Abstract
Purpose
Triple negative breast cancers (TNBC) frequently have high epidermal growth factor receptor (EGFR) expression and are sensitive to DNA-damaging agents. Improved therapies are needed for this aggressive malignancy.
Patients and methods
We performed a phase I trial of bendamustine and erlotinib, an EGFR tyrosine kinase inhibitor, in patients with metastatic TNBC, ECOG performance status ≤2, and ≤1 prior chemotherapy for metastatic disease. Each 28-day cycle included intravenous bendamustine on days 1, 2 and oral erlotinib on days 5–21 with dose escalation according to a 3 + 3 phase I study design. Dose-limiting toxicity (DLT) was determined by toxicities related to study therapy observed during cycle 1.
Results
Eleven patients were treated, 5 on dose level 1 and 6 on dose level 2. One patient had DLT on dose level 2. However, cumulative toxicities were observed, including grade 3/4 lymphopenia in 91 % (95 % CI 0.59–0.998) with progressively decreased CD4 counts and grade ≥3 infections in 36 % (95 % CI 0.11–0.69) of patients.
Conclusions
Combination therapy with bendamustine and erlotinib causes excessive toxicity with severe, prolonged lymphopenia, depressed CD4 counts, and opportunistic infections and should not be pursued further. Future trials of bendamustine combinations in TNBC patients should account for potential cumulative lymphocyte toxicity necessitating patient monitoring during and after treatment.
Keywords: Bendamustine, Erlotinib, Lymphopenia, Triple negative breast cancer, Metastatic breast cancer
Introduction
Triple negative breast cancers (TNBC) are phenotypically estrogen receptor (ER) and progesterone receptor (PR) negative and Her-2 non-overexpressing and account for approximately 12–20 % of breast cancers [1]. These cancers tend to be of higher grade, have more frequent distant metastases with earlier occurrence, and have shorter survival compared with other types of breast cancer [2–4]. In addition, treatment options for TNBC are limited compared with other breast cancer subtypes with no identified effective targeted therapies.
Bendamustine is a unique chemotherapeutic hybrid compound with purine antagonist and alkylating properties. In the United States, it is principally used for hematologic malignancies, while in Europe, it has also been used to treat solid tumors; several studies have revealed breast cancer treatment efficacy [5–8]. Bendamustine causes DNA cross-linking resulting in more extensive, durable single- and double-stranded DNA breaks compared with other compounds [9]. Bendamustine’s mechanisms of action make it a promising treatment for TNBC, which has known sensitivity to DNA-damaging drugs [10].
Erlotinib is an oral epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) used for treatment of non-small cell lung cancer (NSCLC) and pancreatic cancer. EGFR expression in breast cancer has been shown to be an independent poor prognostic factor [11]. Although EGFR over-expression is present in approximately 13 % of all breast cancers [1], the majority of TNBCs express EGFR [3, 12, 13] and many also have EGFR gene amplification [3, 13]. Several early phase clinical trials of EGFR inhibitors combined with chemotherapy in metastatic TNBC patients have been conducted with varying results [14–17].
Given that TNBCs are sensitive to DNA-damaging agents and have high EGFR expression and amplification, we performed a phase I clinical trial to assess the toxicity and tolerability of combination therapy with bendamustine and erlotinib. The study drugs were administered on a sequential, rather than a concurrent schedule given the pre-clinical data, suggesting that concurrent administration may be antagonistic [18–20]. It was hypothesized that a sequential schedule would also limit the toxicity of the therapy.
Pharmacokinetic analyses were performed in multiple studies of erlotinib combined with chemotherapy revealing no significant interactions, supporting the hypothesis that the study drug combination would be safe and tolerable [17, 21]. While significant lymphopenia and low CD4 counts have been observed with bendamustine, serious or opportunistic infections are uncommon [5, 22]. Although lymphopenia was an expected toxicity, severe secondary infections were not anticipated in this study. Overall hematologic toxicity and infection rates were expected to be lower than those observed in treatment for hematologic malignancies, which are more likely to have severe immunosuppression from underlying disease and prior therapy with bone marrow damaging agents.
Patients and methods
The study was a planned Phase I/II trial evaluating the combination of bendamustine and erlotinib for treatment of metastatic TNBC. However, a high degree of cumulative toxicity was observed after 11 patients were enrolled and treated on the Phase I section of the study. Therefore, the study was terminated, and the Phase II study was not performed. The protocol was approved by the Ohio State University Cancer Institutional Review Board. All patients gave informed consent prior to study treatment in accordance with the Declaration of Helsinki. The study was registered at www.clinicaltrials.gov as NCT00834678.
Eligibility criteria
The study enrolled patients ≥18 years with histologically confirmed metastatic TNBC, defined as ER and PR<10 % by immunohistochemistry (IHC), and Her-2 non-amplified on fluorescence in situ hybridization (FISH), or 0–1+ by IHC, or +2 on IHC and non-amplified by FISH. No more than one prior chemotherapy regimen for metastatic breast cancer was permitted. All patients had measurable or evaluable disease. No concurrent anti-neoplastic treatments were administered; bisphosphonate therapy was permitted. Patients could not have symptomatic or progressive central nervous system (CNS) metastases or leptomeningeal disease. Patients with previously treated brain or CNS metastases were permitted provided that radiation was completed ≥8 weeks prior to study registration and they were not receiving steroids.
Patients had Eastern Cooperative Group performance status of 0–2 with an estimated life expectancy ≥6 months. Adequate bone marrow, renal, and hepatic function were required, defined as absolute neutrophil count (ANC) >1.5 × 109/L, platelet >100 × 109/L, estimated creatinine clearance >40 ml/min, bilirubin ≤1.5 times the institutional upper limit of normal (ULN), ALT, AST, and alkaline phosphatase ≤2.5 × ULN. In the presence of documented liver metastases, ALT and AST were to be ≤5 × ULN. Alkaline phosphatase was ≤5 × ULN in the presence of liver or bone metastases. Patients were able to take oral medications and without medical problems or prior surgeries that may interfere with the absorption of oral medications.
Patients with prior treatment with bendamustine or EGFR-directed therapy, known hypersensitivity to bendamustine, mannitol, or erlotinib, uncontrolled intercurrent illness that may interfere with administration of study therapy, active infection requiring systemic therapy, or HIV infection requiring anti-retroviral therapy were excluded from the study. Pregnant and nursing women were ineligible due to the increased risk of fetal harm, including fetal death, from study therapy. All patients of reproductive potential agreed to use an effective barrier contraceptive method or abstinence. Patients could not have history of prior malignancy except for: adequately treated basal cell or squamous cell skin carcinoma or any other cancer from which the patient has been disease free for ≥5 years. All patients were informed of the investigational nature of this study and signed written informed consent prior to enrollment.
Treatment plan
Bendamustine was administered intravenously over 30 min on days 1 and 2 of each cycle. Erlotinib was taken by mouth once per day on days 5–21 (17 days). Each treatment cycle was 28 days. Study drug doses were determined by the dose escalation scheme shown in Table 1. No intra-patient dose escalations or re-escalations were permitted. Therapy was discontinued for disease progression (PD), unacceptable toxicity, or for patient and/or treating physician preference. Patients were permitted to transition to maintenance erlotinib therapy at any time following the administration of ≥6 cycles of therapy, as long as there was not PD or unacceptable toxicity. Maintenance therapy consisted of 150 mg of continuous daily erlotinib. Patients were administered pro-phylactic anti-emetics prior to bendamustine administration and prescribed rescue anti-emetics. Pre-medications for prevention of hypersensitivity reactions were only administered to patients who experienced hypersensitivity to bendamustine. After study termination for severe lymphopenia and opportunistic infections, CD4 monitoring was recommended. Pneumocystis carinii pneumonia (PCP), viral, and mycobacterium avium complex (MAC) prophylaxis were suggested until CD4 recovery.
Table 1.
Drugs and dose escalation scheme
| Dose level | Dose escalation
|
|
|---|---|---|
| Bendamustine | Erlotinib | |
| Level −1 | 100 mg/m2 IV | 100 mg po |
| Level 1 | 120 mg/m2 IV | 100 mg po |
| Level 2 | 120 mg/m2 IV | 150 mg po |
Monitoring of treatment toxicity, including history, physical examination, and laboratory monitoring, was performed on day 1 of each treatment cycle, or more frequently as clinically indicated. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) Version 3. Protocol specified guidelines for dose modifications and interruptions of bendamustine and/or erlotinib were followed. For facilitation and monitoring of treatment compliance, patients were provided with a treatment calendar and log on day 1 of each cycle. Patients were required to bring their treatment logs and erlotinib pill bottles from the prior cycle to the follow-up appointment on day 1 of each cycle. The study coordinator reviewed the log and performed pill counts. Patients were assessed for treatment response by imaging studies and physical examination every 2 cycles. Treatment response was assigned according to RECIST 1.1 criteria [23].
Statistical design and study endpoints
The primary endpoint of the Phase I study was to determine the appropriate Phase II dose of bendamustine and erlotinib through assessment of treatment toxicity during the first cycle of treatment. Dose-limiting toxicity (DLT) was defined based on toxicities possibly, probably, or definitely related to the study therapy observed during the first cycle (first 28 days) and included: ANC<1 × 109/L for >7 days despite use of white blood cell growth factor support; platelet count<25 × 109/L for >7 days, or associated with bleeding, or <10 × 109/L at any time; grade 4 rash or grade 3 rash not controlled with medical therapy; grade 4 diarrhea; or any other non-hematologic toxicity ≥grade 3 by NCI CTCAE version 3.0.
Patients were treated according to a Phase I 3 + 3 study design. The dose escalation scheme is shown in Table 1. All patients in each cohort were observed for the first cycle of treatment (28 days) prior to dose escalation or expansion of a dose level. The Phase II dose was defined as the highest dose tested in which fewer than 2 of 6 patients experienced DLT attributable to the study drug(s).
The primary endpoint of the planned Phase II section of the study was to determine the study therapy efficacy in patients with metastatic TNBC using progression-free survival (PFS) defined as the time from first treatment date until the documented time of disease progression or death. The secondary endpoints included the objective response rate [ORR = complete response (CR) + partial response (PR)] and overall survival rate (OS). As the Phase II study was not conducted, estimates of primary and secondary endpoints are reported with 95 % confidence intervals in the Phase I setting. For the time-to-event endpoints, estimates were obtained by the method of Kaplan–Meier.
Results
Patients
Eleven women with Stage IV TNBC were treated on the study, 5 on dose level 1 and 6 on dose level 2. The median age was 53 years (38–66). The most common sites of metastasis were lymph nodes (82 %), lung/pleura (55 %), and bone (36 %). One patient (9 %) had previously treated brain metastasis. Patient characteristics are summarized in Table 2.
Table 2.
Patient characteristics and outcomes
| Characteristic | All N = 11 |
Dose level 1 N = 5 |
Dose level 2 N = 6 |
|---|---|---|---|
| Age, years | |||
| Median | 53 | 53 | 53 |
| Range | 38–66 | 45–65 | 38–66 |
| Race. No. (%) | |||
| Caucasian | 9 (82) | 5 (100) | 4 (67) |
| African American | 2 (18) | 0 (0) | 2 (33) |
| ECOG performance status, N (%) | |||
| 0 | 4 (36) | 2 (40) | 2 (33) |
| 1 | 6 (55) | 2 (40) | 4 (67) |
| 2 | 1 (9) | 1 (20) | 0 (0) |
| Sites of metastasisa, N (%) | |||
| Lymph nodes | 9 (82) | 4 (80) | 5 (83) |
| Lung/pleura | 6 (55) | 3 (60) | 3 (50) |
| Bone | 4 (36) | 1 (20) | 3 (50) |
| Liver | 3 (27) | 0 (0) | 3 (50) |
| Chest wall/skin | 3 (27) | 1 (20) | 2 (33) |
| Brain | 1 (9) | 1 (20) | 0 (0) |
| Prior chemotherapy, N (%) | |||
| Adjuvant only | 4 (36) | 3 (60) | 1 (17) |
| Metastasis/local recurrence only | 1 (9) | 0 (0) | 1 (17) |
| Both | 6 (55) | 2 (40) | 4 (67) |
| Number of prior regimens, N (%) | |||
| 1 | 4 (36) | 2 (40) | 2 (33) |
| 2 | 7 (64) | 3 (60) | 4 (67) |
| Cycles received | |||
| Median | 2 | 4 | 2 |
| Range | 1–6 | 2–6 | 1–6 |
| Overall response rate, N (%) | 1 (9) | 0 (0) | 1 (17) |
| Progression-free survival | |||
| Median, months | 3.7 | 3.7 | 3.9 |
| 95 % CI | 1.7–5.5 | 1.7–5.5 | 1.7–7.0 |
| Overall survival | |||
| Median, months | 10.8 | 7.6 | 12.8 |
| 95 % CI | 3.6–13.1 | 3.6–10.8 | 2.4–NR |
CI confidence interval, NR not reached
A patient can be represented among multiple sites
All patients received prior chemotherapy in the adjuvant and/or metastatic settings. Ten patients (91 %) had received adjuvant chemotherapy; the 11th patient was diagnosed with metastasis at a time of initial breast cancer diagnosis and had received 1 prior regimen in the metastatic setting; 4/11 (36 %) patients received 1 prior chemotherapy regimen. Seven patients (64 %) received 2 prior regimens; of these, six patients received 1 regimen in the adjuvant setting and 1 regimen for metastatic or locally recurrent cancer, and one patient received 2 adjuvant chemotherapy regimens for 2 separate primary breast cancers.
Toxicity
No patient treated on dose level 1 experienced DLT. One of the 6 patients treated on dose level 2 had DLT with grade 3 fatigue and grade 3 dyspnea during her 1st treatment cycle with no further therapy given. She was hospitalized for progression of her breast mass with superimposed infection in the setting of grade 4 lymphopenia and was transitioned to hospice care. Hence, dose level 2, the highest dose in the treatment plan, would have been the recommended Phase II dose if the Phase II section had been conducted. However, the degree of cumulative toxicities observed prompted early closure of the study and is described below.
Common toxicities included fatigue, constitutional symptoms, and gastrointestinal symptoms (Table 3). One patient (9 %) was hospitalized for grade 3 diarrhea and hypotension, and another patient developed grade 3 nausea; since these toxicities occurred after completion of cycle 1, they were not considered DLTs. Two patients (18 %) developed grade 1/2 hypersensitivity reactions with bendamustine administration, and 4 patients (36 %) had grade 1/2 erlotinib-related rash.
Table 3.
Summary of toxicities
| Toxicity, N (%) | Worst grade N = 11
|
|||
|---|---|---|---|---|
| 1/2 | 3 | 4 | 5 | |
| Leukopenia | 6 (55) | 2 (18) | 1 (9) | |
| Neutropenia | 3 (27) | 1 (9) | ||
| Lymphopenia | 1 (9) | 2 (18) | 8 (73) | |
| CD4 counta | 1 (14) | 6 (86) | ||
| Hemoglobin | 3 (27) | 2 (18) | 1 (9) | |
| Platelets | 6 (55) | 1 (9) | ||
| Infection | 1 (9) | 3 (27) | ||
| Hypersensitivity | 2 (18) | |||
| Rash | 4 (36) | |||
| Diarrhea | 4 (36) | 1 (9) | ||
| Nausea | 4 (36) | 1 (9) | ||
| Vomiting | 2 (18) | |||
| Mucositis | 2 (18) | 1(9) | ||
| Constitutional | 2 (18) | 5 (45) | ||
| Metabolic | 5 (45) | 1 (9) | ||
| Pulmonary | 2 (18) | 2 (18) | ||
1 patient had DLT on dose level 2 (fatigue, dyspnea–grade 3)
Percentages for CD4 Count use a denominator of 7, the number of patients evaluated for CD4 toxicity
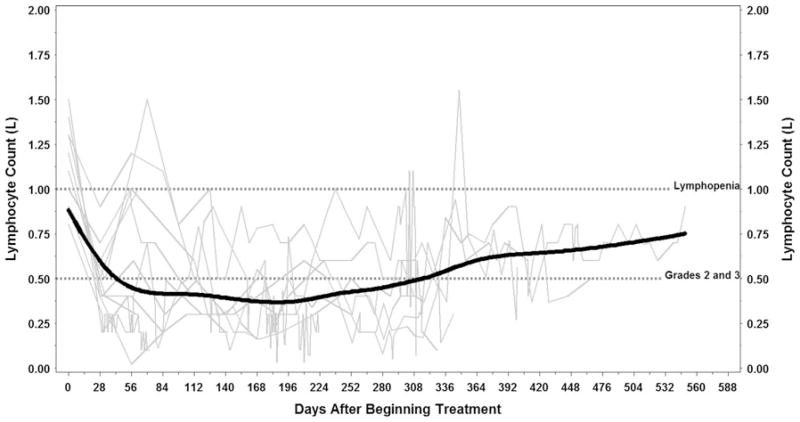
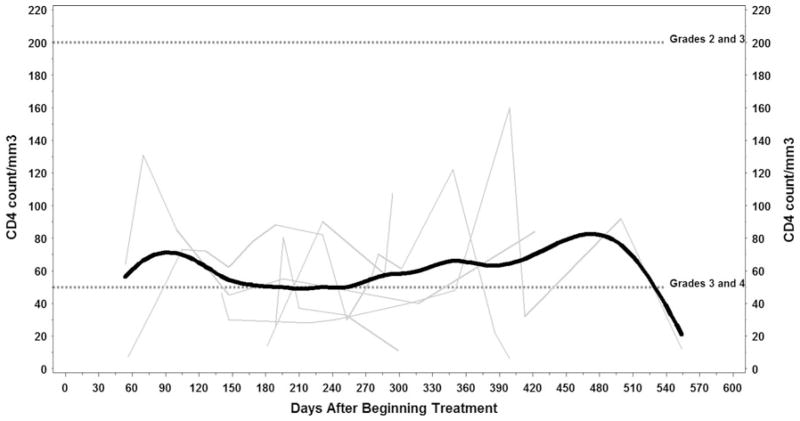
Hematologic toxicity was frequently observed during study therapy and included grade 3/4 leukopenia, neutropenia, lymphopenia, and anemia. Grade 3 thrombocytopenia occurred in 1 patient. Prolonged lymphopenia was observed and persisted after discontinuation of study therapy, for some patients more than 300 days (Fig. 1). Two patients (18 %) had grade 3 lymphopenia, and 8 (73 %) had grade 4 lymphopenia. CD4 counts were obtained in 7 of the 11 study patients. Of these, 1 (14 %) had grade 3 suppression of CD4 counts and 6 (86 %) had grade 4 depressed CD4 counts (Fig. 2).
Fig. 1.
Lymphocyte counts over time. Individual profiles (light gray lines) with average trend line (solid black) of lymphocyte counts. Reference lines (dotted black) are at 1.00 corresponding to presence of lymphopenia and 0.50 corresponding to the division between grade 2 and 3 lymphopenia. Created using Microsoft PowerPoint
Fig. 2.
CD4 counts over time. Individual profiles (light gray lines) with average trend line (solid black) of CD4 counts. Reference lines (dotted black) are at 200 and 50, highlighting areas of grade 3 CD4 counts (<200–50) as well as areas of grade 4 CD4 counts (<50). Created using Microsoft PowerPoint
Serious infections, including opportunistic infections with PCP, occurred in 4 (36 %) study patients and were deemed to be related to study therapy-associated lymphopenia. Three of these patients died as a result of infection. Serious adverse events (SAEs) are summarized in Table 4.
Table 4.
Summary of serious adverse events
| Patient 1 (DL1): <1 month after completing 6 cycles, hospitalized for pneumocystis carinii (PCP) pneumonia with associated grade 4 lymphopenia. She recovered and received additional chemotherapy, but died from sepsis in the setting of persistent lymphopenia >4 months after completing study therapy |
| Patient 5 (DL1): Completed 4 cycles followed by PD and 2 months later received whole brain radiation and steroids for new brain metastases. She was hospitalized 1 month later for presumed PCP pneumonia with grade 4 lymphopenia and subsequently died. No diagnostic procedure was performed |
| Patient 6 (DL 2): <1 month after completion of 6 cycles, she was hospitalized and treated for presumed opportunistic pneumonia with grade 4 lymphopenia and neutropenia, but did not have a diagnostic procedure performed |
| Patient 8 (DL2): After 1 cycle, was hospitalized with progressive breast mass and superimposed infection. She had grade 4 lymphopenia when transitioned to hospice |
| Patient 10 (DL 2): Hospitalized for hypotension and grade 3 diarrhea during cycle 2 |
DL dose level
Efficacy
Efficacy data are summarized in Table 2. Patients received a median of 2 cycles of study therapy (range 1–6). Two patients received all six cycles of treatment, one on dose level 1 and one on dose level 2. No patient attained complete response. One patient (9 %) on dose level 2 had a partial response. Five patients had stable disease (45 %), three on dose level 1 and two on dose level 2. All 11 study patients progressed within 7 months of beginning treatment, with a median time to progression of 3.7 months (95 % CI 1.7–5.5). Nine patients have expired with an estimated median overall survival of 10.8 months (95 % CI 3.6–13.1). Two patients who were treated on dose level 2 are currently alive at 16.2 and 18.9 months following the start of treatment.
Discussion
Although only 1 DLT was observed during the first cycle of therapy, high rates of severe, prolonged lymphopenia resulting in serious opportunistic infections occurred with additional cycles and persisted even long after treatment discontinuation. Grade 3/4 lymphopenia was observed in 91 % (95 % CI 0.59–0.998) of patients, and 4 (36 %, 95 % CI 0.11–0.69) patients had grade ≥3 infections. Unfortunately, 3 (27 %) patients treated with study therapy ultimately died with infectious complications. It is likely that severely depressed CD4 counts contributed to the high rate of serious infections. While it is widely accepted that chemotherapy is associated with myelosuppression, lymphopenia, and infectious complications, the high incidence and severity observed on this study was unexpected.
In the United States, bendamustine is primarily used for treatment of hematologic malignancies. A randomized Phase III trial in untreated chronic lymphocytic leukemia (CLL) patients treated with bendamustine versus chlorambucil revealed grade 3/4 lymphopenia in only 6.2 % and grade 3/4 infections in 8 % of patients treated with bendamustine [22]. Phase II studies in previously treated patients with hematologic malignancies have revealed higher rates of infections [24, 25]. For example, a Phase II trial of bendamustine monotherapy in advanced or refractory CLL patients revealed grade 3/4 lymphopenia in 51 % of patients; three (13 %) patients severely immunocompromised at baseline died from treatment-related sepsis. Decreased CD4/CD8 ratios were observed in all evaluable patients, but did not correlate with increased infectious risk [25]. By nature of their disease, patients with CLL are more prone to hematologic and infectious complications at baseline compared with solid tumor patients. However, unexpectedly higher rates of lymphopenia and serious infections were observed in patients with breast cancer with our study therapy.
Lymphopenia and opportunistic infections have also been reported in patients with solid tumors treated with bendamustine, but without the high infectious complication rates that we observed. A Phase I study of weekly bendamustine in previously treated solid tumors showed grade 3/4 lymphopenia in 11/12 (92 %) patients, but no opportunistic infections [26]. No grade 3/4 infections were observed in two Phase II studies of bendamustine mono-therapy at doses greater than those administered in the current study [5, 6]. However, cases of opportunistic infections, including PCP, associated with bendamustine treatment in metastatic breast cancer have been reported [27]. All patients on our study had received prior chemotherapy, possibly placing them at higher risk for complications related to cumulative bone marrow toxicity when compared with chemotherapy-naïve patients; however, our patients were not heavily pre-treated having received 2 or less prior chemotherapy regimens.
We hypothesize that unexpectedly high rates of lymphopenia and opportunistic infections observed in our study were related to treatment with erlotinib that intensified expected bendamustine toxicity. A Phase III study of erlotinib compared with placebo revealed more infections in patients treated with erlotinib (p < 0.001) [28]. Two Phase III trials evaluating the addition of erlotinib to chemotherapy in patients with advanced NSCLC showed increased overall toxicity with erlotinib, including treatment-related AEs, grade 3/4 AEs, SAEs, AE related deaths, and grade 5 infections [21], [29]. Pharmacokinetic (PK) analyses have been performed in multiple studies with erlotinib combined with chemotherapy revealing no significant PK interaction between erlotinib and chemotherapeutic agents [17, 21, 29]. Since PK analysis was not performed in our study, while unlikely, it is possible that PK interaction contributed to toxicity.
With an aggressive malignancy, such as metastatic TNBC, some treatment-related toxicity is acceptable if it can be appropriately managed and/or prophylaxed. The lymphopenia observed in our study is especially concerning because of the prolonged duration resulting in late serious adverse events. Capture of late toxicity is a weakness of classical Phase I study designs, which typically base DLT on the first 1 or 2 cycles of therapy. Most SAEs observed on our trial were not considered DLTs based on the protocol-defined definition. The decision to terminate the trial was based on delayed SAEs and the prolonged duration of lymphopenia placing patients at high risk of infection over time.
Many study patients were treated with subsequent chemotherapy following study treatment despite persistent lymphopenia. We continued to follow lymphocyte and CD4 counts, and it is possible that these therapies contributed to lack of lymphocyte recovery. It was recommended that patients were placed on PCP, viral, and MAC prophylaxis until CD4 recovery. While infectious prophylaxis is commonly administered in hematologic malignancies, it is less frequent in solid tumors. Prophylaxis in solid tumor patients should also be considered when severe, prolonged lymphopenia is encountered.
The efficacy of the study therapy was disappointing with only 1 patient attaining a partial response. TNBC has historically been difficult to treat, likely because of its inherent genetic instability leading to rapid development of resistance [10]. Our study therapy used erlotinib to target EGFR expression associated with TNBC, but TNBC is not a homogeneous malignancy and EGFR is not uniformly expressed and/or amplified. The cancers treated on this trial may have had low rates of EGFR expression. Correlative studies, including assessment of EGFR expression and gene amplification, were planned, but not performed because of early trial termination.
In conclusion, the combination of bendamustine and erlotinib in TNBC patients is not feasible as it is associated with an unacceptable rate of significant lymphopenia leading to life-threatening infections. The greater severity and duration of lymphopenia than previously observed may relate to potentiation of bendamustine-related lymphopenia by erlotinib. Future trials of bendamustine combinations in TNBC patients should account for potential cumulative toxicity to lymphocytes requiring patient monitoring during and following treatment.
Acknowledgments
This study was approved and funded by the National Comprehensive Cancer Network (NCCN) from general research support provided by Cephalon, Inc. Study supply of bendamustine was supplied by Cephalon, Inc. Study supply of erlotinib was provided by Genentech/OSI Pharmaceuticals.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical standards The clinical trial described in this manuscript complies with the current laws of the country in which it was performed (USA).
Contributor Information
Rachel M. Layman, Email: rachel.layman@osumc.edu, Division of Medical Oncology, Stefanie Spielman Comprehensive Breast Center, The Ohio State University Comprehensive Cancer Center, B411 Starling Loving Hall, 320 West 10th Avenue, Columbus, OH 43210, USA
Amy S. Ruppert, Center for Biostatistics, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA
Melinda Lynn, Department of Hematology/Oncology, Nationwide Children’s Hospital, Columbus, OH, USA.
Ewa Mrozek, Division of Medical Oncology, Stefanie Spielman Comprehensive Breast Center, The Ohio State University Comprehensive Cancer Center, B411 Starling Loving Hall, 320 West 10th Avenue, Columbus, OH 43210, USA.
Bhuvaneswari Ramaswamy, Division of Medical Oncology, Stefanie Spielman Comprehensive Breast Center, The Ohio State University Comprehensive Cancer Center, B411 Starling Loving Hall, 320 West 10th Avenue, Columbus, OH 43210, USA.
Maryam B. Lustberg, Division of Medical Oncology, Stefanie Spielman Comprehensive Breast Center, The Ohio State University Comprehensive Cancer Center, B411 Starling Loving Hall, 320 West 10th Avenue, Columbus, OH 43210, USA
Robert Wesolowski, Division of Medical Oncology, Stefanie Spielman Comprehensive Breast Center, The Ohio State University Comprehensive Cancer Center, B411 Starling Loving Hall, 320 West 10th Avenue, Columbus, OH 43210, USA.
Susan Ottman, Comprehensive Cancer Center Clinical Trials Office, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA.
Sarah Carothers, Comprehensive Cancer Center Clinical Trials Office, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA.
Anissa Bingman, Division of Hematology, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA.
Raquel Reinbolt, Department of Internal Medicine, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA.
Eric H. Kraut, Division of Hematology, The Ohio State University Comprehensive Cancer Center, Columbus, OH, USA
Charles L. Shapiro, Division of Medical Oncology, Stefanie Spielman Comprehensive Breast Center, The Ohio State University Comprehensive Cancer Center, B411 Starling Loving Hall, 320 West 10th Avenue, Columbus, OH 43210, USA
References
- 1.Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 2.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 3.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 4.Rakha EA, El-Sayed ME, Green AR, et al. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 5.Hoffken K, Merkle K, Schonfelder M, et al. Bendamustine as salvage treatment in patients with advanced progressive breast cancer: a phase II study. J Cancer Res Clin Oncol. 1998;124:627–632. doi: 10.1007/s004320050225. [DOI] [PubMed] [Google Scholar]
- 6.Reichmann U, Bokemeyer C, Wallwiener D, et al. Salvage chemotherapy for metastatic breast cancer: results of a phase II study with bendamustine. Ann Oncol. 2007;18:1981–1984. doi: 10.1093/annonc/mdm378. [DOI] [PubMed] [Google Scholar]
- 7.von Minckwitz G, Chernozemsky I, Sirakova L, et al. Bendamustine prolongs progression-free survival in metastatic breast cancer (MBC): a phase III prospective, randomized, multicenter trial of bendamustine hydrochloride, methotrexate and 5-fluorouracil (BMF) versus cyclophosphamide, methotrexate and 5-fluorouracil (CMF) as first-line treatment of MBC. Anti-cancer Drugs. 2005;16:871–877. doi: 10.1097/01.cad.0000175587.31940.19. [DOI] [PubMed] [Google Scholar]
- 8.Zulkowski K, Kath R, Semrau R, et al. Regression of brain metastases from breast carcinoma after chemotherapy with bendamustine. J Cancer Res Clin Oncol. 2002;128:111–113. doi: 10.1007/s00432-001-0303-4. [DOI] [PubMed] [Google Scholar]
- 9.Leoni LM, Bailey B, Reifert J, et al. Bendamustine (Treanda) displays a distinct pattern of cytotoxicity and unique mechanistic features compared with other alkylating agents. Clin Cancer Res. 2008;14:309–317. doi: 10.1158/1078-0432.CCR-07-1061. [DOI] [PubMed] [Google Scholar]
- 10.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–244. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 12.Reis-Filho JS, Milanezi F, Steele D, et al. Metaplastic breast carcinomas are basal-like tumours. Histopathology. 2006;49:10–21. doi: 10.1111/j.1365-2559.2006.02467.x. [DOI] [PubMed] [Google Scholar]
- 13.Reis-Filho JS, Milanezi F, Carvalho S, et al. Metaplastic breast carcinomas exhibit EGFR, but not HER2, gene amplification and overexpression: immunohistochemical and chromogenic in situ hybridization analysis. Breast Cancer Res. 2005;7:R1028–R1035. doi: 10.1186/bcr1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham DL, Hillman DW, Hobday TJ, et al. N0234: Phase II study of erlotinib (OSI-774) plus gemcitabine as first-or-second line therapy for metastatic breast cancer (MBC) J Clin Oncol. 2005;23:Abstract #644. [Google Scholar]
- 15.Kaur H, Silverman P, Singh D, et al. Toxicity and outcome data in a phase II study of weekly docetaxel in combination with erlotinib in recurrent and/or metastatic breast cancer (MBC) J Clin Oncol. 2006;24:Abstract#10623. [Google Scholar]
- 16.Thome S, Hobday T, Hillman D, et al. Translational correlates, including outcome for patients with ER-/PR-/HER2-(triple neg (Tneg)) disease from N0234, a phase II trial of gemcitabine and erlotinib for patients with previously treated meta-static breast cancer (MBC) J Clin Oncol. 2007;25:Abstract #1071. [Google Scholar]
- 17.Twelves C, Trigo JM, Jones R, et al. Erlotinib in combination with capecitabine and docetaxel in patients with metastatic breast cancer: a dose-escalation study. Eur J Cancer. 2008;44:419–426. doi: 10.1016/j.ejca.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Solit DB, She Y, Lobo J, et al. Pulsatile administration of the epidermal growth factor receptor inhibitor gefitinib is significantly more effective than continuous dosing for sensitizing tumors to paclitaxel. Clin Cancer Res. 2005;11:1983–1989. doi: 10.1158/1078-0432.CCR-04-1347. [DOI] [PubMed] [Google Scholar]
- 19.Mahaffey CM, Davies AM, Lara PN, Jr, et al. Schedule-dependent apoptosis in K-ras mutant non-small-cell lung cancer cell lines treated with docetaxel and erlotinib: rationale for pharmacodynamic separation. Clin Lung Cancer. 2007;8:548–553. doi: 10.3816/clc.2007.n.041. [DOI] [PubMed] [Google Scholar]
- 20.Gumerlock PH, Pryde BJ, Kimura T, et al. Enhanced cytotoxicity of docetaxel OSI-774 combination in non-small cell lung carcinoma (NSCLC) Proc Am Soc Clin Oncol. 2003;22:Abstract #2661. [Google Scholar]
- 21.Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 22.Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol. 2009;27:4378–4384. doi: 10.1200/JCO.2008.20.8389. [DOI] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 24.Kahl BS, Bartlett NL, Leonard JP, et al. Bendamustine is effective therapy in patients with rituximab-refractory, indolent B-cell non-Hodgkin lymphoma: results from a Multicenter Study. Cancer. 2010;116:106–114. doi: 10.1002/cncr.24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kath R, Blumenstengel K, Fricke HJ, et al. Bendamustine monotherapy in advanced and refractory chronic lymphocytic leukemia. J Cancer Res Clin Oncol. 2001;127:48–54. doi: 10.1007/s004320000180. [DOI] [PubMed] [Google Scholar]
- 26.Schoffski P, Seeland G, Engel H, et al. Weekly administration of bendamustine: a phase I study in patients with advanced progressive solid tumours. Ann Oncol. 2000;11:729–734. doi: 10.1023/a:1008309911008. [DOI] [PubMed] [Google Scholar]
- 27.Klippstein A, Schneider CP, Sayer HG, et al. Pneumocystis carinii pneumonia as a complication of bendamustine monotherapy in a patient with advanced progressive breast cancer. J Cancer Res Clin Oncol. 2003;129:316–319. doi: 10.1007/s00432-003-0441-y. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 29.Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. doi: 10.1200/JCO.2005.05.1474. [DOI] [PubMed] [Google Scholar]