Abstract
B lymphocytes are known as a potential site for latency and reactivation of the human neurotropic polyomavirus, JC virus (JCV). In light of recent studies on the oncogenicity of JCV and the transforming ability of the JCV early protein, T antigen, we investigated the association of JCV with B-cell lymphomas of the central nervous system. Examination of 27 well-characterized clinical specimens by gene amplification and immunohistochemistry revealed the presence of DNA sequences corresponding to the JCV early genome and the late Agnoprotein in 22 samples and the JCV late genome encoding the viral capsid proteins in 8 samples. Expression of T antigen and that of Agnoprotein by immunohistochemistry were each detected in six specimens. No evidence of the production of viral capsid proteins was observed, ruling out productive infection of JCV in the tumor cells. The results from laser capture microdissection verified the presence of JCV T-antigen sequences in tumor cells with positive immunoreactivity to antibodies against the viral proteins T antigen and Agnoprotein. Due to previous reports demonstrating an association of the Epstein-Barr virus (EBV) with transformation of B lymphocytes, EBV DNA sequences and the EBV transforming protein, latent membrane protein 1 (LMP1), were analyzed in parallel. EBV LMP1 DNA sequences were detected in 16 of 23 samples, and LMP1 expression was detected in 16 samples, 5 of which exhibited positive immunoreactivity to JCV proteins. Double labeling demonstrated coexpression of JCV T antigen and EBV LMP1 in the same cells. The detection of the JCV genome in large numbers of B-cell lymphomas and its coexistence with EBV suggest a potential role for JCV in the pathogenesis of primary CNS lymphoma.
The term primary central nervous system (CNS) lymphoma indicates a set of malignant neoplasias which are of hematological origin that arise in the CNS in patients who display no involvement of lymphoid or other organs at the time of diagnosis. Immunocompetent individuals may present with the disease in the seventh decade of life or greater, whereas immunocompromised patients tend to develop tumors at earlier ages. In fact, the incidence and mortality rate associated with CNS lymphomas has rapidly increased in recent years, most likely due to the fact that a greater number of affected individuals are human immunodeficiency virus type 1 (HIV-1) positive (31, 37). Nearly all primary CNS lymphomas arise from B lymphocytes and, as such, may express one or more B-cell markers, including CD20 (29). Based on genetic analysis, it has been suggested that primary CNS lymphomas may arise in germinal center B cells and that the transformed B cells then seed the CNS (30, 34).
Whereas the cause of B-cell lymphomas is not known, a strong association between lymphomagenesis and the ubiquitous human herpesvirus, Epstein-Barr virus (EBV) has been demonstrated, particularly in immunocompromised individuals, including AIDS patients and transplant recipients (17). In vitro experiments have shown that EBV is capable of immortalizing B cells in vitro and have suggested a role for the viral latent membrane protein 1 (LMP1) in the oncogenic process (8). Studies aimed at determining mechanisms of EBV LMP1 transformation have suggested that LMP1 functions to protect cells from apoptotic death via Bcl-2 and involvement of the NF-κB pathway, thus preserving B cells for viral replication or maintenance of latency (15, 24, 32).
The human polyomavirus, JC virus (JCV), has also been detected in B lymphocytes and mononuclear cells in brain perivascular spaces of patients with progressive multifocal leukoencephalopathy (PML), suggesting that JCV can also infect and possibly remain in a latent state in these cells (20, 23, 41). In fact, JCV has been shown to replicate, albeit to a limited extent, in several human cell lines of B-cell origin and has been detected in a case of CNS lymphoma (4, 19). JC virus is best known as the neurotropic virus responsible for the CNS demyelinating disease, PML, which occurs when JCV productively infects and destroys oligodendrocytes, the myelin-producing cells in the brain (5, 6). PML usually occurs in individuals with underlying deficits in cell-mediated immunity and, like CNS lymphomas, the incidence of PML have dramatically increased in the era of AIDS (6).
Similar to EBV, JCV is a ubiquitous human virus that infects >70% of the population worldwide (36). JCV is thought to infect individuals in early childhood and establish life-long latency in the kidney. The viral genome is comprised of double-stranded DNA that encodes a total of six proteins, including the early proteins, large and small T antigen, and the late structural proteins VP1, VP2, and VP3, as well as Agnoprotein, which is encoded in the leader sequence of the late RNA transcript (18). The early and late genes are separated by a noncoding regulatory region of the virus that contains the origin of DNA replication and transcriptional enhancer elements (Fig. 1A) (38).
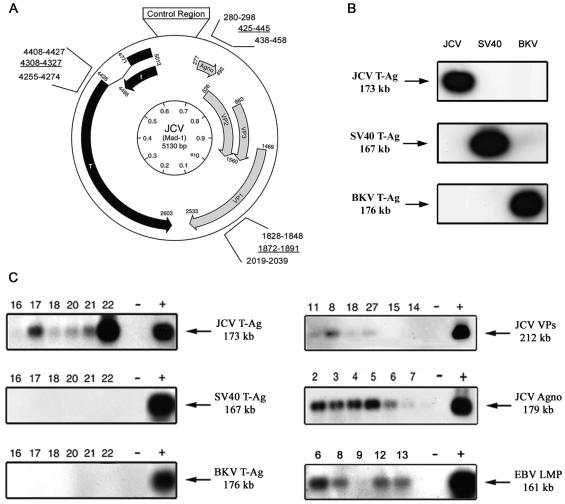
FIG. 1.
PCR amplification and Southern blot hybridization to detect JCV DNA fragments from tissue sections of primary CNS lymphomas. (A) Schematic representation of the JCV genome showing the early and late coding regions separated by the noncoding regulatory region. The location and nucleotide position of the primers used for PCR amplification are noted by using the nomenclature for the Mad-1 reference strain of JCV (GenBank no. J02226). (B) PCR products were analyzed by Southern blotting with three different probes specific for each of the polyomaviruses—JCV, BKV, and SV40—for which representative control data are shown. (C) PCR-Southern blot hybridization of DNA extracted from B-cell lymphoma samples show the presence of JCV T-antigen but not SV40 or BKV T antigens (left). Samples were also analyzed for the presence of JCV VP1, JCV Agnoprotein, and EBV LMP1 (right).
In addition to its role in PML, numerous studies have demonstrated the transforming ability of T antigen in vitro, for which the best-described mechanisms involve T antigen's ability to physically interact with the tumor suppressor proteins pRb and p53 (18). Of note, JCV has been shown to be tumorigenic in several animal models, including rodents and nonhuman primates (reviewed in references 11 and 21). The range of tumors induced by JCV have primarily consisted of neuronal and glial neoplasms resembling medulloblastomas, primitive neuroectodermal tumors, oligodendrogliomas, astrocytomas, and glioblastomas. Analysis of their counterparts in humans has revealed the presence of JCV genomic sequences and expression of the oncogenic T-antigen protein in some neuronal and glial tumors (10, 12, 27). More recently, JCV gene sequences and T-antigen expression have been detected in colon cancer, thus expanding the potential range for this heretofore neurotropic virus (16, 28).
It has been suggested that JC virus migrates in peripheral blood lymphocytes from the kidney, where it remains latent, to the brain, where it can induce PML (2). Many studies have demonstrated that JCV can persistently infect a number of cell types, including immune system cells (13, 20) and that JCV DNA may be commonly detected in peripheral blood mononuclear cells of HIV-1-infected patients (1, 14), HIV-negative immunocompromised patients and, with less frequency, the in lymphocytes of healthy blood donors, indicating that peripheral blood leukocytes could constitute a site of JCV latency after primary infection (14, 33).
Given the ability of JCV to infect and remain latent in B cells, the occurrence of both PML and CNS lymphoma in the setting of AIDS, and the potent transforming potential of JCV T antigen in many different cell types, we examined samples of CNS lymphoma for the presence of viral DNA sequences and expression of the oncogenic viral protein, T antigen.
MATERIALS AND METHODS
Clinical samples.
For the present study, a total of 27 formalin-fixed, paraffin-embedded autopsy cases of primary CNS lymphomas and adjacent brain tissue, which occurred between 1978 and 1992, were collected from the neuropathology archives of the University Institute of Pathology, Lausanne, Switzerland. Table 1 summarizes the clinical data from these patients, including gender and age at time of death, as well as any associated or underlying diseases.
TABLE 1.
Clinical, PCR amplification, and immunohistochemical detection of cellular and viral proteins in CNS lymphomasa
| Patient no. | Age (yr) | Gender | CNS disease | Resultb determined by:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immunohistochemistry
|
PCR-Southern
|
||||||||||||||
| CD79a | CD20 | CD3 | p53 | LMP1 (EBV) | T-Ag | VP1 | Agno | EBV | T-Ag | VP1 | Agno | ||||
| 1 | 47 | F | AIDS | − | ++ | − | − | +++ | − | − | − | + | + | − | + |
| 2 | 38 | M | AIDS | − | ++ | − | − | − | − | − | − | − | + | + | + |
| 3 | 58 | F | AIDS | +f | + | − | +f | + | − | − | − | + | + | − | + |
| 4 | 62 | F | AIDS | − | ++ | − | − | + | − | − | − | + | + | + | + |
| 5 | 35 | M | AIDS | − | + | − | − | − | − | − | − | − | + | + | + |
| 6c | 62 | F | AIDS | − | +++ | − | − | ++ | − | − | − | + | − | − | − |
| 7 | 38 | M | AIDS | + | + | − | − | − | − | − | − | − | + | − | + |
| 8c | 69 | F | AIDS | ++ | ++ | − | − | +++ | − | − | − | + | + | + | + |
| 9 | 27 | M | AIDS | + | ++ | − | − | − | ++ | − | ++ | − | + | − | + |
| 10 | 42 | M | AIDS | + | ++ | − | − | +++ | − | − | − | ND | − | − | − |
| 11 | 39 | M | AIDS | + | +++ | − | + | + | +++ | − | − | ND | + | + | + |
| 12 | 49 | M | AIDS, CMV | − | ++ | − | − | + | − | − | − | + | + | + | + |
| 13 | 77 | F | AIDS, toxoplasmosis | +++ | ++ | − | − | − | − | − | + | + | + | − | + |
| 14 | 80 | M | Alzheimer's disease | + | + | − | − | − | + | − | − | + | + | + | + |
| 15 | 63 | F | Schizophrenia | ++ | ++ | − | − | − | − | − | − | ND | + | − | + |
| 16 | 60 | M | NR | ++ | + | − | + | +++ | ++ | − | + | + | + | − | + |
| 17 | 82 | M | NR | − | +++ | − | ++ | − | − | − | − | − | + | − | + |
| 18 | 69 | M | NR | ++ | +++ | − | − | +++ | − | − | + | + | + | − | + |
| 19 | 67 | M | NR | ++ | ++ | − | − | ++ | ++ | − | + | + | + | − | + |
| 20 | 79 | M | NR | +++ | +++ | − | + | + | +++ | − | − | + | + | − | + |
| 21 | 76 | M | NR | +++ | ++ | − | + | − | − | − | + | − | + | − | + |
| 22 | 72 | F | NR | + | ++ | − | − | + | − | − | − | + | − | − | − |
| 23 | 78 | F | NR | − | +++ | − | − | − | − | − | − | − | − | − | − |
| 24 | 65 | F | NR | ++ | +++ | − | − | + | − | − | − | ND | + | − | + |
| 25 | 86 | M | NR | +++ | +++ | − | − | + | − | − | − | + | + | − | + |
| 26 | 67 | F | NR | + | ++ | − | − | + | − | − | − | + | + | + | + |
| 27 | 69 | F | NR | + | +++ | − | − | − | − | − | − | + | − | − | − |
Diagnosis of primary CNS lymphoma was based on the World Health Organization classification of brain tumors. CMV, cytomegalovirus; T-Ag, T antigen; NR, none reported.
−, Negative reactivity; +, 1 to 30% cell positivity; ++, 31 to 60% cell positivity; +++, >61% cell positivity; +f, focal positivity; ND, not determined.
Leptomeningeal infiltration.
DNA extraction, amplification, and hybridization.
DNA extraction, PCR amplification, and Southern blot hybridization were performed essentially as described previously (10). DNA was extracted from several 10-μm sections from the tissue samples by using the QIAamp tissue kit according to the manufacturer's instructions (Qiagen, Valencia, Calif.). PCR amplification was performed on DNA extracted from the tumors with four individual sets of primers: Pep1 and Pep2, which amplify sequences (nucleotides [nt] 4255 to 4274 and nt 4408 to 4427, respectively) in the N-terminal regions of the T antigen of the polyomaviruses JCV, BK virus (BKV), and simian virus 40 (SV40); VP2 and VP3, which amplify regions of the JCV VP1 capsid protein (nt 1828 to 1848 and nt 2019 to 2039, respectively, of JCV); Agno1 and Agno2, which amplify sequences within the coding region of the JCV Agnogene (nt 279 to 298 and nt 438 to 458, respectively); and LMP1 and LMP2, which amplify portions of the EBV LMP1 gene (nt 168190 to 168209 and nt 168350 to 168331) (9, 22). Amplification was carried out on 500 ng of template DNA with Failsafe Taq polymerase in Failsafe Buffer B (Epicenter) in a total volume of 50 μl in the presence of 0.5 nM concentrations of the primers. After denaturation at 95°C for 10 min, 45 cycles of denaturation at 95°C for 15 s, annealing for 30 s, and extension at 72°C for 30 s, a final extension step of 72°C for 7 min was performed for termination. Annealing temperatures were 55°C for Pep primers, 54°C for VP primers, 57°C for Agno primers, and 55°C for LMP1 primers. In parallel, 500 ng of plasmid DNA containing JCV, SV40, or BKV sequences was amplified to serve as positive and negative controls, while 500 ng of genomic DNA extracted from the EBV-positive cell line Raji was used as a positive control for EBV. Then, 10 μl of the PCR products was separated by 2% agarose gel electrophoresis, depurinated, denaturated, neutralized, and transferred to nylon membranes (Hybond-N; Amersham). The membranes were hybridized with 106 cpm of γ-32P-end-labeled oligonucleotide probes/ml overnight, followed by washing and autoradiography as described previously (27). Oligonucleotides homologous to the following JCV-specific sequences were utilized as probes: JC probe (Pep primers; nt 4303 to 4327), VP probe (nt 1872 to 1891), and Agno probe (nt 425 to 445), as well as LMP1 probe for EBV (nt 168311 to 168330). For samples amplified with the Pep primers, blots were stripped by incubation in 0.1% sodium dodecyl sulfate-0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 65°C for 30 min and then rehybridized with radiolabeled oligonucleotide probes specific for SV40 and BKV.
Histological and immunohistochemical analysis.
Formalin-fixed, paraffin-embedded tumor tissue was sectioned at 4 μm in thickness and stained with hematoxylin and eosin for routine histological characterization. Immunohistochemistry was performed by using an avidin-biotin-peroxidase complex system according to the manufacturer's instructions (Vectastain Elite ABC peroxidase kit; Vector Laboratories, Inc.) Samples were deparaffinized in xylenes, rehydrated through alcohols, and nonenzymatic antigen retrieval was performed in 0.01 M sodium citrate buffer (pH 6.0) for 40 min at 95°C. After the mixture was allowed to cool, peroxidase quenching in MeOH-% H2O2 for 20 min was performed, followed by blocking in 5% normal horse serum for 2 h at room temperature. Primary antibodies against viral and cellular proteins were incubated overnight at room temperature in a humidified chamber. Primary antibodies to detect JCV proteins used in the present study included rabbit anti-JCV Agnoprotein (previously described in reference 12) and anti-SV40 VP1 (clone pAb597 [kindly provided by Walter Atwood, Brown University]); mouse anti-SV40 T-antigen (clone pAb416; Oncogene Science). It should be noted that all three antibodies to detect JCV proteins cross-react with SV40 and BKV (J. Gordon et al., unpublished observations). The primary antibodies to EBV and cellular proteins used in the present study included anti-EBV LMP1 (clone Kal-1; Novocastra Laboratories), anti-CD20 (clone L26; Dako), anti-CD79a (clone HM57; Dako), anti-CD3-ɛ (clone UCH-T1; Dako), and anti-p53 (DO-7; Dako). After incubation with primary antibodies, the sections were incubated in biotinylated secondary anti-mouse or anti-rabbit antibodies for 1 h at room temperature, followed by incubation with avidin-biotin complex according to the manufacturer's instructions (Vector Laboratories) and were developed with diaminobenzidine. Slides were counterstained with hematoxylin, dehydrated, cleared in xylene, and mounted with Permount (Sigma Laboratories).
Laser capture microdissection and DNA isolation.
Representative sections of tissue samples were labeled by immunohistochemistry as described above. Slides were dehydrated, cleared in xylene, and air dried overnight. Afterward, laser capture was performed under direct microscopic visualization of the immunolabeled cells by laser activation of thermoplastic film mounted on optically transparent CapSure HS LCM caps (Arcturus Engineering). The PixCell II LCM System (Arcturus) was set to the following parameters: 15-μm spot size, 40-mW power, and 3.0-ms duration. A total of 50 neoplastic cells per category were captured by focal melting of the membrane through carbon dioxide laser pulse activation. DNA isolation was performed by using an Arcturus PicoPure DNA extraction kit according to the manufacturer's instructions. PCR amplification and analyses were performed as described above.
Double-labeling immunofluorescence.
Deparaffinization, antigen retrieval, and blocking of paraffin-embedded sections were performed as described above. Sections were then incubated in mouse anti-T-antigen antibody (clone 416; Oncogene Science) overnight at room temperature, followed by incubation in anti-mouse fluorescein antibody for 2 h. Next, sections were incubated with mouse anti-EBV LMP protein (clone Kal-1; Novocastra Laboratories) overnight, followed by incubation in anti-mouse rhodamine antibody. Sections were washed and mounted in Vectashield aqueous mounting medium and then visualized by fluorescence microscopy. Black-and-white images were acquired, pseudocolored, and overlaid by using IPLab software.
RESULTS AND DISCUSSION
Given earlier observations on the presence of JCV in B lymphocytes and the oncogenic potential of this virus, we initiated studies to examine the association of JCV with human primary CNS lymphoma in a collection of 27 archival autopsy specimens of primary CNS lymphomas from the University Institute of Pathology, Lausanne, Switzerland. Twelve of the patients were known to be HIV-1 positive, and two of them had other HIV-1-associated opportunistic infections, namely, cytomegalovirus infection and toxoplasmosis. The ages of the patients ranged from 27 to 82 years, and all patients were confirmed as primary CNS lymphoma based on the absence of involvement of other tissues or organs. Extensive neuropathological evaluation of the brain at autopsy revealed no signs of PML in any of the cases. Of note, tumors were observed to have spread through the subarachnoid space (leptomeningeal infiltration) in two of the cases. Table 1 summarizes the clinical data from the patients and any underlying or associated disease, if known.
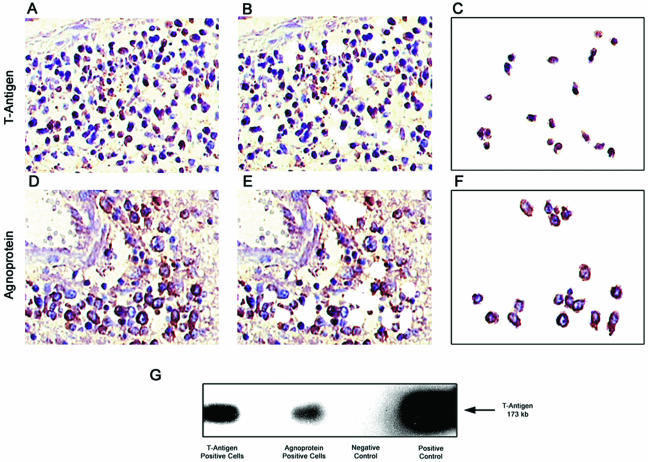
Previous studies have demonstrated the presence of JC virus DNA in both normal lymphocytes and in different varieties of brain tumors of neuronal and glial origin (10, 25, 41). In order to identify the presence of JCV DNA sequences in the tumor samples, PCR amplification studies were performed. As shown in Fig. 1A, three independent regions of the JCV genome were targeted for amplification by using specific primers, including sequences encoding the early gene T antigen and the late genes Agnogene and VP1. To confirm the specificity of the PCR products, Southern blot hybridization was performed with oligonucleotide probes homologous to sequences within each amplicon, representative data for which are shown in Fig. 1B. It should be noted that whereas primers for the T-antigen sequences show significant homology with SV40 and BKV, the probe target is highly specific since it shares 41% homology with SV40 and 59% homology with BKV. These primers and polyomavirus-specific probes have been utilized extensively in many laboratories, including our own, to distinguish JCV from SV40 and BKV by Southern blot hybridization, as shown in Fig. 1B (42). The primer sequences for VP1 and Agnogene are highly specific for JCV and do not detect SV40 and BKV, with Agno probe showing only 24% homology with SV40 and 52% homology with BKV, whereas the VP probe shows 50% homology with SV40 and 52% homology with BKV. With primers targeting sequences within the N-terminal of T-antigen along with JCV-specific probe, viral DNA was found in 22 of 27 cases (81.5%), whereas primers specific for the Agnogene region amplified viral DNA in the same 22 cases in which T antigen was detected (81.5%). DNA encoding the late capsid protein (VP1) was amplified from 8 of the 27 cases (29.6%). Of note, only five samples were negative for all JCV DNA sequences, whereas the eight samples that contained VP1 DNA also contained sequences corresponding to both T antigen and Agnogene. In addition, PCR followed by Southern blot hybridization performed with primers specific for the LMP-1 gene, as described previously, demonstrated the presence of EBV in 16 of 23 samples (70%) (9, 22). All samples were negative for SV40 and BKV T-antigen sequences as determined by Southern blot hybridization with specific probes performed on blots containing sequences amplified with the Pep primers. In order to verify the presence of viral DNA sequences within the tumor cells, immunohistochemistry to detect the JCV proteins, T antigen and Agnoprotein, were performed (Fig. 2A and D), followed by laser capture microdissection (LCM) to dissect T-antigen- or Agnoprotein-positive cells from the tumor tissue (compare Fig. 2B and E to Fig. 2C and F). As shown in Fig. 2G, PCR-Southern blotting performed on the captured cells revealed that sequences homologous with JCV T antigen could be detected in both T-antigen-positive and Agnoprotein-positive tumor cells. Since the CNS lymphoma samples contained both Agnogene and T-antigen sequences, the LCM data suggest the possibility that T antigen and Agnoprotein were expressed within the same tumor cells.
FIG. 2.
LCM and PCR amplification of JCV DNA from immunohistochemically positive tumor cells. Samples immunostained for the presence of JCV T-antigen and Agnoprotein are shown prior to LCM (A and D, respectively) and after LCM (B and E, respectively). The captured T-antigen- and Agnoprotein-positive cells (C and F, respectively) were analyzed by PCR-Southern blotting for JCV T antigen. (G) JCV T antigen could be detected by PCR-Southern blot analysis in both T-antigen-positive and Agnoprotein-positive cells.
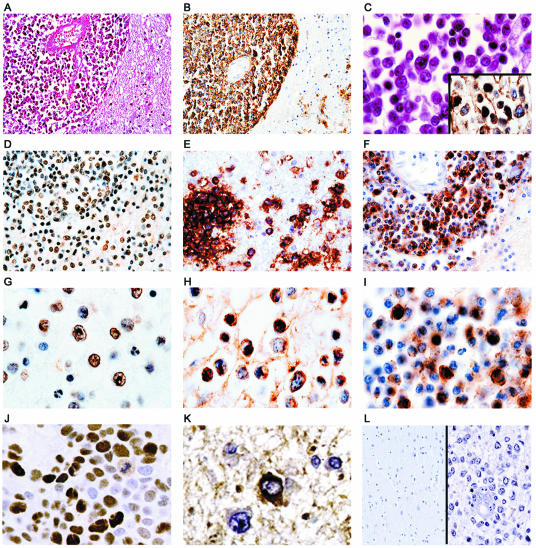
The tumors were histologically and immunohistochemically characterized according to the latest World Health Organization classification of brain tumors (26). In general, tumors were characterized by perivascular accumulation of neoplastic lymphocytes, which in most cases were seen to break through the Virchow-Robin space and infiltrate the brain parenchyma (Fig. 3A). Immunohistochemical evaluation showed strong cytoplasmic reactivity to Pan-B cell markers with all samples exhibiting positivity for CD-20 (Fig. 3B and C [inset]). The majority of the samples also stained positive for the pre-B cell marker CD79a, and all samples were negative for the T-cell marker, CD3 (data not shown; see Table 1 for summary). At a higher magnification, the neoplastic lymphocytes were round with irregular, atypical nuclei. Numerous mitotic figures were observed (3C). JCV T antigen could be detected by immunohistochemistry in the nuclei of the tumor cells in six cases (22.2%) (Fig. 3D and G), whereas the staining pattern exhibited by JCV Agnoprotein in the cytoplasm appeared with a perinuclear pattern in six cases (22.2%) (Fig. 3E and H). In three cases, the samples were positive for both T antigen and Agnoprotein. None of the samples were positive for JCV VP1, indicating an absence of active viral replication (data not shown; see Table 1 for summary). Normal brain tissue adjacent to the CNS lymphomas was negative for viral antigens (Fig. 3L, left). In addition, immunohistochemistry was performed to detect the cell cycle regulatory protein p53, which demonstrated robust positivity in the nuclei of neoplastic lymphocytes in six cases (22.2%), one of which showed focal positivity (data not shown; see Table 1 for summary). These observations are in accord with earlier reports of chromosomal imbalances and low levels of p53 expression in primary CNS lymphomas (7, 39) and speculation on the involvement of JCV in “rogue” cells, lymphocytes that exhibit chromosomal damage, perhaps due to nonfunctional p53 (36).
FIG. 3.
Histological characterization and immunohistochemical detection of JCV and EBV proteins in primary CNS lymphoma samples. (A) A low-power magnification view of the tumor shows abundant neoplastic lymphocytes arranged in a concentric perivascular pattern that diffusely infiltrates the brain parenchyma. (B and C [inset]) Immunohistochemical expression of the Pan-B cell marker, CD20, demonstrates robust cytoplasmic reactivity in the neoplastic cells. At higher magnification, the cells appear round with irregular pleomorphic nuclei and mitotic figures are observed (C). Immunohistochemistry to detect the JCV gene products, T antigen and Agnoprotein, demonstrates intense reactivity for T antigen in the nuclei of neoplastic lymphocytes and Agnoprotein in the cytoplasm of the tumoral cells with a perinuclear pattern (T antigen [D and G]; Agnoprotein [E and H]). (F and I) Samples stained with antibody for the EBV LMP1 show cytoplasmic staining within neoplastic lymphocytes in the cells trapped in the Virchow-Robin space, as well as in cells infiltrating the brain parenchyma. Positive controls for T-antigen and Agnoprotein staining, respectively, included sections of JCV T-antigen-induced murine tumor (J) and a case of PML (K). (L, left and right sides, respectively) Negative controls included sections of normal brain adjacent to CNS lymphoma and tumor sections in which primary antibodies were omitted during the immunohistochemistry. Magnifications: A and B, ×100; D to F, L, ×400; C, G to K, ×1,000.
Since previous reports have detected the presence of EBV within B-cell lymphomas, we analyzed the samples for EBV by performing immunohistochemistry to detect EBV LMP1 within tumor cells. As shown in Fig. 3, LMP1 could be detected in the cytoplasm of neoplastic lymphocytes both in cells trapped in the Virchow-Robin space and in cells infiltrating the brain parenchyma (Fig. 3F and I). LMP1 was detected in 16 samples (59.3%), 4 of which were also positive for JCV T antigen (14.8%). As shown in Table 1, 20 of the 27 samples were positive for either the EBV LMP1, JCV T antigen, or JCV Agnoprotein. Of these 20 positive samples, 5 of the LMP1-positive samples were also positive for one of the JCV proteins, and only 4 samples contained JCV proteins but not EBV LMP1.
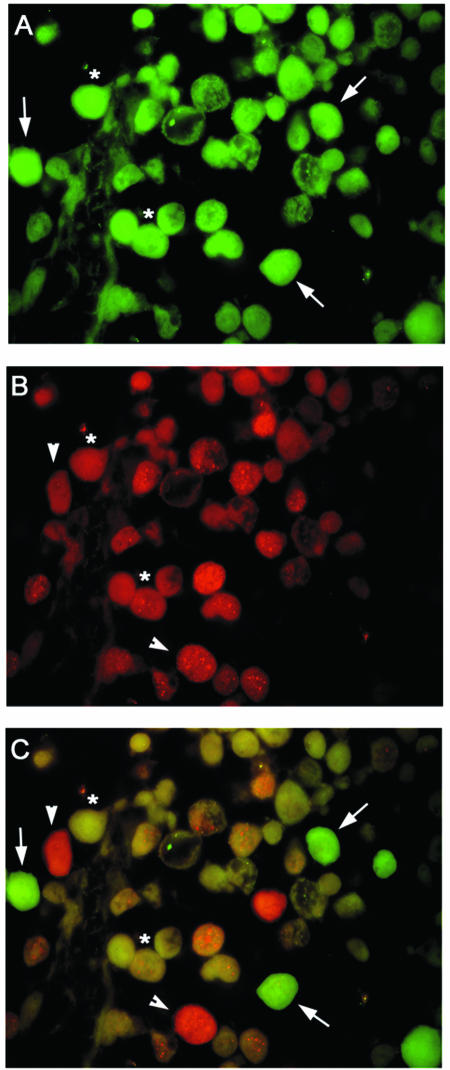
After this observation, double immunofluorescence labeling was undertaken to ascertain whether LMP1 and T antigen could be found localized within the same tumor cells. Immunofluorescence labeling of paraffin-embedded tumor sections demonstrated that a majority of the cells were immunopositive for T antigen (Fig. 4A), while a significant portion of the cells were positive for the LMP1 protein (Fig. 4B). An overlay of these two images illustrates cells in which T antigen alone is detected (arrows), cells in which LMP1 alone can be seen (arrowheads), and cells where both T antigen and LMP1 were observed (asterisks).
FIG. 4.
Double-labeling immunofluorescence for JCV T antigen and EBV LMP1 in primary CNS lymphoma cells. Samples of primary CNS lymphoma were evaluated by double immunolabeling for the presence of JCV T antigen (A) and EBV LMP1 (B). (C) Superimposition of panels A and B in which JCV T-antigen-positive cells are green and LMP1-positive cells are red. Representative tumor cells expressing T antigen alone are indicated with an arrow; arrowheads indicate cells expressing LMP1 only, and cells positive for both T antigen and LMP1 are indicated with an asterisk. Magnification, ×1,000.
It is interesting that a high number of tumor samples contained all three regions of JCV DNA and that all samples containing T-antigen sequences also were positive for DNA representing the Agnogene. Given that JCV has been shown to latently infected B cells and that B-cell lymphomas likely represent tumors of clonal origin, these data suggest that the entire genome of JCV may be intact within cells of some tumors. This finding is in contrast to observations made in neuronal and glial tumors, as well as colorectal tumors in which T-antigen sequences appear with greater frequency than the Agnogene. Consistent with previous observations, VP1 sequences are observed with the least frequency and were not detected in the absence of T-antigen sequences.
The presence of the polyomavirus, SV40, has recently been examined in tumors of B-cell lineage, including Hodgkin's and non-Hodgkin lymphomas. More specifically, Vilchez et al. reported the detection of SV40-specific sequences by PCR-Southern blot and sequence analysis in 64% of non-Hodgkin lymphomas from both HIV-1-infected and uninfected individuals (42). It should be noted that few of the tumor samples were positive for both SV40 and EBV genomic sequences. In a separate study, 43% of non-Hodgkin and 9% of Hodgkin's lymphomas were found to contain SV40 DNA sequences (40). In each of these studies, nonmalignant lymphoid samples and control tissues were negative for SV40 DNA. Taken together, these two reports suggest an association between SV40 and non-Hodgkin lymphoma.
Once classified exclusively as a neurotropic polyomavirus whose genes are expressed in glial-origin cells, thus causing demyelination of the white matter, recent studies have indicated that a broader range of cells, including lymphoid cells, can harbor the JCV genome (4, 20). The detection of JCV proteins, in the absence of viral replication and lytic infection, in a series of primary CNS lymphomas indicates that, under certain physiological conditions, the JC viral genome may be transcribed by a subset of transcription factors in nonglial cells, leading to the accumulation of viral proteins which, in turn, can deregulate several pathways responsible for the control of cellular proliferation. These observations raise several important questions regarding the possible mechanisms for JCV reactivation in non-CNS cells and the potential role of JCV in tumorigenesis in cells which, under normal conditions, do not allow viral gene expression. One can speculate that, as a nontransformed cell enters into a cancerous stage, several inducible and cell-type-specific transcription factors that are not active under normal conditions gain the opportunity to stimulate the expression of cellular genes, as well as latent viral genes such JCV. Not mutually exclusive, one can also hypothesize that reactivation of JCV may occur via cross-communication of JCV with other viruses, such as HIV-1 in AIDS patients and EBV in immunocompromised patients. In fact, earlier studies from our laboratory have demonstrated the ability of HIV-1 Tat in stimulation of the JCV promoter in glial cells, an event that may contribute to the higher incidence of PML observed in AIDS patients (38).
Since the first description of PML by Astrom et al. in 1958 (3), there have been several reports of PML concomitant with CNS lymphoma, the majority of which have been reported in patients with impaired immunity either due to immunosuppressive therapy after renal transplantation or secondary to lymphoproliferative disorders such as Hodgkin's disease (20). In one more recent case, T-antigen expression was detected within the neoplasia by immunohistochemistry (19). Our results, shown in Table 1, reveal no significant difference between the T-antigen-positive cases in HIV-1-positive versus HIV-1-negative patients, nor are there any clear distinctions between AIDS and non-AIDS CNS lymphomas. On the other hand, four of six cases positive for T antigen expressed EBV LMP1 leading to speculation of possible cross-reactivation or cooperation between EBV and JCV in B-cell lymphoma. Given that the majority of the human population are latently infected with EBV, the incidence of CNS lymphoma would be significantly higher if EBV were the sole factor responsible for their development. Although EBV infection may be a prerequisite for the development of CNS lymphoma in most patients, we suggest that JCV may be a cofactor or may provide one of several additional “hits” required for transformation in some CNS lymphomas. These observations invite thorough molecular studies to investigate such cooperation or cross-reactivation. Although the mechanisms whereby JCV exerts its contribution toward the development of primary CNS lymphomas remain unknown, one may anticipate that the interaction of tumor suppressors such as p53 and the pRb family and the dysregulation of signaling pathways such as IGF-1 and Wnt provide potential targets for T-antigen function. At present, experiments are in progress to decipher the molecular mechanisms that may be involved in JCV's association with primary CNS lymphoma.
Acknowledgments
We thank past and present members of the Center for Neurovirology and Cancer Biology for insightful discussion and sharing of ideas and reagents. We thank C. Schriver for editorial assistance and preparation of the manuscript.
This study was made possible by grants awarded by the NIH to K.K.
REFERENCES
- 1.Andreoletti, L., V. Dubois, A. Lescieux, A. Dewilde, L. Bocket, H. J. Fleury, and P. Wattre. 1999. Human polyomavirus JC latency and reactivation status in blood of HIV-1 positive immunocompromised patients with and without progressive multifocal leukoencephalopathy. AIDS 13:1469-1475. [DOI] [PubMed] [Google Scholar]
- 2.Andreoletti, L., A. Lescieux, V. Lambert, A. Si-Mohamed, M. Matta, P. Wattre, and L. Belec. 2002. Semiquantitative detection of JCV DNA in peripheral blood leukocytes from HIV-1-infected patients with or without progressive multifocal leukoencephalopathy. J. Med. Virol. 66:1-7. [DOI] [PubMed] [Google Scholar]
- 3.Astrom, K. E., E. L. Mancall, and E. P. Richardson, Jr. 1958. Progressive multifocal leukoencephalopathy: a hitherto unrecognized complication of chronic lymphatic leukemia and Hodgkin's disease. Brain 81:93-111. [DOI] [PubMed] [Google Scholar]
- 4.Atwood, W. J., K. Amemiya, R. Traub, J. Harms, and E. O. Major. 1992. Interaction of the human polyomavirus, JCV, with human B-lymphocytes. Virology 190:716-723. [DOI] [PubMed] [Google Scholar]
- 5.Berger, J. R., and M. Concha. 1995. Progressive multifocal leukoencephalopathy: the evolution of a disease once considered rare. J. Neurovirol. 1:15-18. [DOI] [PubMed] [Google Scholar]
- 6.Berger, J. R., and E. O. Major. 1999. Progressive multifocal leukoencephalopathy. Semin. Neurol. 19:193-200. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri-Broet, S., A. Martin, A. Moreau, R. Angonin, D. Henin, M. F. Gontier, M. C. Rousselet, S. Caulet-Maugendre, P. Cuilliere, T. Lefrancq, K. Mokhtari, M. Morcos, P. Broet, M. Kujas, J. J. Hauw, B. Desablens, and M. Raphael. 1998. Primary central nervous system lymphomas in 72 immunocompetent patients: pathologic findings and clinical correlations. Am. J. Clin. Pathol. 110:607-612. [DOI] [PubMed] [Google Scholar]
- 8.Cesarman, E. 2002. Epstein-Barr virus (EBV) and lymphomagenesis. Front. Biosci. 7:e58-e65. [DOI] [PubMed] [Google Scholar]
- 9.Chu, P. G., K. L. Chang, Y.-Y. Chen, W.-G. Chen, and L. M. Weiss. 2001. No significant association of Epstein-Barr virus infection with invasive breast carcinoma. Am. J. Pathol. 159:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Valle, L., J. Gordon, Assimakopolou, S. Enam, J. F. Geddes, J. Varakis, C. Katsetos, S. E. Croul, and K. Khalili. 2001. Detection of JC virus DNA sequences and expression of the viral regulatory protein, T-antigen, in tumors of the central nervous system. Cancer Res. 61:4287-4293. [PubMed] [Google Scholar]
- 11.Del Valle, L., J. Gordon, P. Ferrante, and K. Khalili. 2001. JC virus in experimental and clinical brain tumorigenesis, p. 409-430. In K. Khalili and G. L. Stoner (ed.), Human polyomaviruses: molecular and clinical perspectives. Wiley-Liss, New York, N.Y.
- 12.Del Valle, L., J. Gordon, S. Enam, S. Delbue, Croul., S., Abraham, S. S. Radhakrishnan, M. Assimakoupoulou, C. D. Katsetos, and K. Khalili. 2002. Expression of human neurotropic polyomavirus JCV late gene product Agnoprotein in human medulloblastoma. J. Natl. Cancer Inst. 94:267-273. [DOI] [PubMed] [Google Scholar]
- 13.Dorries, K., E. Vogel, S. Gunther, and S. Czub. 1994. Infection of human polyomaviruses JC and BK in peripheral blood leukocytes from immunocompetent individuals. Virology 198:59-70. [DOI] [PubMed] [Google Scholar]
- 14.Dubois, V., M. E. Lafon, J. M. Ragnaud, et al. 1996. Detection of JC virus DNA in the peripheral blood leukocytes of HIV-infected patients. AIDS 10:353-358. [DOI] [PubMed] [Google Scholar]
- 15.Eliopoulos, A. G., M. Stack, C. W. Dawson, K. M. Kaye, L. Hodgkin, S. Sihota, M. Rowe, and L. S. Young. 1997. Epstein-Barr virus-encoded LMP1 and CK40 mediate IL-6 production in epithelial cells via an NF-κB pathway involving TNF receptor-associated factors. Oncogene 14:2899-2916. [DOI] [PubMed] [Google Scholar]
- 16.Enam, S., L. Del Valle, C. Lara, D. D. Gan, C. Ortiz-Hidalgo, J. P. Palazzo, and K. Khalili. 2002. Association of human polyomavirus JCV with colon cancer: evidence for interaction of viral T-antigen and beta-catenin. Cancer Res. 62:7093-7101. [PubMed]
- 17.Faulkner, G. C., A. S. Krajewski, and D. H. Crawford. 2000. The ins and outs of EBV infection. Trends Microbiol. 8:185-189. [DOI] [PubMed] [Google Scholar]
- 18.Frisque, R. J., and F. A. White III. 1992. The molecular biology of JC virus, causative agent of progressive multifocal leukoencephalopathy, p. 25-158. In R. P. Roos (ed.), Molecular neurovirology. Humana Press, Inc., Clifton, N.J.
- 19.Gallia, G. L., L. Del Valle, C. Laine, M. Curtis, and K. Khalili. 2001. Concomitant progressive multifocal leukoencephalopathy and primary central nervous system lymphoma expressing JC virus oncogenic protein, large T antigen. Mol. Pathol. 54:354-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallia, G. L., S. A. Houff, E. O. Major, and K. Khalili. 1997. Review: JC virus infection of lymphocytes-revisited. J. Infect. Dis. 176:1603-1609. [DOI] [PubMed] [Google Scholar]
- 21.Gordon, J., B. Krynska, J. Otte, S. A. Houff, and K. Khalili. 1998. Oncogenic potential of human neurotropic papovavirus, JCV, in CNS. Dev. Biol. Stand. 94:93-101. [PubMed] [Google Scholar]
- 22.Higa, M., T. Kinjo, K. Kamiyama, T. Iwamasa, T. Hamada, and K. Iyama. 2001. Epstein-Barr virus (EBV) subtype EBV related oral squamous cell carcinoma in Okinawa, a subtropical island in southern Japan, compared with Kitakyushu and Kumamoto in mainland Japan. J. Clin. Pathol. 55:414-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houff, S. A., E. O. Major, D. A. Katz, et al. 1988. Involvement of JC virus-infected mononuclear cells from the bone marrow and spleen in the pathogenesis of progressive multifocal leukoencephalopathy. N. Engl. J. Med. 318:301-305. [DOI] [PubMed] [Google Scholar]
- 24.Kenney, J. L., M. E. Guinness, T. Curiel, and J. Lacy. 1998. Antisense to the Epstein-Barr virus (EBV)-encoded latent membrane protein 1 (LMP1) suppresses LMP1 and bcl-2 expression and promotes apoptosis in EBV-immortalized B cells. Blood 92:1721-1727. [PubMed] [Google Scholar]
- 25.Khalili, K., B. Krynska, L. Del Valle, C. Katsetos, and S. E. Croul. 1999. Medulloblastomas and the human neurotropic polyomavirus, JCV. Lancet 353:1152-1153. [DOI] [PubMed] [Google Scholar]
- 26.Kleihues, P., and W. K. Cavenee. 2000. World Health organization classification of tumors: pathology and genetics. Tumors of the nervous system. IARC Press, Lyon, France.
- 27.Krynska, B., L. Del Valle, S. E. Croul, J. Gordon, C. Katsetos, M. Carbone, A. Giordano, and K. Khalili. 1999. Detection of human neurotropic JC virus DNA sequence and expression of the viral oncogenic protein in pediatric medulloblastomas. Proc. Natl. Acad. Sci. USA 96:11519-11524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laghi, L., A. E. Randolph, D. P. Chauhan, G. Marra, E. O. Major, J. V. Neel, and C. R. Boland. 1999. JC Virus DNA is present in the mucosa of the human colon and in colorectal cancers. Proc. Natl. Acad. Sci. USA. 96:7484-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lantos, P. L., D. N. Louis, M. K. Rosenblum, and P. Kleihues. 2002. Tumors of the nervous system, p. 950-959. In D. I. Graham and P. L. Lantos (ed.), Greenfield's neuropathology, 7th ed. Oxford University Press, New York, N.Y.
- 30.Larocca, L. M., D. Capello, A. Rinelli, S. Nori, A. Antinori, A. Gloghini, A. Cingolani, A. Migliazza, G. Saglio, S. Cammilleri-Broet, M. Raphael, A. Carbone, and G. Gaidano. 1998. The molecular and phenotypic profile of primary central nervous system lymphoma identifies distinct categories of the disease and is consistent with histogenetic derivation from germinal center-related B cells. Blood 92:1011-1019. [PubMed] [Google Scholar]
- 31.Mamidi, A., J. A. DeSimone, and R. J. Pomerantz. 2002. Central nervous system infections in individuals with HIV-1 infection. J. Neurovirol. 8:158-167. [DOI] [PubMed] [Google Scholar]
- 32.McFarland, E. D., K. M. Izumi, and G. Mosialos. 1999. Epstein-Barr virus transformation: involvement of the latent membrane protein 1-mediated activation of NF-κB. Oncogene 18:6959-6964. [DOI] [PubMed] [Google Scholar]
- 33.Monaco, M. C., W. J. Atwood, M. Gravell, C. S. Tornatore, and E. O. Major. 1996. JC virus infection of hematopoietic progenitor cells, primary B lymphocytes, and tonsillar stromal cells: implications for viral latency. J. Virol. 70:7004-7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montesinos-Rongen, M., R. Kuppers, D. Schluter, T. Spieker, D. Van Roost, C. Schaller, G. Reifenberger, O. D. Wiestler, and M. Deckert-Schluter. 1999. Primary central nervous system lymphomas are derived from germinal-center B cells and show a preferential usage of the V4-34 gene segment. Am. J. Pathol. 155:2077-2086. [DOI] [PMC free article] [PubMed]
- 35.Neel, J. V., E. O. Major, A. A. Awa, T. Glover, A. Burgess, R. Traub, B. Curfman, and C. Satoh. 1996. Hypothesis: “rogue cell”-type chromosomal damage in lymphocytes is associated with infection with the JC human polyomavirus and has implications for oncogenesis. Proc. Natl. Acad. Sci. USA 93:2690-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padgett, B. L., and D. L. Walker. 1983. Virologic and serologic studies of progressive multifocal leukoencephalopathy. Prog. Clin. Biol. Res. 105:107-117. [PubMed] [Google Scholar]
- 37.Raez, L. E., L. P. Patel, Feun, A. Restrepo, W. A. Raub, Jr., and P. A. Cassileth. 1998. Natural history and prognostic factors for survival in patients with acquired immune deficiency syndrome (AIDS)-related primary central nervous system lymphoma (PCNSL). Crit. Rev. Oncog. 9:199-208. [PubMed] [Google Scholar]
- 38.Raj, G. V., and K. Khalili. 1995. Transcriptional regulation: lessons from the human neurotropic polyomavirus, JCV. Virology 213:283-291. [DOI] [PubMed] [Google Scholar]
- 39.Rickert, C. H., B. Dockhorn-Dworniczak, R. Simon, and W. Paulus. 1999. Chromosomal imbalances in primary lymphomas of the central nervous system. Am. J. Pathol. 155:1445-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shivapurkar, N., K. Harada, J. Reddy, R. H. Scheuermann, Y. Xu, R. W. McKenna, S. Milchgrup, S. H. Kroft, Z. Feng, and A. F. Gazdar. 2002. Presence of simian virus 40 DNA sequences in human lymphomas. Lancet 359:851-852. [DOI] [PubMed] [Google Scholar]
- 41.Tornatore, C., J. R. Berger, S. A. Houff, B. Curfman, K. Meyers, D. Winfield, and E. O. Major. 1992. Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann. Neurol. 31:454-462. [DOI] [PubMed] [Google Scholar]
- 42.Vilchez, R. A., C. R. Madden, C. A. Kozinetz, S. J. Halvorson, Z. S. White, J. L. Jorgensen, C. J. Finch, and J. S. Butel. 2002. Association between simian virus 40 and non-Hodgkin lymphoma. Lancet 359:817-823. [DOI] [PubMed] [Google Scholar]