Abstract
AIM: To evaluate the feasibility of a new computed virtual chromoendoscopy (CVC) device (M i-scan) in the diagnosis of gastric neoplasia.
METHODS: Patients with superficial lesions no larger than 1.0 cm found during high definition endoscopy were included. Those with advanced or obviously protruded or depressed lesions, lesions larger than 1.0 cm and/or lesions which were not amenable to observation by zoom function were excluded. The endoscopist was required to give the real-time descriptions of surface pit patterns of the lesions, based on surface pattern classification of enhanced magnification endoscopy. According to previous reports, types I-III represent non-neoplastic lesions, and types IV-V represent neoplastic lesions. Diagnosis with M i-scan and biopsy was performed before histopathological diagnosis. Magnified images of gastric lesions with and without enhancement were collected for further analysis. The diagnostic yield of real-time M i-scan and effects on magnification image quality by tone enhancement (TE), surface enhancement (SE) and color enhancement (CE) were calculated. The selected images were sent to another endoscopist. The endoscopist rated the image quality of each lesion at 3 levels. Ratings of image quality were based on visualization of pit pattern, vessel and demarcation line.
RESULTS: One hundred and eighty-three patients were recruited. Five patients were excluded for advanced gastric lesions, 1 patient was excluded for poor preparation and 2 patients were excluded for superficial lesions larger than 1.0 cm; 132 patients were excluded for no lesions found by high definition endoscopy. In the end, 43 patients with 43 lesions were included. Histopathology revealed 10 inflammation, 14 atrophy, 10 metaplasia, 1 low grade dysplasia (LGD), 5 high grade dysplasia (HGD) and 3 cancers. For 7 lesions classified into type I, histopathology revealed 6 atrophy and 1 metaplasia; for 10 lesions classified into type II, histopathology revealed 2 inflammation, 7 atrophy and 1 metaplasia; for 10 lesions classified into type III, histopathology revealed 1 inflammation, 8 metaplasia and 1 LGD; for 9 lesions classified into type IV, histopathology revealed 4 inflammation, 1 atrophy and 4 HGD; for 7 lesions classified into type V, histopathology revealed 3 inflammation, 1 HGD and 3 cancers. A total of 172 still images, including 43 images by white light (MWL) and 129 images by M i-scan (43 with TE, 43 with SE and 43 with CE), were selected and sent to the endoscopist who did the analysis. General image quality of M i-scan with TE and SE was significantly better than that of MWL (TE, 4.55 ± 1.07; SE, 4.30 ± 1.02; MWL, 3.25 ± 0.99; P < 0.001). Visualization of pit pattern was significantly improved by M i-scan with SE (1.93 ± 0.25 vs 1.50 ± 0.50, P < 0.001). Microvessel visualization was significantly improved by M i-scan with TE (1.23 ± 0.78 vs 0.76 ± 0.73, P < 0.001). Demarcation line visualization was improved by M i-scan with both TE and SE (TE, 1.75 ± 0.52; SE, 1.56 ± 0.59; MWL, 0.98 ± 0.44; P < 0.001). M i-scan with CE did not show any significant improvements of image quality in general or in the 3 key parameters. Although M i-scan with TE and SE slightly increased the diagnostic yield of MWL, there was no significant difference (P > 0.1).
CONCLUSION: Although digital enhancement improves the image quality of magnification endoscopy, its value in improving the diagnostic yield seems to be limited.
Keywords: Computed virtual chromoendoscopy, i-scan, Gastric neoplasia, Diagnosis
Core tip: In this study, the authors applied a new endoscopic device combining magnification endoscopy and virtual chromoendoscopy, equipped with surface enhancement, tone enhancement and color enhancement (M i-scan), in the diagnosis of 43 patients with small superficial gastric lesions. The results showed that real-time diagnosis of the gastric cancerous lesions by using M i-scan corresponded well with their histopathology. In comparisons between different enhancement capabilities using offline images, images with surface enhancement and tone enhancement were found to be slightly superior to those with color enhancement.
INTRODUCTION
Since conventional endoscopy has limited definition and magnification, detection and characterization of early gastric cancer are still challenging in daily practice. Recently, advanced endoscopy techniques have been introduced to improve the diagnosis of early gastric cancer, such as chromoendoscopy with dyes[1], acetic acid-enhanced endoscopy[2,3], magnification endoscopy[4] and dyeless virtual chromoendoscopy[5]. Incorporation of magnification endoscopy and chromoendoscopy[6] or enhanced endoscopy[7] into one instrument is perfect, because chromoendoscopy and enhanced endoscopy serve as the red flag in detection, while magnification endoscopy serves in characterization. Magnified virtual chromoendoscopy is more preferable than dye spraying magnification chromoendoscopy for efficiency and safety[5]. One example is magnified narrow band imaging (M-NBI)[8-16]. Clinical trials suggest that M-NBI is helpful in the diagnosis of Barrett’s esophagus[17-21], small colorectal lesions[22] and early gastric cancer[8,23]. Along with NBI, multi-band imaging virtual chromoendoscopy, such as Fuji Intelligence Chromoendoscopy (FICE)[24-30] and Pentax i-scan, are also available in clinical practice.
Unlike NBI, FICE and i-scan use reflection band filtering to achieve color enhancement of the mucosa. The instrument in this study not only incorporates color enhancement but also surface enhancement and magnification (M i-scan). The principle of surface enhancement is to adjust the dark-to-light contrast of the nearby pixels in order to show sharper surface details.
The aim of this study is to assess the accuracy of a real-time M i-scan in the diagnosis of gastric neoplasia (primary outcome). A comparison between magnified virtual chromoscopy and non-magnified virtual chromoscopy was made by using post-endoscopy still images (secondary outcome).
MATERIALS AND METHODS
Patients
From January 1st to March 31st 2012, consecutive patients who underwent high definition gastroscopy in Shandong University Qilu Hospital were recruited into this study. Patients aged 18-80 years, having superficial lesions with diameter less than 1 cm were included. Those with advanced or obviously protruded or depressed lesions, lesions larger than 1.0 cm and/or lesions which were not amenable to observation by zoom function (poor preparation, difficult positions, and non-cooperation of patients) were excluded. This study was approved by the local ethics committee (Ethics Committee of Shandong University Qilu Hospital) and adhered to the Declaration of Helsinki for Medical Research involving Human Subjects-Ethical Principles for Medical Research Involving Human Subjects. All the patients who participated in this study have provided their written informed consents.
Endoscopic procedure
The instruments applied in this study were an EG-2990Zi endoscope (Pentax, Tokyo, Japan) and an EPK-i endoscopic system (Pentax, Tokyo, Japan). This high definition endoscope incorporated surface enhancement (at +2, +4 and +6 levels), color enhancement (+4, +5 and +6 levels) and tone enhancement functions. It is also equipped with an adjustable image magnification in a continuous range up to 100-fold. The diameter and the length of the insertion tube of this instrument are the same as those of a standard upper endoscope. To achieve the maximum magnification, a transparent hood was attached to the distal tip of the endoscope to fix the distance between endoscope and gastric mucosa at 2 mm.
All the patients underwent routine preparation before the procedure. The detected lesions were observed with magnification endoscopy in white light (MWL) mode and in enhancement (M i-scan) mode consecutively. The endoscopic procedures were performed by an experienced endoscopist who was familiar with magnification endoscopy diagnosis of early gastric cancer. The endoscopist was required to give the real-time descriptions of surface pit patterns of the lesions, based on surface pattern classification of enhanced magnification endoscopy. The surface pattern classification includes 5 types: type I, small round pits of uniform size and shape; type II, slit-like pits; type III, gyrus and villous patterns; type IV, irregular arrangement and size; and type V, destructive pattern. According to previous reports, types I-III represent non-neoplastic lesions, and types IV-V represent neoplastic lesions[7]. Real-time diagnoses to determine neoplasia or non-neoplasia were not required from the endoscopist. Instead, the diagnoses were made by another investigator according to the diagnostic strategy and real-time description above. Images of MWL [without tone enhancement (TE), surface enhancement (SE) and color enhancement (CE)] and i-scan (with “g” TE, +2 SE or +4 CE) were collected and stored on USB devices during the procedures. Four best quality images per lesion were selected and sorted randomly by the investigator.
Post-endoscopy still image analysis
The selected images were sent to another endoscopist who did not participate in any of the endoscopic procedures. The endoscopist was kept blind to the clinical and endoscopic information of the patients. The endoscopist rated the image quality of each lesion at 3 levels. Ratings of image quality were based on visualization of pit pattern, vessel, and demarcation line[22], which are key parameters to detect and characterize the gastric neoplasia. Rating scales of image quality were: pit pattern, 0 for unassessable, 1 for fine, 2 for excellent; vessel, 0 for invisible, 1 for visible, 2 for clearly visible; demarcation line, 0 for unassessable, 1 for fine, 2 for clear. The endoscopist then recorded the descriptions of the still images according to the same standards as applied in the real time observation[7].
Biopsy and histopathology
The lesions were routinely biopsied, and the specimens were placed in 10% formalin solution and processed in the routine manner. The slices were examined by an experienced pathologist who had specific training in gastrointestinal pathology. The pathologist was kept blind to the clinical and endoscopic information of the patients. The histology report was based on the WHO (World Health Organization) classification of gastrointestinal tumors. The study flow diagram is illustrated in Figure 1.
Figure 1.
Study flow diagram. TE: Tone enhancement; SE: Surface enhancement; CE: Color enhancement.
Statistical analysis
Diagnostic accuracy of gastric neoplasia by using real-time M i-scan was presented with sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and likelihood ratio (LR). The agreement between real time M i-scan and histopathology was presented with kappa values (0.1-0.2 were considered slight agreement, 0.21-0.4 fair agreement, 0.41-0.6 moderate agreement, 0.61-0.8 substantial agreement and 0.81-0.99 almost perfect agreement). Parameters of still image quality were presented as mean ± SD, and differences of magnification image quality between MWL and i-scan were determined by one-way ANOVA test. A P value < 0.05 is considered to be significant. All data were analyzed by SPSS 13.0 (SPSS Inc., Chicago, IL, United States).
RESULTS
Patients
One hundred and eighty-three patients were recruited. Five patients were excluded for advanced gastric lesions, 1 patient was excluded for poor preparation and 2 patients were excluded for superficial lesions larger than 1.0 cm; 132 patients were excluded for no lesions found by high definition endoscopy. In the end, 43 patients with 43 lesions were included. The average age of the patients was 47.5 (18-74) years, of which 32 were males. Locations of the lesions were: 5 in cardia and fundus, 2 in body, 4 on angle and 32 in antrum. All the lesions could be easily identified and zoomed. Histopathology revealed 10 inflammation, 14 atrophy, 10 metaplasia, 1 low grade dysplasia (LGD), 5 high grade dysplasia (HGD) and 3 cancers.
Real-time diagnosis by M i-scan
For 7 lesions classified into type I, histopathology revealed 6 atrophy and 1 metaplasia; for 10 lesions classified into type II, histopathology revealed 2 inflammation, 7 atrophy and 1 metaplasia; for 10 lesions classified into type III, histopathology revealed 1 inflammation, 8 metaplasia and 1 LGD; for 9 lesions classified into type IV, histopathology revealed 4 inflammation, 1 atrophy and 4 HGD; for 7 lesions classified into type V, histopathology revealed 3 inflammation, 1 HGD and 3 cancers. The real-time descriptions of pit patterns and the corresponding histopathology are shown in Table 1. Typical images representing pit patterns of types I-V are illustrated in Figure 2.
Table 1.
Histopathology and pit patterns of the lesions classified by M i-scan
| Pit |
Histology |
Total | |||||
| Inflammation | Atrophy | Metaplasia | LGD | HGD | Cancer | ||
| Type I | 0 | 6 | 1 | 0 | 0 | 0 | 7 |
| Type II | 2 | 7 | 1 | 0 | 0 | 0 | 10 |
| Type III | 1 | 0 | 8 | 1 | 0 | 0 | 10 |
| Type IV | 4 | 1 | 0 | 0 | 4 | 0 | 9 |
| Type V | 3 | 0 | 0 | 0 | 1 | 3 | 7 |
| Total | 10 | 14 | 10 | 1 | 5 | 3 | 43 |
HGD: High grade dysplasia; LGD: Low grade dysplasia.
Figure 2.
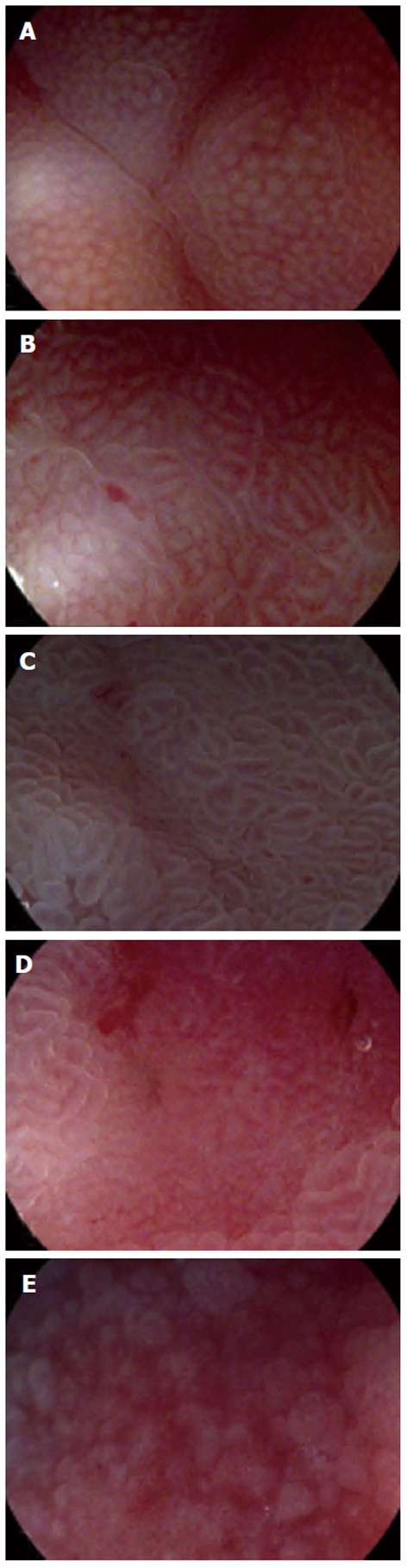
Images representing typical pit pattern classification by M i-scan. A: Type I, small round pits of uniform size and shape; B: Type II, slit-like pits; C: Type III, gyrus and villous patterns; D: Type IV, irregular arrangement and size; E: Type V, destructive pattern.
When the histopathology was re-classified into 2 categories (as non-cancerous lesions including inflammation, atrophy, metaplasia and LGD, or cancerous lesions including HGD and cancer) and the pit patterns re-classified into 2 categories as described above, sensitivity, specificity, PPV, NPV and likelihood ratio of M i-scan regarding gastric neoplasia were 100%, 77.1%, 50%, 100% and 4.37% respectively. Kappa value calculated from agreement between M i-scan and histopathology was 0.557 (moderate agreement). The diagnostic yield after re-classification is shown in Table 2.
Table 2.
Diagnostic yield of gastric neoplasia by real time M i-scan
| M i-scan |
Histopathology |
Total | |
| Cancerous | Non-cancerous | ||
| Neoplasia | 8 | 8 | |
| Non-neoplasia | 0 | 27 | |
| Total | 8 | 35 | 43 |
Sensitivity: 100%; Specificity: 77.1%; Positive predictive value: 50%; Negative predictive value: 100%; Likelihood ratio: 4.37.
Post-endoscopy still image analysis
A total of 172 still images, including 43 images by MWL and 129 images by M i-scan (43 with TE, 43 with SE and 43 with CE), were selected and sent to the endoscopist who did the analysis. General image quality of M i-scan with TE and SE was significantly better than that of MWL (TE, 4.55 ± 1.07; SE, 4.30 ± 1.02; MWL, 3.25 ± 0.99; P < 0.001). Regarding the 3 key parameters, visualization of pit pattern was significantly improved by M i-scan with SE (1.93 ± 0.25 vs 1.50 ± 0.50, P < 0.001). Microvessel visualization was significantly improved by M i-scan with TE (1.23 ± 0.78 vs 0.76 ± 0.73, P < 0.001). Demarcation line visualization was improved by both M i-scan with TE and SE (TE, 1.75 ± 0.52; SE, 1.56 ± 0.59; MWL, 0.98 ± 0.44; P < 0.001). M i-scan with CE did not show any significant improvements of image quality in general or in the 3 key parameters.
Descriptions of the still images based on lesions demonstrated that diagnosis by MWL revealed a sensitivity, specificity, PPV, NPV and LR of 87.5%, 71.4%, 41.2%, 96.2% and 3.06%, respectively. Although M i-scan with TE and SE slightly increased the diagnostic yield, there was no significant difference (P > 0.1). M i-scan with CE did not change the diagnostic yield by MWL. M i-scan with SE perfectly matched the results of real-time descriptions. Representative images showing image quality differences among different modes are illustrated in Figure 3.
Figure 3.
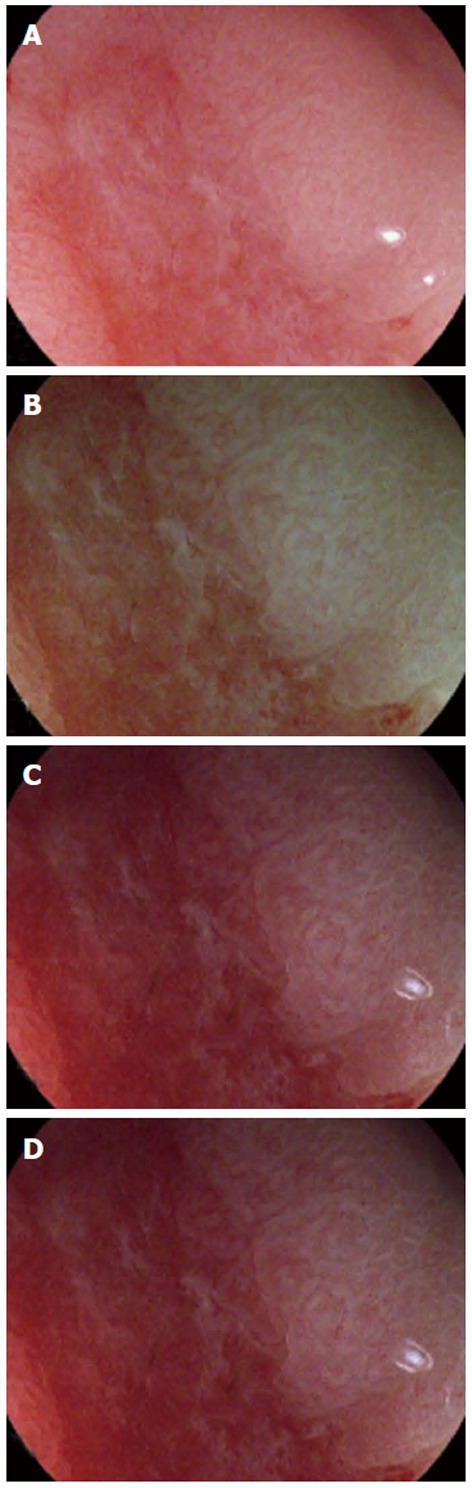
Representative images showing white light (A), M i-scan with tone enhancement (B), surface enhancement (C) and color enhancement (D), respectively.
DISCUSSION
Detection and characterization of early gastric cancer by dyeless virtual chromoendoscopy, such as NBI and computed virtual chromoendoscopy (CVC), are preferable for the endoscopist, because of time, labor and potential risks reduction[31,32]. Virtual chromoendoscopy with magnification is thought to be the dream team, since the former provides the detection “red flag” followed by real-time characterization by the latter. It was reported that real-time characterization of Barrett’s esophagus[33,34], gastric cancer[35,36] and colorectal adenoma[37,38] can be improved by dyeless virtual chromoendoscopy, such as NBI or FICE[25-29,39]. In this pilot feasibility study, we aimed to evaluate application of M i-scan in the diagnosis of small superficial gastric lesions, both in real-time investigation and post-endoscopy still image analysis. The preliminary results showed that M i-scan is helpful for the in vivo prediction of small gastric superficial lesions with excellent sensitivity and NPV, acceptable specificity and LR, and poor PPV. The post-endoscopy still image analysis showed that M i-scan with TE and SE can slightly increase the image quality.
One feature of M i-scan is to mimic the surface enhancement of EME by acetic acid spraying. In this study, the still image analysis showed that SE significantly improves visualization of surface pit pattern and demarcation line compared to MWL. Although there were excellent sensitivity and NPV, and acceptable specificity results, the PPV was poor, just as the results of enhanced magnification endoscopy[7]. This is partly due to the low percentage of neoplastic lesions in the sample (18.6%, 8/43). On the other hand, erosion is sometimes difficult to be differentiated from neoplasia by surface pit pattern evaluation, as in both lesions surface pits could be lost. In these cases, evaluation of microvessel pattern in addition to surface pit pattern may be helpful. However, observation of microvessels is not satisfactory by M i-scan. Although still image analysis shows that TE significantly improves the visualization of microvessels, which only happens in cases with visible microvessels (visible to clearly visible), visualization of those cases with invisible microvessels (41.2%) remains unchanged.
This study has several limitations. Firstly, this is a feasibility study with small sample size and no sample size calculation. Secondly, the detection rate of small superficial gastric lesions was not evaluated. There has not been any report on the detection rate of small gastric lesions by CVC yet. In our own practice, CVC is not suitable for screen gastroscopy with insufficient luminous intensity. Thirdly, only one endoscopist performed the real-time and still image analysis, so there was no interobserver agreement analysis. However, the perfect match between surface classification of real-time and still image with SE suggests an excellent consistency, which should be validated in future studies. Fourthly, there was no comparison between M i-scan and magnification chromoendoscopy with indigo carmine or other contrast agents. And finally (the last may not be the least), gold standard histopathology was only performed by biopsy. Although we only included lesions smaller than 1.0 cm to minimize the heterogeneity, a discrepancy between biopsy and autopsy still remains.
In conclusion, real-time prediction of the histopathology of small superficial gastric lesions by M i-scan is feasible. Although digital enhancement increases image quality, its value in the diagnosis of gastric neoplasia seems to be limited.
COMMENTS
Background
Magnified chromoendoscopy is a promising tool in the surveillance and diagnosis of gastric neoplasia. Enhanced magnification endoscopy is superior to conventional endoscopy with detailed surface characterization.
Research frontiers
Dyeless virtual chromoendoscopy with magnification might be preferable for reduction of labor and health risks. The endoscope used in this study is a magnification endoscope with both color and surface enhancement.
Innovations and breakthroughs
To date, this is the first endoscopic device with surface enhancement mimicking acetic acid spraying enhanced magnification endoscopy. With the surface enhancement, the gastric pit patterns can be classified into 5 categories according to the classification from enhanced magnification endoscopy, which enables the detailed characterization of the gastric mucosa. With classification of gastric pits, different common gastric pathologies such as atrophy, intestinal metaplasia and neoplasia can be identified in real-time procedures or by still image analysis. The margin of gastric lesions can be more easily identified although the differences were not significant.
Terminology
Although digital enhancement improves the image quality of magnification endoscopy, its value in improving the diagnostic yield seems to be limited.
Peer review
This is a quite interesting study on virtual chromoscopy on gastric neoplasia. However, data are limited.
Footnotes
Supported by The National Clinical Project from the Health Ministry of China; the Shandong Province Science and Technology Committee, No. 2010GSF10247; and National Natural Foundation of Science of China, No. NSFC81101098
P- Reviewer Kim BW S- Editor Zhai HH L- Editor Logan S E- Editor Zhang DN
References
- 1.Okabayashi T, Gotoda T, Kondo H, Ono H, Oda I, Fujishiro M, Yachida S. Usefulness of indigo carmine chromoendoscopy and endoscopic clipping for accurate preoperative assessment of proximal gastric cancer. Endoscopy. 2000;32:S62. [PubMed] [Google Scholar]
- 2.Guelrud M, Ehrlich EE. Enhanced magnification endoscopy in the upper gastrointestinal tract. Gastrointest Endosc Clin N Am. 2004;14:461–473, viii. doi: 10.1016/j.giec.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka K, Toyoda H, Kadowaki S, Kosaka R, Shiraishi T, Imoto I, Shiku H, Adachi Y. Features of early gastric cancer and gastric adenoma by enhanced-magnification endoscopy. J Gastroenterol. 2006;41:332–338. doi: 10.1007/s00535-005-1760-3. [DOI] [PubMed] [Google Scholar]
- 4.Ohashi A, Niwa Y, Ohmiya N, Miyahara R, Itoh A, Hirooka Y, Goto H. Quantitative analysis of the microvascular architecture observed on magnification endoscopy in cancerous and benign gastric lesions. Endoscopy. 2005;37:1215–1219. doi: 10.1055/s-2005-870339. [DOI] [PubMed] [Google Scholar]
- 5.Pohl J, May A, Rabenstein T, Pech O, Ell C. Computed virtual chromoendoscopy: a new tool for enhancing tissue surface structures. Endoscopy. 2007;39:80–83. doi: 10.1055/s-2006-945045. [DOI] [PubMed] [Google Scholar]
- 6.Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, Lara-Santos L, Guilherme M, Moreira-Dias L, Lomba-Viana H, Ribeiro A, Santos C, Soares J, et al. Magnification chromoendoscopy for the diagnosis of gastric intestinal metaplasia and dysplasia. Gastrointest Endosc. 2003;57:498–504. doi: 10.1067/mge.2003.145. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka K, Toyoda H, Kadowaki S, Hamada Y, Kosaka R, Matsuzaki S, Shiraishi T, Imoto I, Takei Y. Surface pattern classification by enhanced-magnification endoscopy for identifying early gastric cancers. Gastrointest Endosc. 2008;67:430–437. doi: 10.1016/j.gie.2007.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video) Endoscopy. 2004;36:1080–1084. doi: 10.1055/s-2004-825961. [DOI] [PubMed] [Google Scholar]
- 9.Yao K, Iwashita A, Tanabe H, Nishimata N, Nagahama T, Maki S, Takaki Y, Hirai F, Hisabe T, Nishimura T, et al. White opaque substance within superficial elevated gastric neoplasia as visualized by magnification endoscopy with narrow-band imaging: a new optical sign for differentiating between adenoma and carcinoma. Gastrointest Endosc. 2008;68:574–580. doi: 10.1016/j.gie.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Kaise M, Kato M, Urashima M, Arai Y, Kaneyama H, Kanzazawa Y, Yonezawa J, Yoshida Y, Yoshimura N, Yamasaki T, et al. Magnifying endoscopy combined with narrow-band imaging for differential diagnosis of superficial depressed gastric lesions. Endoscopy. 2009;41:310–315. doi: 10.1055/s-0028-1119639. [DOI] [PubMed] [Google Scholar]
- 11.Kato M, Kaise M, Yonezawa J, Goda K, Toyoizumi H, Yoshimura N, Yoshida Y, Kawamura M, Tajiri H. Trimodal imaging endoscopy may improve diagnostic accuracy of early gastric neoplasia: a feasibility study. Gastrointest Endosc. 2009;70:899–906. doi: 10.1016/j.gie.2009.03.1171. [DOI] [PubMed] [Google Scholar]
- 12.Muto M, Horimatsu T, Ezoe Y, Morita S, Miyamoto S. Improving visualization techniques by narrow band imaging and magnification endoscopy. J Gastroenterol Hepatol. 2009;24:1333–1346. doi: 10.1111/j.1440-1746.2009.05925.x. [DOI] [PubMed] [Google Scholar]
- 13.Kato M, Kaise M, Yonezawa J, Toyoizumi H, Yoshimura N, Yoshida Y, Kawamura M, Tajiri H. Magnifying endoscopy with narrow-band imaging achieves superior accuracy in the differential diagnosis of superficial gastric lesions identified with white-light endoscopy: a prospective study. Gastrointest Endosc. 2010;72:523–529. doi: 10.1016/j.gie.2010.04.041. [DOI] [PubMed] [Google Scholar]
- 14.Kiyotoki S, Nishikawa J, Satake M, Fukagawa Y, Shirai Y, Hamabe K, Saito M, Okamoto T, Sakaida I. Usefulness of magnifying endoscopy with narrow-band imaging for determining gastric tumor margin. J Gastroenterol Hepatol. 2010;25:1636–1641. doi: 10.1111/j.1440-1746.2010.06379.x. [DOI] [PubMed] [Google Scholar]
- 15.Okada K, Fujisaki J, Kasuga A, Omae M, Hirasawa T, Ishiyama A, Inamori M, Chino A, Yamamoto Y, Tsuchida T, et al. Diagnosis of undifferentiated type early gastric cancers by magnification endoscopy with narrow-band imaging. J Gastroenterol Hepatol. 2011;26:1262–1269. doi: 10.1111/j.1440-1746.2011.06730.x. [DOI] [PubMed] [Google Scholar]
- 16.Uedo N, Fujishiro M, Goda K, Hirasawa D, Kawahara Y, Lee JH, Miyahara R, Morita Y, Singh R, Takeuchi M, et al. Role of narrow band imaging for diagnosis of early-stage esophagogastric cancer: current consensus of experienced endoscopists in Asia-Pacific region. Dig Endosc. 2011;23 Suppl 1:58–71. doi: 10.1111/j.1443-1661.2011.01119.x. [DOI] [PubMed] [Google Scholar]
- 17.Anagnostopoulos GK, Yao K, Kaye P, Hawkey CJ, Ragunath K. Novel endoscopic observation in Barrett’s oesophagus using high resolution magnification endoscopy and narrow band imaging. Aliment Pharmacol Ther. 2007;26:501–507. doi: 10.1111/j.1365-2036.2007.03374.x. [DOI] [PubMed] [Google Scholar]
- 18.Curvers W, Baak L, Kiesslich R, Van Oijen A, Rabenstein T, Ragunath K, Rey JF, Scholten P, Seitz U, Ten Kate F, et al. Chromoendoscopy and narrow-band imaging compared with high-resolution magnification endoscopy in Barrett’s esophagus. Gastroenterology. 2008;134:670–679. doi: 10.1053/j.gastro.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Yao K, Takaki Y, Matsui T, Iwashita A, Anagnostopoulos GK, Kaye P, Ragunath K. Clinical application of magnification endoscopy and narrow-band imaging in the upper gastrointestinal tract: new imaging techniques for detecting and characterizing gastrointestinal neoplasia. Gastrointest Endosc Clin N Am. 2008;18:415–433, vii-viii. doi: 10.1016/j.giec.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Singh R, Karageorgiou H, Owen V, Garsed K, Fortun PJ, Fogden E, Subramaniam V, Shonde A, Kaye P, Hawkey CJ, et al. Comparison of high-resolution magnification narrow-band imaging and white-light endoscopy in the prediction of histology in Barrett’s oesophagus. Scand J Gastroenterol. 2009;44:85–92. doi: 10.1080/00365520802400818. [DOI] [PubMed] [Google Scholar]
- 21.Silva FB, Dinis-Ribeiro M, Vieth M, Rabenstein T, Goda K, Kiesslich R, Haringsma J, Edebo A, Toth E, Soares J, et al. Endoscopic assessment and grading of Barrett’s esophagus using magnification endoscopy and narrow-band imaging: accuracy and interobserver agreement of different classification systems (with videos) Gastrointest Endosc. 2011;73:7–14. doi: 10.1016/j.gie.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 22.Zhou QJ, Yang JM, Fei BY, Xu QS, Wu WQ, Ruan HJ. Narrow-band imaging endoscopy with and without magnification in diagnosis of colorectal neoplasia. World J Gastroenterol. 2011;17:666–670. doi: 10.3748/wjg.v17.i5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohnita K, Isomoto H, Shikuwa S, Yamaguchi N, Nakayama T, Nishiyama H, Okamoto K, Fukuda E, Takeshima F, Hayashi T, et al. Magnifying chromoendoscopic findings of early gastric cancer and gastric adenoma. Dig Dis Sci. 2011;56:2715–2722. doi: 10.1007/s10620-011-1638-6. [DOI] [PubMed] [Google Scholar]
- 24.Coriat R, Chryssostalis A, Zeitoun JD, Deyra J, Gaudric M, Prat F, Chaussade S. Computed virtual chromoendoscopy system (FICE): a new tool for upper endoscopy? Gastroenterol Clin Biol. 2008;32:363–369. doi: 10.1016/j.gcb.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Pohl J, Nguyen-Tat M, Pech O, May A, Rabenstein T, Ell C. Computed virtual chromoendoscopy for classification of small colorectal lesions: a prospective comparative study. Am J Gastroenterol. 2008;103:562–569. doi: 10.1111/j.1572-0241.2007.01670.x. [DOI] [PubMed] [Google Scholar]
- 26.Mouri R, Yoshida S, Tanaka S, Oka S, Yoshihara M, Chayama K. Evaluation and validation of computed virtual chromoendoscopy in early gastric cancer. Gastrointest Endosc. 2009;69:1052–1058. doi: 10.1016/j.gie.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Pohl J, Lotterer E, Balzer C, Sackmann M, Schmidt KD, Gossner L, Schaab C, Frieling T, Medve M, Mayer G, et al. Computed virtual chromoendoscopy versus standard colonoscopy with targeted indigocarmine chromoscopy: a randomised multicentre trial. Gut. 2009;58:73–78. doi: 10.1136/gut.2008.153601. [DOI] [PubMed] [Google Scholar]
- 28.Chung SJ, Kim D, Song JH, Park MJ, Kim YS, Kim JS, Jung HC, Song IS. Efficacy of computed virtual chromoendoscopy on colorectal cancer screening: a prospective, randomized, back-to-back trial of Fuji Intelligent Color Enhancement versus conventional colonoscopy to compare adenoma miss rates. Gastrointest Endosc. 2010;72:136–142. doi: 10.1016/j.gie.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 29.dos Santos CE, Lima JC, Lopes CV, Malaman D, Salomão AD, Garcia AC, Teixeira CR. Computerized virtual chromoendoscopy versus indigo carmine chromoendoscopy combined with magnification for diagnosis of small colorectal lesions: a randomized and prospective study. Eur J Gastroenterol Hepatol. 2010;22:1364–1371. doi: 10.1097/MEG.0b013e32833a5d63. [DOI] [PubMed] [Google Scholar]
- 30.Inoue M, Miyake Y, Odaka T, Sato T, Watanabe Y, Sakama A, Zenbutsu S, Yokosuka O. Objective evaluation of visibility in virtual chromoendoscopy for esophageal squamous carcinoma using a color difference formula. J Biomed Opt. 2010;15:056019. doi: 10.1117/1.3502666. [DOI] [PubMed] [Google Scholar]
- 31.Olliver JR, Wild CP, Sahay P, Dexter S, Hardie LJ. Chromoendoscopy with methylene blue and associated DNA damage in Barrett’s oesophagus. Lancet. 2003;362:373–374. doi: 10.1016/s0140-6736(03)14026-3. [DOI] [PubMed] [Google Scholar]
- 32.Dumbarton TC, Gorman SK, Minor S, Loubani O, White F, Green R. Local cutaneous necrosis secondary to a prolonged peripheral infusion of methylene blue in vasodilatory shock. Ann Pharmacother. 2012;46:e6. doi: 10.1345/aph.1Q560. [DOI] [PubMed] [Google Scholar]
- 33.Gorospe EC, Wang KK. Endoscopy: NBI in Barrett esophagus--look more and sample less. Nat Rev Gastroenterol Hepatol. 2012;9:250–251. doi: 10.1038/nrgastro.2012.62. [DOI] [PubMed] [Google Scholar]
- 34.Curvers WL, Herrero LA, Wallace MB, Wong Kee Song LM, Ragunath K, Wolfsen HC, Prasad GA, Wang KK, Subramanian V, Weusten BL, et al. Endoscopic tri-modal imaging is more effective than standard endoscopy in identifying early-stage neoplasia in Barrett’s esophagus. Gastroenterology. 2010;139:1106–1114. doi: 10.1053/j.gastro.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 35.Dutta AK, Sajith KG, Pulimood AB, Chacko A. Narrow band imaging versus white light gastroscopy in detecting potentially premalignant gastric lesions: a randomized prospective crossover study. Indian J Gastroenterol. 2013;32:37–42. doi: 10.1007/s12664-012-0246-5. [DOI] [PubMed] [Google Scholar]
- 36.Pimentel-Nunes P, Dinis-Ribeiro M, Soares JB, Marcos-Pinto R, Santos C, Rolanda C, Bastos RP, Areia M, Afonso L, Bergman J, et al. A multicenter validation of an endoscopic classification with narrow band imaging for gastric precancerous and cancerous lesions. Endoscopy. 2012;44:236–246. doi: 10.1055/s-0031-1291537. [DOI] [PubMed] [Google Scholar]
- 37.Takemura Y, Yoshida S, Tanaka S, Kawase R, Onji K, Oka S, Tamaki T, Raytchev B, Kaneda K, Yoshihara M, et al. Computer-aided system for predicting the histology of colorectal tumors by using narrow-band imaging magnifying colonoscopy (with video) Gastrointest Endosc. 2012;75:179–185. doi: 10.1016/j.gie.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 38.Rotondano G, Bianco MA, Sansone S, Prisco A, Meucci C, Garofano ML, Cipolletta L. Trimodal endoscopic imaging for the detection and differentiation of colorectal adenomas: a prospective single-centre clinical evaluation. Int J Colorectal Dis. 2012;27:331–336. doi: 10.1007/s00384-011-1312-7. [DOI] [PubMed] [Google Scholar]
- 39.Cha JM, Lee JI, Joo KR, Jung SW, Shin HP. A prospective randomized study on computed virtual chromoendoscopy versus conventional colonoscopy for the detection of small colorectal adenomas. Dig Dis Sci. 2010;55:2357–2364. doi: 10.1007/s10620-009-1003-1. [DOI] [PubMed] [Google Scholar]


