Abstract
Background
We have previously demonstrated that heparin-binding EGF-like growth factor (HB-EGF) administration protects the intestines from ischemia/reperfusion (I/R) injury in vivo. We have also shown that HB-EGF promotes mesenchymal stem cell (MSC) proliferation and migration in vitro. The goals of the current study were to examine the effects of HB-EGF and both bone marrow (BM)- and amniotic fluid (AF)-derived MSC on intestinal I/R injury in vivo.
Materials and Methods
MSC were isolated from pan-EGFP mice, expanded, and purified. Pluripotency was confirmed by induced differentiation. Mice were subjected to terminal ileum I/R and received either: 1) no therapy; 2) HB-EGF; 3) BM-MSC; 4) HB-EGF + BM-MSC; 5) AF-MSC; or 6) HB-EGF +AF-MSC. MSC engraftment, histologic injury, and intestinal permeability were quantified.
Results
There was increased MSC engraftment into injured compared to uninjured intestine for all experimental groups, with significantly increased engraftment for AF-MSC + HB-EGF compared to AF-MSC alone. Administration of HB-EGF and MSC improved intestinal histology and intestinal permeability after I/R injury. The greatest improvement was with combined administration of HB-EGF + AF-MSC.
Conclusions
Both HB-EGF alone and MSC alone can protect the intestines from I/R injury, with synergistic efficacy occurring when HB-EGF and AF-MSC are administered together.
Keywords: heparin-binding EGF-like growth factor, mesenchymal stem cells, bone marrow, amniotic fluid, ischemia/reperfusion, intestinal injury
INTRODUCTION
Mesenchymal stem cells (MSC) are non-hematopoietic, pluripotent, self-renewing progenitor cells with a characteristic spindle-shaped morphology, that have been shown to contribute to the maintenance and regeneration of various connective tissues.1 They are mobilized from bone marrow in response to tissue injury and aid in repair after a variety of end-organ injury-models including models of myocardial infarction,2 spinal cord injury,3 renal ischemia/reperfusion (I/R) injury,4 intestinal radiation injury5 and ischemic cerebral injury.6 MSC can engraft into injured tissues where they can differentiate to replace injured cells.7,8 MSC also exert paracrine functions by secreting protective factors that act on injured cells.9,10 The robust, self-renewing, multilineage differentiation potential of MSC makes these cells very desirable candidates for possible clinical cellular therapy.11
Populations of MSC derived from bone marrow (BM-MSC)12 and amniotic fluid (AF-MSC)13 have been isolated. Most previous studies have focused on BM-MSC. Much like BM-MSC, AF-MSC have dynamic differentiation potential and can be induced into cells of all three embryonic lineages.14, 15 AF-MSC may actually have more potent differentiation potential compared to BM-MSC.16 Furthermore, unlike embryonic stem cells, AF-MSC are not tumorigenic.15 These characteristics make AF-MSC a very attractive option for potential cellular therapy.
Heparin-binding EGF-like growth factor (HB-EGF) was initially identified as a secreted product of cultured human macrophages17 that is a member of the epidermal growth factor (EGF) family.18 We have previously demonstrated that HB-EGF promotes angiogenesis,19 and acts as a potent intestinal cytoprotective agent in animal models of intestinal I/R injury,20 hemorrhagic shock and resuscitation (HS/R)21 and experimental necrotizing enterocolitis (NEC).22 We have also demonstrated that HB-EGF can protect native intestinal stem cells (ISC) in a rat model of NEC.23 HB-EGF promotes MSC proliferation and migration, and protects MSC from apoptosis, with a more profound effect on AF-MSC compared to BM-MSC.24 Furthermore, HB-EGF and BM-MSC act synergistically to reduce intestinal injury and improve survival in experimental NEC.25 The goal of the current study was to investigate the interactions between HB-EGF and BM- and AF-MSC in a mouse model of intestinal I/R injury.
METHODS
Isolation of BM-MSC
All procedures were performed with approval from the Institutional Animal Care and Use Committee of the Research Institute of Nationwide Children’s Hospital (protocol #AR06-0002). BM-MSC were harvested from adult pan-EGFP C57/BL6 mice following previously described protocols.12 Briefly, mice were euthanized by cervical dislocation, and the femurs and tibias were removed and dissected free of surrounding tissue using sterile technique. The marrow was flushed out with 2 ml of phosphate-buffered saline (PBS) using a sterile syringe and 20 gauge needle. The marrow pellet was dispersed by gentle pipetting and transferred to uncoated cell culture flasks.
Isolation of AF-MSC
Amniotic fluid was obtained via amniocentesis of pan-EGFP C57/BL6 mice using an adaptation of previously described techniques.13 Female mice at 12.5 days gestation were anesthetized with 2.5% tribromoethanol via intraperitoneal (IP) injection. The abdominal skin was shaved and scrubbed with 70% ethanol. A midline laparotomy was performed and the gravid uterus identified. The uterus was opened and amniocentesis was performed under direct vision of the individual placentas using a 23 gauge needle. Amniotic fluid samples were transferred to uncoated cell culture flasks.
Cell Culture and Differentiation
After harvesting, BM-MSC and AF-MSC were cultured in Dulbecco’s modified Eagle’s medium: Nutrient Mixture F-12 with GlutaMax (DMEM/F12; Invitrogen, Carlsbad, CA) supplemented with 10% MSC Qualified Fetal Bovine Serum (FBS; Invitrogen, Carlsbad, CA) and gentamycin (5 µg/ml) (Invitrogen, Carlsbad, CA) in uncoated cell culture flasks at 37°C in a humidified atmosphere of 5%CO2/95%Nitrogen. After 24 h, non-adherent cells were washed away with PBS and discarded. Adherent MSC were purified and expanded during successive passages. MSC were passaged once they achieved 80% confluence to expand the primary cultures. MSC from passages four through nine were used for all experiments. Differentiation assays were performed with cells at passage 4–5, and the remaining assays were performed using cells at passage 5–9.
MSC pluripotency was confirmed using the STEMPRO Adipogenesis Differentiation Kit (Invitrogen, Carlsbad, CA) and the STEMPRO Osteogenesis Differentiation Kit (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions, as we have described previously.24
Murine Ischemia/Repurfusion Model
All procedures were performed with approval from the Institutional Animal Care and Use Committee of the Research Institute of Nationwide Children’s Hospital (protocol #AR000903). 8 to 10 week old male wild-type FVB mice weighing ~20 grams were used for all experiments. Animals were anesthetized with 3% isoflurane and the abdomen was shaved and rinsed with 70% isopropanol. Anesthesia was maintained using 1% isoflurane and body temperature was maintained with a heating pad.
A midline laparotomy was made and branches of the superior mesenteric artery were identified. Branches supplying the distal six centimeters of the terminal ileum were occluded with non-traumatic vascular clamps. For sham operated animals, no vascular clamps were applied. The abdomen was temporarily closed and segmental mesenteric artery occlusion (sMAO) was maintained for 60 minutes. The vascular clamps were then removed and 0.2 mL of either HB-EGF (1000 µg/kg) or PBS was administered via an intraluminal injection into the more proximal small intestine using a 0.3 mL low-dose U-100 insulin syringe and a 29-guage needle (Becton Dickinson, Franklin Lakes, NJ). The abdominal wall was sutured closed in layers. Animals were kept warm and observed until fully awake and ambulatory. Animals were euthanized 24 h after reperfusion via cervical dislocation under anesthesia.
MSC Preparation and Administration
Adherent BM-MSC or AF-MSC were trypsinized (0.25% trypsin; Cellgro, Manassas, VA) for 5 min and then neutralized with DMEM/F12 supplemented with 10% FBS. Cells were quantified using a hemocytometer. MSC were centrifuged, washed with sterile PBS, re-suspended in PBS to a concentration of 5×106 MSC/mL, and then gently mixed to prevent aggregation. Animals received 0.2 mL of either MSC suspension (1×106 total MSC) or PBS 2 h after reperfusion via IP injection with 29-guage needles.
MSC Intestinal Engraftment
Mice were randomized into eight groups: (1) sham operated mice (sham) with BM-MSC; (2) sham + BM-MSC + HB-EGF; (3) sMAO + BM-MSC; (4) sMAO + BM-MSC + HB-EGF; (5) sham + AF-MSC; (6) sham + AF-MSC + HB-EGF; (7) sMAO + AF-MSC or (8) sMAO + AF-MSC + HB-EGF. There were ≥ 8 mice in each experimental group. Terminal ileum from sham operated mice as well as uninjured jejunum from mice exposed to sMAO were both used as controls. After euthanasia, the terminal ileum and jejunum were harvested and fixed in solution containing 1% paraformaldehyde, 15% picric acid and 0.1 M sodium phosphate buffer (pH 7.0) and shaken gently at 4°C overnight. Thirty µm frozen sections were mounted in DAPI containing mounting media (Vector Laboratories, Burlingame, CA). Fluorescence was observed using a Zeiss Axioskip fluorescent microscope (Carl Zeiss, New York, NY). MSC quantification was performed by counting EGFP-positive cells per crypt-villus axis in three separate intestinal sections per mouse at 200× magnification.
Histologic Injury Score
Mice were randomized into seven groups: (1) sham; (2) sMAO; (3) sMAO + HB-EGF; (4) sMAO + BM-MSC; (5) sMAO + HB-EGF + BM-MSC; (6)sMAO + AF-MSC or (7) sMAO + HB-EGF + AF-MSC. There were ≥ 9 mice in each experimental group. After euthanasia, the terminal ileum was harvested and fixed in 10% formalin overnight. Paraffin-embedded sections were prepared and stained with hematoxylin and eosin. Histologic scoring of the depth of tissue injury was performed as described by Chiu et al.26 with modifications as follows: 0, no damage; 1, subepithelial space at villous tip; 2, loss of mucosal lining of the villous tip; 3, loss of less than half of the villous structure; 4, loss of more than half of villous structure; and 5, transmural necrosis. Sections were evaluated blindly by two observers, with all scores averaged.
Intestinal Permeability Assay
Mice were randomized into the groups shown above for histologic injury scoring. There were seven mice in each experimental group. After euthanasia, small intestinal mucosal barrier function was assessed using the ex vivo isolated everted sac method as we have previously described.27 In brief, six cm segments of terminal ileum were harvested, inverted, and incubated in ice-cold Krebs-Henseleit bicarbonate buffer (KHBB) at pH 7.4. Fluorescein-isothiocynate dextran (FD4, molecular weight; 4000 Da) was used as a permeability probe. Everted gut sacs were gently distended by injecting 0.4 mL of KHBB and suspending the sacs in KHBB with added FD4 (60 µg/mL), representing the FD4muc, for 30 min. The incubation medium was maintained at 37°C and was continuously bubbled with a gas mixture containing 95% O2 and 5% CO2. Gut length (L) and diameter (D) were measured, and the intraluminal KHBB (FD4ser) was collected and measured (intraluminal volume). Both FD4muc and FD4ser were measured with a fluorescence spectrophotometer (SpectraMax Plus, Molecular Devices, CA). Gut permeability was expressed as the mucosal-to-serosal clearance of FD4 as follows:
Clearance (nL/min/cm2) = FD4ser × (intraluminal volume) × 30−1 × FD4muc−1 × (πDL)−1
Statistical Analyses
Experimental data were expressed as mean ± SD. Intragroup statistical differences were determined with ANOVA analysis and statistical significance between individual experimental groups were determined using a post-hoc Student’s t-test. Differences were considered to be statistically significant if p< 0.05.
RESULTS
MSC Preferentially Engraft into Injured Intestine and HB-EGF Promotes AF-MSC Engraftment
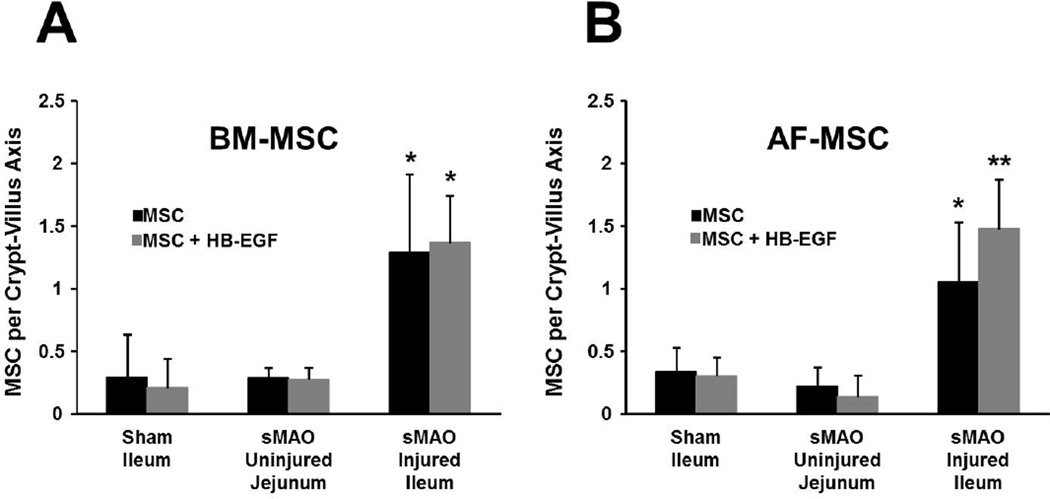
Representative intestinal sections displaying MSC engraftment are shown in Figure 1. There was increased intestinal engraftment into injured terminal ileum compared to either uninjured jejunum from the same animal, or to uninjured terminal ileum from sham operated animals for both BM-MSC (Figures 1, 2A) and AF-MSC (Figures 1, 2B). Administration of HB-EGF led to significantly increased intestinal engraftment of AF-MSC into injured terminal ileum (Figure 2B), but had no effect on the engraftment of BM-MSC into injured ileum or on engraftment of either type of MSC into non-injured intestine.
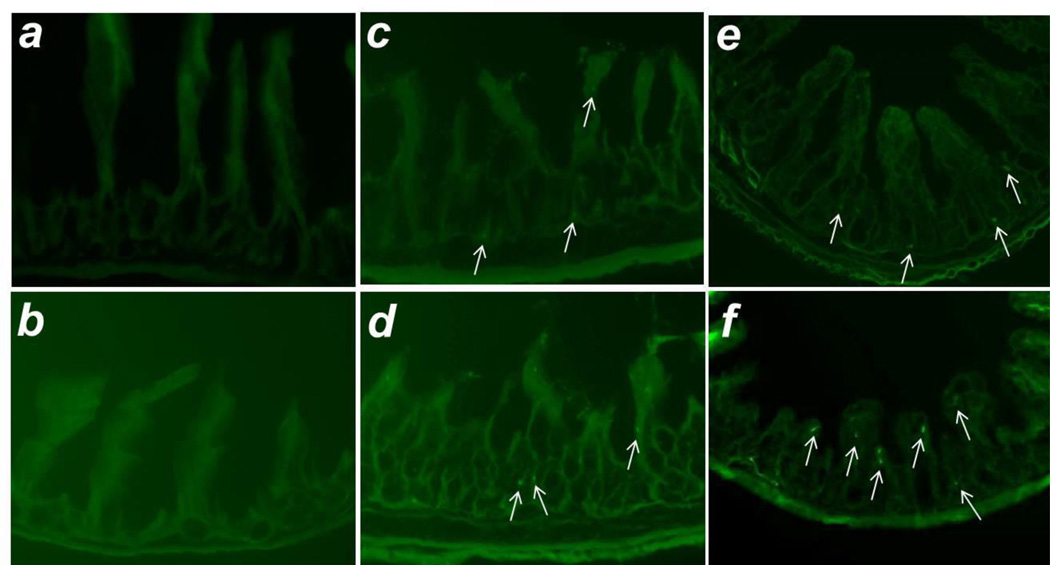
Figure 1. MSC intestinal engraftment.
Shown are representative images of: a) uninjured jejunum from an animal subjected to sMAO and treated with AF-MSC, b) terminal ileum from a sham operated animal treated with AF-MSC, c) terminal ileum from an animal subjected to sMAO + BM-MSC, d) terminal ileum from an animal subjected to sMAO + BM-MSC + HB-EGF, e) terminal ileum from an animal subjected to sMAO + AF-MSC, and f) terminal ileum from an animal subjected to sMAO + AF-MSC + HB-EGF. Intestines were viewed with fluorescent microscopy at 200× magnification. White arrows indicate engrafted MSC.
Figure 2. Quantification of MSC intestinal engraftment.
Mice were subjected to sMAO or sham surgery. A) Increased BM-MSC engraftment to injured ileum vs uninjured jejunum and uninjured ileum. B) Increased AF-MSC engraftment to injured ileum vs uninjured jejunum and uninjured ileum. Values represent mean + SD with ≥ 8 animals per group. *p< 0.01 compared with to jejunum/sham; **p< 0.01 compared with to jejunum/sham and p<0.05 compared to MSC alone.
MSC Transplantation Improves Intestinal Histologic Injury
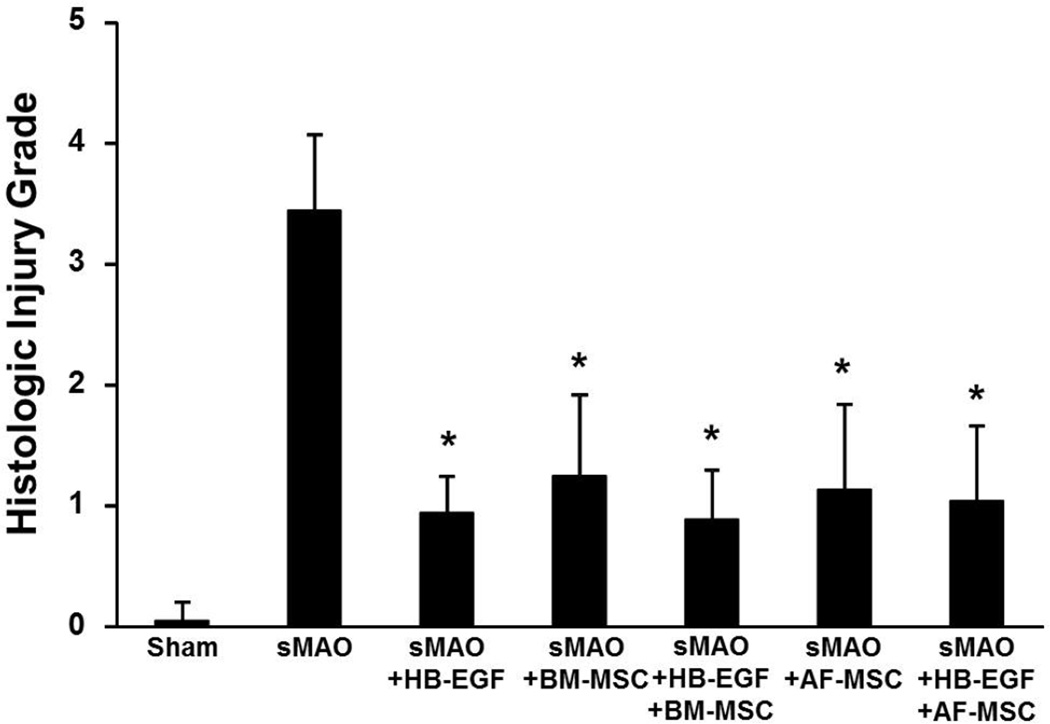
As expected, there was significantly increased intestinal histologic injury in terminal ileum from animals subjected to sMAO compared to sham operated animals (3.44±0.63 vs 0.05±0.15; p<0.01) (Figure 3). Administration of HB-EGF led to significantly decreased terminal ileal histologic injury in animals subjected to sMAO (0.94±0.3 vs 3.44±0.63; p<0.01). Administration of BM-MSC significantly decreased terminal ileal histologic injury in both the absence (1.25±0.68; p<0.01) or presence (0.89±0.42; p<0.01) of HB-EGF, with no significant differences between the individual treatment groups. Administration of AF-MSC also decreased terminal ileal histologic injury in the absence (1.13±0.71; p<0.01) or presence (1.04±0.62; p<0.01) of HB-EGF, again with no significant difference between individual treatment groups.
Figure 3. Intestinal histologic injury.
Mice were subjected to sham surgery or to sMAO and received the treatments indicated. Values represent mean + SD with ≥ 9 animals per group. *p < 0.01 compared to sMAO.
MSC Transplantation and HB-EGF Improve Gut Barrier Function with Synergistic Efficacy
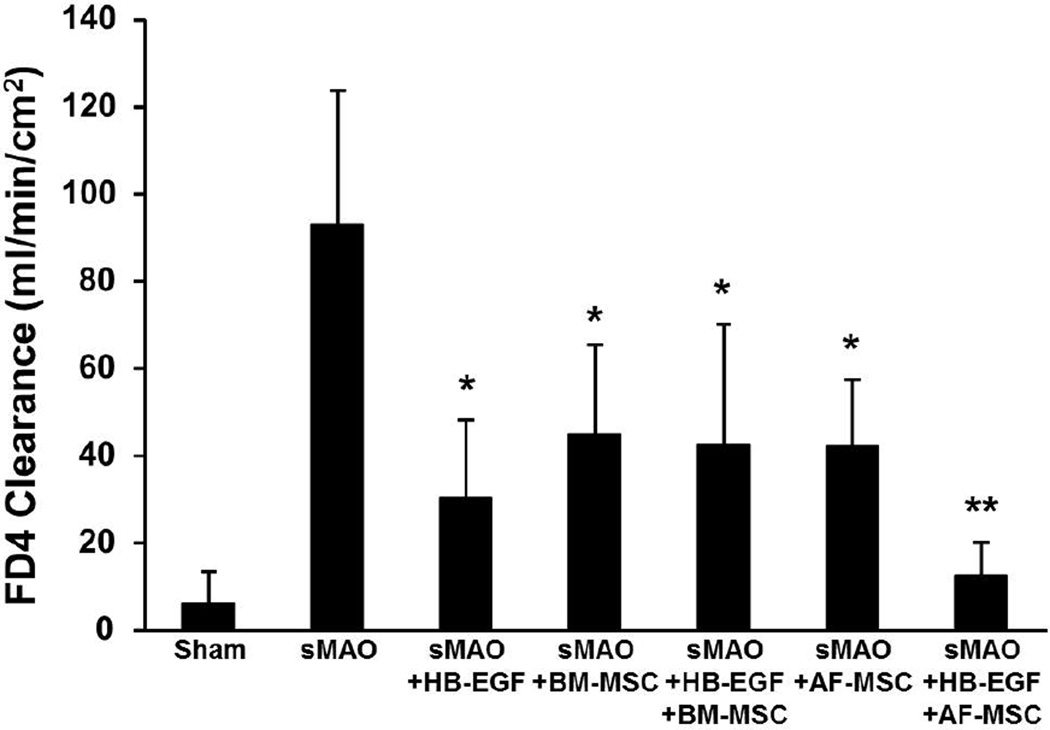
There was significantly increased intestinal permeability, reflecting impaired gut barrier function, in terminal ileum from animals subjected to sMAO compared to sham operated animals (92.9±30.9 vs 6.1±7.3; p<0.01) (Figure 4). Animals subjected to sMAO but treated with HB-EGF had significantly improved gut barrier function compared to non-HB-EGF-treated animals (30.4±17.8 vs 92.9±30.9; p<0.01). Administration of BM-MSC improved gut barrier function in both the absence (44.8±20.6; p<0.01) or presence (42.6±27.6; p<0.01) of HB-EGF, with no significant differences between the individual treatment groups. Administration of AF-MSC improved gut barrier function in both the absence (42.2±15.2; p<0.01) or presence (12.6±7.6; p<0.01) of HB-EGF, with significantly improved gut barrier function for combined therapy with HB-EGF and AF-MSC compared to either HB-EGF alone (p<0.05) or AF-MSC alone (p<0.01).
Figure 4. Intestinal permeability.
Mice were subjected to sham surgery or to sMAO and received the treatments indicated. Values represent mean + SD with 7 animals per group. *p < 0.01 compared to sMAO; **p < 0.01 compared to sMAO and p<0.05 compared to other (non-sham) treatment groups.
DISCUSSION
In recent years, MSC have emerged as an attractive target for regenerative medicine. MSC administration has been shown to be beneficial in multiple end-organ ischemic injury models.2–6 Improving and augmenting MSC transplantation efficacy is a new area of interest.
HB-EGF has previously been demonstrated to have potent mitogenic activity for a variety of cell types, including smooth muscle cells, epithelial cells, fibroblasts, keratinocytes and renal tubular cells,28 and is a known chemotactic agent for various cell types.29,30 It exerts its effects by binding to cell-surface EGFR31,32 and to the HB-EGF-specific receptor Nardilysin (Nrdc).33,34 We have previously characterized the in vitro interactions between HB-EGF and MSC, demonstrating that HB-EGF promotes MSC proliferation and migration, and protects MSC from anoxia-induced apoptosis.24 HB-EGF has a more potent effect on AF-MSC compared to BM-MSC in vitro.24 We have also shown that HB-EGF protects resident intestinal stem cells during NEC23 and that BM-MSC transplantation improves survival in experimental NEC.25 However, differential effects of HB-EGF on sub-populations of MSC in vivo have never been previously explored.
Our results demonstrate that MSC transplantation can protect the intestines from injury after I/R, with improved intestinal histology and gut barrier function. When administered as monotherapy, there were no differences in the ability of BM-MSC or AF-MSC to protect the intestines from injury. No additional improvement in protection from injury occurred when HB-EGF was administered in combination with BM-MSC transplantation. However, when HB-EGF administration was combined with AF-MSC transplantation, there was significantly increased MSC engraftment into injured intestine and significantly improved gut barrier function. This corroborates our in vitro data demonstrating that HB-EGF has a more potent effect on AF-MSC compared to BM-MSC.24
The mechanisms behind this MSC sub-population differential behavior in response to HB-EGF are yet to be elucidated. The specific interactions between HB-EGF and MSC are currently incompletely understood. Others have shown that HB-EGF promotes MSC proliferation and prevents MSC differentiation via the HER-1 cell surface receptor.35 A possible explanation we are currently exploring to explain the different behavior patterns we observed is a differential expression profile of the known HB-EGF cell surface receptor proteins between the two MSC sub-types. Previous observations have demonstrated that hypoxia activates many survival and stress pathways, including down regulation of p38MAPK,36 down regulation of PI3K and ERK 1–2,37 and up regulation of the Akt inhibitor PTEN.38 We have shown that HB-EGF activates these pathways in other cell types,39 and future experiments will clarify the exact mechanisms utilized by HB-EGF in its effects on MSC.
In conclusion, we have demonstrated that MSC transplantation leads to preferential engraftment into injured intestine, and improves intestinal histology and gut barrier function, after intestinal I/R injury. HB-EGF promotes increased AF-MSC engraftment and has synergistic efficacy with AF-MSC transplantation, leading to further improved gut barrier function. These effects were not found with BM-MSC transplantation. Our data support the administration of HB-EGF as a potential method to improve the efficacy of AF-MSC transplantation therapy in the future.
ACKNOWLEDGMENTS
This work was supported by NIH R01 GM61193 (GEB). We would like to thank Amanda Darbyshire for her help with maintaining our animals, and helping with cell culture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Kawada H, Fujita J, Kinjo K, et al. Nonhematopoietic mesenchymal stem cells can be mobilized and differentiate into cardiomyocytes after myocardial infarction. Blood. 2004;104:3581–3587. doi: 10.1182/blood-2004-04-1488. [DOI] [PubMed] [Google Scholar]
- 3.Koda M, Okada S, Nakayama T, et al. Hematopoietic stem cell and marrow stromal cell for spinal cord injury in mice. Neuroreport. 2005;16:1763–1767. doi: 10.1097/01.wnr.0000183329.05994.d7. [DOI] [PubMed] [Google Scholar]
- 4.Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Gong JF, Zhang W, et al. Effects of transplanted bone marrow mesenchymal stem cells on the irradiated intestine of mice. J Biomed Sci. 2008;15:585–594. doi: 10.1007/s11373-008-9256-9. [DOI] [PubMed] [Google Scholar]
- 6.Wei L, Fraser JL, Lu ZY, et al. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhance angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46:635–645. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan Q, Ruan JW, Ding Y, et al. Electro-acupuncture promotes differentiation of mesenchymal stem cells, regeneration of nerve fibers and partial functional recovery after spinal cord injury. ExpToxicolPathol. 2011;63:151–156. doi: 10.1016/j.etp.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Wong CY, Cheong SK, Mok PL, et al. Differentiation of human mesenchymal stem cells into mesangial cells in post-glomerular injury murine model. Pathology. 2008;40:52–57. doi: 10.1080/00313020701716367. [DOI] [PubMed] [Google Scholar]
- 9.Zarjou A, Kim J, Traylor AM, et al. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury requiresheme oxygenase-1. Am J Physiol Renal Physiol. 2011;300:F254–F262. doi: 10.1152/ajprenal.00594.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weil BR, Markel TA, Herrmann JL, et al. Mesenchymal stem cells enhance the viability and proliferation of human fetal intestinal epithelial cells following hypoxic injury via paracrine mechanisms. Surgery. 2009;146:190–197. doi: 10.1016/j.surg.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 11.Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phinney DG, Kopen G, Isaacson RL, et al. Plastic adherent stromal cells from the bone marrow of commonly used strains of inbred mice: variations in yield, growth, differentiation. J Cell Biochem. 1999;72:570–585. [PubMed] [Google Scholar]
- 13.Baghaban EM, Jahangir S, Aghdami N. Mesenchymal stem cells from murine amniotic fluid as a model for preclinical investigation. Arch Iran Med. 2011;14:96–103. [PubMed] [Google Scholar]
- 14.Delo DM, De Coppi P, Bartsch G, Jr, et al. Amniotic fluid and placental stem cells. Methods Enzymol. 2006;419:426–438. doi: 10.1016/S0076-6879(06)19017-5. [DOI] [PubMed] [Google Scholar]
- 15.DeCoppi P, Bartsch G, Jr, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 16.Peister A, Woodruff MA, Prince JJ, et al. Cell sourcing for bone tissue engineering: amniotic fluid stem cells have a delayed, robust differentiation compared to mesenchymal stem cells. Stem Cell Res. 2011;7:17–27. doi: 10.1016/j.scr.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besner G, Higashiyama S, Klasbrun MP. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul. 1990;1:811–819. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higashiyama S, Abraham JA, Miller J, et al. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 19.Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007;25:253–263. doi: 10.1080/08977190701773070. [DOI] [PubMed] [Google Scholar]
- 20.El-Assal ON, Paddock H, Marquez A, et al. Heparin-binding epidermal growth factor-like growth factor gene disruption is associated with delayed intestinal restitution, impaired angiogenesis, and poor survival after intestinal ischemia in mice. J PediatrSurg. 2008;43:1182–1190. doi: 10.1016/j.jpedsurg.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery. 2007;142:234–242. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Yu X, Radulescu A, Zorko N, et al. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology. 2009;137:221–330. doi: 10.1053/j.gastro.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen CL, Yu X, James IO, et al. Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab Invest. 2012;92:331–334. doi: 10.1038/labinvest.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watkins DJ, Zhou Y, Chen CL, et al. Heparin-binding EGF-like growth factor protects mesenchymal stem cells. J Surg Res. doi: 10.1016/j.jss.2012.05.016. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Watkins DJ, Chen CL, et al. Heparin-binding epidermal growth factor-like growth factor and mesenchymal stem cells act synergistically to prevent experimental necrotizing enterocolitis. J Am Coll Surg. doi: 10.1016/j.jamcollsurg.2012.05.037. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu CJ, McArdle AH, Brown R, et al. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Radulescu A, Besner GE. Heparin-binding epidermal growth factor-like growth factor is essential for preservation of gut barrier function after hemorrhagic shock and resuscitation in mice. Surgery. 2009;146:334–339. doi: 10.1016/j.surg.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis-Fleischer KM, Besner GE. Structure and function of heparin-binding EGF-like growth factor (HB-EGF) Front Biosci. 1998;3:d288–d299. doi: 10.2741/a241. [DOI] [PubMed] [Google Scholar]
- 29.Tokumara S, Higashiyama S, Endo T, et al. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. J Cell Biol. 2000;151:209–220. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faull RJ, Stanley JM, Fraser S, et al. HB-EGF is produced in the peritoneal cavity and enhances mesothelial cell adhesion and migration. Kidney Int. 2001;59:614–624. doi: 10.1046/j.1523-1755.2001.059002614.x. [DOI] [PubMed] [Google Scholar]
- 31.Beerli RR, Hynes NE. Epidermal growth factor-related peptides activate distinct subsets of ErbB receptors and differ in their biological activities. J BiolChem. 1996;271:6071–6076. doi: 10.1074/jbc.271.11.6071. [DOI] [PubMed] [Google Scholar]
- 32.Elenius K, Paul S, Allison G, et al. Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. EMBO J. 1997;16:1268–1278. doi: 10.1093/emboj/16.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishi E, Prat A, Hospital V, et al. N-arginine dibasic convertase is a specific receptor for heparin-binding EGF-like growth factor that mediates cell migration. EMBO J. 2001;20:3342–3350. doi: 10.1093/emboj/20.13.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hospital V, Prat A. Nardilysin, a basic residues specific metallopeptidase that mediates cell migration and proliferation. Protein PeptLett. 2004;11:501–508. doi: 10.2174/0929866043406508. [DOI] [PubMed] [Google Scholar]
- 35.Krempera M, Pasini A, Rigo A, et al. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood. 2005;106:59–66. doi: 10.1182/blood-2004-09-3645. [DOI] [PubMed] [Google Scholar]
- 36.Ren H, Accili D, Duan C. Hypoxia converts the myogenic action of insulin-like growth factors into mitogenic action by differentially regulating multiple signaling pathways. ProcNatlAcadSci U S A. 2010;107:5857–5862. doi: 10.1073/pnas.0909570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu R, Chen J, Cong X, et al. Lovastatin protects mesenchymal stem cells against hypoxi- and serum deprivation-induced apoptosis by activation of pi3k/akt and erk1/2. J Cell Biochem. 2008;103:256–289. doi: 10.1002/jcb.21402. [DOI] [PubMed] [Google Scholar]
- 38.Peterson KM, Aly A, Lerman A, et al. Improved survival of mesenchymal stromal cell after hypoxia precontioning: role of oxidative stress. Life Sci. 2011;88:65–73. doi: 10.1016/j.lfs.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129:609–662. doi: 10.1016/j.gastro.2005.05.054. [DOI] [PubMed] [Google Scholar]