Abstract
Viral replication depends on specific interactions with host factors. For example, poliovirus RNA replication requires association with intracellular membranes. Brefeldin A (BFA), which induces a major rearrangement of the cellular secretory apparatus, is a potent inhibitor of poliovirus RNA replication. Most aspects governing the relationship between viral replication complex and the host membranes remain poorly defined. To explore these interactions, we used a genetic approach and isolated BFA-resistant poliovirus variants. Mutations within viral proteins 2C and 3A render poliovirus resistant to BFA. In the absence of BFA, viruses containing either or both of these mutations replicated similarly to wild type. In the presence of BFA, viruses carrying a single mutation in 2C or 3A exhibited an intermediate-growth phenotype, while the double mutant was fully resistant. The viral proteins 2C and 3A have critical roles in both RNA replication and vesicle formation. The identification of BFA resistant mutants may facilitate the identification of cellular membrane-associated proteins necessary for induction of vesicle formation and RNA replication. Importantly, our data underscore the dramatic plasticity of the host-virus interactions required for successful viral replication.
Positive-strand RNA viruses replicate on intracellular membranes. Intriguingly, many of these viruses, including poliovirus, are not enveloped. Presumably, the association with membranes creates an environment that facilitates viral replication. For example, membrane-associated replication may provide an increased local concentration of viral proteins, allow compartmentalization of the different virus replication steps, or avoid triggering cellular double-stranded RNA responses by replication intermediates. In many cases virus replication induces membrane proliferation, vesiculation, and rearrangements. Indeed, poliovirus replication is physically associated with newly formed virus-induced vesicles (4). The source of poliovirus-induced vesicles remains unclear, although endoplasmic reticulum (ER) (3, 45, 40), Golgi (5, 8, 41), and mixed membrane sources (autophagic vesicles) (45) or varied membrane sources (20, 43) have all been suggested. No cellular protein marker has been identified that tightly colocalizes with poliovirus replication complexes (8, 43, 45), although COPII coat proteins appear to colocalize with budding poliovirus-induced vesicles early in infection (40).
Brefeldin A (BFA) strongly inhibits poliovirus RNA replication and the formation of virus-induced vesicles that become the RNA replication complexes (14, 16, 27, 31). BFA blocks the function of cellular protein ADP ribosylation factor 1 (ARF1) (among other ARFs), and ARF1 function is necessary for COPI-coated vesicle formation. BFA inhibits GTP cycling and thus causes the disruption of the Golgi complex (9, 29, 35). Interestingly, BFA does not directly inhibit any poliovirus proteins, since cell types resistant to BFA are capable of supporting poliovirus replication with wild-type kinetics even in the presence of BFA (16). In addition, BFA inhibits poliovirus replication in a cell-free poliovirus replication system (14). In this cell-free system, high concentrations of ARF1 dominant-negative peptides that are capable of inhibiting in vitro host vesicular transport are also able to block poliovirus replication (14). However, because BFA induces a major disruption of the structure and function of the secretory pathway in vivo, the precise mechanism of action in intact cells remains to be elucidated. It is possible that the inhibitory effect of BFA observed in poliovirus replication is the consequence of a pleiotropic effect triggered by the action of BFA on multiple cellular organelles. In addition, it remains intriguing that BFA inhibits poliovirus replication, since in a normal poliovirus infection the Golgi complex is rapidly dispersed (9, 29, 35), the virus does not appear to require Golgi membranes for replication (40, 45), and COPII coat proteins, but not COPI coat proteins, appear to colocalize with budding poliovirus-induced vesicles (40).
In the present study, we report the isolation of polioviruses resistant to BFA that replicate to high titers even in the presence of 2.0 μg of BFA/ml. The virus is able to replicate in the presence of BFA in at least two different cell types. We identified the determinants of BFA resistance within 2C and 3A. Interestingly, the resistant virus appears to induce poliovirus vesicles and produce classic dispersion of the Golgi apparatus both in the presence and in the absence of BFA. These results demonstrate that poliovirus can replicate in cells where the secretory function has been completely disrupted via a large rearrangement of membranous compartments. Thus, our data suggest that BFA inhibits a specific step in poliovirus replication, perhaps involving the direct interaction of 2C and 3A viral proteins with the cellular factor ARF1.
MATERIALS AND METHODS
Cells and viruses.
HeLa S3 cells were used in all experiments (unless 293T cells are indicated) and grown under conditions previously described (12). BFA was obtained from Sigma, and stock solutions were 2.0 mg/ml in 70% ethanol. Starting poliovirus wild-type stock was a Mahoney stock derived from pMoRA (also called pMo rib+polyAlong) (11, 26), which was derived from the pXpA plasmid (38). One-step growth curves (46) and plaque assays (12) were done as described previously.
Replicons.
Replicon RNA was produced by in vitro transcription of linearized plasmids—pRLucRA wild-type plasmid (13, 26) or the pRLuc-3Dpol238A derivative (23)—by using T7 RNA polymerase as described previously (12). Next, 10 μg of each viral RNA transcript was electroporated into 1.2 × 106 HeLa cells, cells were incubated in complete medium at 37°C for the indicated period of time (in the presence or absence of 2.0 μg of BFA/ml), and the luciferase activity was measured as described previously (23).
Selection conditions.
Titration of BFA (Fig. 1C) was done in 10-cm dishes of HeLa cells incubated in 10 ml of 50% Dulbecco modified Eagle medium-50% F12 medium, 10% fetal calf serum, and the appropriate concentration of BFA at 37°C (5 to 7% CO2). Each passage of virus was done by infecting a 10-cm plate containing 3 × 106 to 5 × 106 HeLa cells with 104 PFU of virus for 15 min, followed by a wash with phosphate-buffered saline; then, 5 ml of medium containing the appropriate concentration of BFA was added, and the dishes were incubated at 37°C until 100% lysis was observed. Four control plates were always included: an uninfected plate with no drug, an uninfected plate with BFA, a passaged poliovirus-infected plate without BFA, and a wild-type poliovirus stock-infected plate with BFA. Poliovirus was passaged once in 0.1 μg of BFA/ml to get the P1 stock. Lysis of the P1 plate was observed after 2 days, with a viral titer of 108 PFU/ml. The P1 stock was then passaged in 0.2 μg of BFA/ml to obtain a P2 stock. Lysis of the P2 plate was observed after 3 days, with a titer of 5 × 106 PFU/ml. The P2 stock was then passaged in 0.4 μg of BFA/ml to obtain a P3 stock. Lysis of the P3 plate was observed after 2 days, with a titer of 5 × 107 PFU/ml. The P3 stock was then passaged in 0.6 μg of BFA/ml to obtain a P4 stock. Lysis of the P4 plate was observed after 2 days, with a titer of 1.2 × 108 PFU/ml. The P4 stock was then passaged once in 1.0 μg of BFA/ml to obtain a P5 stock. Lysis of the P5 plate was observed after 2 days, with a titer of 5 × 106 PFU/ml.
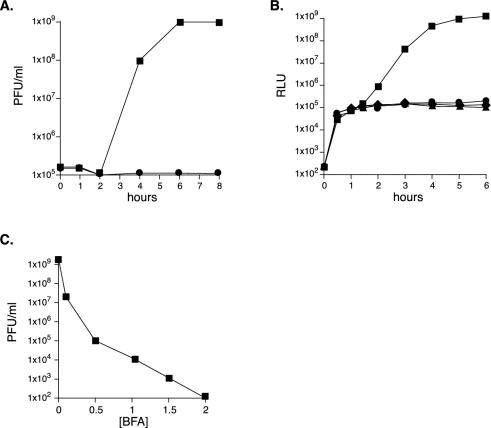
FIG. 1.
BFA specifically inhibits poliovirus RNA replication. (A) Poliovirus growth curve with or without BFA. HeLa cells were infected with wild-type poliovirus at an MOI of 5 PFU/cell in the absence (▪) or presence (•) of 2.0 μg of BFA/ml. Virus was harvested from parallel wells at the time points indicated. Titers of <105 PFU/ml are shown at 105 PFU/ml. (B) Poliovirus replicon translation and replication with or without BFA. Poliovirus replicon (FLuc) RNA has the capsid-coding sequence replaced by the luciferase gene. In the absence of replication, luciferase levels (in relative light units [RLU]) in HeLa cells transfected with replicon RNA are a measure of input poliovirus RNA translation. Translation alone was measured by transfecting cells with a mutant replicon, FLuc-3Dpol238A, possessing an inactive viral polymerase (▴). Normal poliovirus replication was measured by transfecting cells with wild-type FLuc (▪). Luciferase levels in the presence of 2.0 μg of BFA per ml are shown in cells transfected with wild-type FLuc (•) or mutant FLuc-3Dpol238A (♦). (C) Titration of BFA inhibition of poliovirus. HeLa cells were infected at an MOI of 0.001 and incubated with the indicated concentration of BFA (μg/ml). Virus was harvested, and titers were determined at 48 h postinfection.
Cloning and plasmids.
RNA of potential BFA-resistant viruses was obtained from infected HeLa cells by using Qiagen RNeasy. RNA was reverse transcribed with Superscript II RT, and poliovirus genome fragments were amplified by PCR with high-fidelity PfuTurbo polymerase (Stratagene) in standard conditions for 30 cycles. The entire genome (positions 20 to 7425) of clone bfa3 was sequenced directly from the PCR products by using BigDye terminator cycle sequencing, and the electropherograms were analyzed with DNAStar as described previously (13). Other clones were sequenced across the full length of genes 2C and 3A. The bfa3 double point mutation (4361A/5190T) was cloned into a full-length poliovirus plasmid clone by digesting amplified PCR product with NheI and BglII and ligating the fragment (consisting of the poliovirus genome from positions 2470 to 5601) into a NheI-BglII-digested pMoRA plasmid. The resulting plasmid pMo4361A/5190T (also known as pMoBFAr-DB) was sequenced from the NheI site through to the BglII site and confirmed to contain only the desired 4361A and 5190T mutations. To obtain pMo4361A and pMo5190T, the SnaBI-BglII fragment of pMoBFAr-DB was subcloned into pLITMUS28 (New England BioLabs) to give pLITS-BAr-P2. The 1.0-kb BamHI-BglII fragment of wild-type poliovirus (from pMoRA) was then cloned into a BamHI-BglII-digested pLITS-BAr-P2 to give pLITS-4361A. In parallel, the 1.6-kb SnaBI-BamHI fragment of wild-type poliovirus (from pMoRA, also digested with AatII to avoid an extra band) was gel purified and cloned into a SnaBI-BamHI-digested pLITS-BAr-P2 to give pLITS-5190T. The SnaBI-BglII fragments from the pLITS-4361A and pLITS-5190T subcloning plasmids were then cloned into SnaBI-BglII-digested pMoRA to get pMo4361A and pMo5190T containing full-length poliovirus genomes containing the 4361A and 5190T mutations, respectively. Each plasmid was sequenced across the full cloned region to confirm the presence of the desired mutation. All of these plasmids are readily available to any interested investigator. Infectious RNA is derived from these poliovirus plasmids by linearization with ClaI or ApaI, followed by T7 RNA polymerase transcription as described previously (23, 26). The T7 transcripts of the poliovirus genome are infectious upon transfection.
Immunofluorescence and microscopy.
Anti-2C polyclonal antibodies were produced by the inoculation of the 2C C-terminal peptide (CNIGNCMEALFQ) conjugated to keyhole lympet hemocyanin into rabbits (HTI Bio-Products, Ramona, Calif.). The calnexin antibody was purchased from BD Biosciences. GM130 antibody was purchased from Transduction Laboratories. HeLa cells were grown on a coverslip for microscopy experiments. Cells were infected with the appropriate virus at an MOI of 1 and incubated in DMEM-F12-10% FCS, with or without 2.0 μg of BFA/ml for the indicated number of hours. Cells were fixed for 15 min with cold 4% paraformaldehyde in PBS. Incubation with the primary antibody was carried out in PBS plus 0.1% Triton (PBST) solution for 1 h. The cells were then washed three times with PBST and then incubated for 30 min with the secondary antibody in PBST. After three washes with PBST, the slides were mounted with Vectashield (purchased from Vecta). Standard epifluorescence microscopy was performed at ×40 magnification, and digital images were obtained with a Leica fluorescence microscope attached to a Sensicam charge-coupled device camera. The data shown in Fig. 6 were captured on an Olympus fluorescence microscope at ×60 magnification by using Deltavision software. Optical sections were then deconvolved using the same software to exclude out-of-focus information and flattened into a two-dimensional projection for presentation. All images were then imported to and processed in Adobe Photoshop and Adobe Illustrator.
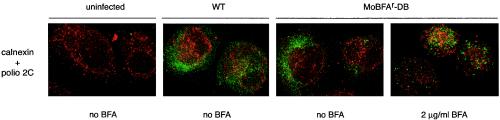
FIG. 6.
Subcellular localization of virus replication. HeLa cells were infected with wild-type poliovirus (WT) or MoBFAr-DB for 7 h in the presence or absence of 2.0 μg of BFA/ml and then fixed and stained for poliovirus protein 2C (green) and ER-resident protein calnexin (red). Cells were imaged by using confocal deconvolution microscopy.
RESULTS
Effects of BFA on poliovirus replication.
Poliovirus growth is completely inhibited by BFA (Fig. 1A) (27, 31). To further confirm this result, we examined the effect of BFA on poliovirus RNA replication by using a poliovirus replicon (Fluc). FLuc is a full-length poliovirus genome containing the luciferase gene instead of the poliovirus capsid genes (26). Transfection of HeLa cells with FLuc replicon RNA results in the production of a polyprotein containing luciferase that is processed by the viral 2A protease to liberate active luciferase, and the replicon translates and replicates comparably to wild-type poliovirus (1). The initial luciferase activity observed between 0 and 1 h corresponds to direct translation of the input RNA (Fig. 1B). Under normal conditions, a 10,000-fold increase in FLuc luciferase activity is observed between 2 and 6 h due to rapid viral RNA replication (Fig. 1B). BFA specifically inhibits RNA replication and has no effect on poliovirus translation (Fig. 1B), a finding in agreement with previous studies (31) (see also Fig. 4C). As a control for translation of the input transfected RNA, we included a mutant poliovirus replicon, FLuc-3Dpol238A, with an inactive polymerase (23). The input FLuc-3Dpol238A RNA translates comparably to wild-type FLuc after transfection, but FLuc-3Dpol238A is completely defective for RNA replication (23). Thus, BFA does not affect poliovirus translation, since FLuc in the presence of BFA produced luciferase at levels comparable to the untreated FLuc-3Dpol238A mutant (Fig. 1B).
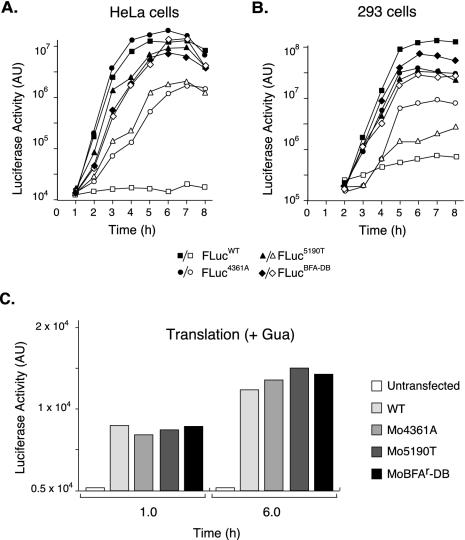
FIG. 4.
Kinetic analysis of BFA-resistant replicons. (A) HeLa cells or (B) 293 cells were transfected with Fluc replicon RNAs corresponding to wild type (WT) or those carrying mutations that confer resistance to BFA (Mo4361A, Mo5190T, or MoBFAr-DB). Transfections were performed in the absence (solid symbols) or presence (open symbols) of 2.0 μg of BFA/ml. Cells were harvested at the indicated time points, and the luciferase activity was determined as described in Materials and Methods. (C) Transfections were carried out in the presence of guanidinium HCl to determined luciferase production in the absence of RNA replication. Luciferase was determined at 1 and 6 h posttransfection.
Isolation of BFA-resistant viruses.
To isolate BFA-resistant poliovirus mutants, we first titrated the concentration of BFA necessary to inhibit poliovirus in HeLa cells. BFA concentrations of 0.1 μg/ml are sufficient to inhibit poliovirus production ≥99%, a concentration of 0.5 μg/ml inhibits poliovirus production 104-fold, and a concentration of 2.0 μg/ml completely inhibits poliovirus production with a >107-fold reduction in titer (Fig. 1C). The concentrations of BFA necessary to inhibit poliovirus replication were consistent with the concentrations of BFA necessary to disrupt the Golgi complexes in a population of HeLa cells (data not shown).
We reasoned that resistance to BFA might require multiple adaptive mutations in the poliovirus genome. Low concentrations of BFA may provide sufficient selective pressure to promote the emergence of partially resistant viruses, and the partially resistant viruses could possibly then evolve into fully drug-resistant strains upon selection in the presence of high concentrations of BFA (>0.5 μg/ml). Poliovirus was therefore passaged once in 0.1 μg of BFA/ml (P1), once in 0.2 μg of BFA/ml (P2), once in 0.4 μg of BFA/ml (P3), once in 0.6 μg of BFA/ml (P4), and then once in 1.0 μg of BFA/ml (P5). The titer of the fifth passage virus was 5 × 106 PFU/ml, a value well above the background level of 104 PFU of virus/ml expected in a concentration of 1.0 μg of BFA/ml.
We then isolated individual virus clones from the P5 population and assessed their growth in the presence of BFA. HeLa cells were infected at a low multiplicity of infection (MOI; 0.001 PFU/cell) and then incubated for 2 days in the presence or absence of 0.5 μg of BFA/ml. All of the viruses grew similar to the wild type in the absence of drug (Fig. 2A). One viral clone (bfa1) exhibited partial resistance to BFA, incompletely lysing HeLa cells in the presence of drug (Fig. 2A). Several other clones exhibited complete resistance to BFA, rapidly spreading throughout the plate and killing all HeLa cells (Fig. 2A, viruses bfa3 and bfa4).
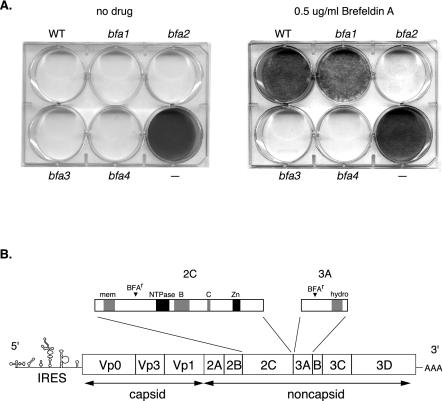
FIG. 2.
Identification of BFA-resistant viruses. (A) Phenotype of BFA-resistant viruses. HeLa cells were infected at a low MOI (0.001 PFU/cell) and then incubated for 2 days in the absence or presence of 0.5 μg of BFA/ml. WT, wild-type poliovirus; −, control uninfected cells; bfa1, bfa2, bfa3, and bfa4, potential drug-resistant mutants tested. All viruses replicated and spread throughout the plate under normal growth conditions (no BFA), causing 100% cytopathic effect (CPE). Wild-type poliovirus did not cause CPE in the presence of 0.5 μg of BFA/ml. Candidate mutant bfa1 caused partial CPE in the presence of 0.5 μg of BFA/ml, since pinpoint plaques are visible. Candidate mutant bfa1 was later confirmed to have a single point mutation in the 2C gene at position 4361. Candidate mutants bfa3 and bfa4 caused 100% CPE in the presence of 0.5 μg of BFA/ml (also in 1.0 μg and 2.0 μg of BFA/ml [data not shown]). Candidate mutants bfa3 and bfa4 were later confirmed to both contain the double point mutations G4361A (in gene 2C) and C5190T (in gene 3A). Mutant bfa2 was not identified in the original screen but is instead the molecular-clone-derived Mo5190T. Mutant bfa2 exhibited significant drug resistance and caused >90% CPE in the presence of 0.5 μg of BFA/ml. (B) Location of BFA resistance (BFAr) mutations in the poliovirus genome. Poliovirus is a positive-strand RNA virus with an ∼7,500-nucleotide genome. The full-length poliovirus genome is diagrammed on the bottom, with expanded versions of the 2C and 3A genes shown above it. 2C is 329 aa long and 3A is 87 aa long. One BFAr mutation was found in replication protein 2C, a valine-to-isoleucine change at aa 80. A second BFAr mutation was found in replication protein 3A, an alanine-to-valine change at aa 27. Known domains of 2C and 3A are indicated. 2C contains an NTPase motif and highly conserved motifs B and C (24), as well as a zinc finger (indicated by “Zn”) (36). 2C also contains at least one membrane-binding domain (mem) (19) and an RNA-binding domain (6, 39). 3A contains a 22-aa hydrophobic stretch (hydro) in its C terminus that is thought to play a role in membrane binding (48).
Location of the BFA-resistant determinants.
The genome of BFA-resistant variant bfa3 was completely sequenced, and two point mutations were identified: G→A at nucleotide 4361 and C→T at nucleotide 5190. The G4361A mutation results in a valine to isoleucine change at amino acid (aa) 80 of poliovirus nonstructural protein 2C (Fig. 2B). The C5190T mutation results in an alanine-to-valine change at aa 27 of poliovirus nonstructural protein 3A (Fig. 2B). The 2C and 3A genes were sequenced in seven additional viral clones, and six of the clones contained both the 2C and the 3A mutations, whereas the one clone exhibiting partial resistance (Fig. 2A, bfa1) contained just the 2C mutation.
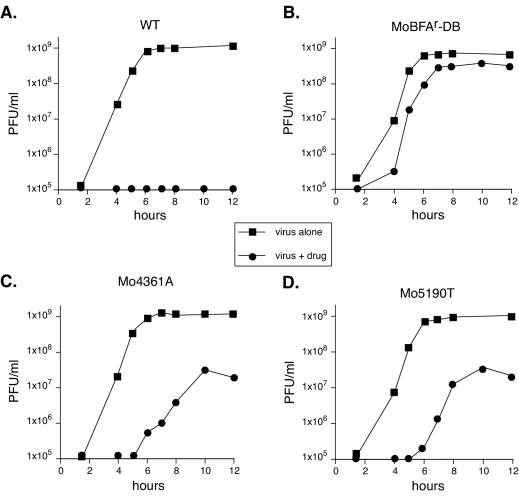
To confirm that these nucleotide changes confer the BFA-resistant phenotype, we introduced the point mutations into poliovirus genome molecular clones, producing plasmid constructs pMo4361A, pMo5190T, and pMo4361A/5190T. Mutant viruses were obtained from the plasmids. The three mutant viruses, derived from molecular clones, Mo4361A, Mo5190T, and MoBFAr-DB (the double mutant Mo4361A/5190T), were analyzed under one-step growth conditions. All three mutants grew with wild-type kinetics in the absence of drug (Fig. 3). As expected, 2.0 μg of BFA/ml blocked wild-type poliovirus replication (Fig. 3A). In contrast, MoBFAr-DB replicated rapidly and to high titers (5 × 108 PFU/ml) in the presence of 2.0 μg of BFA/ml (Fig. 3B). Viruses containing the 2C mutation alone (Mo4361A) or the 3A mutation alone (Mo5190T) had an intermediate resistance phenotype, producing 2 × 107 PFU of virus/ml in the presence of 2.0 μg of BFA/ml but with slower kinetics than the double mutant virus MoBFAr-DB (Fig. 3C and D). Interestingly, the mutant virus with the single change in 3A, Mo5190T, is substantially more cytopathic in tissue culture under low-MOI conditions than mutant Mo4361A (bfa2 versus bfa1 in Fig. 2A). Mutations in 3A have been associated with changes in cytopathogenicity in other picornaviruses (2, 25, 30, 33), and the phenotype of Mo5190T suggests that the 3A protein plays a similar role in poliovirus cytopathogenicity. It should also be noted that MoBFAr-DB was capable of forming plaques in the presence of BFA (data not shown).
FIG. 3.
Kinetic analysis of BFA-resistant virus growth. HeLa cells were infected at an MOI of 5 with the virus strains indicated below in the absence (▪) or presence (•) of 2.0 μg of BFA/ml. Virus was harvested at the indicated time points, and then titers were determined by plaque assay. (A) Wild type (WT); (B) MoBFAr-DB; (C) Mo4361A; (D) Mo5190T. Titers off the bottom of the scale are indicated as 105 PFU.
Replication of BFA-resistant poliovirus replicons.
We next examined the phenotype of poliovirus replicons carrying BFA resistance determinants. The mutations in 2C and 3A observed in BFA-resistant poliovirus were introduced into FLuc replicon plasmids to generated pFLuc4361A, pFLuc5190T, and pFLucBFA-DB. Replication of the single mutation replicons, as well as the double-mutant replicon, was resistant to BFA inhibition in two different cell lines (HeLa and 293 cells). In the absence of BFA, all three mutants replicated with kinetics similar to those of the wild type (Fig. 4A and B). In the presence of BFA wild-type RNA replication was completely inhibited, whereas the mutants carrying single nucleotide alterations replicated with intermediate kinetics (Fig. 4A and B), a finding consistent with the phenotype observed for the resistant viruses (Fig. 3). The double mutant FLucBFA-DB replicated faster and achieved 5- to 10-fold-higher levels of luciferase production, which closely resembled the level produced by FLucBFA-DB in the absence of BFA (Fig. 4A and B). This result is consistent with the fact that MoBFAr-DB replicated rapidly and to high titers in the presence of BFA (Fig. 3B).
Translation efficiencies of wild-type and mutant FLuc RNAs were evaluated by measuring the luciferase activity produced after transfection of RNA into HeLa cells in the presence of guanidinium HCl, a specific inhibitor of poliovirus RNA replication (Fig. 4C). Thus, in these experiments luciferase activity was produced only by the input RNA. As expected, the mutations in 2C and 3A do not affect viral translation (Fig. 4C). We conclude that the observed mutations in 2C and 3A allow poliovirus replicons to replicate in the presence of BFA without affecting translation. These data are consistent with the idea that the major effect of BFA on the virus is inhibition of RNA replication. The BFAr mutations in 2C and 3A appear to release that block.
Cell biology of BFA-resistant viruses.
Given that MoBFAr-DB grew to high titers both in the presence or absence of BFA and that BFA disrupt the Golgi apparatus, we examined whether MoBFAr-DB is able to induce dispersion of the Golgi apparatus. Poliovirus infection normally causes a rapid dispersion of the Golgi via an unknown mechanism (10). We sought to determine whether the development of BFA resistance would alter this phenotype in MoBFAr-DB-infected cells under normal conditions. We inspected Golgi complexes in wild-type and MoBFAr-DB-infected cells and observed no difference; both viruses caused a rapid dispersion of the Golgi (Fig. 5).
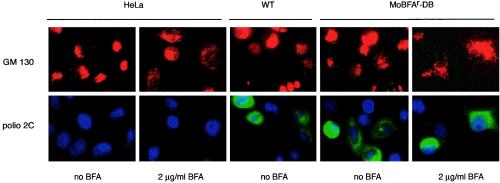
FIG. 5.
Golgi phenotype of BFA-resistant virus. HeLa cells were infected at an MOI of 1 with either wild type (WT) or MoBFAr-DB. Cells were fixed at 7 h postinfection and costained for poliovirus protein 2C (green) and a Golgi marker (GM130, red). The diffuse stain corresponds to Golgi complex dispersion as BFA disrupts the complex, as do both wild-type (WT) and MoBFAr-DB poliovirus infections. Control stains demonstrating the specificity of the anti-poliovirus 2C antibody are also shown.
Poliovirus replication occurs on membranous vesicles, and 2C and 3A are both membrane-associated proteins that are essential components of the poliovirus replication complex (44, 47). BFA appears to prevent the formation of the poliovirus-induced vesicles necessary for replication (31). We therefore analyzed, by deconvolution confocal microscopy, the intracellular distribution of poliovirus replication complexes in cells infected with wild-type or MoBFAr-DB viruses. Immunofluorescent staining for poliovirus protein 2C is a standard marker for poliovirus replication complexes (4, 8, 21). The 2C protein is a multifunctional replication protein with ATPase activity and a domain homologous to the DEAD-family helicases (24, 37). 2C staining is seen on ER early in infection, but the 2C staining becomes more dispersed later in infection. (4). As the infection progresses, poliovirus replication vesicles accumulate, and punctate 2C staining is observed throughout the cell (8, 21). The 2C staining was comparable in both wild-type and MoBFAr-DB virus-infected cells in the absence of BFA (Fig. 6). At 7 h postinfection we observed 2C distribution throughout the cell and no costaining with the ER marker (calnexin). This indicates that under normal growth conditions MoBFAr-DB proteins 2C and 3A traffic, form vesicles, and replicate poliovirus RNA on vesicles in a similar fashion to wild-type virus.
No 2C staining was seen in wild-type virus-infected cells in the presence of 2.0 μg of BFA/ml because replication is completely inhibited and translation of the input RNA does not produce sufficient 2C to be detected (data not shown). In contrast, high levels of 2C expression accumulated in MoBFAr-DB-infected cells in the presence of BFA, since BFA has only minor effects on MoBFAr-DB replication (Fig. 6). Notably, the 2C staining was punctate, similar to that of wild-type virus infection in the absence of BFA. Furthermore, as for mutant and wild-type in the absence of BFA, 2C staining did not colocalize with the ER (red staining, calnexin). We therefore conclude that, even though the Golgi apparatus relocalizes into the ER in the presence of BFA, the mutant replication complexes do not accumulate in the ER and, at least at this level of resolution, these complexes appear to assemble morphologically “normal” poliovirus replication vesicles.
DISCUSSION
We have identified a BFA-resistant poliovirus that rapidly replicates to high titers even in the presence of 2.0 μg of BFA/ml. The identification of BFA resistance mutations in 2C and 3A is quite intriguing. Poliovirus proteins 2C and 3A are major components of the poliovirus replication complex (8, 21, 43, 47). When expressed individually they are each membrane associated (10, 17), and 2C is capable of inducing vesicle formation (10, 19a). Coexpression of 2C and 3A is sufficient to produce vesicles similar in appearance to the poliovirus-induced vesicles seen in infected cells (45). 3A is responsible for blocking the secretory pathway (15). Recently, it was shown that a rhinovirus 16 variant, adapted to efficient replication in mouse cells, has alterations in protein 2C (25a). This observation suggests that changes in the interaction of 2C with cellular factors could determine species and cellular tropism. Thus, the functions of 2C and 3A have been the subject of extensive investigation; however, although several activities have been described, the precise roles of these two multifunctional proteins remain unclear. It is possible that the BFA resistance mutants described here will facilitate the identification of cellular membrane-associated proteins that interact with 2C and 3A and that are necessary for induction of vesicle formation and/or RNA replication.
Mechanism of resistance to BFA.
Since (i) the 2C and 3A proteins are both required for the formation of poliovirus replication complex-like vesicles, (ii) substitutions observed in the BFAr mutants are conservative amino acid changes, and (iii) the BFA-resistant mutant viruses appear to replicate normally in the absence of BFA, we propose the following hypothetical model to explain our findings: the BFAr mutations observed permit a more promiscuous interaction of the virus replication machinery with alternative components of the intracellular membranes. Thus, even though the mutant 2C and 3A proteins may still interact with and recruit ARF1 into the replication complex, the mutations allow interaction with other ARF1-like proteins that functionally replace ARF1 and assist the virus in the replication process in the presence of BFA. There are several highly homologous ARF-like proteins described in human cells (i.e., ARF1 to ARF5); therefore, any of these other ARF1 homologs may take the functional role of ARF1 in the presence of BFA.
However, it is still unclear what the precise relationship is between poliovirus replication complexes and the canonical vesicles of the secretory apparatus. Although COPII, but not COPI, coat proteins appear to be involved in the formation of poliovirus replication complex (19a, 40), BFA specifically targets COPI formation and, since point mutations can overcome this block, this observation suggests that the COPI vesicular trafficking system also participates in the poliovirus replication complex, perhaps via ARF1 but independent of COPI coats.
Antivirus drug development targeting cellular proteins involved in viral replication.
Although much effort has been devoted to developing antiviral drugs, very few are effective. One of the major problems is that viruses rapidly mutate into drug-resistant variants. For most known antiviral drugs capable of completely inhibiting their target virus (>99% inhibition of virus production), the virus can become fully resistant to the drug with a single point mutation (7, 22, 28). Human immunodeficiency virus is well known for its astonishing genetic flexibility, and other viruses are comparably flexible (18, 34).
In general, antiviral drugs have been developed to target viral components or enzymes. However, because RNA viruses have stringent restrictions on their genetic coding capacity, they carry only the most essential replication components in their genomes and rely on a large set of cellular proteins to accomplish their full cycle of replication. It has therefore been proposed that targeting antiviral drugs at cellular proteins necessary for virus replication could avoid the problem of drug resistance because the virus could not become resistant to a drug that targets a cellular protein (32, 42). Our data suggest that this is an erroneous assumption. We discovered that poliovirus could become completely resistant to BFA by acquiring only two point mutations. This exceptional plasticity in the host-virus interactions may be a central feature of the virus's long-term survival strategy.
Acknowledgments
We thank Judith Frydman for helpful advice and comments.
This study was supported by NIH grant AI40085. S.C. was supported in this study as a Howard Hughes Medical Institute doctoral fellow.
REFERENCES
- 1.Andino, R., G. E. Rieckhof, P. L. Achacoso, and D. Baltimore. 1993. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5′ end of viral RNA. EMBO J. 12:3587-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beneduce, F., G. Pisani, M. Divizia, A. Pana, and G. Morace. 1995. Complete nucleotide sequence of a cytopathic hepatitis A virus strain isolated in Italy. Virus Res. 36:299-309. [DOI] [PubMed] [Google Scholar]
- 3.Bienz, K., D. Egger, and L. Pasamontes. 1987. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology 160:220-226. [DOI] [PubMed] [Google Scholar]
- 4.Bienz, K., D. Egger, T. Pfister, and M. Troxler. 1992. Structural and functional characterization of the poliovirus replication complex. J. Virol. 66:2740-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bienz, K., D. Egger, Y. Rasser, and W. Bossart. 1983. Intracellular distribution of poliovirus proteins and the induction of virus-specific cytoplasmic structures. Virology 131:39-48. [DOI] [PubMed] [Google Scholar]
- 6.Bienz, K., D. Egger, M. Troxler, and L. Pasamontes. 1990. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region. J. Virol. 64:1156-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blair, E. 1998. Antiviral therapy. Springer, New York, N.Y.
- 8.Bolten, R., D. Egger, R. Gosert, G. Schaub, L. Landmann, and K. Bienz. 1998. Intracellular localization of poliovirus plus- and minus-strand RNA visualized by strand-specific fluorescent in situ hybridization. J. Virol. 72:8578-8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chardin, P., and F. McCormick. 1999. Brefeldin A: the advantage of being uncompetitive. Cell 97:153-155. [DOI] [PubMed] [Google Scholar]
- 10.Cho, M. W., N. Teterina, D. Egger, K. Bienz, and E. Ehrenfeld. 1994. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells. Virology 202:129-145. [DOI] [PubMed] [Google Scholar]
- 11.Crotty, S., C. E. Cameron, and R. Andino. 2001. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc. Natl. Acad. Sci. USA 98:6895-6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crotty, S., B. L. Lohman, F. X. Lu, S. Tang, C. J. Miller, and R. Andino. 1999. Mucosal immunization of cynomolgus macaques with two serotypes of live poliovirus vectors expressing simian immunodeficiency virus antigens: stimulation of humoral, mucosal, and cellular immunity. J. Virol. 73:9485-9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crotty, S., D. Maag, J. J. Arnold, W. Zhong, J. Y. Lau, Z. Hong, R. Andino, and C. E. Cameron. 2000. The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat. Med. 6:1375-1379. [DOI] [PubMed] [Google Scholar]
- 14.Cuconati, A., A. Molla, and E. Wimmer. 1998. Brefeldin A inhibits cell-free, de novo synthesis of poliovirus. J. Virol. 72:6456-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deitz, S. B., D. A. Dodd, S. Cooper, P. Parham, and K. Kirkegaard. 2000. MHC I-dependent antigen presentation is inhibited by poliovirus protein 3A. Proc. Natl. Acad. Sci. USA 97:13790-13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doedens, J., L. A. Maynell, M. W. Klymkowsky, and K. Kirkegaard. 1994. Secretory pathway function, but not cytoskeletal integrity, is required in poliovirus infection. Arch. Virol. Suppl. 9:159-172. [DOI] [PubMed]
- 17.Doedens, J. R., T. H. Giddings, Jr., and K. Kirkegaard. 1997. Inhibition of endoplasmic reticulum-to-Golgi traffic by poliovirus protein 3A: genetic and ultrastructural analysis. J. Virol. 71:9054-9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domingo, E., J. J. Holland, and P. Ahlquist. 1988. RNA genetics. CRC Press, Inc., Boca Raton, Fla.
- 19.Echeverri, A. C., and A. Dasgupta. 1995. Amino-terminal regions of poliovirus 2C protein mediate membrane binding. Virology 208:540-553. [DOI] [PubMed] [Google Scholar]
- 19a.Egger, D., R. Gosert, and K. Bienz. 2002. Role of cellular structures in viral RNA replication, p. 247-254. In B. L. Semler and E. Wimmer (ed.), Molecular biology of picornaviruses. ASM Press, Washington, D.C.
- 20.Egger, D., L. Pasamontes, R. Bolten, V. Boyko, and K. Bienz. 1996. Reversible dissociation of the poliovirus replication complex: functions and interactions of its components in viral RNA synthesis. J. Virol. 70:8675-8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger, D., N. Teterina, E. Ehrenfeld, and K. Bienz. 2000. Formation of the poliovirus replication complex requires coupled viral translation, vesicle production, and viral RNA synthesis. J. Virol. 74:6570-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galasso, G. J., R. J. Whitley, and T. C. Merigan. 1997. Antiviral agents and human viral diseases, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 23.Gohara, D. W., S. Crotty, J. J. Arnold, J. D. Yoder, R. Andino, and C. E. Cameron. 2000. Poliovirus RNA-dependent RNA polymerase (3Dpol): structural, biochemical, and biological analysis of conserved structural motifs A and B. J. Biol. Chem. 275:25523-25532. [DOI] [PubMed] [Google Scholar]
- 24.Gorbalenya, A. E., E. V. Koonin, and Y. I. Wolf. 1990. A new superfamily of putative NTP-binding domains encoded by genomes of small DNA and RNA viruses. FEBS Lett. 262:145-148. [DOI] [PubMed] [Google Scholar]
- 25.Graff, J., C. Kasang, A. Normann, M. Pfisterer-Hunt, S. M. Feinstone, and B. Flehmig. 1994. Mutational events in consecutive passages of hepatitis A virus strain GBM during cell culture adaptation. Virology 204:60-68. [DOI] [PubMed] [Google Scholar]
- 25a. Harris, J. R., and V. R. Racaniello. 2003. Changes in rhinovirus protein 2C allow efficient replication in mouse cells. J. Virol. 77:4773-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herold, J., and R. Andino. 2000. Poliovirus requires a precise 5′ end for efficient positive-strand RNA synthesis. J. Virol. 74:6394-6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irurzun, A., L. Perez, and L. Carrasco. 1992. Involvement of membrane traffic in the replication of poliovirus genomes: effects of brefeldin A. Virology 191:166-175. [DOI] [PubMed] [Google Scholar]
- 28.Jeffries, D. J., and E. DeClercq. 1995. Antiviral chemotherapy. Wiley, Chichester, England.
- 29.Klausner, R. D., J. G. Donaldson, and J. Lippincott-Schwartz. 1992. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116:1071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lama, J., M. A. Sanz, and L. Carrasco. 1998. Genetic analysis of poliovirus protein 3A: characterization of a non-cytopathic mutant virus defective in killing Vero cells. J. Gen. Virol. 79:1911-1921. [DOI] [PubMed] [Google Scholar]
- 31.Maynell, L. A., K. Kirkegaard, and M. W. Klymkowsky. 1992. Inhibition of poliovirus RNA synthesis by brefeldin A. J. Virol. 66:1985-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meijer, L., S. Leclerc, and M. Leost. 1999. Properties and potential: applications of chemical inhibitors of cyclin-dependent kinases. Pharmacol. Ther. 82:279-284. [DOI] [PubMed] [Google Scholar]
- 33.Morace, G., G. Pisani, F. Beneduce, M. Divizia, and A. Pana. 1993. Mutations in the 3A genomic region of two cytopathic strains of hepatitis A virus isolated in Italy. Virus Res. 28:187-194. [DOI] [PubMed] [Google Scholar]
- 34.Morse, S. S. 1994. The evolutionary biology of viruses. Raven Press, New York, N.Y.
- 35.Peyroche, A., B. Antonny, S. Robineau, J. Acker, J. Cherfils, and C. L. Jackson. 1999. Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol. Cell 3:275-285. [DOI] [PubMed] [Google Scholar]
- 36.Pfister, T., K. W. Jones, and E. Wimmer. 2000. A cysteine-rich motif in poliovirus protein 2C(ATPase) is involved in RNA replication and binds zinc in vitro. J. Virol. 74:334-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfister, T., and E. Wimmer. 1999. Characterization of the nucleoside triphosphatase activity of poliovirus protein 2C reveals a mechanism by which guanidine inhibits poliovirus replication. J. Biol. Chem. 274:6992-7001. [DOI] [PubMed] [Google Scholar]
- 38.Racaniello, V. R., and D. Baltimore. 1981. Cloned poliovirus complementary DNA is infectious in mammalian cells. Science 214:916-919. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez, P. L., and L. Carrasco. 1995. Poliovirus protein 2C contains two regions involved in RNA-binding activity. J. Biol. Chem. 270:10105-10112. [DOI] [PubMed] [Google Scholar]
- 40.Rust, R. C., L. Landmann, R. Gosert, B. L. Tang, W. Hong, H. P. Hauri, D. Egger, and K. Bienz. 2001. Cellular COPII proteins are involved in production of the vesicles that form the poliovirus replication complex. J. Virol. 75:9808-9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandoval, I. V., and L. Carrasco. 1997. Poliovirus infection and expression of the poliovirus protein 2B provoke the disassembly of the Golgi complex, the organelle target for the antipoliovirus drug Ro-090179. J. Virol. 71:4679-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 2000. Roscovitine, a specific inhibitor of cellular cyclin-dependent kinases, inhibits herpes simplex virus DNA synthesis in the presence of viral early proteins. J. Virol. 74:2107-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlegel, A., T. H. Giddings, Jr., M. S. Ladinsky, and K. Kirkegaard. 1996. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J. Virol. 70:6576-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlegel, A., and K. Kirkegaard. 1995. Cell biology of enterovirus infection, p. 135-154. In H. Rotbart (ed.), Human enterovirus infections. ASM Press, Washington, D.C.
- 45.Suhy, D. A., T. H. Giddings, Jr., and K. Kirkegaard. 2000. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles. J. Virol. 74:8953-8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang, S., R. van Rij, D. Silvera, and R. Andino. 1997. Toward a poliovirus-based simian immunodeficiency virus vaccine: correlation between genetic stability and immunogenicity. J. Virol. 71:7841-7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tershak, D. R. 1984. Association of poliovirus proteins with the endoplasmic reticulum. J. Virol. 52:777-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Towner, J., and B. L. Semler. 1996. Determinants of membrane association on poliovirus protein 3AB. J. Biol. Chem. 271:26810-26818. [DOI] [PubMed] [Google Scholar]