Abstract
AIM: To study the effects of low-dose amitriptyline (AMT) on gastrointestinal function and brain-gut peptides in healthy Chinese volunteers.
METHODS: This was a double-blind, randomised, placebo-controlled, two-period cross-over trial. Twenty-eight healthy volunteers were randomised and administered 1-wk treatments of AMT (12.5 mg tid) or placebo. Before and during the final two days of treatment, gastric emptying, proximal gastric accommodation and visceral sensitivity were measured by drinking-ultrasonography test; the orocecal transit time (OCTT) was measured by lactulose hydrogen breath test, and fasting blood was collected. Plasma levels of ghrelin, motilin and neuropeptide Y (NPY) were measured by enzyme-linked immunosorbent assay kits.
RESULTS: AMT slowed the OCTT (109.2 ± 29.68 min vs 96.61 ± 23.9 min, P = 0.004) but did not affect liquid gastric emptying and had no effect on proximal gastric accommodation. AMT resulted in decreases in the visual analogue scale (VAS) for difficulty in drinking 600 and 800 mL of water (3.57 ± 0.94 vs 2.98 ± 0.85, 5.57 ± 0.82 vs 4.57 ± 0.98, P < 0.01 for both), although it had no significant effect on the VAS for difficulty in drinking 200 mL and 400 mL of water. AMT significantly increased the plasma ghrelin level (442.87 ± 176.79 pg/mL vs 526.87 ± 158.44 pg/mL, P = 0.04) and the neuropeptide-Y level (890.15 ± 131.46 pg/mL vs 965.64 ± 165.63 pg/mL, P = 0.03), whereas it had no effect on the MTL level.
CONCLUSION: Low-dose AMT could slow OCTT, make the stomach less sensitive and increase the plasma levels of ghrelin and NPY. Thus, we recommend the use of low-dose AMT for functional gastrointestinal disorders.
Keywords: Amitriptyline, Orocecal transit time, Visceral hypersensitivity, Gastric emptying, Brain-gut peptides
Core tip: Low-dose amitriptyline has been used to treat functional gastrointestinal disorders for many years, but the precise mechanism is still not clear. Brain-gut peptides, such as motilin, ghrelin and neuropeptide Y, may regulate gastrointestinal functions. However, evidence indicating the possible effects of amitriptyline on the levels of brain-gut peptides in healthy Chinese volunteers is limited. In this study, we conclude that low-dose amitriptyline can slow orocecal transit time, make the stomach less sensitive and increase the plasma levels of ghrelin and neuropeptide Y. Thus, we recommend the use of low-dose amitriptyline for functional gastrointestinal disorders.
INTRODUCTION
Functional dyspepsia (FD) and irritable bowel syndrome (IBS) are the most common functional gastrointestinal disorders (FGIDs). The aetiology of FGIDs is unclear, and treatment options are limited[1,2]. Low-dose amitriptyline (AMT) is a tricyclic antidepressant that has been used to treat FGIDs for many years[3]; however, the exact mechanism of action is not clear.
Brain-gut peptides, including motilin (MTL), ghrelin, neuropeptide Y (NPY) and so on, also known as peptide hormones, can be found in the cerebral nervous system, enteric nervous system and endocrine cells in the gastrointestinal tract. Brain-gut peptides, can be neuropeptides and neuroendocrine and paracrine substances, regulate the secretory and motor functions of the gastrointestinal tract. MTL can reportedly accelerate gastric emptying and reduce the proximal gastric volume in patients with FD[4,5]. Ghrelin, the closest family member of MTL, was reported to be abnormal in FD[6]. NPY is a 36 amino-acid peptide in the central and peripheral nervous systems that can inhibit gastric emptying and stimulate colonic transit[7]. However, as far as we know, evidence indicating the possible effects of AMT on the levels of brain-gut peptides in healthy Chinese volunteers is limited.
We hypothesised that low-dose AMT is beneficial for FGIDs because of the changes in the gastrointestinal sensor, motor function and plasma levels of brain-gut peptides. Therefore, we aimed to explore the effects of low-dose AMT on liquid gastric emptying, proximal gastric accommodation, proximal gastric sensitivity, orocecal transit time (OCTT) and the plasma levels of MTL, ghrelin and NPY in healthy Chinese volunteers.
MATERIALS AND METHODS
Methods and drugs
This study was a randomised, double-blind, placebo-controlled, two-period cross-over trial in healthy Chinese volunteers (Clinical trial number: ChiCTR-TTRCC-12001967), which was approved by the ethics committee of the hospital. Written informed consent was obtained from healthy volunteers, which conformed to the Declaration of Helsinki.
Twenty-eight healthy volunteers were randomised to the two therapies: group A was treated for 1 wk with 12.5 mg AMT tid and then with placebo, while group B was treated with the opposite sequence. There was a 2-wk washout phase, followed by a crossover to the alternate treatment (Figure 1). AMT hydrochloride tablets were purchased from HuNan DongTing Pharmaceutical Co. Ltd. of China (batch number: B110824). The placebo was supplied by ShenZhen WanHe Pharmaceutical Co. Ltd. of China. AMT and placebo tablets were similar, and the strength of each tablet was 25 mg. The investigators and patients were blinded to the treatment. The results were analysed by the investigators, and the original randomisation scheme was released after all of the analyses were performed.
Figure 1.
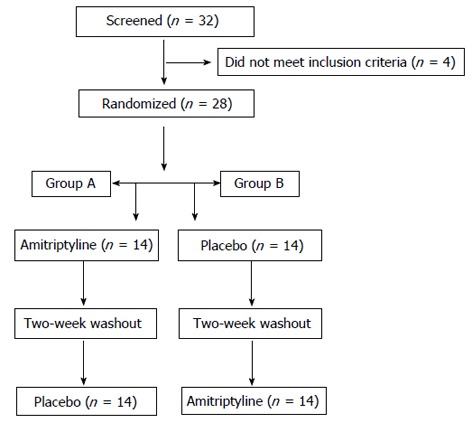
Consort diagram.
Healthy volunteers
The exclusion criteria of healthy volunteers included: (1) history of FGIDs (in line with the definition of the Rome III criteria) that may affect gastrointestinal motility; hypersensitivity or allergy to any tricyclic drug; (2) history of gastrointestinal surgery and psychiatric illness; (3) pregnancy or breast feeding; (4) use of medications that may affect gastrointestinal motor function (e.g., prokinetics and anti-spasmotic agents) or the effect of AMT; (5) concomitant therapy with a monoamine oxidase inhibitor, history of urinary retention, known glaucoma, history of seizures and thyroid or liver dysfunction; and (6) participation in another clinical trial during the last two weeks.
Endpoints of the study
All healthy volunteers completed the Hamilton Anxiety and Depression Rating Scale before the treatment; a score less than 7 was defined as no anxiety or depression[8,9]. During the two days before therapy and the final two days of treatment, we assessed the following endpoints: (1) liquid gastric emptying, proximal gastric relaxation and visceral hypersensitivity by drinking- ultrasonography test; (2) OCTT by lactulose hydrogen breath test; and (3) plasma MTL, ghrelin and NPY levels by ELISA.
Drinking-ultrasonography test
The drinking-ultrasonography test was performed according to the method of Kato et al[10]. After an overnight fast, healthy volunteers ingested 200 mL of water (approximately 28 °C) in 2 min for a total of four times with 2-min intervals. The subjects were in the supine position and ingested water through a straw. The emptying periods were calculated to be 5 and 10 min (by measuring the time) after drinking the total 800 mL of water. All of these examinations were performed by a single ultrasonography technician using a Philips IU22 ultrasound scanner (Philips Medical Systems, Bothell, Washington) and a Convex-type 5-20 MHz probe. The spleen served as an echo window, and the cross-section of the proximal stomach was measured via the 10th inter-costal space. The mucosal surface of the gastric lumen was traced from images acquired before the test at each 2 min interval after water consumption and 5 and 10 min after the end of the water consumption. The cross-sectional area was also calculated. Frozen images were saved on a hard disk. Before the test and every time after ingestion of water, abdominal symptoms were self-evaluated and recorded on a questionnaire using a visual analogue scale from 0 to 10 to investigate the difficulty (such as abdominal fullness) in drinking water.
Lactulose hydrogen breath test for OCTT
Subjects were placed on a low fibre diet 3 d before the test. After a 12-h overnight fast, two end-expiratory breath H2 samples were collected as base values using a HHBT-01 breath hydrogen detector (Hydeway, China). After the subjects ingested 15 mL of lactulose syrup containing 10 g of lactulose, exhaled H2 was recorded every 15 min for a total of three hours. OCTT was defined as the duration from the moment of lactulose administration to the moment when exhaled H2 was increased over 12 ppm from the baseline[11].
Plasma ghrelin, MTL and NPY levels
After twelve-hours of fasting, the blood samples were collected and centrifuged at 3000 g for 10 min. Plasma samples were collected and stored at -70 °C until the procedure. We measured plasma levels of ghrelin, MTL and NPY using commercial ELISA kits (Shanghai Bluegene Biotech Co., Ltd., China).
Statistical analysis
Data analysis was performed using SPSS 13.0 software (SPSS Inc., Chicago IL, United States), and the measurement data are reported as the mean ± SD; baseline parameters and differences between the two treatments were compared using Student’s t test. Differences between the baseline and AMT or placebo treatment were compared by paired t test. P < 0.05 was considered statistically significant.
RESULTS
Study participants
Thirty-two healthy volunteers were selected initially by public advertisement. After a screening visit, four subjects were not appropriate for the study by the exclusion criteria (two subjects had a history of gastrointestinal surgery and two subjects experienced abdominal pain during the last three months). Twenty-eight subjects completed the study. There were no statistically significant differences in age, gender, body mass index, Hamilton depression scale, Hamilton anxiety scale scores, proximal gastric accommodation, liquid gastric emptying, proximal gastric sensitivity, OCTT or the levels of MTL, ghrelin and NPY between group A and group B (P > 0.05) (Table 1).
Table 1.
Demographic and baseline characteristics of study in healthy volunteers
| Variable | Group A (n = 14) | Group B (n = 14) | P value |
| Age (yr) | 27.71 ± 8.56 | 32.5 ± 13.36 | 0.26 |
| Sex (male) | 7 | 7 | 1 |
| BMI (kg/m2) | 20.09 ± 1.41 | 20.21 ± 1.42 | 0.82 |
| HAMD | 2.36 ± 1.28 | 1.71 ± 1.20 | 0.29 |
| HAMA | 2.71 ± 1.73 | 3.36 ± 1.45 | 0.18 |
| Cross-sectional area of the proximal stomach (cm2) | |||
| 200 mL | 17.13 ± 4.53 | 18.75 ± 2.30 | 0.08 |
| 400 mL | 29.19 ± 6.24 | 30.76 ± 6.59 | 0.95 |
| 600 mL | 40.92 ± 11.5 | 40.66 ± 7.28 | 0.35 |
| 800 mL | 46.21 ± 12.16 | 46.46 ± 6.81 | 0.24 |
| Difficulty in drinking water VAS | |||
| 200 mL | 0.79 ± 0.58 | 1.07 ± 0.62 | 0.23 |
| 400 mL | 1.79 ± 0.58 | 1.86 ± 0.54 | 0.71 |
| 600 mL | 3.21 ± 0.80 | 3.50 ± 0.65 | 0.31 |
| 800 mL | 5.50 ± 0.76 | 5.43 ± 0.76 | 0.82 |
| Gastric emptying | |||
| 5 min | 77.65% ± 6.5% | 81.46% ± 5.81% | 0.67 |
| 10 min | 62.61% ± 9.85% | 65.18% ± 6.77% | 0.55 |
| OCTT (min) | 88.93% ± 19.03% | 81.43% ± 20.14% | 0.46 |
| Plasma levels (pg/mL) | |||
| MTL | 502.66 ± 127.52 | 440.85 ± 123.25 | 0.20 |
| Ghrelin | 460.06 ± 146.25 | 444.94 ± 202.43 | 0.82 |
| NPY | 888.88 ± 154.52 | 913.46 ± 139.32 | 0.66 |
Values are represented as mean ± SD. Symptom scores on 10-cm visual analogue scale. HAMD: Hamilton depression rating scale; HAMA: Hamilton anxiety rating scale; BMI: Body mass index; OCTT: Orocecal transit time; NPY: Neuropeptide Y; MTL: Motilin; VAS: Visual analogue scale.
Proximal accommodation, visceral hypersensitivity and gastric emptying using the drinking-ultrasonography test
There was no statistically significant difference in the proximal gastric accommodation between the AMT and placebo groups after consumption of 200, 400, 600 or 800 mL water (P > 0.05). Similarly, no differences were found in the gastric emptying rate (%) at 5 and 10 min after the completion of the drinking test (all P > 0.05). Moreover, there were no statistically significant differences between the two groups for the VAS test for difficulty in drinking 200 or 400 mL water (all P > 0.05). However, there were significant differences in VAS results for difficulty in drinking 600 and 800 mL between the two groups (all P = 0.001) (Table 2).
Table 2.
Effects of amitriptyline on gastrointestinal function and brain-gut peptides
| Variable | Amitriptyline (n = 28) | Placebo (n = 28) | P value |
| Cross-sectional area of the proximal stomach (cm2) | |||
| 200 mL | 16.51 ± 3.78 | 16.56 ± 3.98 | 0.97 |
| 400 mL | 27.14 ± 5.71 | 27.84 ± 5.95 | 0.49 |
| 600 mL | 34.11 ± 6.11 | 34.85 ± 6.61 | 0.39 |
| 800 mL | 39.58 ± 7.35 | 40.86 ± 8.45 | 0.34 |
| Difficulty in drinking water VAS | |||
| 200 mL | 0.93 ± 0.65 | 0.96 ± 0.56 | 0.58 |
| 400 mL | 1.93 ± 0.46 | 1.82 ± 0.54 | 0.29 |
| 600 mL | 2.98 ± 0.85 | 3.57 ± 0.94 | 0.001 |
| 800 mL | 4.57 ± 0.98 | 5.57 ± 0.82 | 0.001 |
| Gastric emptying | |||
| 5 min | 78.40 ± 11.71 | 78.84 ± 7.47 | 0.87 |
| 10 min | 66.72 ± 11.63 | 64.54 ± 10.29 | 0.47 |
| OCTT (min) | 109.29 ± 29.68 | 96.61 ± 23.9 | 0.004 |
| Plasma levels (pg/mL) | |||
| MTL | 461.88 ± 129.66 | 473.40 ± 122.75 | 0.61 |
| Ghrelin | 526.87 ± 158.44 | 442.87 ± 176.79 | 0.04 |
| NPY | 965.64 ± 165.63 | 890.15 ± 131.46 | 0.03 |
Values are represented as mean ± SD. Symptom scores on 10-cm visual analogue scale. OCTT: Orocecal transit time; MTL: Motilin; NPY: Neuropeptide Y; VAS: Visual analogue scale.
There were no significant differences between the baseline and placebo treatment in proximal accommodation, gastric emptying and VAS results for difficulty in drinking 200, 400, 600 or 800 mL of water (P > 0.05) (Table 3).
Table 3.
Baseline and after treatment with placebo
| Variable | Baseline (n = 28) | Placebo (n = 28) | P value |
| Cross-sectional area of the proximal stomach (cm2) | |||
| 200 mL | 17.95 ± 3.62 | 16.56 ± 3.98 | 0.19 |
| 400 mL | 29.97 ± 6.35 | 27.84 ± 5.95 | 0.09 |
| 600 mL | 40.78 ± 9.54 | 34.85 ± 6.61 | 0.06 |
| 800 mL | 46.34 ± 9.67 | 40.86 ± 8.45 | 0.06 |
| Difficulty in drinking water VAS | |||
| 200 mL | 0.93 ± 0.59 | 0.96 ± 0.56 | 0.70 |
| 400 mL | 1.82 ± 0.54 | 1.82 ± 0.54 | 1.00 |
| 600 mL | 3.36 ± 0.72 | 3.57 ± 0.94 | 0.34 |
| 800 mL | 5.46 ± 0.73 | 5.57 ± 0.82 | 0.56 |
| Gastric emptying | |||
| 5 min | 79.55 ± 6.35 | 78.84 ± 7.47 | 0.47 |
| 10 min | 63.89 ± 8.39 | 64.54 ± 10.29 | 0.79 |
| OCTT (min) | 85.18 ± 19.60 | 96.61 ± 23.90 | 0.07 |
| Plasma levels (pg/mL) | |||
| MTL | 471.75 ± 127.02 | 473.40 ± 122.75 | 0.75 |
| Ghrelin | 452.50 ± 173.46 | 442.87 ± 176.79 | 0.35 |
| NPY | 901.17 ± 144.91 | 890.15 ± 131.46 | 0.12 |
Values are represented as mean ± SD. Symptom scores on 10-cm visual analogue scale. OCTT: Orocecal transit time; MTL: Motilin; NPY: Neuropeptide Y; VAS: Visual analogue scale.
There were no significant differences between the baseline and AMT treatment in the cross-sectional area of the proximal stomach (cm2) after drinking 200, 400, 600 or 800 mL of water (P > 0.05) (Table 4). Similarly, no significant differences were found between the baseline and AMT treatment in the VAS after drinking 200 or 400 mL of water (P > 0.05). However, the VAS results significantly dropped from baseline in response to AMT treatment after drinking 600 and 800 mL water (P = 0.001) (Table 4). No differences in gastric emptying were observed between the baseline and AMT treatment (P > 0.05; Table 4).
Table 4.
Baseline and after treatment with amitriptyline
| Variable | Baseline (n = 28) | Amitriptyline (n = 28) | P value |
| Cross-sectional area of the proximal stomach (cm2) | |||
| 200 mL | 17.95 ± 3.62 | 16.51 ± 3.78 | 0.22 |
| 400 mL | 29.97 ± 6.35 | 27.14 ± 5.71 | 0.09 |
| 600 mL | 40.78 ± 9.54 | 34.11 ± 6.11 | 0.06 |
| 800 mL | 46.34 ± 9.67 | 39.58 ± 7.35 | 0.06 |
| Difficulty in drinking water VAS | |||
| 200 mL | 0.93 ± 0.59 | 0.93 ± 0.65 | 0.99 |
| 400 mL | 1.82 ± 0.54 | 1.93 ± 0.46 | 0.36 |
| 600 mL | 3.36 ± 0.72 | 2.98 ± 0.85 | 0.01 |
| 800 mL | 5.46 ± 0.73 | 4.57 ± 0.98 | 0.001 |
| Gastric emptying | |||
| 5 min | 79.55 ± 6.35 | 78.40 ± 11.71 | 0.96 |
| 10 min | 63.89 ± 8.39 | 66.72 ± 11.63 | 0.17 |
| OCTT (min) | 85.18 ± 19.60 | 109.29 ± 29.68 | 0.001 |
| Plasma levels (pg/mL) | |||
| MTL | 471.75 ± 127.02 | 461.88 ± 129.66 | 0.11 |
| Ghrelin | 452.50 ± 173.46 | 526.87 ± 158.44 | 0.001 |
| NPY | 901.17 ± 144.91 | 965.64 ± 165.63 | 0.001 |
Values are represented as mean ± SD. Symptom scores on 10-cm visual analogue scale. OCTT: Orocecal transit time; MTL: Motilin; NPY: Neuropeptide Y; VAS: Visual analogue scale.
OCTT with lactulose hydrogen breath test
AMT slowed the OCTT, and there was a significant difference between the AMT and placebo groups (P = 0.004; Table 2). OCTT was not different between the baseline and placebo treatment (Table 3), although there was a significant difference between the baseline and treatment with AMT (P = 0.001; Table 4).
Plasma levels of MTL, ghrelin and NPY using ELISA
The fasting plasma concentration of MTL was similar in the AMT and placebo groups (P = 0.61; Table 2). There were no significant differences in the MTL levels between the baseline and treatment with placebo (P = 0.75; Table 3) or between the baseline and treatment with AMT (P = 0.11; Table 4). However, in the AMT group, the fasting plasma ghrelin concentration was significantly greater than the placebo group (P = 0.04; Table 2). There was no difference in the ghrelin level between the baseline and treatment with placebo (P = 0.35; Table 3), but the ghrelin concentration was elevated following AMT treatment compared to the baseline values (P = 0.001; Table 4). Compared with the placebo group, the AMT group had higher fasting plasma NPY levels (P = 0.03; Table 2). There was no significant difference between the baseline and treatment with placebo (P = 0.12; Table 3), but the NPY level was significantly elevated after AMT treatment (P = 0.001; Table 4).
Adverse effects and safety
Table 5 shows the adverse effects that occurred during the treatment. There were no adverse effects which required emergency evaluation or hospitalisation. No subjects dropped out of the study.
Table 5.
Adverse effects of amitriptyline and placebo
| Adverse effect | Amitriptyline (n = 28) | Placebo (n = 28) |
| Sleepiness | 10 | 2 |
| Bitter taste | 7 | 2 |
| Dry mouth | 6 | 3 |
| Tired in early morning | 2 | 1 |
| Dizziness | 2 | 0 |
| Constipation | 1 | 1 |
DISCUSSION
In previous studies, low-dose AMT was useful for FD and IBS, especially for the improvement of abdominal pain[12-15], possibly because AMT reduces the visceral sensitivity and increases the pain threshold in FGID patients, although this is still controversial. Mertz et al[12] suggested that after AMT (50 mg/d) treatment for 4 wk in FD, the perception of gastric distension using the barostat test was not different from the placebo treatment. Conversely, Thoua et al[13] demonstrated that after 3 mo of treatment with AMT (25-50 mg/d) in IBS, the rectal hypersensitivity to electrical current stress was decreased, however the study was uncontrolled. Obviously, it is not due to the antidepressant effect of AMT as the doses were below the effective doses of the antidepressant; the benefits are in patients who are not depressive, with responses occurring before the antidepressant effect[16].
Here, we measured visceral sensitivity using the noninvasive drinking-ultrasonography test in healthy volunteers, which is different from previous studies. We found that low-dose AMT reduced gastric sensitivity immediately after the volunteers ingested 600 and 800 mL water, which was consistent with the result of Thoua et al[13]. This might contribute to the potential centrally mediated visceral analgesic properties of AMT. As Morgan et al[16] suggested, low-dose AMT has a central effect on pain-related cerebral activation in the anterior cingulate cortex and left posterior parietal complex in IBS patients during mental stress.
A variety of gastrointestinal motility disturbances have been implicated in FGIDs[17]. Bouras et al[18] demonstrated that low-dose AMT could slow solid gastric emptying in healthy individuals. Vahedi et al[14] observed that low-dose AMT was effective for the treatment of diarrhoea-predominant IBS; the reason might be the anti-cholinergic effect of the drug. In our research, AMT did not affect liquid gastric emptying but did significantly prolong the OCTT (which is also reflects the small bowel transit time[19]). This is consistent with previous investigations in which imipramine delayed OCTT in controls and IBS patients[20]. The reason for the differences in the current results compared to previous studies might be that liquid emptying is related to the proximal portion or fundus relaxation, but solid emptying is associated with the distal stomach[21]. In the current study, there were no effects on proximal gastric accommodation with low-dose AMT. Similar conclusions have been previously reported and showed that AMT had no effect on drinking capacity in healthy volunteers[18]. The gold standard for the measurement of proximal gastric accommodation is gastric barostat[22], although this procedure is invasive. In this study, we used the new drinking-ultrasonography test, which is non-invasiveness, safe, reproducible, better accepted by volunteers and relatively simple to administer.
MTL levels were not significantly different between the placebo and AMT groups. However MTL levels have been reported to be significantly elevated in patients with constipation who are receiving tricyclic antidepressant drugs[23]. It is possible that we evaluated healthy volunteers rather than patients in this study. In healthy individuals, AMT might not have any effect on the normal levels of MTL because of intact reflex mechanisms. Ghrelin plays a role in regulating appetite[24]. Lee et al[6] reported that unusually low preprandial ghrelin levels occur in FD patients due to dysmotility. It is possible that FD patients with dysmotility may respond to AMT effectively. Caproni et al[25] found that the plasma levels of NPY were markedly increased in migraine patients receiving AMT treatment (25 mg/d) for 3 mo. The present study extends the previous finding by showing that the plasma level of NPY was significantly increased with low-dose AMT treatment. A previous study showed that NPY may help patients with stress on the gut-brain axis[26], so the increase in NPY levels might be a reason for the treatment of FD and IBS patients who are often hypersensitive to stress[27].
This study included a small sample size of healthy volunteers. Further studies consisting of larger sample sizes that are powered to find smaller differences may be required. As the duration of AMT administration in the clinic is typically 4-12 wk[12-15], it is possible that the course of medication in our study was too short. A longer trial might have different effects on gastrointestinal function and brain-gut peptides.
In summary, low-dose AMT slows OCTT, decreases gastric sensitivity and increases the plasma levels of ghrelin and neuropeptide Y in healthy Chinese individuals, which may be the cause of the beneficial effects of low-dose AMT in FGID patients.
ACKNOWLEDGMENTS
We thank Ming-Zhi Xu, Professor of the Guangdong Mental Disorder Research Institute, for his role in the application and quality control of the anxiety and depression scale.
COMMENTS
Background
Low-dose amitriptyline (AMT) has been used to study functional gastrointestinal disorders for many years, although the precise mechanism of the action is still not clear. Evidence indicating the possible effects of AMT on gastrointestinal function and brain-gut peptides in healthy Chinese volunteers is limited.
Research frontiers
Therapeutic options for functional gastrointestinal disorders are limited. Antidepressant agents such as low dose AMT are effective in functional gastrointestinal disorders.
Innovations and breakthroughs
Based on previous data, this study first explored the possible effects of low dose AMT on gastrointestinal function and brain-gut peptides in healthy Chinese volunteers. The results of the present study revealed that low-dose AMT could slow orocecal transit time (OCTT), decrease gastric sensitivity and increase the plasma levels of ghrelin and neuropeptide Y in healthy Chinese individuals, which may be the cause of the beneficial effects of low-dose AMT in functional gastrointestinal disorders (FGID) patients.
Applications
Low dose AMT plays a role in regulating gastrointestinal function, supporting its clinical applicability for gastrointestinal disorders in China.
Terminology
The drinking-ultrasonography test is a novel method to measure proximal accommodation, visceral hypersensitivity and gastric emptying. The test is non-invasive, safe, better accepted by volunteers, reproducible and relatively simple to administer.
Peer review
This manuscript has originality so far. Low-dose AMT slows OCTT, decreases gastric sensitivity and increases the plasma levels of ghrelin and neuropeptide Y in healthy Chinese individuals, which may be the cause of the beneficial effects of low-dose AMT in FGID patients.
Footnotes
P- Reviewer Kim N S- Editor Gou SX L- Editor A E- Editor Ma S
References
- 1.Ohman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7:163–173. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M, Tack JF. Current medical treatments of dyspepsia and irritable bowel syndrome. Gastroenterol Clin North Am. 2010;39:481–493. doi: 10.1016/j.gtc.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Rahimi R, Nikfar S, Rezaie A, Abdollahi M. Efficacy of tricyclic antidepressants in irritable bowel syndrome: a meta-analysis. World J Gastroenterol. 2009;15:1548–1553. doi: 10.3748/wjg.15.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annese V, Janssens J, Vantrappen G, Tack J, Peeters TL, Willemse P, Van Cutsem E. Erythromycin accelerates gastric emptying by inducing antral contractions and improved gastroduodenal coordination. Gastroenterology. 1992;102:823–828. doi: 10.1016/0016-5085(92)90164-t. [DOI] [PubMed] [Google Scholar]
- 5.Kamerling IM, Van Haarst AD, Burggraaf J, Schoemaker RC, Biemond I, Heinzerling H, Jones R, Cohen AF, Masclee AA. Motilin effects on the proximal stomach in patients with functional dyspepsia and healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2003;284:G776–G781. doi: 10.1152/ajpgi.00456.2002. [DOI] [PubMed] [Google Scholar]
- 6.Lee KJ, Cha DY, Cheon SJ, Yeo M, Cho SW. Plasma ghrelin levels and their relationship with gastric emptying in patients with dysmotility-like functional dyspepsia. Digestion. 2009;80:58–63. doi: 10.1159/000215389. [DOI] [PubMed] [Google Scholar]
- 7.Forbes S, Herzog H, Cox HM. A role for neuropeptide Y in the gender-specific gastrointestinal, corticosterone and feeding responses to stress. Br J Pharmacol. 2012;166:2307–2316. doi: 10.1111/j.1476-5381.2012.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballesteros J, Bobes J, Bulbena A, Luque A, Dal-Ré R, Ibarra N, Güemes I. Sensitivity to change, discriminative performance, and cutoff criteria to define remission for embedded short scales of the Hamilton depression rating scale (HAMD) J Affect Disord. 2007;102:93–99. doi: 10.1016/j.jad.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Matza LS, Morlock R, Sexton C, Malley K, Feltner D. Identifying HAM-A cutoffs for mild, moderate, and severe generalized anxiety disorder. Int J Methods Psychiatr Res. 2010;19:223–232. doi: 10.1002/mpr.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato M, Nishida U, Nishida M, Hata T, Asaka R, Haneda M, Yamamoto K, Imai A, Yoshida T, Ono S, et al. Pathophysiological classification of functional dyspepsia using a novel drinking-ultrasonography test. Digestion. 2010;82:162–166. doi: 10.1159/000308363. [DOI] [PubMed] [Google Scholar]
- 11.Rana S, Bhansali A, Bhadada S, Sharma S, Kaur J, Singh K. Orocecal transit time and small intestinal bacterial overgrowth in type 2 diabetes patients from North India. Diabetes Technol Ther. 2011;13:1115–1120. doi: 10.1089/dia.2011.0078. [DOI] [PubMed] [Google Scholar]
- 12.Mertz H, Fass R, Kodner A, Yan-Go F, Fullerton S, Mayer EA. Effect of amitriptyline on symptoms, sleep, and visceral perception in patients with functional dyspepsia. Am J Gastroenterol. 1998;93:160–165. doi: 10.1111/j.1572-0241.1998.00160.x. [DOI] [PubMed] [Google Scholar]
- 13.Thoua NM, Murray CD, Winchester WJ, Roy AJ, Pitcher MC, Kamm MA, Emmanuel AV. Amitriptyline modifies the visceral hypersensitivity response to acute stress in the irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:552–560. doi: 10.1111/j.1365-2036.2008.03918.x. [DOI] [PubMed] [Google Scholar]
- 14.Vahedi H, Merat S, Momtahen S, Kazzazi AS, Ghaffari N, Olfati G, Malekzadeh R. Clinical trial: the effect of amitriptyline in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;27:678–684. doi: 10.1111/j.1365-2036.2008.03633.x. [DOI] [PubMed] [Google Scholar]
- 15.Braak B, Klooker TK, Wouters MM, Lei A, van den Wijngaard RM, Boeckxstaens GE. Randomised clinical trial: the effects of amitriptyline on drinking capacity and symptoms in patients with functional dyspepsia, a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34:638–648. doi: 10.1111/j.1365-2036.2011.04775.x. [DOI] [PubMed] [Google Scholar]
- 16.Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601–607. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camilleri M, McKinzie S, Busciglio I, Low PA, Sweetser S, Burton D, Baxter K, Ryks M, Zinsmeister AR. Prospective study of motor, sensory, psychologic, and autonomic functions in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2008;6:772–781. doi: 10.1016/j.cgh.2008.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouras EP, Talley NJ, Camilleri M, Burton DD, Heckman MG, Crook JE, Richelson E. Effects of amitriptyline on gastric sensorimotor function and postprandial symptoms in healthy individuals: a randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2008;103:2043–2050. doi: 10.1111/j.1572-0241.2008.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodé S, Dreyer M, Greisen G. Gastric emptying and small intestinal transit time in preterm infants: a scintigraphic method. J Pediatr Gastroenterol Nutr. 2004;39:378–382. doi: 10.1097/00005176-200410000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Gorard DA, Libby GW, Farthing MJ. Effect of a tricyclic antidepressant on small intestinal motility in health and diarrhea-predominant irritable bowel syndrome. Dig Dis Sci. 1995;40:86–95. doi: 10.1007/BF02063948. [DOI] [PubMed] [Google Scholar]
- 21.Ziessman HA, Okolo PI, Mullin GE, Chander A. Liquid gastric emptying is often abnormal when solid emptying is normal. J Clin Gastroenterol. 2009;43:639–643. doi: 10.1097/mcg.0b013e318181b42f. [DOI] [PubMed] [Google Scholar]
- 22.Sarnelli G, Vos R, Cuomo R, Janssens J, Tack J. Reproducibility of gastric barostat studies in healthy controls and in dyspeptic patients. Am J Gastroenterol. 2001;96:1047–1053. doi: 10.1111/j.1572-0241.2001.03520.x. [DOI] [PubMed] [Google Scholar]
- 23.Allen JM, Christofides ND, Cramer PA, Steinert J, Bloom SR. Elevated motilin levels in patients treated with antidepressant and neuroleptic drugs. Br J Psychiatry. 1982;141:27–29. doi: 10.1192/bjp.141.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Kojima M, Hosoda H, Kangawa K. Clinical endocrinology and metabolism. Ghrelin, a novel growth-hormone-releasing and appetite-stimulating peptide from stomach. Best Pract Res Clin Endocrinol Metab. 2004;18:517–530. doi: 10.1016/j.beem.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Caproni S, Corbelli I, Pini LA, Cupini ML, Calabresi P, Sarchielli P. Migraine preventive drug-induced weight gain may be mediated by effects on hypothalamic peptides: the results of a pilot study. Cephalalgia. 2011;31:543–549. doi: 10.1177/0333102410392605. [DOI] [PubMed] [Google Scholar]
- 26.Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides. 2012;46:261–274. doi: 10.1016/j.npep.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mearin F. Postinfectious functional gastrointestinal disorders. J Clin Gastroenterol. 2011;45 Suppl:S102–S105. doi: 10.1097/MCG.0b013e31821fbf58. [DOI] [PubMed] [Google Scholar]


