Abstract
Hepatocellular carcinoma (HCC) represents one of the most common neoplasms worldwide. Surgical resection and local ablative therapies represent the most frequent first lines therapies adopted when liver transplantation can not be offered or is not immediately accessible. Hepatic resection (HR) is currently considered the most curative strategy, but in the last decade local ablative therapies have started to obtain satisfactory results in term of efficacy and, of them, radiofrequency ablation (RFA) is considered the reference standard. An extensive literature review, from the year 2000, was performed, focusing on results coming from studies that directly compared HR and RFA. Qualities of the studies, characteristics of patients included, and patient survival and recurrence rates were analyzed. Except for three randomized controlled trials (RCT), most studies are affected by uncertain methodological approaches since surgical and ablated patients represent different populations as regards clinical and tumor features that are known to affect prognosis. Unfortunately, even the available RCTs report conflicting results. Until further evidences become available, it seems reasonable to offer RFA to very small HCC (< 2 cm) with no technical contraindications, since in this instance complete necrosis is most likely to be achieved. In larger nodules, namely > 2 cm and especially if > 3 cm, and/or in tumor locations in which ablation is not expected to be effective or safe, surgical removal is to be preferred.
Keywords: Hepatocellular carcinoma, Hepatic resection, Surgical therapy, Ablation techniques, Survival, Liver failure
Core tip: The present review shows the lights and shadows of the comparative literature regarding hepatic resection and radiofrequency ablation for hepatocellular carcinoma. Nineteen studies that directly compared these two therapies were found through an extensive literature review; of them, three randomized controlled trial were available for comparison whereas the remaining studies were represented by retrospective observational studies. Results are often conflicting and further randomized controlled trial are warranted; otherwise, retrospective observational studies should include in their analyses statistical approaches aimed at reduce possible confounding sources at a minimum.
INTRODUCTION
Hepatocellular carcinoma (HCC) represents one of the most common primary malignancies of the liver worldwide, with an incidence that varies in the different geographic areas as a consequence of the regional variations in exposure to risk factors for this tumor[1,2]. The increasing use of surveillance in clinical practice, and the advancements in diagnostic and therapeutic abilities achieved in the last decades have greatly improved patient survival[3-5]. Liver resection and radiofrequency ablation represent the most common first-line therapies adopted when HCC is diagnosed at early stages[6]. Liver resection still remains a mainstay of HCC treatment, and thanks to the considerable improvements in surgical techniques and peri-operative care, the rates of death and complications after liver resection have remarkably decreased over time, giving the procedure added value[7,8]. However, surgery can negatively impact on the already compromised function of cirrhotic livers and, on the other hand, radiofrequency ablation seems safer but its ability to achieve complete and sustained tumor necrosis can be less predictable, and technical feasibility may be sub-optimal. For these reasons, the choice between hepatic resection and radiofrequency ablation for HCC is still a matter of debate. The aim of the present review is to examine the available literature that directly compares these two therapeutic strategies. The qualities and flaws of each included study were highlighted in the attempt to reach conclusions regarding the effectiveness of one treatment with respect to the other and to make suggestions for future research on this debated topic.
LITERATURE STRATEGY SEARCH
A systematic search within the Medline and Embase databases, in the period between 1 January 2000 and 1 December 2012, was performed with the MeSH terms “hepatocellular carcinoma” and (“hepatectomy” or “surgical therapy”) and “ablation techniques”. The keywords “hepatocellular carcinoma”, “partial hepatectomy”, “hepatic resection”, “radiofrequency ablation” or “percutaneous ablation” and “survival” were used to supplement the literature search. The reference lists of retrieved publications were reviewed for other relevant papers. Only articles involving human subjects and that directly compared radiofrequency ablation vs hepatic resection for HCC were considered for the present review. The quality of the selected articles was attributed on the basis of their level of evidence and by means of the Newcastle-Ottawa quality assessment scale for observational studies[9]. The Newcastle-Ottawa Scale (NOS) is a score system that was developed to assess the quality of non-randomized studies, in which a study is judged on three broad perspectives: (1) the selection of the study groups; (2) the comparability of the groups; and (3) the ascertainment of either the exposure or outcome of interest for case-control or cohort studies, respectively.
WHAT GUIDELINES RECOMMEND
Clinical practice guidelines should be evidence-based and should represent the consensus of expert committees. However, it is often very difficult to reach a consensus in the field of HCC, especially as regards the therapeutic approach, given the extremely limited availability of high quality trials. Table 1 reports a summary of the levels of evidence and the strength of recommendations from three published guidelines, namely, the European Association for the Study of the Liver (EASL-EORTC), updated in 2012[10], the American Association for the Study of Liver Diseases (AASLD), updated in 2010[11], and the Asian Pacific Association for the Study of the Liver (APASL), updated in 2010[12]. The EASL and AASLD guidelines are mainly based on the Barcelona Clinic Liver Cancer (BCLC) algorithm for staging and treatment of HCC and represent the most popular treatment algorithms in Western countries[13], however, the BCLC algorithm is not very popular in Asia. There are two important aspects of these guidelines that deserve attention and that are strictly related to each other. The first is represented by the role of the “alternative strategy” of ablation, with respect to resection, and the second is the recommended selection criteria for surgery. It can be immediately noted that radiofrequency ablation is always considered as a strategy alternative, and not competitive, to resection: the EASL recommends ablative therapies “for patients with BCLC 0-A tumours not suitable for surgery”, the AASLD suggests that ablative therapy is “effective for patients who cannot undergo resection” and the APASL recommends local ablation as “an acceptable alternative to resection”. These recommendations mainly derive from indirect comparisons of the results from the two treatments. In brief, modern standards of HCC resection in cirrhotic patients call for a peri-operative mortality < 3% and an expected 5-year survival rate above 60%[10,14-18], whereas, on the other hand, mortality after RFA has been reported to range between 0.9% and 7.9% and the 5-year survival rate to range between 40% and 70%[19-25]. Most of the uncertainties are related to the efficacy of ablation techniques, since response to ablative therapies is strongly influenced by tumor size and location[19,26-29]. In addition, patients allocated to ablation tend to suffer from a more advanced degree of liver dysfunction in comparison to those undergoing surgery, and this feature can negatively impact the observed results. On the other hand, strict selection criteria for hepatic resection can ameliorate patient survival after surgery and this is especially true as regards liver reserve. These two features are obviously related to each other, since at varying criteria for resection, different patients will be shifted to ablation techniques and this represents the second aspect that deserves attention. For example, a selection of candidates for hepatic resection strictly based on the hepatic vein pressure gradient (HVPG), as recommended by the EASL[10], could exclude several patients from surgery, shifting them to RFA. Specifically, HVPG should be < 10 mmHg to allow a safe resection[13], but the evidence for this recommendation is not very strong since it was based on data obtained in a very small cohort studied in the 1990s[30] and surgical techniques have substantially improved since then. Only one recent external validation was conducted on only 39 patients[31], whereas other studies could not confirm the influence of portal hypertension[32]. HVPG measurement can probably help to select surgical candidates, with a very low or null probability of post-operative liver failure, but it probably also excludes patients that can still benefit from surgery and that will be submitted to RFA with a lower chance of cure[32]. Thus, more restrictive criteria for resection result in a better outcome after surgery and a worse outcome after ablation that represents the alternative therapy to be adopted. It can be concluded that such discrepancies, evident even in international guidelines, are attributable to the relatively low level of evidence that can be obtained from the literature, as is pointed out in the following paragraphs.
Table 1.
Proposed evidences and recommendations from international guidelines
| Guidelines | Hepatic resection | Radiofrequency ablation |
| EASL | Resection is the first-line treatment option for patients with solitary tumors and very well-preserved liver function, defined as normal bilirubin with either hepatic venous pressure gradient ≤ 10 mmHg or platelet count ≥ 100000 (evidence 2A; recommendation 1B) | Local ablation with radiofrequency or percutaneous ethanol injection is considered the standard of care for patients with BCLC 0-A tumors not suitable for surgery (evidence 2A; recommendation 1B) |
| EORTC[9] | Additional indications for patients with multifocal tumors meeting Milan criteria (≤ 3 nodules ≤ 3 cm) or with mild portal hypertension not suitable for liver transplantation require prospective comparisons with loco-regional treatments. (evidence 3A; recommendation 2C) | In tumors < 2 cm, BCLC 0, Ethanol injection and radio-frequency ablation achieve complete responses in more than 90% of cases with good long-term outcome [evidence 1(i)A; recommendation 1C] |
| AASLD[10] | Patients who have a single lesion can be offered surgical resection if they are non-cirrhotic or have cirrhosis but still have well preserved liver function, normal bilirubin and hepatic vein pressure gradient < 10 mmHg (recommendation 2) | Local ablation is safe and effective therapy for patients who cannot undergo resection, or as a bridge to transplantation (recommendation 2); Alcohol injection and radiofrequency are equally effective for tumors < 2 cm. However, the necrotic effect of radiofrequency ablation is more predictable in all tumor sizes and its efficacy is clearly superior to that of alcohol injection in larger tumors (recommendation 1) |
| APASL[11] | Liver resection is a first-line curative treatment of solitary or multifocal HCC confined to the liver, anatomically resectable, and with satisfactory liver function reserve (evidence 2B, recommendation B) | Local ablation is an acceptable alternative to resection for small HCC (< 3 cm) in Child-Pugh A cirrhosis (evidence 2B, recommendation B); Local ablation is a first-line treatment of unresectable, small HCC with 3 or fewer nodules in Child-Pugh A or B cirrhosis (evidence 2B, recommendation B) |
Strength of evidence according to study design: Level 1, Randomized controlled clinical trials or meta-analyses of randomized studies; Level 2, Non-randomized controlled clinical trials; Level 3, Case series. Strength of evidence according to end-points: A, Total mortality; B, Cause-specific mortality; C, Carefully assessed quality of life; D, Indirect surrogates. Grading of recommendation: 1, Strong recommendation warranted; 2, Weaker recommendation. Grading of recommendation: A, Further research is very unlikely to change out confidence in the estimate of effect; B, Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; C, Further research is very likely to have an important impact on our confidence in the estimate of effect. BCLC: Barcelona Clinic Liver Cancer; HCC: Hepatocellular carcinoma; EASL: European Association for the Study of the Liver; EORTC: European Organisation For Research And Treatment Of Cancer; AASLD: American Association for the Study of Liver Diseases; APASL: Asian Pacific Association for the Study of the Liver.
COMPARATIVE STUDIES ON RESECTION VS ABLATION
The literature review retrieved 19 studies that directly compared resection and radiofrequency ablation; of them, three RCTs were available for comparison whereas the remaining studies were represented by retrospective observational studies. Randomized controlled studies were reviewed separately from observational studies. As can be noted from Table 2, the NOS scale of observational studies ranged from 5 to 8, none of them reached the maximum quality assessment of 9 and most of them had a quality scale below 8. In fact, the review of these studies showed that for the two treatment arms patients often have significant differences regarding most clinical and tumor variables, that are able to confound results. Thus, stratification for tumor size was attempted in order to reduce to a minimum potential biases resulting from covariate distribution. Differences observed between the two treatment arms were also highlighted. Characteristics of RCTs are reported in Table 3 and of observational studies in Table 4.
Table 2.
Summary of published articles that directly compared hepatic resection and radio-frequency ablation identified through literature search
| Ref. | Study period | Type of study | NOS |
| Feng et al[35] | 2005-2008 | RCT | - |
| Peng et al[36] | 2003-2008 | Retrospective | 7 |
| Wang et al[37] | 2002-2009 | Retrospective | 6 |
| Ruzzenente et al[47] | 1995-2009 | Retrospective | 8 |
| Nishikawa et al[42] | 2004-2010 | Retrospective | 7 |
| Hung et al[38] | 2002-2007 | Retrospective | 7 |
| Takayama et al[39] | 2000-2003 | Retrospective | 5 |
| Huang et al[34] | 2003-2005 | RCT | - |
| Ueno et al[41] | 2000-2005 | Retrospective | 7 |
| Abu-Hilal et al[48] | 1991-2003 | Retrospective | 8 |
| Guglielmi et al[43] | 1996-2006 | Retrospective | 7 |
| Hiraoka et al[40] | 2000-2007 | Retrospective | 7 |
| Hasegawa et al[46] | 2000-2003 | Survey | 6 |
| Lupo et al[45] | 1999-2006 | Retrospective | 8 |
| Chen et al[33] | 1999-2004 | RCT | - |
| Ogihara et al[49] | 1995-2003 | Retrospective | 7 |
| Montorsi et al[50] | 1997-2003 | Retrospective | 6 |
| Hong et al[51] | 1999-2001 | Retrospective | 6 |
| Vivarelli et al[44] | 1998-2002 | Retrospective | 5 |
The Newcastle-Ottawa Score (NOS) scale can range from 5 to 9. RCT: Randomized controlled trials.
Table 3.
Characteristics of randomized controlled studies that compared hepatic resection vs radiofrequency ablation
| Ref. | Liver function | Tumor features | Treatment | Study characteristics and main findings |
| Chen et al[33] | Child-Pugh class AICG-R15 < 30%PLT > 40000/mm3 | Single < 5 cm | HR: 90RFA: 71 | 21% of patients randomized to RFA withdrew their consent. The 1-, 3-, and 4-year overall survival rates after RFA and surgery were 95.8%, 71.4%, 67.9% and 93.3%, 73.4%, 64.0%, respectively. The corresponding DFS rates were 85.9%, 64.1%, 46.4% and 86.6%, 69%, 51.6%, respectively. Statistically, there was no difference. The 5-year rates were not reported |
| Single tumor ≤ 3 cm | HR: 42RFA: 37 | Authors stated that patient survival and DFS did not change in tumors < 3 cm but survival rates and P-values were not provided (only Kaplan-Meier curves were reported) | ||
| Single 3.1-5.0 cm | HR: 48RFA: 34 | Authors stated that patient survival and DFS did not change in tumors between 3.1 and 5.0 cm but survival rates and P-values were not provided (only Kaplan-Meier curves were reported) | ||
| Huang et al[34] | Child-Pugh class A/BICG-R15 < 20%PLT > 50000/mm3 | Single ≤ 5 cm or up to 3 nodules < 3 cm | HR: 115RFA: 115 | Despite randomization, RFA patients had higher prevalence of nodules ≤ 3 cm (P = 0.021). The 3- and 5-year survival rates for the RFA group and the HR group were 69.6%, 54.8% and 92.2%, 75.7%, respectively (P = 0.001). The corresponding RFS rates were 46.1%, 28.7% and 60.9%, 51.3%, respectively (P = 0.017) |
| Single tumor ≤ 3 cm | HR: 45RFA: 57 | The 3- and 5-year survival rates for the RFA group and the HR group were 77.2%, 61.4% and 95.6%, 82.2%, respectively (P = 0.030). Neither DFS nor RFS for this subgroup were provided | ||
| Single 3.1-5.0 cm | HR: 44RFA: 27 | The 3- and 5-year survival rates for the RFA group and the HR group were 66.7%, 51.5% and 95.5%, 72.3%, respectively (P = 0.046). Neither DFS nor RFS for this subgroup were provided | ||
| Multifocal < 3 cm | HR: 26RFA: 31 | The 3- and 5-year survival rates for the RFA group and the HR group were 58.1%, 45.2% and 80.8%, 69.2%, respectively (P = 0.042). Neither DFS nor RFS for this subgroup were provided | ||
| Feng et al[35] | Child-Pugh class A/BICG-R15 < 30%PLT > 50000/mm3 | Up to 2 nodules < 4 cm | HR: 84RFA: 84 | The 1- and 3-year survival rates for HR and RFA groups were 96.0%, 74.8% and 93.1%, 67.2%, respectively (P = 0.342). The corresponding RFS rates were 90.6%, 61.1% and 86.2%, 49.6%, respectively (P = 0.122). Results at 5-year not reported (or not reached). On the basis of this lack of evidence, the authors did not include treatment as a variable in multivariate analysis |
Other inclusion criteria common to all randomized controlled trials (RCTs): no radiological evidence of invasion into the portal/hepatic vein branches, no extra-hepatic metastases, no previous treatment of hepatocellular carcinoma (HCC), patient should be suitable to be treated by surgical resection and radiofrequency ablation. HR: Hepatic resection; RFA: Radiofrequency ablation; DFS: Disease-free survival; PLT: Platelet.
Table 4.
Characteristics of observational studies that compared hepatic resection vs radiofrequency ablation
| Ref. | Liver function | Tumor features | Treatment | Study characteristics and main findings |
| Peng et al[36] | Child-Pugh class A | Single tumor ≤ 2 cm | HR: 74RFA: 71 | RFA patients showed lower prothrombin activity (P = 0.001) and lower platelet count (P = 0.010). Other features were similar between the two groups |
| The 3-, and 5-year survival rates were 87.7% and 71.9%, respectively, after RFA and 70.9% and 62.1% after HR (P = 0.048). The corresponding RFS rates were 65.2% and 59.8% with RFA and 56.1%, and 51.3% after HR (P = 0.548) | ||||
| Wang et al[37] | Child-Pugh class A and B | BCLC early stage | HR: 208RFA: 254 | Patient characteristics were considerably different between the two treatments. RFA patients were significantly older, anti-HCV+, in Child-Pugh class B, with lower platelet count, with smaller and multifocal tumors than HR patients (P = 0.001 in all cases) |
| The 3- and 5-year survival rates were 87.8% and 77.2% for HR, and 73.5% and 57.4% for RFA (P = 0.001). The 3- and 5-year DFS rates were 59.9% and 50.8% for HR and 28.3% and 14.1% for RFA, respectively (P < 0.001) | ||||
| BCLC early stage after PS match | HR: 208RFA: 208 | Patient characteristics were different between the two treatment arms. RFA patients were significantly older, anti-HCV+, in Child-Pugh class B, with lower platelet count, with smaller and multifocal tumors than HR patients (P = 0.001 in all cases). Patient and DFS rates not provided for this subgroup | ||
| Single tumor < 2 cm | HR: 52RFA: 91 | Patient characteristics were different between the two treatment arms. RFA patients were significantly older, anti-HCV+, with lower platelet count than HR patients (P < 0.050). No Child-Pugh stratification was provided | ||
| The 3- and 5-year survival rates were 98% and 91.5% for HR, and 80.3% and 72% for RFA (P = 0.073). The 3- and 5-year DFS rates were 62.1% and 40.7% for HR and 39.8% and 29.3% for RFA, respectively (P = 0.006) | ||||
| Single tumor < 2 cm after PS match | HR: 52RFA: 52 | Patient characteristics seem similar between the two treatments. The 3- and 5-year survival rates were 98% and 91.5% for HR, and 82.8% and 82.8% for RFA, respectively (P = 0.269). The 3- and 5-year DFS rates were 62.1% and 40.7% for HR and 46.8% and 38.0% for RFA (P = 0.031) | ||
| Ruzzenente et al[47] | Child-Pugh class A and B | Up to 3 tumors ≤ 6 cm after PS match | HR: 88RFA: 88 | Patient characteristics seem similar between the two treatments. The 3- and 5-year survival rates were 68.7% and 59.3% for HR, and 50.1% and 27.7% for RFA (P = 0.012). The 3- and 5-year DFS rates were 50.4% and 27.1% for HR and 30.2% and 18.6% for RFA, respectively (P = 0.001) |
| Child-Pugh class A and B | Single tumor < 5 cm | HR: 45RFA: 40 | The 3- and 5-year survival rates were 66.1% and 54.5% for HR, and 63.7% and 43.8% for RFA (P = 0.633). The 3- and 5-year DFS rates were 42.4% and 22.6% for HR and 30.7% and 23.0% for RFA, respectively (P = 0.644). Patient and disease-free survival after HR were significantly superior to RFA, in patients with tumors ≥ 5 cm | |
| Further stratifications lead to very small groups (n < 10) | ||||
| Nishikawa et al[42] | Child-Pugh class A and B | Single tumor ≤ 3 cm | HR: 78RFA: 92 | RFA patients had smaller tumors (P = 0.001) and lower platelet count (P = 0.004) in comparison to HR patients |
| The 5-year overall survival rates after RFA and HR were 63.1% and 74.6%, respectively (P = 0.259). The corresponding RFS rates were 18.0% and 26.0%, respectively (P = 0.324). In the multivariate analysis treatment was not an independent risk factor for overall and RFS | ||||
| Hung et al[38] | Child-Pugh class A and B | Up to 3 tumors ≤ 5 cm | HR: 229RFA: 190 | RFA patients were significantly older, anti-HCV+, with lower albumin and platelet count (P < 0.050) in comparison to HR patients |
| The 3- and 5-year survival rates were 88.2% and 79.3% for HR, and 77.3% and 67.4% for RFA, respectively (P = 0.009). The 3- and 5-year RFS rates were 56.1% and 40.9% for HR and 29.0% and 20.5% for RFA (P = 0.001) | ||||
| Up to 3 tumors ≤ 5 cm after PS match | HR: 84RFA: 84 | Patient characteristics seem similar between the two treatments | ||
| Patient and DFS rates not provided but only reported in Kaplan-Meier graphs. For patient survival no difference was found (P = 0.519); RFS was significantly worse after RFA (P < 0.001) | ||||
| Single tumor < 2 cm | HR: 50RFA: 66 | RFA patients were significantly older, anti-HCV+, with lower albumin and platelet count, higher bilirubin, AST and ICG-R15 and with smaller tumors (P = 0.001) in comparison to HR patients | ||
| The 3- and 5-year survival rates were 91.1% and 84.6% for HR, and 86.5% and 77.8% for RFA, respectively (P = 0.358). The 3- and 5-year RFS rates were 42.6% and 21.8% for HR and 59.5% and 45.2% for RFA (P = 0.104) | ||||
| Takayama et al[39] | Child-Pugh class A and B | Single tumor ≤ 2 cm | HR: 1235RFA: 1315 | Data from the Liver Cancer Study Group of Japan database. Results were reported in the form of brief communication. RFA patients were significantly more frequently in Child-Pugh class B, had higher ICG-R15 and smaller tumor size (P = 0.001 in all cases) in comparison to HR patients |
| The 1- and 2-year survival rates were 98% and 94% for HR, and 99% and 95% for RFA, respectively (P = 0.280). The 1- and 2-year DFS rates were 91% and 70% for HR and 84% and 58% for RFA, respectively (P = 0.001) | ||||
| Multivariate analysis on DFS confirmed alpha-fetoprotein, therapy and Child-Pugh class as independent factors | ||||
| Ueno et al[41] | Child-Pugh class A and B | BCLC early stage | HR: 123RFA: 155 | RFA patients were significantly more frequently in Liver Damage class B or C, had higher ICG-R15, MELD score and smaller tumor size (P = 0.001 in all cases) in comparison to HR patients |
| The 3- and 5-year survival rates were 92% and 80% for HR, and 92% and 63% for RFA, respectively (P = 0.06). The 3- and 5-year DFS rates were 47% and 38% for HR and 36% and 20% for RFA (P = 0.02) | ||||
| Single tumor ≤ 3 cm | HR: 78RFA: 92 | The 3- and 5-year survival rates were 95% and 95% for HR, and 90% and 60% for RFA, respectively (P = 0.01). The 3- and 5-year DFS rates were 56% and 44% for HR and 37% and 11% for RFA (P = 0.02) | ||
| Single tumor 3.1-5.0 cm | HR: 32RFA: 9 | The 3- and 5-year survival rates were 92% and 72% for HR, and 73% and 73% for RFA, respectively (P = 0.15). The 3- and 5-year DFS rates were 33% and 25% for HR and 14% and 14% for RFA (P = 0.12) | ||
| 2 or 3 nodules ≤ 3 cm | HR: 13RFA: 54 | The 3- and 5-year survival rates were 67% and not reached for HR, and 93% and 63% for RFA, respectively (P = 0.002). The 3- and 5-year DFS rates were 29% and not reached for HR and 35% and 22% for RFA (P = 0.59) | ||
| Abu-Hilal et al[48] | Child-Pugh class A and B | Single tumor ≤ 5 cm | HR: 34 | This was a matched analysis for age, sex, tumor size, and Child-Pugh grade |
| RFA: 34 | The 5-year survival was 56% for HR, and 57% for RFA (P = 0.302). The 5-year DFS was 28% for HR and 21% for RFA (P = 0.028) | |||
| Guglielmi et al[43] | Child-Pugh class A and B | Up to 3 tumors ≤ 6 cm | HR: 91RFA: 109 | RFA patients were significantly older, belonged more frequently to Child-Pugh class B and more frequently had multinodular tumors (P = 0.010) in comparison to HR patients |
| The 3- and 5-year survival rates were 64% and 48% for HR, and 42% and 20% for RFA, respectively (P = 0.010). The 3- and 5-year DFS rates were 56% and 27% for HR and 22% and 22% for RFA (P = 0.001) | ||||
| Superiority of HR was confined to patients in Child-Pugh class A. Further stratifications resulted in groups of patients not large enough (n < 10) to obtain realistic comparisons | ||||
| Type of treatment was significantly related to survival and DFS at multivariate analyses | ||||
| Child-Pugh class A | Single tumor ≤ 3 cm | HR: 20RFA: 11 | The 3- and 5-year survival rates were 93% and 71% for HR, and 50% and not reached for RFA, respectively (P = 0.060) | |
| Child-Pugh class A | Single tumor > 3 cm | HR: 33RFA: 23 | The 3- and 5-year survival rates were 64% and 55% for HR, and 63% and 45% for RFA, respectively (P = 0.700) | |
| Hiraoka et al[40] | Child-Pugh class A and B | Single tumor ≤ 3 cm | HR: 59RFA: 105 | RFA patients belonged more frequently to Child-Pugh class B (P = 0.011), more frequently had tumors < 2 cm (P = 0.001), and had worse ICG-R15 (P = 0.026) in comparison to HR patients |
| The 3- and 5-year survival rates were 91.4% and 59.4% for HR, and 87.8% and 59.3% for RFA, respectively (P = NS). The 3- and 5-year DFS rates were 64.3% and 22.4% for HR and 58.7% and 24.6% for RFA (P = NS) | ||||
| No multivariate analysis provided | ||||
| Hasegawa et al[46] | Child-Pugh class A and B | Up to 3 tumors ≤ 3 cm | HR: 2857RFA: 3022 | Data were analyzed together with a population of 1306 patients submitted to percutaneous ethanol injection. RFA patients were significantly older, belonged more frequently to Child-Pugh class B, had lower serum albumin, higher bilirubin, worse ICG-R15 and more frequently had multinodular and smaller tumors (P < 0.001 in all cases) in comparison to HR patients |
| Results were limited to 24 mo. The 1- and 2-year survival rates were 98.3% and 94.5% for HR, and 98.5% and 93.0% for RFA, respectively (P = 0.640) | ||||
| The 1- and 2-year recurrence rates were 17.0% and 35.5% for HR and 26.0% and 55.4% for RFA (P < 0.001) | ||||
| At multivariate analysis, type of treatment did not affect overall survival but affected recurrence rate | ||||
| Lupo et al[45] | Child-Pugh class A and B | Single tumor 3-5 cm | HR: 42RFA: 60 | The groups were similar in terms of median age, Child-Pugh score and tumor size |
| The 3- and 5-year survival rates were 57% and 43% for HR, and 53% and 32% for RFA, respectively (P = 0.824). The 3- and 5-year DFS rates were 35% and 14% for HR and 18% and 0% for RFA (P = 0.283) | ||||
| No multivariate analyses were performed | ||||
| Ogihara et al[49] | Child-Pugh class A and B | Single tumor without size limit | HR: 47RFA: 40 | RFA patients were significantly older, belonged more frequently to Child-Pugh class B and had smaller tumors (P < 0.001 in all cases) in comparison to HR patients |
| The 3- and 5-year survival rates were 65% and 31% for HR, and 58% and 39% for RFA, respectively (P = NS). DFS not provided. No multivariate analysis was provided | ||||
| Child-Pugh class A and B | Single tumor ≤ 5 cm | HR: 18RFA: 26 | In these subgroups, RFA patients were still significantly older and belonged more frequently to Child-Pugh class B (P < 0.050) in comparison to HR patients | |
| The 3- and 5-year survival rates were 64% and 21% for HR, and 53% and 32% for RFA, respectively (P = NS). The 3- and 5-year DFS rates were 37% and 37% for HR and 31% and 23% for RFA (P = NS) | ||||
| Results did not change in single tumors > 5 cm | ||||
| Montorsi et al[50] | Child-Pugh class A and B | Single tumor ≤ 5 cm | HR: 40RFA: 58 | All RFA were performed with laparoscopic approach. RFA patients had significantly worse INR and higher AST (P < 0.050). A trend toward higher bilirubin, lower platelet count and higher ALT was also reported (P < 0.10) |
| The 3- and 4-year survival rates were 73% and 61% for HR, and 61% and 42% for RFA, respectively (P = 0.139). The RFS rates were not reported and only plotted in a Kaplan-Meier curve reporting a P = 0.024. Five-year rates not reported. Multivariate analysis on survival did not include the primary exposure variable (HR vs RFA) | ||||
| Hong et al[51] | Child-Pugh class A | Single tumor ≤ 4 cm | HR: 93RFA: 55 | RFA patients were significantly older (P < 0.001) but the other characteristics reported were not statistically different between the two groups |
| The 1- and 3-year survival rates were 97.9% and 83.9% for HR, and 100% and 72.7% for RFA, respectively (P = 0.24). The 1- and 3-year RFS rates were 75.9% and 54.7% for HR and 74.1% and 40.2% for RFA (P = 0.54). Five-year rates not reported. Results did not change when patients were stratified by AJCC or CLIP stages | ||||
| No multivariate analyses were performed | ||||
| Vivarelli et al[44] | Child-Pugh class A and B | No inclusion criteria specified | HR: 79RFA: 79 | RFA patients belonged more frequently to Child-Pugh class B and more frequently had multinodular tumors (P < 0.001 in both cases) |
| The 1- and 3-year survival rates were 83% and 65% for HR, and 78% and 33% for RFA, respectively (P = 0.002). The 1- and 3-year DFS rates were 79% and 50% for HR and 60% and 20% for RFA (P = 0.001). Five-year rates not reported. No multivariate analyses were performed | ||||
| Child-Pugh class A and B | Single tumor ≤ 3 cm | HR: 21RFA: 22 | The 1- and 3-year survival rates were 89% and 79% for HR, and 89% and 50% for RFA, respectively (P = NS). The 1- and 3-year DFS rates were 84% and 67% for HR and 70% and 34% for RFA (P = NS). Five-year rates not reported | |
| Child-Pugh class A and B | Single tumor > 3 cm | HR: 58RFA: 57 | The 1- and 3-year survival rates were 81% and 59% for HR, and 74% and 24% for RFA, respectively (P = 0.007). The 1- and 3-year DFS rates were 77% and 43% for HR and 56% and 12% for RFA (P = 0.003). Five-year rates not reported. These differences were confirmed when the analyses were confined to Child-Pugh class A patients |
HR: Hepatic resection; RFA: Radiofrequency ablation; RFS: Recurrence-free survival; DFS: disease-free survival; PS: Propensity score; AST: Aspartate aminotransferase; NS: Not significant; BCLC: Barcelona Clinic Liver Cancer; HCV: Hepatitis C virus; AJCC: American Joint Committee on Cancer; CLIP: Cancer of the Liver Italian Program; MELD: Model for end-stage liver disease.
Randomized controlled studies
At December 2012, three RCTs were available for review and all were from Eastern countries[33-35] (Table 3). The first RCT was published by Chen et al[33]. Tumor recurrence rate at 2 years after treatment was used as the primary outcome measure to estimate the sample size of the study. After post-randomization exclusion, the study involved 71 patients submitted to ablation and 90 submitted to resection. The results showed that the 3-year overall survival was 71.4% after ablation and 73.4% after surgery. The corresponding disease-free survival rates were 64.1% and 69.0%, respectively. No statistical difference was observed and no differences were observed when patients were stratified by tumor size (P-values not provided). The authors concluded that the overall and disease-free survivals were the same for patients with a single tumor ≤ 5 cm treated with either ablation or resection; however, ablation showed an advantage over surgical resection in causing less post-treatment complications, less pain, and a shorter in-hospital stay[33].
The second RCT was published by Huang et al[34]. The 5-year overall survival rate after treatment was used as the primary outcome measure to estimate the sample size of the study. After post-randomization exclusion, the study involved 115 patients submitted to ablation and 115 submitted to resection. Results showed that the 5-year overall survival rates was 54.8% after ablation and 75.7% after surgery (P = 0.001). The corresponding recurrence-free survival rates were 28.7% and 51.3%, respectively (P = 0.017). The benefit of resection was maintained when patients were stratified by tumor size and number. The authors concluded that surgical resection may provide better survival and lower tumor recurrence rates than ablation for HCC within Milan criteria[34].
The third, and last, RCT was published by Feng et al[35]. The 3-year overall survival rate after treatment was used as the outcome measure to estimate the sample size of the study. After post-randomization exclusion, the study involved 84 patients submitted to ablation and 84 submitted to resection. Results showed that the 3-year overall survival rates was 67.2% after ablation and 74.8% after surgery (P = 0.342). The corresponding recurrence-free survival rates were 49.6% and 61.1%, respectively (P = 0.122). No stratification for tumor stage was provided. The authors concluded that percutaneous radiofrequency ablation may provide therapeutic effects similar to those of hepatic resection[35].
Thus, the available RCTs report different results and only Huang demonstrated a superiority of hepatic resection over ablation. Even if higher survival rates after resection were also observed in the analyses of Chen and Feng, they did not find a statistically significant superiority of surgery over ablation, leaving the question regarding the best therapeutic approach to be adopted unsolved. It should be noted, however, that the different proportions of HCC beyond the very early stage can, at least in part, explain the conflicting results, since it is known that ablation beyond this stage is less able to achieve complete tumor necrosis, thus biasing the final results[19,28,29]. Hence, a further review of the available observational studies is necessary to obtain more clinical, useful information.
Single tumors less or equal to 2 cm
Four observational retrospective studies analyzed outcomes of resection and ablation in single tumors ≤ 2 cm[36-39] (Table 4) while none of the previous reported RCTs analyzed this specific tumor stage. None of the observational studies reported a convincing comparability between the two treatment arms, and the most frequent differences observed between ablated and surgical patients were that RFA patients were older than surgical patients, had a lower platelet count, belonged more frequently to Child-Pugh class B and were affected by smaller tumors (P < 0.050). Thus, results in terms of both patient survival and recurrence rate can be biased by covariate distribution. Three articles deserve some discussion for different reasons. The first article derives from a multi-institutional database of the Liver Cancer Study Group of Japan involving 2550 patients[39]. In this report, disease-free survival (DFS) was significantly better (P = 0.001) after resection (n = 1235) than after RFA (n = 1315), but patient survival was similar (P = 0.280). Ablated patients were more frequently in Child-Pugh class B, had higher ICG-R15 and smaller tumor size in comparison to resected patients (P = 0.001 in all cases). Therapy and Child-Pugh class were independent prognostic factors of DFS at Cox regression analysis but regression on patient survival was not performed. This report represents the largest series published in the literature that analyzed this specific tumor stage. It can be speculated that patient survival after RFA could be under-estimated, because of more advanced hepatic dysfunction, and, on the contrary, recurrence rate over-estimated because of smaller tumor size. These observations support the hypothesis that patient survival after ablation can be similar to that of surgery for tumors < 2 cm; unfortunately the choice of a composite end-point, as DFS is (in which the event is death or recurrence), does not allow a similar conclusion for just recurrence rate.
In a more recent report by Wang et al[37], the authors tried to handle the different covariate distribution by means of propensity score one-to-one match. In their sub-analysis of 104 matched patients with single tumor < 2 cm (52 patients for each arm), the authors reported that resection and RFA provide similar patient survival (P = 0.296), but that DFS of surgical patients was significantly better than that of RFA patients (P = 0.031). Unfortunately, the match was unconvincing and the inaccuracy of the match procedure is reinforced by the match provided in the same manuscript for patients with tumors < 3 cm, where covariates were still significantly different, after matching, among the two treatment arms (P < 0.001 in some cases)[37]. This work highlights the need for a rigorous statistical approach in the presence of significant covariate differences; without such an approach, the results can remain difficult to interpret with some degree of certainty.
The third report was published by Peng[36] in 2012, and involved 145 patients, submitted to resection, or ablation, for single tumor ≤ 2 cm. The authors found that overall survival was better after RFA (P = 0.048) but that recurrence-free survival (RFS) was unaffected (P = 0.548). The results are intriguing since, when looking at the baseline characteristics, the two groups were quite similar as regards clinical and demographical covariates, except for lower prothrombin time and platelet count in the RFA arm. Thus, supposing an effect of worse liver function on survival, this would have to be shown in patients undergoing RFA, returning to an under-estimation of survival after ablation. Multivariate regression analyses showed that treatment allocation was the only significant prognostic factor for overall survival (P = 0.046). If a conclusion, regarding comparative analyses in this HCC stage, is to be drawn, it can be said that there is some evidence that for single nodules, not larger than 2 cm, RFA can provide survival similar to that of resection[24]. An increased recurrence rate, however, has to be expected after RFA even if the tumor is small but this could theoretically be the subject of re-treatment, justifying comparable survivals. For very early HCC, dedicated RCTs are warranted.
Single tumors less than or equal to 3 cm
There is greater experience published in the literature when this size threshold was selected as an inclusion criterion. Overall, seven studies were found to analyze ablation vs resection in single tumors ≤ 3 cm, or that included a sub-analysis in this specific tumor stage (Tables 3, 4)[33,34,40-44]. Two of these studies were the previously cited RCTs by Chen et al[33] and Huang et al[34], which contained a sub-analysis for this specific tumor stage. In the RCT by Chen et al[33], the authors stated that both patient survival and DFS did not change in single tumors < 3 cm, but, unfortunately, both survival rates and P-values were not provided. The RCT by Huang et al[34] reported a survival advantage of surgery: in the subgroups of 45 resected patients vs 57 ablated patients with a solitary nodule ≤ 3 cm, the 5-year survival after surgery was 82.2%, significantly higher than the 61.4% after RFA of (P = 0.030). Disease-free or recurrence-free survivals were not analyzed. One limitation is represented by the fact that covariate distribution among the two treatment arms was not provided for these specific subgroups of patients; however, since in the whole study population tumor size was the only variable that proved to be slightly different among the two groups, this sub-analysis seems quite realistic and is, at present, the most robust evidence of the superiority of one treatment (surgery) over the competing one (ablation)[34].
Similar comments regarding covariate distribution, made for single tumors < 2 cm, can be repeated for analyses on single tumors < 3 cm. Of the five retrospective studies found, two series deserve particular discussion. In 2008, Hiraoka published results from a population of 59 surgical and 105 RFA patients: no significant differences were found in terms of both patient survival and DFS[40]. However, the magnitude of the differences observed between the two treatment arms, in terms of Child-Pugh class, ICG-R15, serum albumin, bilirubin, and tumor size that were all in favor of resection, was so large that the comparison was evidently unrealistic. Furthermore, the authors did not provide an inferential analysis, leaving the doubt unsolved[40]. In 2009, Ueno et al[41] published a report from the Kagoshima Liver Cancer Study Group reporting that patients with a single nodule ≤ 3 cm achieved a 5-year survival of 95% after resection (n = 78), significantly higher than that of 60% after RFA (n = 92; P = 0.010), but 75.6% of the resected patients had a Liver damage A whereas 66.3% of ablated patients had a Liver damage B or C (P = 0.001). Stratification of survival for Liver damage returned to non-significant differences in terms of both patient and disease-free survivals and these results did not help clarify, with a convincing degree of evidence, the real superiority of resection over ablation[41]. The remaining studies report results on very small subgroups, often less than 10 patients[42-44], or suffered from wrongful comparison[37], making it hard to consider findings to provide enough degree of evidence.
Single tumors 3-5 cm
Four articles were identified that analyzed comparative results of surgery and ablation in single nodules between 3 and 5 cm or that included a sub-analysis in this specific tumor stage (Tables 3 and 4)[33,34,41,45]. Two of these studies were, again, the RCT by Chen et al[33] and the one by Huang et al[34], which contained a sub-analysis for this specific tumor stage. In the RCT by Chen et al[33], the authors stated that both patient survival and DFS did not change between treatment arms but survival rates and p-values were again not provided. Huang’s results reported a 5-year survival after surgery of 72.3% vs 51.5% after ablation (P = 0.046); neither DFS nor RFS were provided (Table 3)[34]. Thus, with the limitations of subgroup analyses, the available RCTs reported a limited difference between surgery and ablation for single nodules between 3 and 5 cm. When observational studies were analyzed, the findings became very difficult to interpret. In a subgroup analysis by Ueno et al[41] (resection: 32 patients; RFA: 9 patients), no differences were found in terms of either patient survival (5-year rate after resection: 72%; ablation: 73%; P = 0.15) or DFS (5-year rate after resection: 25%; ablation: 14%; P = 0.15) but, as can be immediately noted, the sample size was very small. Another retrospective study published by Lupo et al[45] reported that resection and ablation provide very similar results. In particular, the 5-year survival was 43% after resection (n = 42) and 32% after ablation (n = 60; P = 0.824), and the corresponding DFS rates were 14% and 0% (P = 0.283). Thus, it must be noted that resection repeatedly leads to better patient survival and recurrence-rate, but the inability to detect a statistical difference between the two treatments leaves the question of the superiority of surgery unsolved. It could be speculated that it is paradoxical for ablation to be inferior to resection for nodules < 3 cm and equivalent for larger tumors, since the ability of RFA to achieve tumor necrosis decreases with the increase in tumor size[19,28,29,46]. Thus, for this single HCC 3-5 cm, it can be said that the literature consistently reports higher patient and disease-free survival rates that do not achieve statistical significance likely only for the small sample size of study populations. This specific tumor stage also probably deserves dedicated studies.
Multiple tumors
The presence of multiple tumors, at diagnostic evaluation prior to treatment, represents the most frequent indication for radiofrequency ablation. Except for the three RCTs and the studies conducted on solitary tumors, multifocal tumor prevalence was almost always higher in ablated patients in comparison to surgical ones[37,43,44,46]. Only two studies reported a subgroup analysis on two or three nodules less than 3 cm, thus within BCLC early stage, excluding single nodules[34,41]. The RCT by Huang reported a survival advantage of surgery (P = 0.042): in the subgroups of 26 resected patients vs 31 ablated patients with a solitary nodule ≤ 3 cm, the 5-year survival after surgery was 69.2%, significantly higher than the 45.2% after RFA of[34]. Disease-free or recurrence-free survivals were not analyzed. In the report by Ueno et al[41], the 5-year survival was not reached for surgical patients (n = 13) and the 3-year survival was in favor of RFA (n = 54; P = 0.002), while DFS was similar (P = 0.590). The difficulty to obtain a comparison within this stage was highlighted by the sub-analysis by Guglielmi et al[43] who tried to stratify for Child-Pugh class 11 ablated patients (6 in Child-Pugh class A) vs 7 resected patients (all belonging to Child-Pugh class A) without obtaining any reliable result. For multiple tumors, the current comparative literature leaves the impression that the prognosis will be relatively lower despite the treatment adopted.
Other studies
Five studies remain to be briefly discussed[47-51]. The reports from Ruzzenente et al[47], in 2012, and from Abu-Hilal et al[48], in 2008, are examples of the attempt to account for confounding variables through matching. The first study used a propensity score match to select patients, submitted to surgery or RFA, having similar covariate distributions[47], and the second used an “a-priori” match based on age, sex, tumor size and Child-Pugh grade[48]. Both studies included tumors larger than 2 cm in both arms and reported an advantage of surgery in determining DFS over ablation but not in terms of patient survival that was similar for single tumors < 5 cm. Of note, the study by Abu-Hilal included only 34 patients for each arm and of the one by Ruzzenente included Child-Pugh B patients. The remaining articles reported results from comparative analyses without tumor size limit[49], or with large (up to 5 cm) size limit, but unfortunately they lack inference analyses[50,51].
DISCUSSION
The present review shows the lights and shadows of the comparative literature regarding hepatic resection and radiofrequency ablation for HCC. It is evident that most studies are affected by questionable methodological approaches since surgical patients and ablated patients represent patient populations that appear quite different as regards clinical and tumor features that are known to affect prognosis. Despite the inconclusive results and the interest in understanding which treatment strategy is best, it is worthwhile pointing out that the situations in which surgery and ablation would be both really equally feasible, and in which they could thus truly compete, occur in less than half of the cases seen in daily clinical practice. In fact, most studies did not report how many patients were excluded from either surgery or ablation, because of the presence of absolute or relative contraindications to one or the other treatment, which might differ in the case of one or the other therapy (thus these patients were most likely offered the alternative therapy). Patients might not be considered suitable for surgery because of liver dysfunction and/or portal hypertension, according to the individual center’s strategy, as well as the presence of comorbidities or advanced age contraindicating general anesthesia. Some clinical examples can be found in Figures 1 and 2. In some cases, surgery might not be considered because of the hepatic location of the tumor, which would require very extensive parenchymal sacrifice. Conversely, a subcapsular anterior location exposes the patient to a higher risk of bleeding and/or peritoneal seeding[52], unless a direct puncture of the tumor could be avoided[53], which is however not always possible. Moreover, complete necrosis of lesions close to the gallbladder is less often possible to achieved because of the potential risk of gallbladder wall damage[26]. Similarly, complete necrosis of lesions abutting the diaphragm may not be possible[27]. Finally, patients with compromised prothrombin time (prolonged International Normalized Radio) are invariably excluded from surgery because this alteration indicates liver dysfunction; similarly, a very low platelet count (< 50.000) is often also considered a contraindication to surgery since it indicates portal hypertension. Such patients should therefore be treated with ablation, as the first alternative therapy, but a clotting impairment might also contraindicate percutaneous ablation or at least increase the risk of adverse events, despite the possibility of preliminary transfusions. All these different variables affecting the choice between resection and ablation most likely justify the difference in the clinical covariates found in the various non randomized studies, as commented above, leading to rather heterogeneous patient populations. This is in keeping with the hypothesis that patients were not randomly allocated to one or the other treatment, but following clear preferences, so that in each case either surgery or ablation was specifically preferred on the basis of clinical variables. Only in rather a few remaining cases of early HCC within the Milan criteria might hepatic resection and radiofrequency ablation be considered truly competitive, and no definitive evidence exists strongly favoring one or the other technique.
Figure 1.
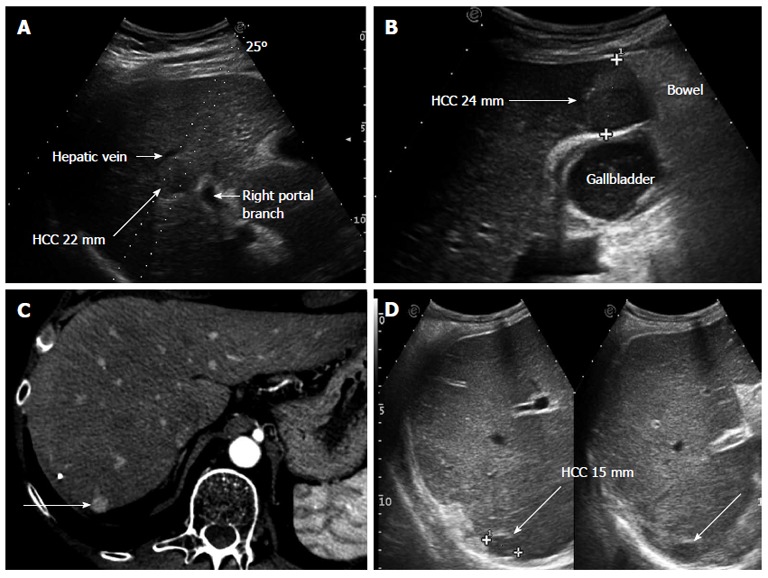
Clinical cases in which performing hepatic resection or radiofrequency ablation had to be decided. A: Small hepatocellular carcinoma (HCC), 22 mm in diameter, located centrally in the right liver lobe in a patient with MELD 10 and clinical signs of portal hypertension. Surgery would have required a right hepatectomy, thus, radiofrequency ablation was preferred even if a reduced rate of complete necrosis could be expected due to the possible heat sink effect of the nearby large vessels; B: The tumor is located sub-capsular, close to the bowel loops and in strict contact with the gallbladder, implying various technical contraindications to percutaneous ablation. Open surgery was the strategy adopted; C: The tumor (long arrow), shown in the arterial phase of contrast enhancement at computed tomography scan, is located sub-capsular at the liver dome; D: Ultrasonography confirms the tumor (long arrow) to lie very deep and without a safe needle track; in fact, these images are taken in deep inspiration, the lesion being hardly visible during normal breathing. The location was considered to contraindicate percutaneous ablation and surgery was performed.
Figure 2.
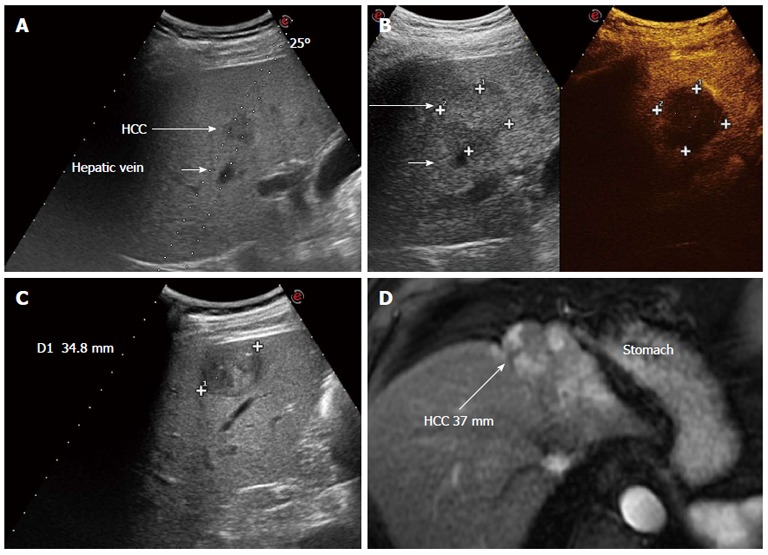
Clinical cases in which performing hepatic resection or radiofrequency ablation had to be decided. A: Ultrasonography through a right inter-costal scan shows a very early hepatocellular carcinoma (HCC) in segment 5 that can be reached with a safe needle track for thermal ablation. Given the small size and easy access, radiofrequency ablation was carried out; B: Post treatment assessment with contrast enhanced ultrasound shows a necrotic devascularized area (34 mm × 35 mm) that includes the tumor with a safety margin > 5 mm; C: Superficial HCC of 35 mm in hepatitis B virus related cirrhosis with preserved liver function. This lesion could be treated by either ablation or resection, but resection is preferable given the superficial location in segment 5 and the size > 3 cm; D: Tumor lesion partially treated by a previous trans-catheter arterial chemoembolization performed in another hospital, in a sub-capsular location close to the stomach. The theoretical path for radiofrequency ablation would lead the needle to puncture the tumor directly and thermal ablation would imply a risk of heat damage to the stomach wall. Laparoscopic resection was the strategy adopted. The long arrow indicates the HCC after treatment.
However, based on the results reported and commented on above, we can conclude that, until further studies become available, it seems reasonable to offer radiofrequency ablation to very small HCC (< 2 cm) which present an easy access, with no technical contraindications, since in this instance complete necrosis, including the desired safety margin, is most likely to be achieved. At variance, in larger nodules, namely > 2 cm and especially if > 3 cm, and/or in tumor locations in which ablation is not expected to be effective or safe (which often correspond to subcapsular locations, which instead make atypical resections possible), surgical removal is to be preferred in our opinion. For future explorative research, it can be suggested that: (1) intention-to-treat analysis should be included in the studies; (2) further RCTs are warranted, especially for single tumors < 2 cm in which ablation can achieve a sustained pathological response; (3) retrospective observational studies should include in their analyses an inference approach that includes the primary exposure variable (that is resection vs ablation) regardless of its statistical difference at univariate analysis; and (4) retrospective observational studies should include stratification for tumor size and liver degree dysfunction together with an attempt at matching, as propensity score can provide.
Footnotes
Supported by A speaker fee from Siemens, research contracts with Esaote; and advisory board and speaker fee from Bayer to Fabio Piscaglia
P- Reviewers Carrilho FJ, Streba CT S- Editor Wen LL L- Editor A E- Editor Zhang DN
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka M, Katayama F, Kato H, Tanaka H, Wang J, Qiao YL, Inoue M. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol. 2011;21:401–416. doi: 10.2188/jea.JE20100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davila JA, Kramer JR, Duan Z, Richardson PA, Tyson GL, Sada YH, Kanwal F, El-Serag HB. Referral and receipt of treatment for hepatocellular carcinoma in United States veterans: effect of patient and nonpatient factors. Hepatology. 2013;57:1858–1868. doi: 10.1002/hep.26287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sangiovanni A, Del Ninno E, Fasani P, De Fazio C, Ronchi G, Romeo R, Morabito A, De Franchis R, Colombo M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology. 2004;126:1005–1014. doi: 10.1053/j.gastro.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 5.Han KH, Kim do Y, Park JY, Ahn SH, Kim J, Kim SU, Kim JK, Lee KS, Chon CY. Survival of Hepatocellular Carcinoma Patients May be Improved in Surveillance Interval not More Than 6 Months Compared With More Than 6 Months: A 15-Year Prospective Study. J Clin Gastroenterol. 2013;47:538–544. doi: 10.1097/MCG.0b013e3182755c13. [DOI] [PubMed] [Google Scholar]
- 6.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 7.Kamiyama T, Nakanishi K, Yokoo H, Kamachi H, Tahara M, Yamashita K, Taniguchi M, Shimamura T, Matsushita M, Todo S. Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211:443–449. doi: 10.1016/j.jamcollsurg.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Cucchetti A, Zanello M, Cescon M, Ercolani G, Del Gaudio M, Ravaioli M, Grazi GL, Pinna AD. Improved diagnostic imaging and interventional therapies prolong survival after resection for hepatocellular carcinoma in cirrhosis: the university of bologna experience over 10 years. Ann Surg Oncol. 2011;18:1630–1637. doi: 10.1245/s10434-010-1463-8. [DOI] [PubMed] [Google Scholar]
- 9.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available from: http: //www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Google Scholar]
- 10.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Omata M, Lesmana LA, Tateishi R, Chen PJ, Lin SM, Yoshida H, Kudo M, Lee JM, Choi BI, Poon RT, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4:439–474. doi: 10.1007/s12072-010-9165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 14.Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, Llovet JM, Schwartz ME. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602–611. doi: 10.1097/00000658-200211000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli G, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543–1554. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]
- 17.Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- 18.Cucchetti A, Piscaglia F, Cescon M, Ercolani G, Terzi E, Bolondi L, Zanello M, Pinna AD. Conditional survival after hepatic resection for hepatocellular carcinoma in cirrhotic patients. Clin Cancer Res. 2012;18:4397–4405. doi: 10.1158/1078-0432.CCR-11-2663. [DOI] [PubMed] [Google Scholar]
- 19.Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 20.Omata M, Tateishi R, Yoshida H, Shiina S. Treatment of hepatocellular carcinoma by percutaneous tumor ablation methods: Ethanol injection therapy and radiofrequency ablation. Gastroenterology. 2004;127:S159–S166. doi: 10.1053/j.gastro.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 21.Machi J, Bueno RS, Wong LL. Long-term follow-up outcome of patients undergoing radiofrequency ablation for unresectable hepatocellular carcinoma. World J Surg. 2005;29:1364–1373. doi: 10.1007/s00268-005-7829-6. [DOI] [PubMed] [Google Scholar]
- 22.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 23.Cabassa P, Donato F, Simeone F, Grazioli L, Romanini L. Radiofrequency ablation of hepatocellular carcinoma: long-term experience with expandable needle electrodes. AJR Am J Roentgenol. 2006;186:S316–S321. doi: 10.2214/AJR.05.0243. [DOI] [PubMed] [Google Scholar]
- 24.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 25.Yan K, Chen MH, Yang W, Wang YB, Gao W, Hao CY, Xing BC, Huang XF. Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol. 2008;67:336–347. doi: 10.1016/j.ejrad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Kim SW, Rhim H, Park M, Kim H, Kim YS, Choi D, Lim HK. Percutaneous radiofrequency ablation of hepatocellular carcinomas adjacent to the gallbladder with internally cooled electrodes: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009;10:366–376. doi: 10.3348/kjr.2009.10.4.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang TW, Rhim H, Kim EY, Kim YS, Choi D, Lee WJ, Lim HK. Percutaneous radiofrequency ablation for the hepatocellular carcinoma abutting the diaphragm: assessment of safety and therapeutic efficacy. Korean J Radiol. 2009;10:34–42. doi: 10.3348/kjr.2009.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262:43–58. doi: 10.1148/radiol.11110144. [DOI] [PubMed] [Google Scholar]
- 29.Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, Tong MJ, Amado RG, Busuttil RW. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954–960. doi: 10.1148/radiol.2343040153. [DOI] [PubMed] [Google Scholar]
- 30.Bruix J, Castells A, Bosch J, Feu F, Fuster J, Garcia-Pagan JC, Visa J, Bru C, Rodés J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 31.Stremitzer S, Tamandl D, Kaczirek K, Maresch J, Abbasov B, Payer BA, Ferlitsch A, Gruenberger T. Value of hepatic venous pressure gradient measurement before liver resection for hepatocellular carcinoma. Br J Surg. 2011;98:1752–1758. doi: 10.1002/bjs.7672. [DOI] [PubMed] [Google Scholar]
- 32.Cucchetti A, Cescon M, Trevisani F, Pinna AD. Current concepts in hepatic resection for hepatocellular carcinoma in cirrhotic patients. World J Gastroenterol. 2012;18:6398–6408. doi: 10.3748/wjg.v18.i44.6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252:903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- 35.Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, Dong J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57:794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, Chen MS. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262:1022–1033. doi: 10.1148/radiol.11110817. [DOI] [PubMed] [Google Scholar]
- 37.Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412–418. doi: 10.1016/j.jhep.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Hung HH, Chiou YY, Hsia CY, Su CW, Chou YH, Chiang JH, Kao WY, Huo TI, Huang YH, Su YH, et al. Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. Clin Gastroenterol Hepatol. 2011;9:79–86. doi: 10.1016/j.cgh.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 39.Takayama T, Makuuchi M, Hasegawa K. Single HCC smaller than 2 cm: surgery or ablation?: surgeon’s perspective. J Hepatobiliary Pancreat Sci. 2010;17:422–424. doi: 10.1007/s00534-009-0239-7. [DOI] [PubMed] [Google Scholar]
- 40.Hiraoka A, Horiike N, Yamashita Y, Koizumi Y, Doi K, Yamamoto Y, Hasebe A, Ichikawa S, Yano M, Miyamoto Y, et al. Efficacy of radiofrequency ablation therapy compared to surgical resection in 164 patients in Japan with single hepatocellular carcinoma smaller than 3 cm, along with report of complications. Hepatogastroenterology. 2008;55:2171–2174. [PubMed] [Google Scholar]
- 41.Ueno S, Sakoda M, Kubo F, Hiwatashi K, Tateno T, Baba Y, Hasegawa S, Tsubouchi H. Surgical resection versus radiofrequency ablation for small hepatocellular carcinomas within the Milan criteria. J Hepatobiliary Pancreat Surg. 2009;16:359–366. doi: 10.1007/s00534-009-0069-7. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa H, Inuzuka T, Takeda H, Nakajima J, Matsuda F, Sakamoto A, Henmi S, Hatamaru K, Ishikawa T, Saito S, et al. Comparison of percutaneous radiofrequency thermal ablation and surgical resection for small hepatocellular carcinoma. BMC Gastroenterol. 2011;11:143. doi: 10.1186/1471-230X-11-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guglielmi A, Ruzzenente A, Valdegamberi A, Pachera S, Campagnaro T, D’Onofrio M, Martone E, Nicoli P, Iacono C. Radiofrequency ablation versus surgical resection for the treatment of hepatocellular carcinoma in cirrhosis. J Gastrointest Surg. 2008;12:192–198. doi: 10.1007/s11605-007-0392-8. [DOI] [PubMed] [Google Scholar]
- 44.Vivarelli M, Guglielmi A, Ruzzenente A, Cucchetti A, Bellusci R, Cordiano C, Cavallari A. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240:102–107. doi: 10.1097/01.sla.0000129672.51886.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lupo L, Panzera P, Giannelli G, Memeo M, Gentile A, Memeo V. Single hepatocellular carcinoma ranging from 3 to 5 cm: radiofrequency ablation or resection? HPB (Oxford) 2007;9:429–434. doi: 10.1080/13651820701713758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasegawa K, Makuuchi M, Takayama T, Kokudo N, Arii S, Okazaki M, Okita K, Omata M, Kudo M, Kojiro M, et al. Surgical resection vs. percutaneous ablation for hepatocellular carcinoma: a preliminary report of the Japanese nationwide survey. J Hepatol. 2008;49:589–594. doi: 10.1016/j.jhep.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 47.Ruzzenente A, Guglielmi A, Sandri M, Campagnaro T, Valdegamberi A, Conci S, Bagante F, Turcato G, D’Onofrio M, Iacono C. Surgical resection versus local ablation for HCC on cirrhosis: results from a propensity case-matched study. J Gastrointest Surg. 2012;16:301–311; discussion 311. doi: 10.1007/s11605-011-1745-x. [DOI] [PubMed] [Google Scholar]
- 48.Abu-Hilal M, Primrose JN, Casaril A, McPhail MJ, Pearce NW, Nicoli N. Surgical resection versus radiofrequency ablation in the treatment of small unifocal hepatocellular carcinoma. J Gastrointest Surg. 2008;12:1521–1526. doi: 10.1007/s11605-008-0553-4. [DOI] [PubMed] [Google Scholar]
- 49.Ogihara M, Wong LL, Machi J. Radiofrequency ablation versus surgical resection for single nodule hepatocellular carcinoma: long-term outcomes. HPB (Oxford) 2005;7:214–221. doi: 10.1080/13651820510028846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montorsi M, Santambrogio R, Bianchi P, Donadon M, Moroni E, Spinelli A, Costa M. Survival and recurrences after hepatic resection or radiofrequency for hepatocellular carcinoma in cirrhotic patients: a multivariate analysis. J Gastrointest Surg. 2005;9:62–67; discussion 67-68. doi: 10.1016/j.gassur.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 51.Hong SN, Lee SY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC, Rhee JC, Choi D, Lim HK, et al. Comparing the outcomes of radiofrequency ablation and surgery in patients with a single small hepatocellular carcinoma and well-preserved hepatic function. J Clin Gastroenterol. 2005;39:247–252. doi: 10.1097/01.mcg.0000152746.72149.31. [DOI] [PubMed] [Google Scholar]
- 52.Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124–1129. doi: 10.1053/jhep.2001.24233. [DOI] [PubMed] [Google Scholar]
- 53.Bolondi L, Gaiani S, Celli N, Piscaglia F. Tumor dissemination after radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2001;34:608; author reply 610–611. doi: 10.1002/hep.510340325. [DOI] [PubMed] [Google Scholar]


