Consistent with several reports demonstrating the presence of antigen-specific T cells in many tissues that may harbor the infection (43, 44, 61), the migration of virus-specific T cells from lymphoid tissues through the blood to nonlymphoid tissues proceeded rapidly, resulting in efficient control of the infection. As shown in Fig. 1, the kinetics of proliferative expansion and memory T-cell development were similar for virus-specific CD8+ T-cell populations isolated from lymphoid and nonlymphoid tissues. Consistent with that observed for the spleen and PLN, an equal distribution of epitope hierarchies among virus-specific CD8+ T-cell populations was observed for nonlymphoid tissues.
FIG. 1.
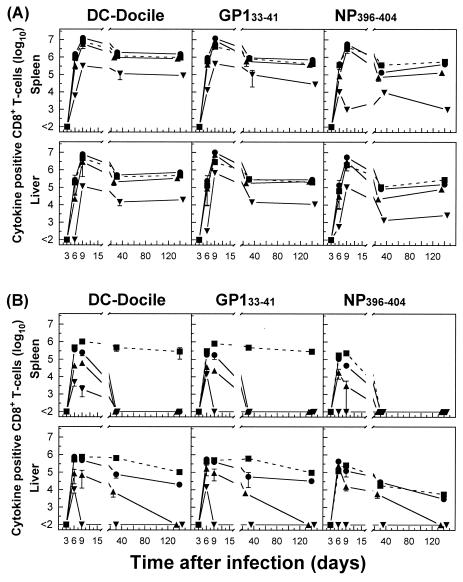
Persistence of virus-specific CD8+ T cells at high stable memory levels in nonlymphoid tissues during an acute viral infection. Analyses were performed to correlate the kinetics of virus replication (A and D) with the kinetics of virus-specific CD8+ T-cell responses (B, C, E, and F). C57BL/6 mice were infected with 102 PFU of LCMV-Docile, and virus titers in different tissues were measured at the indicated times. Data shown are means ± standard errors of the means (SEM) of log10 PFU/g of tissue for 5 to 10 mice. Parallel total numbers of GP133-41 or NP396-404 peptide-specific CD8+ T cells were determined by staining with H-2Db tetramers (•) or measuring intracellular IFN-γ (○) production after the stimulation of cells with the appropriate peptide. Values were derived by multiplying the percentages of total tetramer-positive cells by the total numbers of lymphocytes isolated from a given tissue. Data shown are means ± SEM of log10 virus-specific T cells per spleen for 5 to 10 mice.
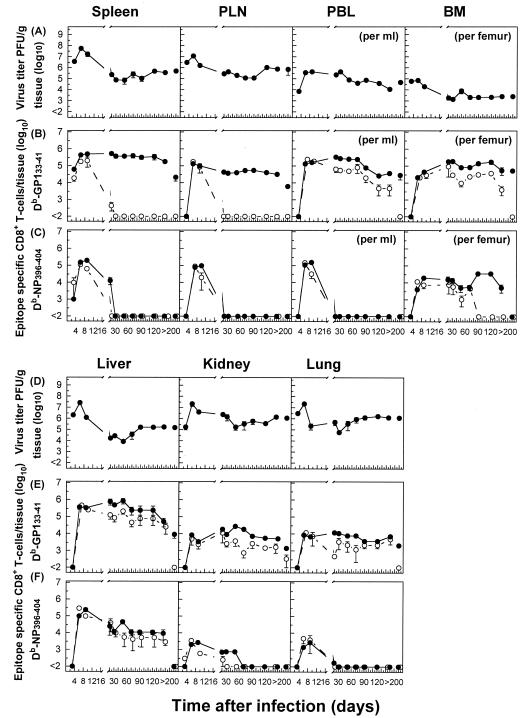
The infection of B6 mice with 2 × 106 PFU of LCMV-Docile resulted in a long-term persistence of the infection in different host tissues (Fig. 2A and D). The expansion of GP133-41- or NP396-404-specific CD8+ T cells, initially exhibiting a functional phenotype, proceeded rapidly in lymphoid tissues (spleen and PLN), but their function (IFN-γ secretion) was progressively lost (by day 20) and the cells either persisted at high levels in an anergic stage (GP133-41), as indicated by a lack of IFN-γ secretion upon restimulation, or underwent physical elimination (NP396-404). In striking contrast, a considerable fraction (10 to 20%) of virus-specific T cells that migrated to nonlymphoid tissues with intact antiviral functions retained their functional phenotypes for prolonged times. Moreover, the fate of NP396-404-specific CD8+ T cells in lymphoid tissues followed a distinct pattern compared to that for nonlymphoid tissues. While in the blood and lungs the initial expansion of NP396-404-specific CD8+ T-cell populations was followed by their rapid physical elimination, in other tissues (BM, liver, and kidney) they persisted for prolonged periods, with a fraction of them retaining functional activity. However, antigen-specific CD8+ T cells with a functional phenotype were inefficient at viral control, and they eventually (by day 250) lost their antiviral properties and either persisted (GP133-41) or underwent deletion (NP396-404). An extended analysis of the Kb-restricted GP134-43 peptide-specific CD8+ T-cell response that is prominent during acute infections revealed comparable kinetics of functional inactivation and physical elimination to those observed for NP396-404 peptide-specific T cells in mice with chronic LCMV infections (data not shown). Thus, the impact of a chronic infection on the dynamics and functioning of the antiviral CD8+ T-cell population differs markedly in different tissues. Lymphoid tissues provide an environment for effective and rapid down-regulation of the antiviral CD8+ T-cell response during chronic infections. In contrast, the mechanisms of T-cell exhaustion operate less efficiently in nonlymphoid tissues.
FIG. 2.
Differential regulation of virus-specific CD8+ T-cell responses in lymphoid versus nonlymphoid tissues during a persistent infection. C57BL/6 mice were infected with 2 × 106 PFU of LCMV-Docile, and virus titers in different tissues were measured at the indicated times (A and D). Data shown are means ± SEM of log10 PFU/g of tissue for 3 to 5 mice. Total numbers of GP133-41 or NP396-404 peptide-specific CD8+ T cells were determined by staining with H-2Db tetramers (•) or measuring intracellular IFN-γ (○) production after the stimulation of cells with the appropriate peptide (B, C, E, and F). Data shown are means ± SEM of log10 virus-specific T cells per spleen for 5 to 10 mice.
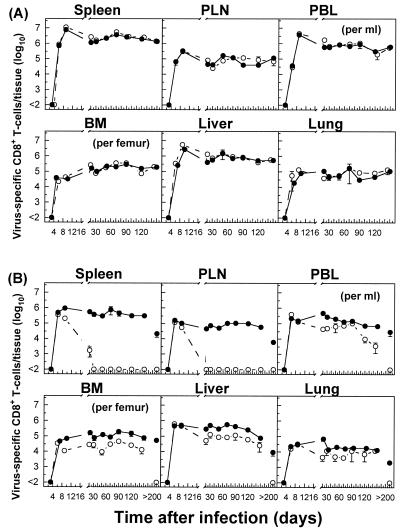
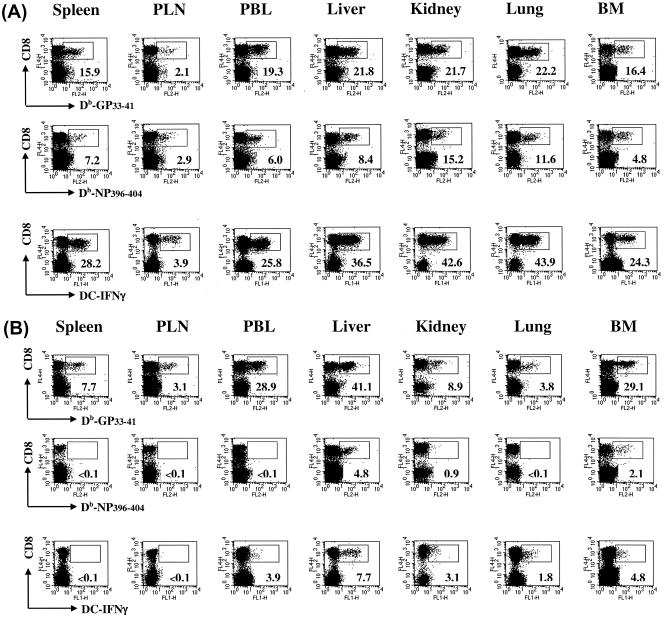
To test whether the distinct kinetics of epitope-specific CD8+ T-cell populations observed in lymphoid versus nonlymphoid tissues in mice with acute or persistent infections (Fig. 1 and 2) reflected the overall response to the virus, we determined the kinetics of virus-specific CD8+ T-cell populations by using virus-infected DCs (DC2.4) in combination with intracellular IFN-γ staining. As expected, in mice with acute infections, the sum of GP133-41- and NP396-404-specific CD8+ T-cell populations, as detected by tetramer staining, essentially comprised the overall LCMV-specific CD8+ T-cell response measured by the IFN-γ secretion assay (Fig. 3). In addition, the data confirmed the distinct pattern of functional loss and deletion during the course of persistent infections that was observed for individual epitope-specific CD8+ T-cell populations. Collectively, these data demonstrate that LCMV infection induces a robust antiviral CD8+ T-cell response in multiple host tissues. An illustration of the massive expansion and tissue distribution of GP133-41 or NP396-404 tetramer-positive CD8+ T cells in different tissues in relation to the total IFN-γ-positive CD8+ T cells on day 20 after an acute or persistent infection with LCMV-Docile is presented in Fig. 4.
FIG. 3.
Kinetics of total virus-specific CD8+ T-cell response in different tissues based on tetramer versus intracellular IFN-γ secretion after stimulation of lymphocytes with DC2.4 virus-infected cells during acute or persistent infections. C57BL/6 mice were infected with 102 (A) or 2 × 106 (B) PFU of LCMV-Docile, and total numbers of virus-specific CD8+ T cells (sum of GP133-41 and NP396-404 peptide-specific T cells) were determined by staining with Db/GP133-41 and Db/NP396-404 tetramers (•). The total numbers of virus-specific CD8+ T cells from tissues were tested for the ability to produce IFN-γ after short-term culturing with virus-infected DC2.4 cells on the indicated days after infection (○). Data shown are means ± SEM of log10 virus-specific T cells per tissue for 3 to 6 mice.
FIG. 4.
Expansion and distribution of epitope-specific CD8+ T cells in lymphoid and nonlymphoid tissues in relation to the total numbers of virus-specific IFN-γ-producing CD8+ T cells during acute versus persistent infections. C57BL/6 mice were infected with 102 (A) or 2 × 106 (B) PFU of LCMV-Docile, and lymphocytes were isolated from the indicated tissues 20 days after infection. The percentage of antigen-specific CD8+ T cells was assessed by staining with Db/GP133-41 (top panels) or Db/NP396-404 (middle panels) tetramers and antibody against CD8α. Plots shown are gated on live cells. Total numbers of virus-specific CD8+ T cells producing IFN-γ after stimulation with virus-infected DC2.4 cells were determined by concurrent analyses (bottom panels). The percentages of CD8+ T cells staining positive for Db/GP133-41 or Db/NP396-404 tetramers or producing IFN-γ are indicated in the lower right corners of the corresponding panels. Results are representative of several separate experiments.
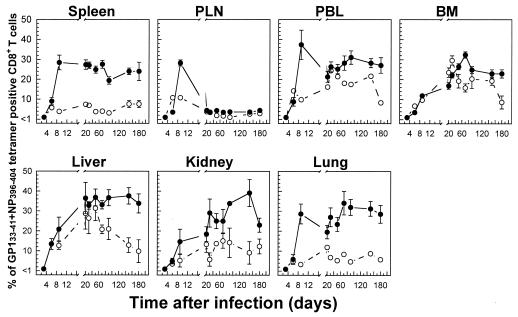
To further examine whether virus-specific CD8+ T-cell populations are preferentially accumulated and/or retained in tissues of infected mice, we evaluated the recruitment kinetics of specific CD8+ T cells by calculating the percentages of tetramer-positive T cells within the lymphocyte population in each tissue as a function of time. As a percentage of CD8+ T cells, the number of tetramer-positive cells (the sum of GP133-41- and NP396-404-specific cells) was higher during an acute infection than during a persistent infection. Furthermore, tissue-specific differences were observed in both the magnitude and kinetics of the LCMV-specific CTL response in each case (Fig. 5). Thus, the percentages of tetramer-positive CD8+ T cells increased rapidly in lymphoid tissues and more slowly in nonlymphoid tissues, including blood, over the course of acute infections before reaching relatively high stable levels (30 to 40%). Surprisingly, in the PLN there was a transient increase in the number of tetramer-positive CD8+ T cells, which peaked by day 9 (around 30%) but then declined rapidly to relatively low but stable levels (4 to 5%). In mice with persistent LCMV infections, the percentages of tetramer-positive CD8+ T cells varied significantly between different tissue compartments. While in the spleen, PLN, kidney, and lung, 5 to 10% of CD8+ T cells were tetramer positive, in the blood, BM, and liver, this population was more prominent (peak values around 30%). This distinct kinetic pattern for the percentages of tetramer-positive CD8+ T cells in different tissues and the fact that tissues such as the kidney, lung, and liver were perfused extensively before the isolation of lymphocytes exclude the possibility that such virus-specific CD8+ T cells were derived from blood contamination. When the total numbers of virus-specific CD8+ T cells per tissue were compared with the cumulative sum of their numbers in nonlymphoid tissues (liver, kidney, lung, BM, and blood), the figures were comparable to or even exceeded that of the spleen at both the peak of the response and the memory phase for both acute and persistent infections (data not shown).
FIG. 5.
Tissue-specific kinetics of the virus-specific CD8+ T-cell response based on percentages of CD8+ T cells that were tetramer positive during acute versus persistent infections. The percentages of total CD8+ T cells specific for GP133-41 or NP396-404 in the indicated tissues, as determined by tetramer staining, were calculated based on the data presented in Fig. 1 for acute infections (•) or in Fig. 2 for persistent infections (○) of C57BL/6 mice with LCMV-Docile. Data shown are means ± SEM of log10 virus-specific T cells per tissue for 5 to 10 mice.
Distinct patterns of virus-specific CD8+ CTL activity in lymphoid versus nonlymphoid tissues during acute or persistent LCMV infections.
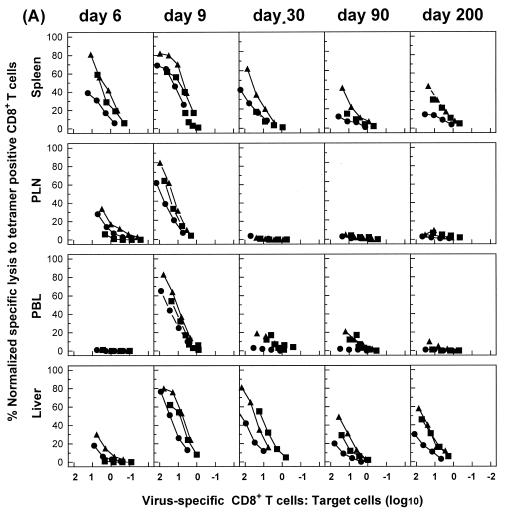
To better characterize virus-specific CD8+ T-cell populations during acute or chronic LCMV infections, we tested for possible functional differences, in addition to IFN-γ production, between antigen-specific CD8+ T cells in different tissues. When we performed direct ex vivo antigen-specific CTL assays, striking differences were observed among T cells isolated from different tissues. Nine days after acute infection with LCMV, a high level of lytic activity measured on virus-infected or peptide-loaded target cells at identical E:T cell ratios was exhibited by cells from all tissues examined (Fig. 6A and data not shown). However, although cells isolated from the spleen and liver exhibited significant antigen-specific lytic activity at later times after infection (up to day 200), cells from PLN and blood lost their lytic activities by day 30 after infection. In mice with chronic infections, direct ex vivo lytic activity was detected in lymphoid tissues at early times (days 6 and 9) but was lost by day 30. In fact, antigen-specific lytic activity was readily detected with both virus-infected and peptide-loaded (GP133-41 or NP396-404) target cells at day 90, although this activity was subsequently lost (by day 200). Similar results were observed for the kidney and lung (data not shown).
FIG. 6.
Virus-specific CD8+ T cells from nonlymphoid tissues preserve their ex vivo lytic activity for prolonged periods during persistent infections. C57BL/6 mice were infected with 102 (A) or 2 × 106 (B) PFU of LCMV-Docile, and lymphocytes were isolated from tissues at the indicated times. Direct ex vivo CTL activity was measured on virus-infected MC57G cells (•) or on EL-4 cells loaded with a peptide GP133-41 (▴) or NP396-404 (▪) target at an E:T ratio of 200:1 for all tissues. The E:T cell values shown are corrected for the number of GP133-41 and NP396-404 tetramer-positive cells in the total virus-specific CTL population (virus-infected targets) or for the number of GP133-41 or NP396-404 tetramer-positive cells in each epitope-specific CTL population (peptide-loaded targets). Lysis of untreated target cells was usually ≤5% at the highest E:T ratio. However, in a few cases, nonspecific lysis exceeding the 5% level was subtracted from corresponding lysis values. Results are representative of three separate experiments.
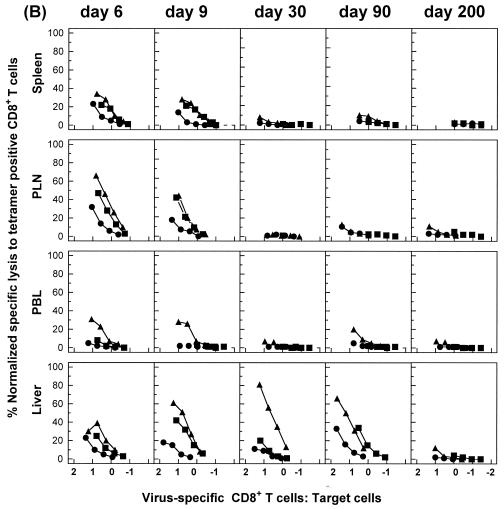
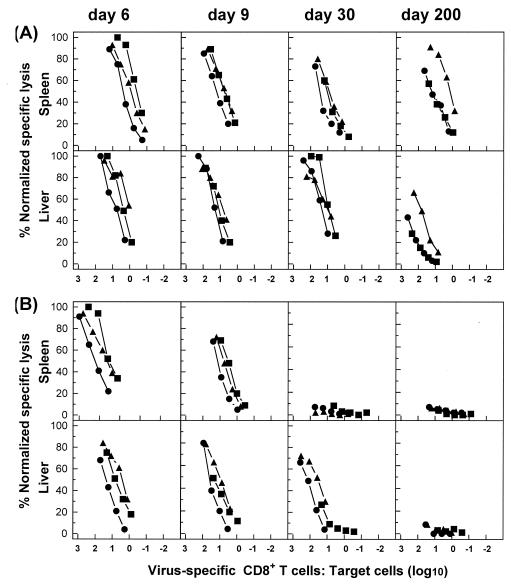
For an independent assay of functional activity, we also compared the abilities of antigen-specific CD8+ T-cell populations in different tissues to develop lytic activity after stimulation in vitro. This approach allows for direct functional comparisons between antigen-specific CD8+ T-cell populations in different tissues with regard to the capacity for proliferation, differentiation to effector cells, and acquisition of cytolytic activity after stimulation with a viral antigen. For this analysis, cytolytic activity was expressed as a function of the E:T ratio corrected for the number of tetramer-positive cells initially added to each culture well, as described in Materials and Methods. Cells isolated from the spleens and livers of acutely infected mice at early times (days 6 and 9) had rapidly up-regulated their lytic activity, and they sustained this response at relatively high levels after virus elimination over a period of 200 days (Fig. 7A). However, at comparable E:T ratios, antigen-specific CD8+ T cells isolated from the spleen were more efficient at lysis of either peptide-loaded or virus-infected target cells than those isolated from the liver, except for on day 6 after infection. As expected, cells from the spleens of mice with chronic infections exhibited substantial cytolytic activity during the initial phase of infection but lost their lytic activities rapidly (by day 30) (Fig. 7B). Experiments performed with cells from the liver confirmed the results of IFN-γ secretion assays, as these cells had substantial antigen-specific cytolytic activity on days 30 and 90, but not day 200, after infection (Fig. 7 and data not shown).
FIG. 7.
Virus-specific CD8+ T cells in nonlymphoid tissues during chronic infection preserve the ability to up-regulate lytic activity after antigenic stimulation in vitro. C57BL/6 mice were infected with 102 (A) or 2 × 106 (B) PFU of LCMV-Docile, and lymphocytes isolated from the spleen and liver at the indicated times after infection were stimulated with virus-infected macrophages as described in Materials and Methods. The cytolytic activity of restimulated lymphocytes cultured at a density of 4 × 106/well was measured in a 51Cr release assay using virus-infected MC57G cells (•) or EL-4 cells loaded with peptide GP133-41 (▴) or NP396-404 (▪) as a target. Cultured cells were resuspended in 1 ml of medium per culture well, and serial threefold dilutions of effector cells were performed. E:T cell ratios shown in the blot are corrected for the total number of tetramer-positive cells per culture well of the original lymphocyte populations (prior to culture), as described in Materials and Methods. Results are representative of three separate experiments.
Hierarchical functional impairment of CD8+ T cells during a chronic viral infection.
To further evaluate the functional properties of virus-specific CD8+ T-cell populations during acute versus chronic infections, we performed analyses of cells isolated from the liver or spleen to establish the kinetics of cytokine responses by comparing numbers of IFN-γ-, TNF-α-, and IL-2-producing CD8+ T cells with the numbers of tetramer-positive CD8+ T cells with the same specificity by restimulating cells with either virus (using DC2.4-infected cells to stimulate cytokine secretion) or peptide (GP133-41 or NP396-404). As shown in Fig. 8A for mice with acute infections, nearly the entire population of tetramer-positive cells from the spleen produced IFN-γ during the course of infection. The number of tetramer-positive CD8+ T cells producing TNF-α varied from 20 to 80% of the total number of tetramer-positive cells, but the percentages became higher during the memory phase of infection (days 30 and 140). In contrast, only 5 to 10% of the tetramer-positive population produced IL-2 upon stimulation with virus or peptide. Costaining for IFN-γ and TNF-α or IFN-γ and IL-2 during the response to acute infections revealed that TNF-α and IL-2 were produced by antigen-specific CD8+ T cells producing IFN-γ (data not shown). Similar kinetic profiles for virus- or peptide-specific CD8+ T-cell populations were observed for cells isolated from the liver.
FIG. 8.
Virus-specific CD8+ T cells in nonlymphoid tissues experience functional exhaustion with a hierarchical loss of IL-2, TNF-α, and IFN-γ during chronic infections. C57BL/6 mice were infected with 102 (A) or with 2 × 106 (B) PFU of LCMV-Docile, and lymphocytes were isolated from the spleen and liver at the indicated times. The total numbers of virus- or GP133-41 or NP396-404 epitope-specific CD8+ T cells producing IFN-γ (•), TNF-α (▴), or IL-2 (▾) after short-term culturing with virus-infected DC2.4 cells or peptide, respectively, are shown. For a comparison, the numbers of total (sum of GP133-41 and NP396-404) (left panels), GP133-41 (middle panels), or NP396-404 (right panels) peptide-specific CD8+ T cells were determined by staining with H-2Db tetramers (▪). Data shown are means ± SEM of log10 virus-specific T cells per tissue for 5 to 10 mice.
To examine how chronic infections impacted the ability of antigen-specific CD8+ T cells to up-regulate cytokine production upon antigen stimulation in vitro, similar analyses were performed with mice that were persistently infected with LCMV. As shown in Fig. 8B, during the initial phase of infection (day 6) the entire population of tetramer-positive CD8+ T cells isolated from the spleen possessed the capacity to produce IFN-γ, while only a fraction of them produced TNF-α (10 to 20%) or IL-2 (1 to 2%). However, this ability was progressively lost by day 20 after infection, with similar kinetics for the individual cytokines. Interestingly, the ability of NP396-404-specific CD8+ T cells to produce IL-2 was completely suppressed, even at early times of infection. An essentially similar pattern of cytokine production was observed at day 6 after infection for antigen-specific T cells isolated from the liver. However, marked qualitative differences were observed further in the course of infection. Thus, while the capacity for IFN-γ production during the initial phase of infection was lost for the majority of virus- or peptide-specific CD8+ T cells, a substantial fraction of them (10 to 20%) preserved this function at stable levels (until at least 140 days after infection). However, the ability to produce IFN-γ was also compromised at day 250 after infection (data not shown). In striking contrast to this, the frequency of antigen-specific CD8+ T cells producing TNF-α progressively declined and was essentially absent by day 120 after infection. The capacity for IL-2 production was abolished more rapidly, during the initial phase of infection. Thus, these findings suggest that antigen-specific CD8+ T cells in nonlymphoid tissues experience functional exhaustion with a hierarchical level of secretion and subsequent loss of IL-2, TNF-α, and finally, IFN-γ production. In contrast, in the environment of lymphoid tissues (e.g., the spleen), T cells experience a rapid functional inactivation with similar kinetics for the individual cytokines.
Phenotypic analysis of antigen-specific CD8+ T cells during acute versus chronic infections.
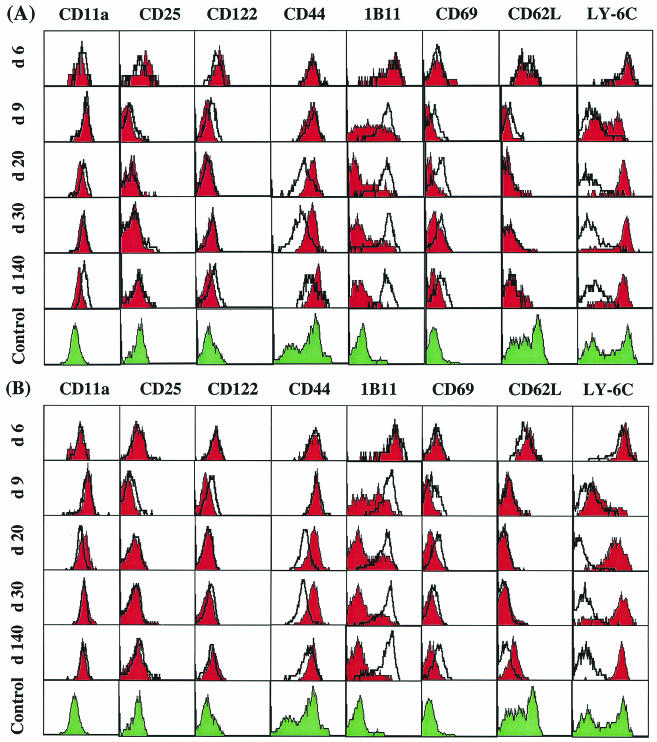
The differentiation of naïve CD8+ T cells into effector cells by their acquisition of antiviral properties and their relocation to different tissues is a complex process involving a structural reorganization of the cellular membrane and cytoskeleton associated with the expression of activation markers and an alteration in cell adhesion and homing receptor expression (6, 42, 66). To examine the pattern of expression of such molecules during acute versus persistent infections, we longitudinally analyzed activation and adhesion molecules on CD8+ T cells (specific for GP133-41) isolated from the spleen and liver. As shown in Fig. 9A, in the spleen CD11a (LFA-1) expression was up-regulated on antigen-specific CD8+ T cells during the early phase of an acute or persistent infection (day 6) and remained at elevated levels throughout the infection. At a very late stage of infection (day 140), however, CD11a expression was higher on antigen-specific T cells from a persistent infection than on those from an acute infection. The expression of CD25 (IL-2-Rα) and CD122 (IL-2-Rβ) molecules was transiently up-regulated during the proliferative phase (day 6 after infection) of antigen-specific T-cell responses for both acute and persistent infections. By day 20 after infection, the expression returned to the levels detected with control CD8+ T-cell populations from uninfected mice. GP133-41-specific CD8+ T cells expressed high levels of CD44, which plays a role in lymphocyte migration, throughout the course of infection. Interestingly, although similar expression levels were observed for specific T-cell populations from acute and persistent infections on days 6 and 9, later in the course of infection CD44 expression was markedly lower for persistent infections. The 1B11 determinant, a carbohydrate epitope expressed on CD43 and CD45 epitopes, was up-regulated on activated antigen-specific CD8+ T cells at the onset of acute infections (day 6), followed by down-regulation during the transition from the effector to the memory phase (days 9 to 20). However, 1B11 was constitutively expressed at high levels during persistent infections. CD69, an early activation marker, was transiently up-regulated on day 6 during acute infections. However, this expression level remained somewhat higher during persistent infections, as was reported previously (80). The expression of the lymph node homing receptor CD62L was rapidly down-regulated by day 9 after infection during both acute and persistent infections, although the down-regulation appeared less complete at day 9 during persistent infections. Finally, staining for the expression of Ly-6C, a membrane-anchored glycoprotein involved in cell adhesion and homing, revealed distinct expression patterns for T cells during acute versus persistent infections. During acute infections, Ly-6C was highly expressed on antigen-specific CD8+ T cells during the initial proliferative phase (day 6), followed by a transient down-regulation during the effector phase (day 9) and reexpression at high levels during the memory phase (from day 20 on). In striking contrast, during persistent infections, antigen-specific T cells lost expression of Ly-6C during the transition from the effector to the memory phase. Similar patterns of CD11a, CD25, CD122, CD44, 1B11, CD69, CD62L, and Ly-6C expression were observed with GP133-41-specific CD8+ T cells isolated from the livers of mice with acute or persistent infections (Fig. 9B). In conclusion, a phenotypic analysis of antigen-specific CD8+ T cells in the spleen and liver revealed similar expression patterns for several molecules that are broadly used to define effector and memory T-cell populations. However, comparative analyses with regard to the expression of these molecules during acute versus persistent infections revealed striking differences.
FIG. 9.
Phenotypic analysis of virus-specific CD8+ T cells from the spleen and liver during acute versus persistent infections. Lymphocytes isolated from the spleens (A) or livers (B) of C57BL/6 mice infected with 102 PFU (red histograms) or 2 × 106 PFU (open, thickly lined histograms) of LCMV-Docile at the indicated times after infection were triple stained with anti-CD8α, an antibody specific for CD11a (LFA-1), CD25 (IL-2-Rα), CD122 (IL-2-Rβ), CD44, 1B11 (CD43), CD69, CD62L, or Ly-6C, and the Db/GP133-41 tetramer. As a control, cells from uninfected mice were double stained for CD8α and the activation markers listed above (green histograms). Histograms are gated on cells that were positive for CD8α and Db/GP133-41 tetramer. These results are representative of three separate experiments.
Comparable “functional avidity” and TCR affinity profiles of CD8+ T cells in lymphoid versus nonlymphoid tissues during acute or chronic infection.
To further investigate whether the differential susceptibilities of antigen-specific CD8+ T-cell populations to clonal exhaustion (anergy and/or physical elimination) observed for the spleen and liver were associated with the selection of particular CD8+ T-cell clones, we measured function-based avidities and performed comparative TCR repertoire analyses within GP133-41- and NP396-404-specific CD8+ T-cell populations.
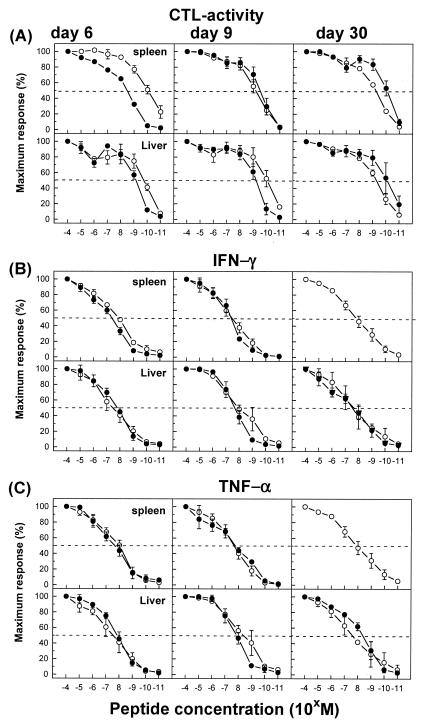
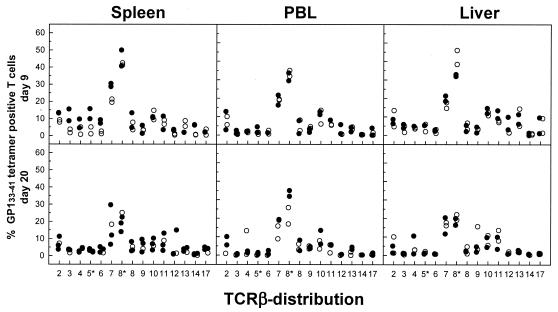
To measure function-based avidities of GP133-41-specific CD8+ T-cell populations, we quantified the amount of peptide required to induce critical effector functions, such as lytic activity and cytokine production (IFN-γ and TNF-α) (Fig. 10). In general, avidities, defined as the peptide concentrations required to obtain 50% maximal lysis of target cells or to induce IFN-γ or TNF-α production in 50% of the GP133-41-specific CD8+ T cells isolated from the spleen and liver on day 6, 9, or 30 after an acute or persistent infection, did not differ significantly. Interestingly, GP133-41-specific CD8+ T cells from the spleens of acutely infected mice on day 6 after infection had a 10-fold higher avidity, as determined by the CTL assay, than corresponding cells from mice with persistent infections. However, this difference could be not verified by the peptide-induced IFN-γ or TNF-α production analysis. The absence of a difference in functional avidity between GP133-41-specific CD8+ T cells isolated from the spleen and liver during acute versus persistent infections was further supported by analyses of the Vβ TCR repertoires, which showed comparable distribution profiles of Vβ usage by GP133-41-specific CD8+ T cells isolated from the spleen, PBL, and liver between days 6 and 100 after infection. Representative distribution profiles of Vβ usage for days 9 and 20 after infection are shown in Fig. 11. Concurrent analyses of NP396-404-specific CD8+ T-cell populations on days 6 and 9 after infection confirmed the above conclusion (data not shown).
FIG. 10.
Functional avidity of virus-specific CD8+ T cells in the spleens and livers of mice with acute versus persistent infections. C57BL/6 mice were infected with 102 (○) or 2 × 106 (•) PFU of LCMV-Docile, and lymphocytes were isolated from the spleen and liver at the indicated times. Function-based avidities of GP133-41-specific CD8+ T cells were determined by quantifying the amount of peptide required to induce ex vivo lytic activity against EL-4 target cells loaded with the indicated concentrations of GP133-41 (KAVYNFATM) peptide (A). Antigen-specific lysis of target cells at each peptide concentration is shown as a percentage of the maximum response obtained with a 10−4 M peptide concentration. In parallel analyses, IFN-γ (B) or TNF-α (C) production was measured after direct ex vivo stimulation with graded doses of peptide. The results were expressed as percentages of the maximum response attained with a saturating peptide concentration (10−4 M). Data shown are means ± SEM of log10 virus-specific T cells per tissue for three experiments.
FIG. 11.
Distribution profiles of Vβ usage by GP133-41-specific CD8+ T cells isolated from the spleen and liver during acute versus chronic infections. C57BL/6 mice were infected with 102 (○) or 2 × 106 (•) PFU of LCMV-Docile, and lymphocytes isolated from the spleen, blood, and liver at the indicated times were triple stained with an anti-CD8α antibody, the Db/GP133-41 tetramer, and an antibody specific for Vβ segments. Results from individual mice are shown as percentages of the GP133-41-specific CD8+ T-cell populations for each Vβ segment.
By extended analyses, the TCR affinities of NP396-404 versus GP133-41 peptide-specific CD8+ T cells were compared. Although there was a small but reproducible increase in TCR affinity (increased t1/2 values) over the course of the infection, no apparent differences in TCR affinity were observed between the NP396-404- or GP33-41-specific CD8+ T-cell populations isolated from the spleens and livers of mice with acute or persistent viral infections (Table 1). Thus, the susceptibility to deletion of virus-specific CD8+ T cells in mice with chronic LCMV infections is unlikely to be an intrinsic property of the TCR affinity of each population.
TABLE 1.
TCR affinities of virus-specific CD8 T cells in the spleens and livers of mice during acute or persistent LCMV-Docile infections
| Dose of LCMV-Docile (PFU) | Time after infection (days) |
t1/2 of TCR-peptide-H-2Db interactiona
|
|||
|---|---|---|---|---|---|
| Db-GP133-41
|
Db-NP396-404
|
||||
| Spleen | Liver | Spleen | Liver | ||
| 2 × 106 | 7 | 24.9 ± 0.3 | 38.8 ± 3.1 | 18.7 ± 0.5 | 18.2 ± 2.9 |
| 9 | 37.2 ± 3.2 | 40.7 ± 3.2 | 48.5 ± 8.7 | 23.3 ± 2.0 | |
| 30 | 31.0 ± 0.9 | 48.2 ± 12.7 | ND | ND | |
| 102 | 7 | 37.1 ± 2.1 | 31.2 ± 3.9 | 20.7 ± 1.5 | 18.4 ± 1.7 |
| 9 | 57.2 ± 6.8 | 57.7 ± 7.0 | 56.7 ± 3.3 | 26.8 ± 3.5 | |
| 30 | 43.9 ± 5.4 | 56.1 ± 4.5 | 61.6 ± 5.3 | 60.5 ± 3.2 | |
GP133-41 or NP396-404 peptide-specific CD8+ T cells in the spleens and livers of persistently (2 × 106 PFU) or acutely (102 PFU) infected mice exhibited similar TCR affinities, as measured by a dissociation assay. Lymphocytes isolated on days 7, 9, and 30 after the infection of C57BL/6 mice were stained with the Db-GP133-41 or Db-NP396-404 peptide tetramer at room temperature. Cells were washed twice and incubated in the presence of a saturating amount of Db monoclonal antibody. Dissociation was monitored for 0 to 180 min. The natural logarithm of the percentage of tetramer-positive T cells at each time point (compared to 0 min) was plotted against time. The half-life of each tetramer was derived from the slope by the equation t1/2 = ln 2/slope, as described in Materials and Methods. Data shown are means ± SEM of t1/2 expressed in minutes for three or four individual mice. ND, not done.
Link between persistence of virus-specific CD8+ T-cell response and liver injury.
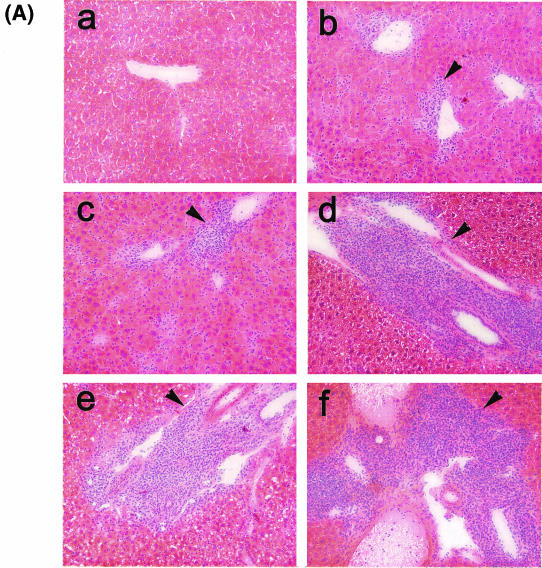
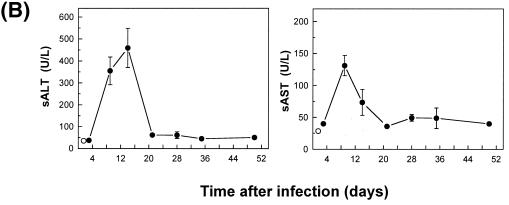
A characteristic feature of many chronic infections in humans is the persistence of a virus-specific CD8+ T-cell response in the presence of relatively high levels of virus which can be associated with the emergence of viral escape mutants in class I MHC-restricted CD8+ T-cell epitopes and/or with tissue injury (e.g., hepatitis). The persistence of a viral infection despite the presence of functional CD8+ T cells in nonlymphoid tissues could be explained by the emergence of T-cell escape viral mutants. For an exploration of this possibility, sequencing was performed on the LCMV genomes of several bulk virus isolates recovered from the spleen, liver, kidney, and lung on day 30 of a persistent infection. The results did not support this possibility, as no mutations in regions encoding dominant CD8+ T-cell epitopes were detected (data not shown). The possibility that the persistence of functional CD8+ T cells in nonlymphoid tissues, especially the liver, may result in disease was examined by an immunohistochemical analysis of liver tissues and by measurement of the serum transaminase activity over the course of a persistent infection. A histological examination of liver sections revealed signs of extensive inflammatory reactions at all times evaluated (9, 15, 21, 28, and 35 days) (Fig. 12A). .During the early stage of infection (9, 14, and 21 days), inflammatory cells, consisting mostly of lymphocytes, were found distributed over the entire liver parenchyma and were associated with signs of liver cell destruction. Thereafter, the inflammation became more focal, with the prominent formation of periportal mononuclear and lymphocytic infiltrates, associated with a decrease in liver pathology. An immunohistochemical analysis revealed that the infiltrates contained T and B lymphocytes (positive for CD4, CD8, or B220 antigen) and were colocalized with liver cells stained with an antibody specific for the LCMV NP antigen (data not shown). Liver tissue damage was also reflected by a drastic increase in transaminase activities in serum; they peaked on day 9 for sAST or day 15 for sALT and fell rapidly after day 21 (Fig. 12B). Because liver disease during LCMV infections is largely dependent on the activity of virus-specific CD8+ T cells, these results indicate that virus-specific CD8+ T cells detected in the liver were active at least during the initial stage of persistent infection, although they were not adequate for the resolution of the LCMV infection.
FIG. 12.
Immune system-mediated liver injury during chronic infection of mice with LCMV. (A) C57BL/6 mice were infected with 2 × 106 PFU of LCMV-Docile, and liver tissues recovered on days 9 (b), 15 (c), 21 (d), 28 (e), and 35 (f) after infection were fixed in acetone, sectioned, and stained with hematoxylin and eosin. Liver tissues from uninfected mice (a) were used as a control. Arrows indicate periportal mononuclear infiltrates in tissues of infected mice. Original magnification, ×200. (B) Liver-specific enzyme activities in serum (sALT and sAST) were measured over a period of 35 days after the infection of mice with 2 × 106 PFU of LCMV-Docile (•). Control values from sera of uninfected mice are also shown (○). Data shown are means ± SEM of enzymatic activities (units per liter) of three individual mice.
Regulation of virus-specific CD8+ T-cell response by CD4+ T cells in lymphoid and nonlymphoid tissues during chronic LCMV infection.
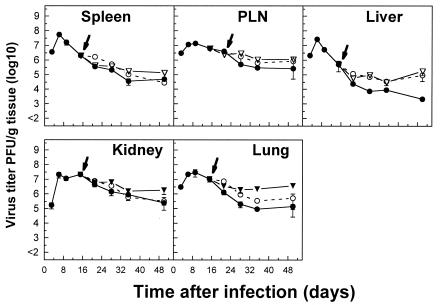
Evidence that a robust CD4+ T-cell response may be essential for effective antiviral CD8+ T-cell immunity has been obtained in several experimental settings. The induction of virus-specific CD4+ T cells that persist and retain their functions for several weeks during chronic LCMV infections has been well documented (54). In this context, we were interested to know whether the prolonged persistence of virus-specific CD8+ T cells is dependent on CD4+ T-cell help. The induction of virus-specific CD8+ T cells in CD4−/− mice during the initial stage of persistent LCMV infections was comparable to that observed with control mice (Fig. 13). However, in the absence of CD4+ T-cell help, virus-specific CD8+ T cells lost their antiviral functions rapidly (within 20 days) after infections in different tissues. This was in marked contrast to what was observed for control mice with intact CD4+ T-cell populations and was reflected by significantly higher levels of persisting virus titers in different tissues than those in control tissues (compare Fig. 2 and 13). GP133-41 peptide-specific T cells that were resistant to deletion were physically eliminated more rapidly in the absence of CD4+ T cells than in control mice in all tissues studied. As is evident from Fig. 14, an increase in viral titers, especially in the liver, in chronically infected mice depleted of CD4+ T cells by an antibody treatment on day 15 after infection, when virus-specific T cells with intact functions were present in nonlymphoid tissues, provided further evidence of a role for CD4+ T cells in the control of virus levels in different tissues. We therefore postulate that a persistent CD4+ T-cell helper response may be essential for effective antiviral CD8+ T-cell responses during chronic infections.
FIG. 13.
Rapid functional loss and physical elimination of virus-specific CD8+ T cells in lymphoid versus nonlymphoid tissues in the absence of CD4+ T-cell help during chronic LCMV infection. C57BL/6-CD4−/− mice were infected with 2 × 106 PFU of LCMV-Docile, and virus titers in different tissues were measured at the indicated times (A and D). Data shown are means ± SEM of log10 PFU/g of tissue for 3 to 5 mice. Total numbers of GP133-41 or NP396-404 peptide-specific CD8+ T cells were determined by staining with H-2Db tetramers (•) or measuring intracellular IFN-γ (○) production after stimulation of cells with the appropriate peptide (B, C, E, and F). Data shown are means ± SEM of log10 virus-specific T cells per spleen for 5 to 10 mice.
FIG. 14.
Capacity of virus-specific CD8+ T cells and of CD4+ T cells to control virus levels in different tissues of mice with persistent LCMV infections. Kinetics of viral titers in different tissues of C57BL/6 mice infected with 2 × 106 PFU of LCMV-Docile and depleted of CD8+ T cells (○) or CD4+ T cells (▴) by an antibody treatment on day 15 after infection (indicated by arrow) are shown. Infected but untreated C57BL/6 mice were used as a control (•). Data shown are means ± SEM of log10 virus-specific T cells per spleen for 3 to 5 mice.
DISCUSSION
One of the challenges faced in contemporary viral vaccine development concerns the induction of antigen-specific CD8+ T-cell responses that are both qualitatively and quantitatively able to deal with a pathogen in different tissue compartments of the host. This consideration is especially critical for immunological therapy of persistent viral infections. Although the development of the T-cell-mediated immune response to viruses has been well characterized in several systems, the mechanisms that determine when and to what extent an effective CD8+ T-cell response develops against a viral pathogen remain largely unknown. In this study, we used a murine LCMV model to better understand the regulation of virus-specific CD8+ T-cell responses in different tissues, with particular reference to the outcome of infection. We demonstrated that a critical feature for viral persistence is the rapid inactivation of CD8+ T cells in the microenvironment of lymphoid tissues. Thus, the degree and kinetics of T-cell exhaustion of antigen-specific CD8+ T cells in infected lymphoid tissues determine whether the viral infection will be acute or will persist for life. The process of clonal exhaustion in the lymphoid tissues is progressive, with two characteristic phases. Initially, there is a clonal expansion of virus-specific CD8+ T cells exhibiting a fully functional phenotype and expressing homing receptors to enable them to migrate to nonlymphoid tissues. This is followed by the down-regulation of antiviral functions, which may eventually be followed by the physical elimination of expanded clonal populations. Therefore, it is not surprising that a considerable population of virus-specific CD8+ T cells with intact antiviral phenotypes escaping clonal exhaustion in lymphoid tissues at the onset of infection can be found distributed in different nonlymphoid tissues. However, although these surviving T cells are present for a prolonged period of time at relatively high levels and although a fraction of them exhibit antiviral properties associated with liver damage during the early stage of infection, they are not able to control the infection and are eventually eliminated. This may reconcile the presence of functionally active virus-specific CD8+ T cells in the circulation of patients with chronic viral infections (e.g., HIV or hepatitis B or C) (7, 18, 40, 78) by the prediction from these findings that functional virus-specific CTLs are preferentially sequestered in different tissues (e.g., the liver for HBV or HCV) without complete virus eradication. The model developed based on the data presented here makes three further predictions.
The first is that a continuous supply of antiviral effector cells from lymphoid tissues is required for the efficient control of infection before the pathogen disseminates to different tissues. During an acute infection, the proliferation and differentiation of virus-specific CD8+ T cells and subsequent development of memory T cells apparently represent an optimal situation in which antigen-specific T cells with homing properties are continuously produced in lymphoid tissues. These cells leave the lymphoid organs via the blood and access all peripheral tissues, enabling efficient control of the infection. The presence of virus-specific CD8+ T cells at high levels long after the resolution of infection (for over 8 months of this study) in all tissues tested is of particular interest. The continued stimulation of memory CD8+ T cells by antigen in lymphoid and nonlymphoid tissues represents a plausible mechanism by which virus-specific CD8+ T cells might be sustained. Perhaps a subset of infected cells that are inefficiently recognized by CD8+ T cells provides a reservoir of persistent antigen. Indeed, there is good evidence in the LCMV literature to suggest that LCMV can persist in minute amounts in different tissues even under conditions of an acute infection (11, 34). Consistent with this possibility is the fact that ex vivo CTL activity was detectable in the spleen and nonlymphoid tissues (e.g., the liver) after the resolution of the infection (day 200), suggesting that at least a fraction of virus-specific CD8+ T-cell populations retained an effector phenotype. In striking contrast, ex vivo CTL activity in the PLN was detectable at high levels during the effector phase but was subsequently lost during the memory phase. This functional distinction of antigen-specific CD8+ T-cell populations resident in lymph nodes versus nonlymphoid tissues is reminiscent of the two main subsets of memory T cells in humans, namely the central memory T-cell population, which traffics through secondary lymphoid tissues and lacks an immediate effector ability (identified by the presence of CCR7), and the effector memory T-cell population, which is located in extra-lymphoid tissues (identified by the absence of CCR7) (65). However, our data suggest a more complex system because, in contrast to the case for lymph nodes, the virus-specific memory CD8+ T-cell population in the spleen retained an effector phenotype, possessing ex vivo CTL activity for prolonged period of times. The generation of heterogenous effector and memory CD8+ T-cell populations could provide a plausible explanation for the divergent functional phenotype we observed (63). The emergence of memory cells with such discrepancies in function perhaps occurs as a consequence of variable stimulation conditions due to prolonged or transient viral an-tigen presentation in the spleen and nonlymphoid tissues versus that in lymph nodes. Furthermore, such an interpretation could also reconcile studies conducted with vesicular stomatitis virus, which has been broadly used as a model of a nonpersisting pathogen. With this model, CD8+ memory T cells isolated from nonlymphoid tissues exhibited effector levels of ex vivo CTL activity for prolonged periods (several months after viral challenge), in contrast to their counterparts in the spleen, which lost their activity by day 20 after infection (44). An alternative explanation that cannot be excluded based on our data is that effector memory T cells may undergo tissue-specific regulation independent of antigen persistence.
A second prediction of our model is that under conditions of chronic LCMV infection, exhaustion of antigen-specific CD8+ T cells in the lymphoid tissues substantially limits the rate at which virus-specific effector T cells are generated in the lymphoid tissues and migrate through the blood to reach the sites of viral replication in different tissue compartments. The critical question raised by these data relates to the disparity in the kinetics and extent of functional inactivation and physical elimination of epitope-specific CD8+ T cells in different tissues. One possibility is that differences in virus titers, which are indicative of the antigenic load, may differentially impact the fate of antigen-specific T cells in different tissues. Based on our observations in this study, the level of antigenic stimulation that T cells receive upon interaction with virally infected APCs is likely the most relevant parameter in driving T-cell dysfunction and apoptotic deletion. In principle, this is determined at the single-cell level by the concentration of viral peptide-MHC complexes on the surface of the APC, the concentration of costimulatory molecules, and the duration of interactions between T cells and the APC (33). However, at the more complex tissue level, the amount of antigen presentation likely depends on additional factors, such as the cellular microenvironment and the quantity of infected cells as a consequence of viral spread in that tissue. The fact that nonlymphoid tissues contained equal or even higher viral titers than lymphoid tissues did (e.g., spleen versus kidney in Fig. 2) suggests that the overall virus-specific CD8+ T-cell response is critically regulated by the tissue-specific cellular environment. Regarding the differential regulation of epitope-specific CD8+ T-cell populations in chronically infected mice, in which NP396-406-specific CD8+ T cells are sensitive to deletion whereas GP133-41-specific T cells are relatively resistant to deletion, the density of the peptide-MHC complexes on infected cells may differ for individual epitopes. Consistent with this, recent studies have provided direct evidence that differences in the kinetics of viral gene expression in different cell types and amounts of the viral glycoprotein and nucleoprotein produced at different stages of the viral replication cycle can impact the fate of virus-specific T cells (59, 77). Alternatively, the different fates of virus-specific CD8+ T-cell populations may result from differences in TCR affinities and dissociation rates of epitope-specific T cells. This possibility is unlikely, because we found no differences in TCR affinity between GP133-41- and NP396-406-specific CD8+ T-cell populations.
A second question raised is as follows: why are virus-specific CD8+ T cells localized in nonlymphoid tissues unable to accomplish viral clearance? We propose that the population of virus-specific CD8+ T cells that homes to nonlymphoid tissues at the onset of infection represents an effector cell population that is capable of causing liver disease but eventually ineffective at virus resolution, having not fully acquired the protective qualities of memory CD8+ T cells. The limited capacity of effector cells compared to memory T cells to proliferate and survive in response to viral antigen could account for this phenomenon, which was originally described about 40 years ago in the LCMV literature (26, 74). However, changes in the ability of virus-specific CD8+ T cells with intact antiviral functions to migrate through nonlymphoid tissues likely also play a role in this lack of viral clearance. Such sequestration of effector cells within tissues at sites distinct from those of virus replication would explain this apparent disparity. A comparison of T-cell expression profiles for activation and homing receptors of functional CD8+ T cells from an acute infection versus dysfunctional CD8+ T cells from a persistent infection revealed striking differences in the expression of CD44, CD43, and Ly-6C molecules. Decreased levels of CD44 expression on dysfunctional virus-specific CD8+ T cells are compatible with the hypothesis that T-cell migration within tissues is impaired. The expression of the lymph node homing molecule L-selectin (CD62L), which regulates effector migration, remained down-regulated on anergic GP133-41-specific CD8+ T cells from persistently infected mice, possibly allowing cells to escape lymphoid tissues and migrate to nonlymphoid sites. The consequences of Ly-6C down-regulation are unclear, although this molecule has been shown to regulate CD8+ T-cell adhesion to endothelial cells and subsequent extravasation (25). In addition, the high-level expression of 1B11 (CD43) on antigen-specific T cells during persistent infections is likely to play a role in the maintenance of numbers and the dysfunctional phenotype of virus-specific CD8+ T cells (22, 51). The remarkable detection of NP396-404-specific CD8+ T-cell populations in the liver and BM during persistent infections while they are undetectable in lymphoid tissues and blood is consistent with the hypothesis that virus-specific CD8+ T cells which enter nonlymphoid tissues are impaired in the ability to traffic between different tissue compartments. Finally, a recent report demonstrated that the inhibition of HBV by the treatment of chronically infected patients with lamivudine results in the reconstitution of a circulating population of HBV-specific CD8+ T cells. These virus-specific T cells seem not to be derived from cells present in the liver but rather originate in the PLN, which supports our model (41).
A third prediction of our model is that under conditions of chronic LCMV infection, the functional inactivation of virus-specific CD8+ T cells proceeds with different kinetics in lymphoid versus nonlymphoid tissues. In the latter compartments, functional exhaustion follows a characteristic pattern in that the ability of virus-specific CD8+ T cells to up-regulate IL-2 production after antigenic stimulation in vitro is lost first, followed by TNF-α regulation and finally IFN-γ production and lytic activity. In contrast, the functional inactivation in lymphoid tissues proceeds rapidly and with similar kinetics for cytokines and CTL activity. The existence of CD8+ T cells with distinct functional phenotypes within the broader population of virus-specific CD8+ T cells during chronic infections provides critical information regarding the tissue-specific regulation of the antiviral immune response. In this respect, our results confirm and extend recent studies (13, 30) indicating that during prolonged chronic infections with LCMV Cl-13 Armstrong, virus-specific CD8+ T-cell effector functions in the spleen are progressively lost. The intriguing question raised by these findings regards the molecular basis for the disparity in kinetics of functional inactivation for virus-specific T cells in different tissue compartments and the biological significance of a hierarchical loss of effector functions. It is well recognized that the interaction between T cells and APCs can result in T-cell activation, anergy, or deletion, depending on the level of TCR engagement (32, 37, 73). Likewise, the levels of TCR engagement can trigger a spectrum of biological responses, such as cytotoxicity or cytokine secretion (24). According to this argument, it is conceivable that there is a hierarchical threshold in strength of activation (TCR signaling) required for the down-regulation of transcriptional programs for cytokines and lytic activity. Thus, under high levels of stimulation delivered in a relatively short time, as in the microenvironment of infected lymphoid tissues, a diverse array of functions are lost with similar kinetics. However, under conditions of subsaturated signal strength and long-term stimulation, for example, in nonlymphoid tissues, different threshold levels for the down-regulation of IL-2, TNF-α, and IFN-γ production and for lytic activity become apparent by differences in the kinetics with which these effector functions are lost. We speculate that the initial down-regulation of IL-2 production may prevent excessive proliferation and differentiation of virus-specific CTLs, which in the context of excessive viral antigen would otherwise cause irreparable tissue damage. Likewise, TNF-α overproduction can cause fever, cachexia, and septic shock, and rapid down-regulation of this cytokine's production may be critical for the prevention of such side effects (23). This is apparent in light of recent reports that, in contrast to IFN-γ, which seems to be less harmful, TNF-α production by CD8+ T cells ceases after a short period even when antigen contact is sustained (4, 69). Sustained lytic activity by CD8+ T cells can have severe consequences for the host. The lack of apparent clinical disease may be explained by the fact that this function is completely lost in lymphoid tissues during the onset phase of chronic infections, despite reduced but detectable levels of ex vivo CTL activity in other tissues throughout the course of infection.
Do these observations help us to understand how viruses persist in humans? Physical elimination, anergy, and an array of functional impairments of antigen-specific CD8+ T cells can negatively impact the efficient control or eradication of persistent viruses, such as HBV, HCV, and HIV in humans (3, 16, 19, 28, 36, 38, 39, 60) or SIV in primates (79). Moreover, in addition to CD8+ T cells, a crucial function for virus-specific CD4+ helper T cells in the containment of virus has been clearly demonstrated, including in this report (58, 64). Thus, the clonal exhaustion of virus-specific CD8+ T cells either in isolation or in the context of impaired virus-specific T-helper activity may in large part explain the lack of virus clearance in individuals with chronic HIV infections. The rapid disappearance of initially expanded HIV-specific CD8+ T-cell clones during primary HIV infections, despite the retention of measurable virus-specific cytotoxic functions, suggests that it is likely that deletion or exhaustion has a major impact on the chronic course of infection (55). Indeed, HIV-specific CD8+ T-cell and CD4+ T-cell unresponsiveness has been observed for a cohort of HIV-infected patients (16). In addition, massive Vβ-specific and clonotypic expansion has been demonstrated for acute HIV infections, whereas CTL activity of the same magnitude has not been described, suggesting that the clonal exhaustion of T cells may play a substantial role during the acute phase of this infection (70). Interestingly, individuals who have successfully cleared HCV infections develop strong CTL responses against multiple viral epitopes. However, such responses do not occur, or are not sustained, in all patients and become scarce once a persistent infection is established (35, 36). Similarly, virus-specific CD4+ T-cell responses were initially detected in many patients who subsequently failed to eliminate acute HCV infections, but these responses appeared to be short-lived (15). It is important to underscore that clonal exhaustion may involve individual virus-specific T-cell clones and thus does not necessarily result in the total loss of virus-specific T cells.
In conclusion, our results illustrate that in addition to being the site where a primary antiviral T-cell response develops, the lymphoid tissue environment may also be critical for the silencing of the virus-specific T-cell response by clonal exhaustion. These findings may help us to understand the variable course of persistent viral infections in humans. A further challenge for future studies is to understand the molecular mechanisms regulating virus-specific CD8+ T-cell responses during persistent infections in different tissue microenvironments. In particular, it is important to understand the factors involved in both the positive and negative regulation of T-cell-specific functions during acute and persistent viral infections. These data should prove useful not only for understanding the regulation of virus-specific T-cell responses, but also for defining measures to prevent the physical deletion of virus-specific T cells during persistent viral infections, which is a critical aspect for developing immunotherapeutic strategies to curtail such infections.
.
Acknowledgments
This work was supported by NIH grant AI42114 to D.M.
REFERENCES
- 1.Ahmed, R., L. A. Morrison, and D. M. Knipe. 1996. Persistence of viruses, p. 219-249. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, third ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 2.Altman, J. D., P. A. H. Moss, P. J. R. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 3.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. A. Gillesie, T. Dong, A. King, G. S. Ogg, M. L. Spiegel, C. Conlon, C. A. Spina, et al. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badovinac, V. P., G. A. Corbin, and J. T. Harty. 2000. Off cycling of TNF production by antigen-specific CD8+ T cells is antigen independent. J. Immunol. 165:5387-5391. [DOI] [PubMed] [Google Scholar]
- 5.Battegay, M., S. Cooper, A. Althage, H. Hengartner, and R. M. Zinkernagel. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24 and 96 well plates. J. Virol. Methods 33:191-198. [DOI] [PubMed] [Google Scholar]
- 6.Bauch, A., F. W. Alt, G. R. Crabtree, and S. B. Snapper. 2000. The cytoskeleton in lymphocyte signaling. Adv. Immunol. 75:89-114. [DOI] [PubMed] [Google Scholar]
- 7.Boni, C., A. Penna, G. S. Ogg, A. Bertoletti, M. Pilli, C. Cavallo, A. Cavalli, S. Urbani, R. Boehme, R. Panebianco, F. Fiaccardori, and C. Ferrari. 2001. Lamivudine treatment can overcome cytotoxic T-cell hyporesponsiveness in chronic hepatitis B: new perspectives for immune therapy. Hepatology 33:963-971. [DOI] [PubMed] [Google Scholar]
- 8.Borrow, P., and G. M. Shaw. 1998. Cytotoxic T-lymphocyte escape viral variants: how important are they in viral evasion of immune clearance in vivo? Immunol. Rev. 164:37-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butcher, E. C., and L. J. Picker. 1996. Lymphocyte homing and homeostasis. Science 272:60-66. [DOI] [PubMed] [Google Scholar]
- 10.Chisari, F. V., and C. Ferrari. 1995. Hepatitis B virus immunopathology. Springer Semin. Immunopathol. 17:261-281. [DOI] [PubMed] [Google Scholar]
- 11.Ciurea, A., P. Klenerman, L. Hunziker, E. Horvath, B. Odermatt, A. F. Ochsenbein, H. Hengartner, and R. M. Zinkernagel. 1999. Persistence of lymphocytic choriomeningitis virus at very low levels in immune mice. Proc. Natl. Acad. Sci. USA 96:11964-11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty, P. C., and J. P. Christensen. 2000. Accesing complexity: the dynamics of virus-specific T cell responses. Annu. Rev. Immunol. 18:561-592. [DOI] [PubMed] [Google Scholar]
- 13.Fuller, M. J., and A. J. Zajac. 2003. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170:477-486. [DOI] [PubMed] [Google Scholar]
- 14.Gallimore, A., A. Glithero, A. Godkin, A. C. Tissot, A. Pluckthun, T. Elliott, H. Hengartner, and R. M. Zinkernagel. 1998. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187:1383-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerlach, J. T., H. M. Diepolder, M. C. Jung, N. H. Gruener, W. W. Schraut, R. Zachoval, R. Hoffmann, C. A. Schirren, T. Santantonio, and G. R. Pape. 1999. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T- cell response in acute hepatitis C. Gastroenterology 117:933-941. [DOI] [PubMed] [Google Scholar]
- 16.Goepfert, P. A., A. Bansal, B. H. Edwards, G. D. Ritter, I. Tellez, S. A. McPherson, S. Sabbaj, and M. J. Mulligan. 2000. A significant number of human immunodeficiency virus epitope-specific cytotoxic T lymphocytes detected by tetramer binding do not produce gamma interferon. J. Virol. 74:10249-10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldrath, A. W., and M. J. Bevan. 1999. Selecting and maintaining a diverse T-cell repertoire. Nature 402:255-262. [DOI] [PubMed] [Google Scholar]
- 18.Goulder, P. J. R., Y. Tang, C. Bander, M. R. Betts, M. Altfeld, K. Annamalai, A. Trocha, et al. 2000. Functionally inert HIV-specific cytotoxic T lymphocytes do not play a major role in chronically infected adults and children. J. Exp. Med. 192:1819-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruener, N. H., F. Lechner, M.-C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 21.Harty, J. T., A. R. Tvinnereim, and D. W. White. 2000. CD8+ T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275-308. [DOI] [PubMed] [Google Scholar]
- 22.He, Y.-W., and M. J. Bevan. 1999. High level expression of CD43 inhibits T cell receptor/CD3-mediated apoptosis. J. Exp. Med. 190:1903-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbein, G., and W. A. O'Brien. 2000. Tumor necrosis factor (TNF)-alpha and TNF receptors in viral pathogenesis. Proc. Soc. Exp. Biol. Med. 223:241-257. [DOI] [PubMed] [Google Scholar]
- 24.Itoh, Y., and R. N. Germain. 1997. Single cell analysis reveals regulated hierarchical T cell antigen receptor signaling thresholds and intraclonal heterogeneity for individual cytokine responses of CD4+ T cells. J. Exp. Med. 186:757-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaakkola, I., M. Merinen, S. Jalkanen, and A. Hanninen. 2003. Ly6C induces clustering of LFA-1 (CD11a/CD18) and is involved in subtype-specific adhesion of CD8 T cells. J. Immunol. 170:1283-1290. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, E. D., and G. A. Cole. 1975. Functional heterogeneity of lymphocytic choriomeningitis virus-specific T lymphocytes. I. Identification of effector and memory subsets. J. Exp. Med. 141:866-881. [PMC free article] [PubMed] [Google Scholar]
- 27.Kagi, D., B. Ledermann, K. Burki, R. M. Zinkernagel, and H. Hengartner. 1996. Molecular mechanisms of lymphocyte-mediated cytotoxicity and their role in immunological protection and pathogenesis in vivo. Annu. Rev. Immunol. 14:207-232. [DOI] [PubMed] [Google Scholar]
- 28.Kostense, S., G. S. Ogg, E. H. Manting, G. Gillespie, J. Jolling, K. Vandenberghe, E. Veenhof, D. Von Baarle, S. Jurriaans, M. R. Klein, and F. Miedema. 2001. High viral burden in the presence of major HIV-specific CD8+ T cell expansion: evidence for impaired CTL effector function. Eur. J. Immunol. 31:677-686. [DOI] [PubMed] [Google Scholar]
- 29.Koszinowski, U. H., and M. J. Reddehase. 1993. The role of T-lymphocyte subsets in the control of cytomegalovirus infection, p. 429-445. In D. B. Thomas (ed.), Viruses and the cellular immune response. Marcel Dekker Inc., New York, N.Y.
- 30.Kristensen, N. N., J. P. Christensen, and A. R. Thompsen. 2002. High numbers of IL-2-producing CD8+ T cells during viral infection: correlation with stable memory development. J. Gen. Virol. 83:2123-2133. [DOI] [PubMed] [Google Scholar]
- 31.Lanzavecchia, A., and F. Sallusto. 2000. Dynamics of T lymphocyte responses: intermediates, effector, and memory cells. Science 290:92-97. [DOI] [PubMed] [Google Scholar]
- 32.Lanzavecchia, A., G. Lezzi, and A. Viola. 1999. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell 96:1-4. [DOI] [PubMed] [Google Scholar]
- 33.Lanzavecchia, A., and F. Sallusto. 2002. Progressive differentiation and selection of the fittest in the immune response. Nat. Rev. Immunol. 2:982-987. [DOI] [PubMed] [Google Scholar]
- 34.Larsen, J. H. 1968. Studies on immunological tolerance to LCM virus. 9. Induction of immunological tolerance to the virus in the adult mouse. Acta Pathol. Microbiol. Scand. 73:106-114. [DOI] [PubMed] [Google Scholar]
- 35.Lechner, F., N. H. Gruener, S. Urbani, J. Uggeri, T. Santantonio, A. R. Kammer, A. Cerny, R. Phillips, C. Ferrari, G. R. Pape, and P. Klenerman. 2000. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur. J. Immunol. 30:2479-2487. [DOI] [PubMed] [Google Scholar]
- 36.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lezzi, G., K. Karjalainen, and A. Lanzavecchia. 1998. The duration of antigenic stimulation determines the fate of naive and effector cells. Immunity 8:89-95. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman, J., P. Shankar, and J. Andersson. 2001. Dressed to kill? A review of why antiviral CD8 T lymphocytes fail to prevent progressive immunodeficiency in HIV-infection. Blood 98:1667-1677. [DOI] [PubMed] [Google Scholar]
- 39.Liu, H., S. Andreansky, G. Diaz, T. Hogg, and P. C. Doeherty. 2002. Reduced functional capacity of CD8+ T cells expanded by post-exposure vaccination of γ-herpesvirus infected CD4-deficient mice. J. Immunol. 168:3477-3483. [DOI] [PubMed] [Google Scholar]
- 40.Maini, M. K., C. Boni, C. K. Lee, J. R. Larrubia, S. Reignat, G. S. Ogg, A. S. King, et al. 2000. The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191:1269-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malacarne, F., G. J. M. Webster, S. Reignat, J. Gotto, S. Behboudi, A. K. Burroughs, G. M. Dusheiko, R. Williams, and A. Bertoletti. 2003. Tracking the source of the hepatitis B virus-specific CD8 T cells during lamivudine treatment. J. Infect. Dis. 187:679-682. [DOI] [PubMed] [Google Scholar]
- 42.Mantoya, M. C., D. Sancho, M. Vincente-Manzanares, and F. Sanchez-Madrid. 2002. Cell adhesion and polarity during immune interactions. Adv. Immunol. 186:68-82. [DOI] [PubMed] [Google Scholar]
- 43.Marshall, D. R., S. J. Turner, G. T. Belz, S. Wingo, S. Andreansky, M. Y. Sangster, J. M. Riberdy, T. Liu, M. Tan, and P. C. Doherty. 2001. Measuring the diaspora for virus-specific CD8+ T cells. Proc. Natl. Acad. Sci. USA 98:6313-6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissues. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 45.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune response to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 46.Moskophidis, D., S. P. Cobbold, H. Waldmann, and F. Lehmann-Grube. 1987. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J. Virol. 61:1867-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moskophidis, D., F. Lechner, H.-P. Pircher, and R. M. Zinkernagel. 1993. Virus persistence in acutely infected mice by exhaustion of antiviral cytotoxic T cells. Nature 362:758-761. [DOI] [PubMed] [Google Scholar]
- 48.Moskophidis, D., and R. M. Zinkernagel. 1995. Immunobiology of cytotoxic T-cell escape mutants of lymphocytic choriomeningitis virus. J. Virol. 69:2187-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. D. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 50.Oldstone, M. B. A. 2002. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr. Top. Microbiol. Immunol. 262:83-111. [DOI] [PubMed] [Google Scholar]
- 51.Onami, T. M., L. E. Harrington, M. A. Williams, M. Galvan, C. P. Larsen, T. C. Pearson, N. Manjunath, L. G. Baum, B. D. Pearce, and R. Ahmed. 2002. Dynamic regulation of T cell immunity by CD43. J. Immunol. 168:6022-6031. [DOI] [PubMed] [Google Scholar]
- 52.Ou, R., S. Zhou, L. Huang, and D. Moskophidis. 2001. A critical role for interferon alpha/beta and gamma in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T-cells. J. Virol. 75:8407-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oxenius, A., R. M. Zinkernagel, and H. Hengartner. 1998. CD4+ T-cell induction and effector functions: a comparison of immunity against soluble antigens and viral infections. Adv. Immunol. 70:313-367. [DOI] [PubMed] [Google Scholar]
- 54.Oxenius, A., R. M. Zinkernagel, and H. Hengartner. 1998. Comparison of activation versus induction of unresponsiveness of virus-specific CD4+ and CD8+ T cells upon acute versus persistent viral infection. Immunity 9:449-457. [DOI] [PubMed] [Google Scholar]
- 55.Pantaleo, G., H. Soudeyns, J. F. Demarest, M. Vaccarezza, C. Graziosi, S. Paolucci, M. Daucher, O. J. Cohen, F. Denis, W. E. Biddison, R. P. Sekaly, and A. S. Fauci. 1997. Evidence for rapid disappearance of initially expanded HIV-specific CD8+ T cell clones during primary HIV infection. Proc. Natl. Acad. Sci. USA 94:9848-9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pfau, C. J., J. K. Valenti, D. C. Pevear, and K. D. Hunt. 1982. Lymphocytic choriomeningitis virus killer T cells are lethal only in weakly disseminated murine infections. J. Exp. Med. 156:79-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pircher, H.-P., D. Moskophidis, U. Rohrer, K. Burki, H. Hengartner, and R. M. Zinkernagel. 1990. Viral escape by selection of cytotoxic T cell-resistant virus variants in vivo. Nature 346:629-633. [DOI] [PubMed] [Google Scholar]
- 58.Pitcher, C. J., C. Quittner, D. M. Peterson, M. Connors, R. A. Koup, V. C. Maino, and L. J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518-525. [DOI] [PubMed] [Google Scholar]
- 59.Probst, H. C., K. Tschannen, A. Gallimore, M. Martinic, M. Basler, T. Dumrese, and M. F. Van den Broek. 2003. Immunodominance of an antiviral cytotoxic T cell response is shaped by the kinetics of viral protein expression. J. Immunol. 171:5415-5422. [DOI] [PubMed] [Google Scholar]
- 60.Reignat, S., G. J. M. Webster, D. Brown, G. S. Ogg, A. King, S. L. Seneviratne, G. Dusheiko, R. Wiliams, M. K. Maini, and A. Beroletti. 2002. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J. Exp. Med. 195:1089-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reinhardt, R. L., A. Khoruts, R. Merica, T. Zell, and M. K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410:101-105. [DOI] [PubMed] [Google Scholar]
- 62.Rickinson, A. B., and D. J. Moss. 1997. Human T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 15:405-431. [DOI] [PubMed] [Google Scholar]
- 63.Roman, E., E. Miller, A. Harmsen, J. Wiley, U. H. Von Andrian, G. Huston, and S. L. Swain. 2002. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J. Exp. Med. 196:957-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 65.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712. [DOI] [PubMed] [Google Scholar]
- 66.Sallusto, F., C. R. Mackay, and A. Lanzavecchia. 2000. The role of chemokine receptors in primary, effector, and memory immune response. Annu. Rev. Immunol. 18:593-620. [DOI] [PubMed] [Google Scholar]
- 67.Savage, P. A., J. J. Boniface, and M. M. Davis. 1999. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity 10:485-492. [DOI] [PubMed] [Google Scholar]
- 68.Shen, Z., G. Reznikoff, G. Dranoff, and K. L. Rock. 1997. Cloned dendritic cells can present antigen on both MHC class I and class II molecules. J. Immunol. 158:2723-2730. [PubMed] [Google Scholar]
- 69.Slifka, M. K., and J. L. Whitton. 2000. Antigen-specific regulation of T cell-mediated cytokine production. Immunity 12:451-457. [DOI] [PubMed] [Google Scholar]
- 70.Soudeyns, H., G. Campi, G. P. Rizzardi, C. Lenge, J. F. Demarest, G. Tambussi, A. Lazzarin, D. Kaufmann, G. Casorati, L. Corey, and G. Pantaleo. 2000. Initiation of antiretroviral therapy during primary HIV-1 infection induces rapid stabilization of the T-cell receptor beta chain repertoire and reduces the level of T-cell oligoclonality. Blood 95:1743-1751. [PubMed] [Google Scholar]
- 71.Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multiple paradigm. Cell 76:301-314. [DOI] [PubMed] [Google Scholar]
- 72.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Shust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 73.Valitutti, S., S. Mueller, M. Dessing, and A. Lanzavecchia. 1996. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J. Exp. Med. 183:1917-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Volkert, M., O. Marker, and K. Bro-Jorgensen. 1974. Two populations of T lymphocytes immune to the lymphocytic choriomeningitis virus. J. Exp. Med. 139:1329-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walker, C. M. 1999. Hepatitis C virus, p. 94-115. In R. Ahmed and I. Chen (ed.), Persistent viral infections. John Wiley and Sons Ltd., New York, N.Y.
- 76.Welsh, R. M. 2001. Assessing CD8 T cell number and dysfunction in the presence of antigen. J. Exp. Med. 193:19-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. Van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wong, D. K. H., D. D. Dudley, N. H. Afdhal, J. Dienstag, C. M. Rice, L. Wang, M. Houghton, B. D. Walker, and M. J. Koziel. 1998. Liver-derived CTL in hepatitis C virus infection: breadth and specificity of responses in a cohort of persons with chronic infection. J. Immunol. 160:1479-1488. [PubMed] [Google Scholar]
- 79.Xiong, Y., M. A. Luscher, J. D. Altman, M. Husley, H. L. Robinson, M. Ostrowski, B. H. Barber, and K. S. MacDonald. 2001. Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8+ T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J. Virol. 75:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. D. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou, S., R. Ou, L. Huang, and D. Moskophidis. 2002. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection. J. Virol. 76:829-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zinkernagel, R. M., and H. Hengartner. 2001. Regulation of the immune response by antigen. Science 293:251-253. [DOI] [PubMed] [Google Scholar]
- 83.Zinkernagel, R. M. 1996. Immunology taught by viruses. Science 271:173-178. [DOI] [PubMed] [Google Scholar]