Summary
LEM domain (LEM-D) proteins are components of an extensive protein network that assembles beneath the inner nuclear envelope. Defects in LEM-D proteins cause tissue-restricted human diseases associated with altered stem cell homeostasis. Otefin (Ote) is a Drosophila LEM-D protein that is intrinsically required for female germline stem cells (GSCs) maintenance. Previous studies linked Ote loss with transcriptional activation of the key differentiation gene, bag-of-marbles (bam), leading to the model that Ote tethers the bam gene to the nuclear periphery for gene silencing. Using genetic and phenotypic analyses of multiple ote−/− backgrounds, we obtained evidence that is inconsistent with this model. We show that bam repression is maintained in ote−/− GSCs and that germ cell loss persists in ote−/−, bam−/− mutants, together demonstrating that GSC loss is independent of bam transcription. We show the primary defect in ote−/− GSCs is a block of differentiation, which ultimately leads to germ cell death.
Keywords: Drosophila, emerin, germline stem cell, laminopathies, LEM domain, nuclear lamina, stem cells
Introduction
The nuclear lamina is a filamentous protein network located beneath the nuclear envelope (NE) that contributes to the organization of interphase chromatin. The major constituents of the nuclear lamina are the A- and B-type lamins, which establish an extensive scaffold for protein interactions (Wilson and Foisner, 2010). One important family of lamin-interacting proteins is the LEM domain (LEM-D) family, named for LAP2, emerin and MAN1 proteins. These proteins share an ~40 amino acid domain, called the LEM domain (LEM-D) (Lin et al., 2000; Mansharamani and Wilson, 2005; Wagner and Krohne, 2007), which interacts with Barrier-to-Autointegration Factor (BAF), a small, conserved chromatin protein that binds DNA and histones (Cai et al., 2001; Zheng et al., 2000). Interactions between LEM-D proteins and BAF promote tethering of chromatin to the nuclear periphery, a nuclear compartment commonly associated with low levels of transcription (Geyer et al., 2011). Outside of the LEM-D, proteins in this family have little amino acid similarity. Unique protein domains in LEM-D proteins direct interactions with transcriptional repressors (Bakay et al., 2006; Haraguchi, 2004; Holaska et al., 2003; Mansharamani and Wilson, 2005; Melcon et al., 2006), splicing factors (Wilkinson et al., 2003) and nuclear effectors of signaling cascades, such as Smads and β-catenin (Jiang et al., 2008; Lin et al., 2005; Markiewicz et al., 2006). These observations suggest that LEM-D proteins make broad contributions to nuclear organization and gene regulation.
Mutations in LEM-D proteins cause several age-enhanced human diseases (Worman et al., 2010). These diseases display tissue-restricted pathology, even though LEM-D proteins are globally expressed. For example, mutations in the gene encoding emerin (EMD or STA) are associated with the recessive X-linked form of Emery-Dreifuss muscular dystrophy (EDMD).
These patients display progressive contractures and muscle weakness, with associated dilated cardiomyopathy (Wheeler and Ellis, 2008). How mutations in LEM-D proteins cause tissue-specific disease is unclear. The common mesenchymal origin of affected tissues, coupled with the age-enhanced pathology, has led to suggestions that LEM-D-associated diseases share a common etiology involving regulation of stem cell homeostasis in adult tissues (Meshorer and Gruenbaum, 2008; Wheeler and Ellis, 2008).
The Drosophila LEM-D protein Otefin (Ote) is a homologue of emerin (Padan et al., 1990; Wagner et al., 2004). An ote allele was identified in an ethyl methanesulfonate (EMS) screen for female sterile mutations (Jiang et al., 2008), indicating that Ote is required for oogenesis. In Drosophila, ovaries are divided into sixteen to twenty ovarioles, each containing a specialized structure called the germarium (Figure 1). Each germarium contains a highly organized stem cell niche comprised of terminal filament and cap cells (Chen et al., 2011; Harris and Ashe, 2011; Losick et al., 2011). Cap cells produce Bone Morphogenetic Protein (BMP) ligands that bind receptors on the surface of germline stem cells (GSCs) (Song et al., 2004; Xie and Spradling, 1998). Receptor activation leads to phosphorylation of the receptor-activated (R)-Smad, Mothers against Dpp (Mad), and association with the common mediator (co)-Smad, Medea, resulting in nuclear accumulation of the complex. The nuclear Mad-Medea complex confers transcriptional repression of the key differentiation gene bag-of-marbles (bam) (Chen and McKearin, 2003a; Song et al., 2004; Song and Xie, 2002; Xie and Spradling, 1998). Upon GSC division, one daughter remains intimately associated with the niche and receives the BMP signals to maintain bam repression and a stem cell fate. The other daughter is displaced from the niche and no longer receives BMP signals, resulting in de-repression of bam transcription and initiation of germ cell differentiation. This differentiating daughter, termed a cystoblast (CB), undergoes four rounds of mitosis with incomplete cytokinesis to form a sixteen-cell cyst. Further germ cell maturation involves envelopment of the sixteen-cell cyst by somatic follicle cells to form an egg chamber, wherein fifteen germ cells become the polyploid nurse cells and one germ cell becomes the oocyte. Maintenance of the GSC population sustains oocyte production for over two months (Pan et al., 2007). Females homozygous for the EMS-induced ote allele have small ovaries (Jiang et al., 2008), indicating that Ote is required for GSC maintenance.
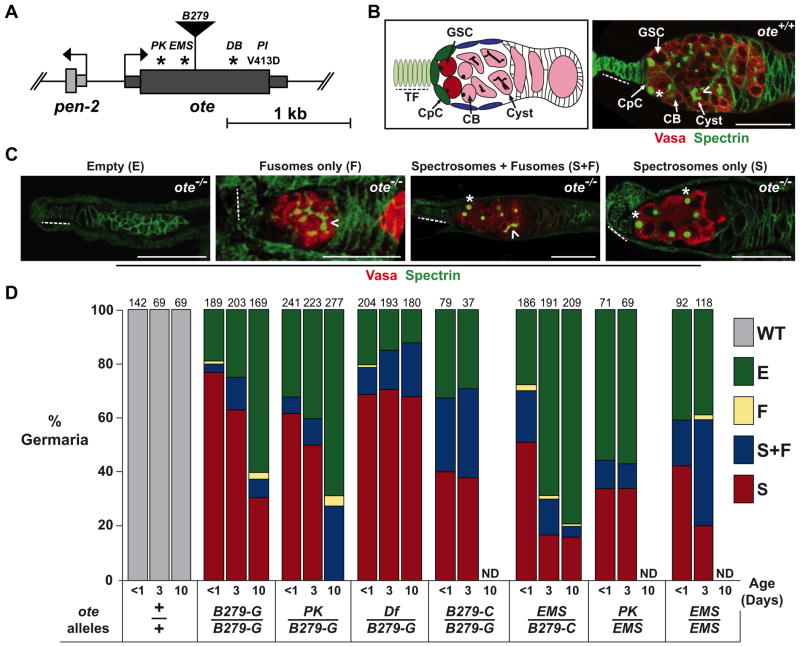
Figure 1. Loss of Otefin causes a complex GSC phenotype.
A. Schematic of the ote gene structure. The thick rectangle represents the ote coding region with the positions of the missense (PK, EMS, DB, PI) mutations and transposon insertion (B279) indicated. Asterisks indicate premature stop codons. B. Left: Shown is a schematic of a germarium. Terminal filament (TF) cells (light green, dashed line) and cap cells (CpC) (dark green) comprise the germline stem cell (GSC) niche. The GSCs (dark red) are positioned adjacent to the niche. Cystoblasts (CB) and cysts (pink) are positioned distal to the niche. Right: Shown is a wild type (ote+/+) germaria stained for Vasa (red) and Spectrin (green). A GSC is indicated by an asterisk and identified by the position near the niche and the presence of a spectrosome. A developing cyst is indicated by a chevron and identified by the presence of a fusome. C. Shown are representative images of each ote−/− germaria class in one-day-old ovaries stained for Vasa (red) and Spectrin (green), with class names noted above the image. All images show germaria oriented with anterior to the left. Scale bars represent 25 μm. D. Quantification of germarial class prevalence in several ote−/− backgrounds. WT: germaria with germ cells with spectrosomes, followed directly by germ cells with fusomes and then egg chambers. E: Empty germaria lacking germ cells, F: germaria with only fusome-containing germ cells, S+F: germaria with germ cells containing both spectrosomes and fusomes, and S: germaria with only spectrosome-containing germ cells. The total number of germaria counted is shown above each bar. In each case, ten or more ovaries were studied, obtained in two independent experiments. ND (Not Determined). See also Figure S1.
Following the isolation of an ote allele, the Chen laboratory conducted genetic and molecular analyses to investigate the role of this LEM-D protein in GSC maintenance (Jiang et al., 2008). Based on analyses of two ote mutations, these investigators reported that the majority of germaria in newly eclosed ote−/− ovaries lacked germ cells or contained only a few differentiated germ cells attached to one or two abnormal egg chambers, while a minority of germaria had one or two GSCs with sickly, undifferentiated germ cells and differentiated cysts. Germ cell loss was associated with altered regulation of bam transcription, founded on studies of expression of the P[bam-GFP] transgene in ote−/− germ cells, a transgene that has been widely used as a reporter of expression of the endogenous bam gene (Chen and McKearin, 2003b). Finally, biochemical data was obtained from somatic S2 cells that showed that Ote interacts with the co-Smad Medea at the silencer element on the bam gene. From these investigations, a model emerged that suggests that interaction of Ote with the Smad complex tethers the bam gene to the nuclear periphery to confers its transcriptional repression. Thus, loss of Ote would result in de-repression of bam transcription, resulting in GSC loss due to differentiation. This model is particularly significant, as it implies that a component of the nuclear lamina is required to scaffold effectors of the BMP signaling at the nuclear periphery, thereby silencing a critical developmental gene in an adult stem cell population.
Here we describe our results that show GSC loss in ote mutants is independent of the transcriptional regulation of bam. These data were obtained through extensive quantitative phenotypic analyses of ovaries obtained from females carrying several independently isolated ote alleles within different genetic backgrounds. Our studies show that in ote−/− ovaries, the majority of germaria contain expanded numbers of GSCs, not lost or inappropriately differentiating germ cells. Our studies of ote−/− developing gonads and adult ovaries indicate that transcription of bam remains repressed and germ cell differentiation is blocked in ote−/− GSCs. As a definitive test of the Chen model, we generated ote−/−, bam−/− double mutants. Analyses of these mutants revealed that ote−/− GSC loss persists, even though differentiation is prevented by deletion of the bam gene. Our studies demonstrate that ote−/− GSC loss results from GSC death, not differentiation.
Results
The major phenotype of ote−/− germaria is GSC expansion
To understand the effects of Ote loss on GSC maintenance, we analyzed phenotypes in ovaries obtained from ote+/+ and ote−/− females. These studies included ote alleles that we identified based upon our recognition that the ote genomic region includes the previously isolated female sterile gene called halted (hal) (Schupbach and Wieschaus, 1991). Using genetic complementation, we demonstrated that hal and ote mutations are allelic (data not shown). Subsequent molecular analyses revealed that all hal alleles contained point mutations in the ote open reading frame (Figure 1A), resulting in a complete loss of Ote protein in protein extracts from dissected ovaries (Supplemental Figure 1A). We have renamed the hal alleles otePK, oteDB and otePI.
We defined phenotypes of ovaries from ote+/+ and ote−/− three-day-old females. Ovaries were stained with antibodies against the well-characterized markers Vasa, a germline-specific RNA helicase (Lasko and Ashburner, 1988) and Spectrin, a cytoskeletal protein that shows a distinct subcellular localization depending upon cell type (Lin et al., 1994). In somatic cells, Spectrin localizes to the cytoplasmic periphery. In GSCs and their daughter CBs, Spectrin forms a spherical structure called the spectrosome, while in differentiating cysts, spectrin forms a branched structure called the fusome. Vasa and Spectrin staining showed that all ote+/+ germaria contained germ cells with spectrosomes, followed directly by germ cells with fusomes and developing egg chambers (Figure 1B). The average number of spectrosome-containing germ cells in ote+/+ germaria was ~5.7 (n=98, data not shown), consistent with previous reports(Song et al., 2007; Song et al., 2002). Immunohistochemical analyses of ote −/− germaria revealed that each ovary carried a range of mutant germarial phenotypes (Figure 1C), including 1) germaria with no germ cells (the empty or E class), 2) germaria with germ cells containing fusomes only (the F class), 3) germaria with germ cells containing both spectrosomes and fusomes (the S+F class), with the vast majority carrying a single developing cyst connected by an incompletely branched fusome, and 4) germaria with germ cells containing spectrosomes only (the S class). In the S class of germaria, the number of spectrosome-containing germ cells was higher than in ote+/+ germaria, with an average of ~24 spectrosome-containing germ cells per germaria (Supplemental Figure 1B). Quantification of the abundance of each phenotypic class demonstrated that the E and S classes of germaria were recovered at high frequency, with the S class representing as much as ~75% of all germaria studied (Figure 1D). Importantly, all of the ote−/− phenotypes were rescued by a transposon carrying only the ote gene (data not shown), confirming that the complex phenotypic defects resulted from loss of Ote.
The prevalence of the S class of germaria in all ote−/− ovaries was unexpected for several reasons. First, this phenotypic class was not reported previously (Jiang et al., 2008), even though these studies also utilized stocks carrying the oteB279 allele. Second, we expected that a loss of bam regulation would result in three-day-old ovaries having fewer germ cells than one-day-old ovaries, because more time would have elapsed to allow differentiating egg chambers to complete development. Together, these surprising observations prompted us to complete a careful developmental analysis of phenotypes present in ote−/− ovaries.
Vasa and Spectrin staining was performed on newly eclosed (less than one-day-old) and ten-day-old females obtained from seven different ote−/− genotypes. Females were generated from crosses involving stocks provided by the Chen lab (oteB279-C and oteEMS) and stocks maintained in the Geyer lab (oteB279-G, oteDf and otePK). Wild type newly eclosed ovaries contain egg chambers that have advanced to only mid-stages in oogenesis, because of the time required to complete egg chamber development. As such, we reasoned that if differentiation were responsible for ote−/− GSCs loss and the production of empty germaria, then newly eclosed ote−/− ovaries would contain increased numbers of differentiating mid-stage egg chambers relative to the numbers found in three-day-old ote−/− ovaries. However, this prediction was not met. Instead, newly eclosed ote−/− ovaries displayed the same range of germarial phenotypes as was seen in three-day-ovaries (Supplemental Figure 1C, Figure 2C). The newly eclosed ote−/− ovaries had several noteworthy characteristics. First, all ote−/− ovaries showed a high prevalence of S class germaria (Figure 1B, C). Notably, ovaries obtained from females carrying alleles from the Chen stock (oteB279-C and oteEMS alleles) had a lower prevalence of this class than ovaries obtained from females carrying Geyer stock alleles. Nonetheless, the S class of germaria represented 50% of total germaria in oteEMS/B279-C ovaries, emphasizing the consistent prominence of this phenotypic class. Second, fewer germ cells were present in S class germaria of newly eclosed relative to three-day-old ovaries (Supplemental Figure 1B). These observations indicate that ote−/− GSC-like cells continue to divide, at least in young females. Third, the prevalence of S class germaria was higher in newly eclosed ovaries and decreased in ten-day-old females, while the prevalence of empty germaria was lower in newly eclosed ovaries and increased in ten-day-old females (Figure 1C). These observations suggest that the empty class of germaria might arise from the S class. Taken together, our data demonstrate that loss of Ote causes a complex ovary phenotype, which is sensitive to genetic background. Despite this sensitivity, a prominent characteristic of the ote−/− phenotype is GSC expansion without germ cell differentiation.
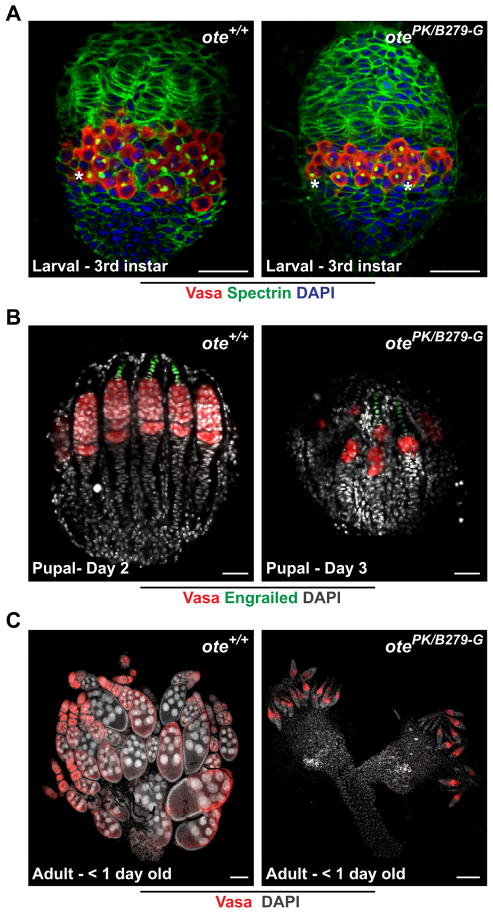
Figure 2. Germ cell differentiation is blocked in ote−/− ovaries.
A. Shown are developing gonads obtained from female wandering 3rd instar larvae stained for Vasa (red), Spectrin (green) and DAPI (blue). Scale bars represent 25 μm. B. Shown are developing gonads obtained from females aged two and three days after pupation. Gonads were stained for Vasa (red), Engrailed (green) and DAPI (blue). Scale bars represent 25 μm. C. Shown are ovaries obtained from newly eclosed females stained for Vasa (red) and DAPI (grey). Scale bars represent 100 μm. All gonads are oriented with anterior towards the top. Asterisks: spectrosome.
Mutant germline cells display an early block of germ cell differentiation
Transcription of bam is repressed during gonad formation. Ectopic bam expression promotes differentiation, as evidenced by the formation of fusomes (Gilboa and Lehmann, 2004). We studied the development of ote−/− larval and pupal gonads, to determine whether premature germ cell differentiation is observed at these stages, as predicted by the bam-repression model of Ote function. Wild type larval gonads contain a medial layer comprised primordial germ cells (PGCs) that are intermingled with somatic inner germarial sheath cells (IGS) (Dansereau and Lasko, 2008). To analyze larval phenotypes, we stained ote+/+ (n=29) and ote−/− (n=35) gonads with Vasa antibodies. We found that ote−/− gonads displayed a normal cellular organization, with ~30% fewer PGCs than age-matched ote+/+ gonads (n= 29; Figure 2A). Among these, five gonads from each genotype were co-stained with Spectrin. Importantly, all ote−/− PGCs in larval gonads contained spectrosomes with no evidence of branching, implying that ote−/− PGCs are not prematurely differentiating in the larval gonad. During pupal development, wild type gonads are divided into ovarioles that each contain an established stem cell niche (Sahut-Barnola et al., 1995). To analyze pupal phenotypes, we stained ote+/+ (n=10) and ote−/− (n=24) gonads with Vasa and Engrailed, a transcription factor expressed only in niche cells of the germaria (Forbes et al., 1996). We found that in ote+/+ and ote−/− pupal gonads, niches were formed and occupied by germ cells (Figure 2B). These findings imply that empty germaria found in adult ovaries do not result from an inability of PGCs to enter the niche. Additionally, our data shows that ote−/− pupal gonads do not contain differentiated egg chambers (Figure 2B), even though egg chambers were established in younger ote+/+ pupal gonads. Taken together, our studies of larval and pupal ote−/− gonads indicate that ote−/− germ cells are blocked in differentiation and fail to support the bam-repression model of Ote function.
Germ cells lacking Ote exhibit bam repression
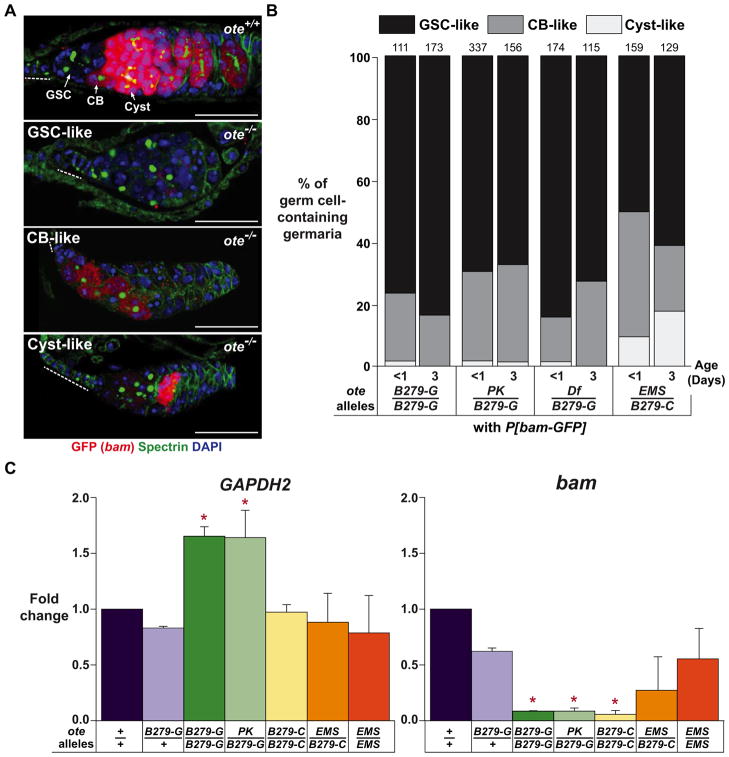
The absence of differentiation phenotypes in ote−/− larval and pupal gonads motivated our investigation of bam transcription. To this end, we generated ote+/+, P[bam-GFP] and ote−/−, P[bam-GFP] females and assessed bam expression in the ovary using histochemical analyses with GFP antibodies. In ote+/+ germaria, GFP staining was undetectable in GSCs, at low levels in CBs and at high levels in dividing germ cells (Figure 3A). In ote−/− germaria that contained germ cells, GFP staining varied and was sensitive to genotype. We found that the majority of ote−/− germaria of all genotypes showed undetectable or CB-like GFP staining (Figure 3B, Supplemental Figure 2). These data indicate that bam transcription is not activated in most ote−/− germ cells. To examine expression of the endogenous bam gene, we isolated RNAs from newly eclosed ote+/+ and ote−/− ovaries and used quantitative real time PCR to measure accumulation of bam RNA. We found that levels of bam RNA were significantly lower in ote−/− RNA isolated from oteB279G/B279G, otePK/B279G and oteDf/B279G ovaries relative to that found in ote+/+ RNA (Figure 3C). In ovaries from oteEMS/B279C and oteEMS/EMS females, bam levels were lower that wild type, but did not reach significance (Figure 3C). These data are consistent with the slightly higher bam-GFP expression levels seen in oteEMS/B279C ovaries (Figure 3A). Interestingly, all cases of higher bam expression were seen in females carrying the oteEMS allele, suggesting that the chromosome carrying this allele might also carry a second site modifier that affects bam expression. Regardless, the majority of ote−/− germ cells in ovaries of any genotype display bam repression, implying that the loss of Ote does not cause activation of bam transcription in GSCs.
Figure 3. Germ cells lacking Ote exhibit repression of bam transcription.
A. Shown are germaria from less than one-day-old ote+/+ and ote−/− females carrying the P[bam-GFP] reporter construct that were stained for GFP (red), Spectrin (green) and DAPI (blue). Top Panel: The level of GFP signal found in ote+/+ GSCs, CBs and Cysts is indicated. Bottom Panels: Three GFP signal intensities observed in ote−/− germaria are listed in the top left corner of each image and are categorized as GSC-like, CB-like and Cyst-like. The GSC-like category of ote−/− germaria lacked detectable GFP signal in all germ cells. Scale bars represent 25 μm. Dashed line: TF. B: Quantification of ote−/− germaria categorized by GFP signal. Genotypes are listed below each bar. The total number of germaria counted from ten or more ovaries in two independent experiments is shown above each bar. C. Graphs showing quantitative real time PCR analyses of RNAs obtained from two hour old wild type (ote+/+and oteB279-G/+) and ote−/− ovaries. Fold change is set relative to value obtained in ote+/+ ovary RNA. Error bars indicate standard deviation from three biological replicates. (*, P<0.05, Student’s t-test). See also Figure S2.
Ectopic bam expression accelerates ote−/− germ cell loss
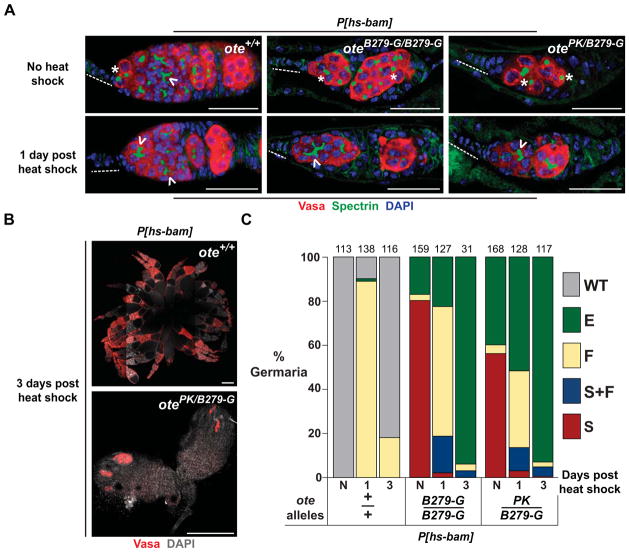
Bam is necessary and sufficient for germ cell differentiation (McKearin and Spradling, 1990; Ohlstein and McKearin, 1997). Based on our findings that loss of Ote is associated with blocked differentiation and repressed bam expression, we wondered whether increased expression of bam in ote−/− germ cells might promote differentiation. To this end, we generated ote−/−, P[hs-bam] females, which permits BMP-independent bam expression at elevated temperatures. The ote+/+, P[hs-bam] and ote−/−, P[hs-bam] females were heat shocked for one hour and ovaries were removed one or three days later for analysis. Vasa and Spectrin staining showed that one day after heat shock, ote+/+ germ cells contained fusomes, consistent with GSC differentiation (Figure 4A, C). Within three days after heat shock, ote+/+ GSCs had re-populated the niche, consistent with previous studies (Kai and Spradling, 2004). In ote−/− ovaries, germ cells with fusomes are found one day after heat shock, indicating that ectopic production of Bam promotes differentiation of ote−/− germ cells into multi-cellular cysts (Figure 4A, C). Surprisingly, three days after heat shock, ote−/− germaria were largely devoid of germ cells, displaying a greater amount of germ cell loss than ote−/− germ cells that had not expressed bam (Figure 4C). Further, a very low number of egg chambers were found in these ovaries (Figure 4B, C), indicating that bam expression is not sufficient for normal germ cell differentiation in ote−/− germ cells. These findings imply that ote−/− germ cells fail to undergo normal differentiation after bam expression. Instead, Bam production increased the rate of germ cell loss.
Figure 4. Ectopic bam expression accelerates ote−/− germ cell loss.
A. Shown are confocal images of germaria obtained from ote+/+ and ote−/− females carrying the P[hs-bam] transgene that were stained for Vasa (red), Spectrin (green) and DAPI (blue). The top panels show germaria from non-heat shocked females and the bottom panels show germaria from females that were heat shocked one day prior to ovary staining. Genotypes are listed in the top right corner of each image. Scale bars represent 25 μm. Dashed line: TF. Asterisk: spectrosome. Chevron: fusome. B. Shown are ovaries obtained from ote+/+ (top) and ote−/− (bottom) females carrying P[hs-bam] stained for Vasa (red) and DAPI (grey) three days after heat shock. Scale bars represent 250 μm. C. Shown is quantification of germarial class prevalence in females carrying the P[hs-bam] transgene. The germarial class designations are the same as described in Figure 1. N: non-heat shocked females aged for two days. The number of germaria scored from ten or more ovaries in two independent experiments is shown above each bar.
Loss of Bam does not rescue germ cell loss in ote−/− germaria
As a final test of whether mis-regulation of bam is responsible for germ cell loss in ote mutants, we generated ote−/−, bam−/− mutant females. We reasoned that if bam activation caused ote−/− GSC loss by differentiation, then deletion of the bam gene would prevent GSCs loss in ote−/− ovaries.
In total, females corresponding to four different ote−/−, bam−/− genetic backgrounds were generated. Ovaries were isolated from one- and three-day-old females and stained for Vasa and Spectrin. As in bam−/− ovaries (McKearin and Spradling, 1990), ovaries from one-day-old ote+/−, bam−/− females contained germaria with large GSC tumors and no empty germaria (Figure 5A). In contrast, ovaries from one-day-old ote−/−, bam−/− females of all genotypes displayed empty germaria at a prevalence that was similar to that of ote−/− ovaries (Figure 5B). In germ cell containing ote−/−, bam−/− germaria, germ cell numbers were similar to those in S class germaria of ote−/− ovaries but lower than those in age matched ote+/−; bam−/− sibling controls. Notably, the age-dependent germ cell loss seen in ote−/− ovaries persists, as the prevalence of empty germaria increased in three-day-old ote−/−, bam−/− ovaries (Figure 5B). Together, these data establish that the loss of GSCs in ote−/− mutants is not due to activation of bam expression in GSCs.
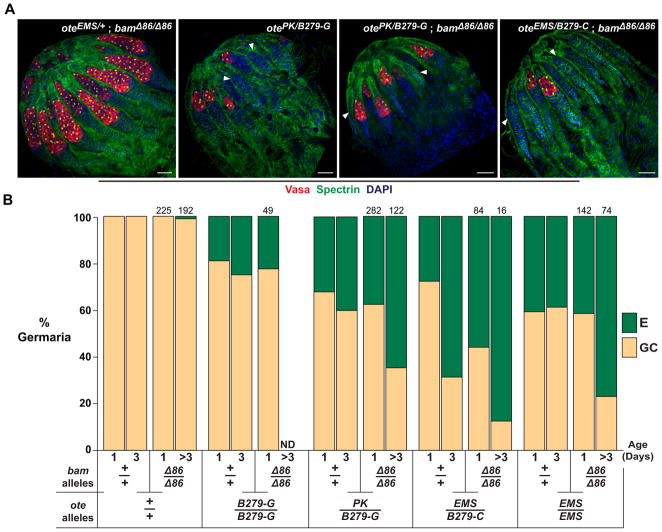
Figure 5. Loss of Bam does not rescue GSC loss in ote−/− ovaries.
A. Shown are confocal images of ovaries obtained from one-day-old females stained for Vasa (red), Spectrin (green) and DAPI (blue). The anterior of each ovary is oriented toward the top left corner. Arrowheads indicate empty germaria. Genotypes are listed in the top right corner. Scale bars represent 25 μm. B. Shown is a graph of the quantification of the prevalence of germaria without (empty, E) or with germ cells (germ cell containing, GC) in ovaries of wild type and mutant females. Ovary genotypes are listed below each bar. Data for the ote+/+, bam+/+ and ote−/−, bam+/+ genotypes represent a re-categorization of data presented in Figure 1D, wherein the WT, F, S+F and S classes of germaria are encompassed in the GC category. The total number of bam−/− germaria scored is indicated above each bar. ND (Not Determined).
Discussion
Otefin is required for GSC maintenance in females. Based on studies of ote mutants, the Chen laboratory proposed that Ote is required for peripheral localization and silencing of the key germline differentiation gene, bam (Jiang et al., 2008). This model makes several predictions. First, prominent GSC loss should be found in ote−/− ovaries of any null background. Second, ote−/− germ cells in developing gonads and young females should display signs of differentiation, because bam repression is required during gonad development (Gilboa and Lehmann, 2004). Third, bam should be constitutively de-repressed in ote−/− germ cells. Fourth, loss of Bam should rescue the loss of ote−/− GSCs. Studies described herein address these predictions.
Building from studies using five null ote alleles, we obtained several lines of evidence that fail to support the model that Ote is required for transcriptional repression of bam. First, we discovered that ote−/− germaria frequently contain excess numbers of GSC-like germ cells (the S class of germaria, Figure 1C, D), suggesting that loss of Ote causes defects in germ cell differentiation and not inappropriate GSC differentiation. Second, we found no evidence of PGC differentiation in developmental analyses of ote−/− larval and pupal gonads (Figure 2A, B), suggesting that bam transcription is repressed at these stages. Third, we demonstrate that as ote−/− females aged, the prevalence of the S class of germaria decreased, while the prevalence of the E germaria class increased without an increase in the F class (Figure 1D). These data suggest that ote−/− GSCs die prematurely, without differentiation. This postulate is supported by the inability of ectopic production of Bam to produce viable egg chambers in ote mutants (Figure 4B). Fourth, we completed two independent assessments of bam expression in adult ovaries, with both demonstrating that transcriptional silencing of bam is maintained in ote−/− ovaries (Figure 3). These findings are consistent with the observation that artificial tethering of the bam locus to the nuclear periphery in germ cells is insufficient for bam gene silencing (Sui and Yang, 2011). Fifth, we found that germ cell loss was unchanged in ote−/−, bam−/− ovaries, with age-dependent germ cell loss in the absence of bam function (Figure 5). Our studies reveal that young ote−/−, bam−/− ovaries carry empty germaria, with continued germ cell loss in adult females. These data demonstrate that empty germaria in ote−/− ovaries does not result from inappropriate GSC differentiation. Together, these studies show that of Ote does not cause germ cell loss through activation of bam transcription. Instead, our studies suggest that Ote loss causes GSC death.
Several factors may explain the different conclusions obtained in our studies and those performed previously (Jiang et al., 2008). First, our studies used quantitative phenotypic analyses of several developmental stages of ote−/− ovaries, which revealed the presence of a previously unreported class of germaria, the S class. Additionally, this approach allowed us to understand the age-dependence of the ote mutant phenotype. Second, we studied ote−/− ovaries from females of multiple genetic backgrounds. These analyses reveal that the complex ote−/− phenotype is sensitive to genetic background. Interestingly, females carrying the EMS allele, oteEMS, show an ote−/− phenotype with a higher prevalence of the E class of germaria and a lower, but significant, prevalence of the S class (Figure 1D). Further, ovaries obtained from females carrying oteEMS showed higher levels of bam expression (Figure 3). We speculate that these skewed phenotypes might be caused by a second site modifier mutation on the EMS chromosome that affects the oteEMS phenotype. Third, ote−/− GSCs differentiation was inferred from studies employing clonal analyses that showed a faster rate of loss of ote−/− GSCs than ote+/+ GSCs, without corresponding evidence of apoptosis. Our findings that ote−/−, bam−/− GSCs are lost in the absence of an ability to differentiate imply that ote−/− GSCs die. These observations indicate that conventional methods to detect GSC death in the germaria may not be informative. This suggestion is supported by several observations. First, over-expression of Drosophila p53 leads to loss of ovarian stem cells, which is not suppressed by over-expression of caspase inhibitors or associated with TUNEL labeling indicative of DNA fragmentation (Bakhrat et al., 2010). Second, other instances of germ cell death have been reported, wherein germ cell loss occurred in the absence of standard markers of apoptosis (Hanyu-Nakamura et al., 2004; Sano et al., 2005).
Our studies imply two requirements for Ote. First, Ote may be required for the timely progression through the cell cycle. This prediction is suggested by our findings that ote−/− gonads contain fewer PGCs compared to ote+/+ gonads (Figure 2, data not shown) and that bam tumors in the absence of Ote are small compared to bam tumors with Ote (Figure 5A). A role of Ote in the cell cycle may be linked to its requirement in nuclear envelope reformation following mitosis (Ashery-Padan et al., 1997) or its localization to the centrosome (Habermann et al., 2012). Second, Ote may be required for germ cell differentiation. We note that in the absence of Ote, germ cell differentiation is defective (Figure 1, 2). We found that ectopic production of Bam promoted limited germ cell differentiation, but these germ cells were rapidly lost (Figure 4B, C). These latter observations suggest that forced differentiation of ote−/− GSCs is detrimental to survival.
Ote is a member of the LEM-D family of nuclear lamina proteins. Mutations in genes encoding LEM-D proteins cause a broad spectrum of human diseases, known as laminopathies (Worman et al., 2010). Interestingly, the phenotypic variation observed in ote mutants is reminiscent of the extreme phenotypic diversity and non-penetrance that has been reported in sporadic and familial laminopathy cases (Higuchi et al., 2005; Mercuri et al., 2004; Rankin et al., 2008). For example, several cases have been reported where patients who carry an identical mutation in the gene encoding lamins A/C (LMNA) display a wide variety of clinical symptoms. Lamin A/C associates with a large number of proteins at the nuclear envelope, suggesting that phenotypic expressivity may be influenced by differences in these lamin associated components. A feature shared among laminopathies is that affected tissues arise from mesenchymal lineages that are replenished by adult stem cells (Willis et al., 2008). Emerging evidence suggests that these stem cell populations are compromised in laminopathies, but how these cells are uniquely compromised remains unclear (Maraldi et al., 2010; Wheeler and Ellis, 2008; Willis et al., 2008). Further studies of Ote will provide needed insights related to disease mechanisms caused by the loss of LEM-D proteins.
Experimental Procedures
Drosophila stocks and culture conditions
All Drosophila stocks and crosses were incubated at 25°C at 70% humidity on standard cornmeal agar medium (yeast, cornmeal, and sugar), with p-hydroxybenzoic acid methyl ester as a mold inhibitor. The mutant and transgenic stocks used in this study include: oteB279-Geyer (B279-G) (Bloomington stock no. 16189), oteB279-Chen (B279-C) (D. Chen (Jiang et al., 2008)), oteEMS (D. Chen), oteDB (Bloomington Stock no. 5092), otePK (T. Schupbach, (Schupbach and Wieschaus, 1991), oteDf corresponding to Df(2R)ED3636 (Bloomington Stock no. 9413), bamΔ86 (M. Busczcak and Bloomington Stock no. 5427), P[bam-GFP] (Chen and McKearin, 2003b) and P[hs-bam] (Chen and McKearin, 2003a).
Immunohistochemistry
Drosophila ovaries were stained and images were collected and processed according to (Baxley et al., 2011). The three- and ten-day-old females were cultured in vials supplemented with yeast pellets in the presence of males. To ensure an unbiased sampling of germarial phenotypes, we analyzed at least ten ovaries per experiment, and completed a minimum of two independent experiments. As an additional measure to decrease sampling bias, we took 40X images of each ovary and quantified all of the germaria within that image. Primary antibodies include: rabbit α-Vasa (Santa Cruz, sc-30210) at 1:300 or 1:1000, mouse α-Spectrin [Developmental Studies Hybridoma Bank (DSHB, 3A9) ] at 1:100, mouse α-Engrailed (DSHB, 4D9) at 1:10, rabbit α-GFP (Invitrogen, A-11122) at 1:5000, mouse α-Ote (Y. Gruenbaum) at 1:10 and mouse α-tubulin (Sigma, T5168) at 1:40,000. Images were collected on a Bio-Rad Radiance 2100 Multiphoton/Confocal Microscope or a Zeiss 710 Confocal Microscope. All images were processed using ImageJ.
Ectopic production of Bam
Less than one day old P[hs-bam] females were heat shocked twice at 37°C for one hour, separated by a two hour recovery period (Chen and McKearin, 2003a). Flies were recovered and grown at 25°C for either one or three days on yeasted food. Non-heat shock control flies were kept at 25°C on yeasted food.
qPCR analysis of bam gene expression
Twenty five pairs of two-hour-old ovaries per biological replicate were dissected in PBS and frozen at −80°C. This age was chosen because such ovaries are closer in size and development content to ote mutant ovaries. RNA was isolated from frozen ovaries using TRIzol (Invitrogen). Total RNA was DNAse I treated using DNA-free (Ambion) and reverse-transcribed using High Capacity cDNA kit with random hexamer primers (Applied Biosystems). Cycle threshold values were normalized to housekeeping gene, RpL32. Fold-enrichment was calculated using ΔΔCt method (Livak and Schittegen, 2001). Primer pairs for RNA quantification are listed 5′ to 3′ for RpL32 (forward: AAGATGACCATCCGCCCAGCATAC and reverse: ACGCACTCTGTTGTCGATACCCTTG), GAPDH (forward: CACTCGTCGGTGTTCGATGCCAAG and reverse: TCGATGACGCGGTTGGAGTAGC) and bam (forward: GACGAGGTGGCGATGATGGCAC and reverse: TTCCTCTCCGCTCTGATCGCCAATC).
Generation of ote, bam double mutant females
To generate ote and bam double mutants, lethal mutations on the bamΔ86 chromosome were removed, using a crosses scheme that permitted recombination on the third chromosome. Briefly, ote mutant females (y1 w67c23; ote−/CyO y+) were crossed to bam mutant males (y+ w+; Sp/CyO; ry506 e1 bamΔ86/TM3 ryRK Sb1 Ser1) (M. Busczcak). Female F1 progeny (y1 w67c23/y+ w+; ote−/CyO; ry 506 e1 bamΔ86/ry+ e+ bam+) were crossed to male F1 progeny (y1 w67c23/y+ w+; ote−/CyO; ry 506 e1 bamΔ86/ry+ e+ bam+) carrying a different ote− allele. Non-CyO (ote−/−) female F2 progeny homozygous for e1 were selected for further analysis. Ovaries were dissected and individually stained, while the DNA was isolated from the remaining carcass. This DNA was genotyped using PCR to distinguish bam+ from bamΔ86, as bamΔ86 is a structurally distinct allele that carries an ~1.2 kb deletion. Primer pairs listed 5′ to 3′ for bam genotyping included forward: GAGTTGCGAAGCGAGTGAGGTG and reverse: TCTTTAAATGCGCCCGGGTGAATG.
Supplementary Material
Supplemental Figure 1: Germline phenotypes in ote−/− females change with age, related to Figure 1.
Supplemental Figure 2: The majority of bam-positive ote−/− germaria exhibit blocked differentiation, related to Figure 3.
Highlights.
Germaria in young ote mutant ovaries display GSC loss and expanded GSC numbers.
A primary defect in ote−/− germ cells is a block in differentiation.
Transcription of the bam differentiation gene is not activated in ote−/− GSCs.
Age-dependent ote−/− GSC loss results from cell death, not differentiation.
Acknowledgments
We thank Trudi Schupbach, Dahua Chen, Dennis McKearin, Michael Busczcak and Yosef Gruenbaum for generously providing fly stocks and antibodies. We are grateful for technical assistance provided by Eric Schultz, Emma Hornick, Cathy Staloch and members of the University of Iowa Central Microscopy Facility. We thank members of the Geyer laboratory for comments on the manuscript. A Muscular Dystrophy grant (MDA4221) and a NIH R01 (GM087341) to P.K.G. supported this research. B.S.P was supported by a pre-doctoral fellowship from the American Heart Association (0910129G).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashery-Padan R, Ulitzur N, Arbel A, Goldberg M, Weiss AM, Maus N, Fisher PA, Gruenbaum Y. Localization and posttranslational modifications of otefin, a protein required for vesicle attachment to chromatin, during Drosophila melanogaster development. Mol Cell Biol. 1997;17:4114–4123. doi: 10.1128/mcb.17.7.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakay M, Wang Z, Melcon G, Schiltz L, Xuan J, Zhao P, Sartorelli V, Seo J, Pegoraro E, Angelini C, et al. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb-MyoD pathways in muscle regeneration. Brain. 2006;129:996–1013. doi: 10.1093/brain/awl023. [DOI] [PubMed] [Google Scholar]
- Bakhrat A, Pritchett T, Peretz G, McCall K, Abdu U. Drosophila Chk2 and p53 proteins induce stage-specific cell death independently during oogenesis. Apoptosis. 2010;15:1425–1434. doi: 10.1007/s10495-010-0539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxley RM, Soshnev AA, Koryakov DE, Zhimulev IF, Geyer PK. The role of the Suppressor of Hairy-wing insulator protein in Drosophila oogenesis. Dev Biol. 2011;356:398–410. doi: 10.1016/j.ydbio.2011.05.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, Huang Y, Ghirlando R, Wilson KL, Craigie R, Clore GM. Solution structure of the constant region of nuclear envelope protein LAP2 reveals two LEM-domain structures: one binds BAF and the other binds DNA. Embo J. 2001;20:4399–4407. doi: 10.1093/emboj/20.16.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003a;13:1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Chen D, McKearin DM. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 2003b;130:1159–1170. doi: 10.1242/dev.00325. [DOI] [PubMed] [Google Scholar]
- Chen S, Wang S, Xie T. Restricting self-renewal signals within the stem cell niche: multiple levels of control. Curr Opin Genet Dev. 2011;21:684–689. doi: 10.1016/j.gde.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Dansereau DA, Lasko P. The development of germline stem cells in Drosophila. Methods Mol Biol. 2008;450:3–26. doi: 10.1007/978-1-60327-214-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes AJ, Spradling AC, Ingham PW, Lin H. The role of segment polarity genes during early oogenesis in Drosophila. Development. 1996;122:3283–3294. doi: 10.1242/dev.122.10.3283. [DOI] [PubMed] [Google Scholar]
- Geyer PK, Vitalini MW, Wallrath LL. Nuclear organization: taking a position on gene expression. Curr Opin Cell Biol. 2011;23:354–359. doi: 10.1016/j.ceb.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa L, Lehmann R. Repression of primordial germ cell differentiation parallels germ line stem cell maintenance. Curr Biol. 2004;14:981–986. doi: 10.1016/j.cub.2004.05.049. [DOI] [PubMed] [Google Scholar]
- Habermann K, Mirgorodskaya E, Gobom J, Lehmann V, Muller H, Blumlein K, Deery MJ, Czogiel I, Erdmann C, Ralser M, et al. Functional analysis of centrosomal kinase substrates in Drosophila melanogaster reveals a new function of the nuclear envelope component otefin in cell cycle progression. Mol Cell Biol. 2012;32:3554–3569. doi: 10.1128/MCB.00814-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyu-Nakamura K, Kobayashi S, Nakamura A. Germ cell-autonomous Wunen2 is required for germline development in Drosophila embryos. Development. 2004;131:4545–4553. doi: 10.1242/dev.01321. [DOI] [PubMed] [Google Scholar]
- Haraguchi T, Holaska JM, Yamane M, Koujin T, Hashiguchi N, Mori C, Wilson KL, Hiraoka Y. Emerin binding to Btf, a death-promoting transcriptional repressor, is disrupted by a missense mutation that causes Emery-Dreifuss muscular dystrophy. Eur J Biochem. 2004;275:1035–1045. doi: 10.1111/j.1432-1033.2004.04007.x. [DOI] [PubMed] [Google Scholar]
- Harris RE, Ashe HL. Cease and desist: modulating short-range Dpp signalling in the stem-cell niche. EMBO Rep. 2011;12:519–526. doi: 10.1038/embor.2011.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi Y, Hongou M, Ozawa K, Kokawa H, Masaki M. A family of Emery-Dreifuss muscular dystrophy with extreme difference in severity. Pediatr Neurol. 2005;32:358–360. doi: 10.1016/j.pediatrneurol.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Holaska JM, Lee KK, Kowalski AK, Wilson KL. Transcriptional repressor germ cell-less (GCL) and barrier to autointegration factor (BAF) compete for binding to emerin in vitro. J Biol Chem. 2003;278:6969–6975. doi: 10.1074/jbc.M208811200. [DOI] [PubMed] [Google Scholar]
- Jiang X, Xia L, Chen D, Yang Y, Huang H, Yang L, Zhao Q, Shen L, Wang J, Chen D. Otefin, a nuclear membrane protein, determines the fate of germline stem cells in Drosophila via interaction with Smad complexes. Dev Cell. 2008;14:494–506. doi: 10.1016/j.devcel.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- Lasko PF, Ashburner M. The product of the Drosophila gene vasa is very similar to eukaryotic initiation factor-4A. Nature. 1988;335:611–617. doi: 10.1038/335611a0. [DOI] [PubMed] [Google Scholar]
- Lin F, Blake DL, Callebaut I, Skerjanc IS, Holmer L, McBurney MW, Paulin-Levasseur M, Worman HJ. MAN1, an inner nuclear membrane protein that shares the LEM domain with lamina-associated polypeptide 2 and emerin. J Biol Chem. 2000;275:4840–4847. doi: 10.1074/jbc.275.7.4840. [DOI] [PubMed] [Google Scholar]
- Lin F, Morrison JM, Wu W, Worman HJ. MAN1, an integral protein of the inner nuclear membrane, binds Smad2 and Smad3 and antagonizes transforming growth factor-beta signaling. Hum Mol Genet. 2005;14:437–445. doi: 10.1093/hmg/ddi040. [DOI] [PubMed] [Google Scholar]
- Lin H, Yue L, Spradling AC. The Drosophila fusome, a germline-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansharamani M, Wilson KL. Direct binding of nuclear membrane protein MAN1 to emerin in vitro and two modes of binding to barrier-to-autointegration factor. J Biol Chem. 2005;280:13863–13870. doi: 10.1074/jbc.M413020200. [DOI] [PubMed] [Google Scholar]
- Maraldi NM, Capanni C, Cenni V, Fini M, Lattanzi G. Laminopathies and lamin-associated signaling pathways. J Cell Biochem. 2010;112:979–992. doi: 10.1002/jcb.22992. [DOI] [PubMed] [Google Scholar]
- Markiewicz E, Tilgner K, Barker N, van de Wetering M, Clevers H, Dorobek M, Hausmanowa-Petrusewicz I, Ramaekers FC, Broers JL, Blankesteijn WM, et al. The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus. Embo J. 2006;25:3275–3285. doi: 10.1038/sj.emboj.7601230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKearin DM, Spradling AC. bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990;4:2242–2251. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- Melcon G, Kozlov S, Cutler DA, Sullivan T, Hernandez L, Zhao P, Mitchell S, Nader G, Bakay M, Rottman JN, et al. Loss of emerin at the nuclear envelope disrupts the Rb1/E2F and MyoD pathways during muscle regeneration. Hum Mol Genet. 2006;15:637–651. doi: 10.1093/hmg/ddi479. [DOI] [PubMed] [Google Scholar]
- Mercuri E, Poppe M, Quinlivan R, Messina S, Kinali M, Demay L, Bourke J, Richard P, Sewry C, Pike M, et al. Extreme variability of phenotype in patients with an identical missense mutation in the lamin A/C gene: from congenital onset with severe phenotype to milder classic Emery-Dreifuss variant. Arch Neurol. 2004;61:690–694. doi: 10.1001/archneur.61.5.690. [DOI] [PubMed] [Google Scholar]
- Meshorer E, Gruenbaum Y. Gone with the Wnt/Notch: stem cells in laminopathies, progeria, and aging. J Cell Biol. 2008;181:9–13. doi: 10.1083/jcb.200802155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, McKearin D. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development. 1997;124:3651–3662. doi: 10.1242/dev.124.18.3651. [DOI] [PubMed] [Google Scholar]
- Padan R, Nainudel-Epszteyn S, Goitein R, Fainsod A, Gruenbaum Y. Isolation and characterization of the Drosophila nuclear envelope otefin cDNA. J Biol Chem. 1990;265:7808–7813. [PubMed] [Google Scholar]
- Pan L, Chen S, Weng C, Call G, Zhu D, Tang H, Zhang N, Xie T. Stem cell aging is controlled both intrinsically and extrinsically in the Drosophila ovary. Cell Stem Cell. 2007;1:458–469. doi: 10.1016/j.stem.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Rankin J, Auer-Grumbach M, Bagg W, Colclough K, Nguyen TD, Fenton-May J, Hattersley A, Hudson J, Jardine P, Josifova D, et al. Extreme phenotypic diversity and nonpenetrance in families with the LMNA gene mutation R644C. Am J Med Genet A. 2008;146A:1530–1542. doi: 10.1002/ajmg.a.32331. [DOI] [PubMed] [Google Scholar]
- Sahut-Barnola I, Godt D, Laski FA, Couderc JL. Drosophila ovary morphogenesis: analysis of terminal filament formation and identification of a gene required for this process. Dev Biol. 1995;170:127–135. doi: 10.1006/dbio.1995.1201. [DOI] [PubMed] [Google Scholar]
- Sano H, Renault AD, Lehmann R. Control of lateral migration and germ cell elimination by the Drosophila melanogaster lipid phosphate phosphatases Wunen and Wunen 2. J Cell Biol. 2005;171:675–683. doi: 10.1083/jcb.200506038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics. 1991;129:1119–1136. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Call GB, Kirilly D, Xie T. Notch signaling controls germline stem cell niche formation in the Drosophila ovary. Development. 2007;134:1071–1080. doi: 10.1242/dev.003392. [DOI] [PubMed] [Google Scholar]
- Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, Xie T. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- Song X, Xie T. DE-cadherin-mediated cell adhesion is essential for maintaining somatic stem cells in the Drosophila ovary. Proc Natl Acad Sci U S A. 2002;99:14813–14818. doi: 10.1073/pnas.232389399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Zhu CH, Doan C, Xie T. Germline stem cells anchored by adherens junctions in the Drosophila ovary niches. Science. 2002;296:1855–1857. doi: 10.1126/science.1069871. [DOI] [PubMed] [Google Scholar]
- Sui L, Yang Y. Distinct effects of nuclear membrane localization on gene transcription silencing in Drosophila S2 cells and germ cells. J Genet Genomics. 2011;38:55–61. doi: 10.1016/j.jcg.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Wagner N, Krohne G. LEM-Domain proteins: new insights into lamin-interacting proteins. Int Rev Cytol. 2007;261:1–46. doi: 10.1016/S0074-7696(07)61001-8. [DOI] [PubMed] [Google Scholar]
- Wagner N, Schmitt J, Krohne G. Two novel LEM-domain proteins are splice products of the annotated Drosophila melanogaster gene CG9424 (Bocksbeutel) Eur J Cell Biol. 2004;82:605–616. doi: 10.1078/0171-9335-00350. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Ellis JA. Molecular signatures of Emery-Dreifuss muscular dystrophy. Biochem Soc Trans. 2008;36:1354–1358. doi: 10.1042/BST0361354. [DOI] [PubMed] [Google Scholar]
- Wilkinson FL, Holaska JM, Zhang Z, Sharma A, Manilal S, Holt I, Stamm S, Wilson KL, Morris GE. Emerin interacts in vitro with the splicing-associated factor, YT521-B. Eur J Biochem. 2003;270:2459–2466. doi: 10.1046/j.1432-1033.2003.03617.x. [DOI] [PubMed] [Google Scholar]
- Willis ND, Wilson RG, Hutchison CJ. Lamin A: a putative colonic epithelial stem cell biomarker which identifies colorectal tumours with a more aggressive phenotype. Biochem Soc Trans. 2008;36:1350–1353. doi: 10.1042/BST0361350. [DOI] [PubMed] [Google Scholar]
- Wilson KL, Foisner R. Lamin-binding Proteins. Cold Spring Harb Perspect Biol. 2010;2:a000554. doi: 10.1101/cshperspect.a000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worman HJ, Ostlund C, Wang Y. Diseases of the nuclear envelope. Cold Spring Harb Perspect Biol. 2010;2:a000760. doi: 10.1101/cshperspect.a000760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- Zheng R, Ghirlando R, Lee MS, Mizuuchi K, Krause M, Craigie R. Barrier-to-autointegration factor (BAF) bridges DNA in a discrete, higher-order nucleoprotein complex. Proc Natl Acad Sci U S A. 2000;97:8997–9002. doi: 10.1073/pnas.150240197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Germline phenotypes in ote−/− females change with age, related to Figure 1.
Supplemental Figure 2: The majority of bam-positive ote−/− germaria exhibit blocked differentiation, related to Figure 3.