Abstract
Purpose
Survival from Wilms Tumor (WT) exceeds 90% at 5 years in developed nations, whereas at last report, 2-year event-free survival (EFS) in Kenya reached only 35%. To clarify factors linked to these poor outcomes in Kenya, we established a comprehensive web-based WT registry, comprised of patients from the four primary hospitals treating childhood cancers.
Materials and Methods
WT patients diagnosed between January 2008 and January 2012 were identified. Files were abstracted for demographic characteristics, treatment regimens, and enrollment in the Kenyan National Hospital Insurance Fund (NHIF). Children under 15 years of age having both a primary kidney tumor on imaging and concordant histology consistent with WT were included.
Results
Two-year event-free survival (EFS) was 52.7% for all patients (n=133), although loss to follow up (LTFU) was 50%. For the 33 patients who completed all scheduled standard therapy, 2-year EFS was 94%. Patients enrolled in NHIF tended to complete more standard therapy and had a lower hazard of death (Cox 0.192, p <0.001).
Conclusion
Survival of Kenyan WT patients has increased slightly since last report. Notably, WT patients completing all phases of standard therapy experienced 2-year survival approaching the benchmarks of developed nations. Efforts in Kenya should be made to enhance compliance with WT treatment through NHIF enrollment.
Keywords: Wilms tumor, global health, pediatric cancer, Kenya, race disparity
Nephroblastoma or Wilms Tumor (WT) is the most common childhood kidney cancer worldwide, yet its incidence, behavior, and outcome vary according to ethnicity and geographic location(1). WT poses a disease burden to black sub-Saharan Africans and ranks second or third among the most frequently diagnosed childhood cancers (2,3). This frequency differs from North American childhood cancer incidences, which place WT fifth behind leukemia, lymphoma, CNS tumors, and neuroblastoma (4).
Although WT is often curable in developed nations, children affected in resource-constrained regions of Sub-Saharan Africa experience poor outcomes. Kenya is one such country particularly burdened by this childhood cancer(5). The last analysis of WT survival among Kenyan patients reported a two-year event-free survival (EFS) of only 34.7% (6), which stands in contrast to recent survival rates exceeding 90% at 5 years in developed countries (7,8). WT therefore occurs commonly among Kenyan children and continues to produce an unacceptably high mortality.
Interestingly, this increased predisposition for children of black African ancestry to develop WT persists despite geographic location and generations of genetic distance from sub-Saharan origin. Black African-American children have an increased incidence of WT (11 cases per million children <15 y of age) compared with whites (8 cases per million) (1,9). Among children presenting with WT in Tennessee, a greater proportion were black, and differences in molecular profiling of WT were observed between race groups, which may in part contribute to these ethnic variations in both incidence and outcome(10,11).
Curious about the possibility of a biological basis for these disparities, we recently conducted an exploratory study to characterize the molecular phenotype in Kenyan Wilms Tumor (KWT) (11). KWT specimens showed many of the classical and typical features of WT. Disease aggressiveness, treatment resistance, and a distinct molecular signature suggested a unique tumor phenotype in these patients. However, interpretation of these results was limited by high numbers of patients who became lost to follow up or abandoned care; in addition, clinical data were incomplete in nearly every case. Sample sizes were too small to make generalizable statements.
To refine and clarify the clinical significance of these preliminary molecular observations, a partnership was established between researchers at Vanderbilt University Medical Center (VUMC), Meharry Medical School in Nashville TN, and the four highest volume pediatric surgical hospitals in Kenya: Kenyatta National Hospital (KNH) in Nairobi, AIC Kijabe Hospital in Kijabe, Tenwek Mission Hospital in Bomet, and Moi Teaching and Referral Hospital (MTRH) in Eldoret. The purposes of these collaborations are: 1) to create a comprehensive Kenyan WT registry (KWTR) for gathering clinical data and for facilitating ongoing patient treatment; 2) to create a WT tissue repository for parallel biological studies, and 3) to assign molecular profiles more reliably to those WT patients having completed cancer care, which will yield greater prognostic accuracy than the alternative and less precise scenario if patients remain undertreated.
We hypothesized that event-free survival (EFS) for children diagnosed with and treated for WT had improved since last report, but that a higher death rate from WT would persist when compared to that in more developed countries. Secondarily, we hypothesized that completion of all phases of therapy would result in greater EFS and that greater access to care through national health insurance coverage would improve completion of therapy and thereby decrease the risk of death.
1. Materials and Methods
1.1. Study Design and Definitions
To initiate the KWTR, we performed a retrospective chart review of all patients diagnosed with WT beginning Jan 1, 2008. All patients under 15 years of age having imaging or operative findings of a primary kidney tumor were included. We excluded patients with a pathologic diagnosis of a tumor other than WT. Several definitions are important to understand this study. Loss to follow-up (LTFU) was defined as any patient missing his or her next scheduled treatment or follow-up appointment and further not having a scheduled follow-up appointment. Study patients were classified as active in therapy if currently receiving chemotherapy, radiation therapy, or surgical planning and who were not LTFU. Patients were classified as having completed all therapy if all therapeutic interventions, including preoperative chemotherapy, operative resection, postoperative chemotherapy, and radiation, had been documented as finished. Patients were considered to have received a blood transfusion if any blood product (packed red blood cells, plasma, platelets, etc.) had been administered outside of the operating room. Nearly every patient received some type of blood transfusion in the operating theatre and so these cases were not considered among the blood transfusion status variable.
1.2. Patient Cohort
To initiate and establish the KWTR, two American authors (JA & MA) traveled to Kenya. After obtaining Ethics Review Committee (ERC) or Institutional Review Board (IRB) permissions at each of the four collaborating Kenyan centers, patient lists were created first by querying discharge diagnosis records. At the two larger institutions, KNH and MTRH, the authors manually reviewed all histology records for the past 4 years. Any patient with a histopathologic diagnosis of WT or nephroblastoma was added to this list. At the Kijabe and Tenwek mission hospitals, digital histopathologic records were available for the study period. Candidates identified by a digital query of these databases were also added to the list of potential registrants. Outpatient and inpatient cancer registries maintained at MTRH were reviewed for potential registry candidates. File clerks at each location attempted to locate records for patients on each list. Multiple visits were made over six weeks to maximize the number of patient files. Of 221 potential KWTR entrants on the initial list, 184 files were located. Of these, 133 met the inclusion criteria. We were unable to determine any characteristics of patients with missing files. They were excluded from further analysis.
1.3. Kenyan Wilms Tumor Registry
The authors wished to create a sustainable Wilms tumor registry that would provide expandability, electronic stability, and equal access for all research partners. It was also essential that the registry be simple to access, operate, and maintain at minimal cost. We hoped that the registry could provide a framework upon which future low cost and sustainable registries for trauma, clinical care, and other cancers in both pediatrics and adults could be built. To fulfill these goals, the consortium hospitals jointly created the KWTR by utilizing REDCap (Research Electronic Data Capture) tools hosted at VUMC(12). REDCap is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources. Although hosted initially at VUMC, data hosting may eventually reside within Kenya as the tool is applied to other disease entities.
Using an existing institutional tumor registry at MTRH, previous studies, and individual clinical experiences of coauthors, we developed a data collection instrument that could capture past, current, and ongoing therapy. After a two-week trial, modifications were made to accommodate the realities of data collection in Kenya.
1.4. Minimum Staging
Minimum staging was estimated using Children’s Oncology Group (COG) criteria(13). Initial staging was based on imaging and then updated according to intraoperative findings, including tumor spillage, visible lymph node involvement, and metastases. Staging was finalized after review of pathological reports, such as of lymph node positivity and margins.
1.5. Statistics
End points for our primary analysis were overall survival, and 2-year event free survival (EFS). The overall survival time for each subgroup of interest was estimated using the Kaplan-Meier method with 95% confidence intervals. Log-rank test was used to compare the equality of survival curves for two subgroups. To investigate potential prognostic factors, such as sex, age, the status of completing all therapy, NHIF membership, tribal membership, and blood transfusion status, we performed multivariable survival analysis by applying Cox proportional hazards model on the survival data. The adjusted p values as well as the adjusted 95% confidence intervals from the Cox model were reported. To assess whether our analysis had potential bias in estimating patient survival due to a large proportion LTFU, we conducted statistical tests to evaluate the relationship between risk factors for patients having detailed follow-up information and those LTFU. For continuous variables, two-sample T test was applied; for categorical variables, Fisher’s exact test was applied. In addition, secondary analysis of tribal data was compared to current state ethnographics using one sample test of proportion with Yates’s correction for continuity. Descriptive percentages are reported as the percentage of patients for whom that data point was available, since complete data were not available for all patients. All tests were conducted at the 0.05 significance level. STATA version 11.2 (StataCorp LP; College Station, TX) was used for statistical analyses.
2. Results
2.1 Demographics and Treatment Regimens
Of 133 analyzed patients, 53.4% were male, and the mean age at diagnosis was 41 months (95% CI 36.7 – 45.3). Fifty-four percent (95% CI 44.4 – 62.7) were enrolled in NHIF (1). Presenting symptoms were similar to those reported in other studies, but also reflect the more advanced stage of many of these tumors (Table 2). The average child presented to medical attention 3.1 months after initial symptoms. Diagnosis was established on average 15.5 days after presentation, but the first therapeutic intervention (operation or chemotherapy) was not initiated until 41.2 days after presentation.
Table 2.
Presenting symptoms, complications, and mode of diagnosis for patients in the Kenyan Wilms Tumor Registry.
| Presenting Symptoms: (n = 133)* | Percent | 95% CI |
|---|---|---|
| Abdominal Distention | 97.7 | 93.5 – 99.5 |
| Weight Loss | 34.5 | 26.6 – 43.3 |
| Decreased Appetite | 33.1 | 25.1 – 41.7 |
| Fever | 16.5 | 10.7 – 24.0 |
| Nausea and Vomiting | 10.5 | 5.9 – 17.0 |
| Hematuria | 6.8 | 3.1 – 12.5 |
| Method of Diagnosis (n = 133) | ||
| Fine Needle Aspiration | 39.8 | 31.5 – 48.7 |
| Ultrasound | 36.1 | 27.9 – 44.9 |
| Computed Tomography | 19.5 | 13.2 – 27.3 |
| Intravenous Urography | 7.4 | 0 – 4.1 |
| Open Biopsy | 2.3 | 0.5 – 6.5 |
| Complications n = 133† | ||
| Wound Infection‡ | 33.8 | 25.9 – 42.5 |
| Blood Transfusion§ | 19.5 | 13.2 – 27.3 |
| Respiratory Distress | 18.8 | 12.5 – 26.5 |
| Therapy Delay Due To Lack Of Funds** | 15.8 | 10.0 – 23.1 |
| Pneumonia | 15.0 | 9.4 – 22.2 |
| Urinary Tract Infection | 14.3 | 8.8 – 21.4 |
| Neutropenia | 10.5 | 5.9 – 17.0 |
| Drug Not Available | 9.0 | 4.7 – 15.2 |
| Chicken Pox | 8.3 | 4.2 – 14.3 |
| Renal Insufficiency/Failure | 5.3 | 2.1 – 10.5 |
| Seizure | 5.3 | 2.1 – 10.5 |
| Therapy Related Cardiomyopathy | 1.5 | 0.2 – 5.3 |
A patient may have more than one presenting symptom (Percentages sum to > 100%).
A patient may have more than one complication (Percentages sum to > 100%).
Includes postoperative infection and soft tissue infections.
Transfusion of blood, or blood product outside of the operating room.
Documented delay of therapy or imaging due to lack of funding.
WT therapy has many variations between the four treating institutions in Kenya. North American COG protocols guide therapy in the two mission hospitals, whereas the International SIOP (Société Internationale d’Oncologie Pédiatrique) based protocols are followed in the state-funded hospitals. This variation in treatment style can be seen in the description of treatment completion (Table 1). For the total study cohort, twenty-eight percent of patients had up-front resection before chemotherapy, while 74% received preoperative chemotherapy; overall, resection was completed in 69.9% of cases. Radiotherapy was administered in full courses to 16.8% of diagnosed children. Overall, 24.8% of patients (n=33) completed all prescribed therapy.
Table 1.
Cohort description and loss to follow up analysis for patients in the Kenyan Wilms Tumor Registry.
| Overall | Loss to Follow Up (LTFU) Analysis | ||||
|---|---|---|---|---|---|
| Statistic (n) | 95% CI | Not LTFU | LTFU | p* | |
| Male, % | 53.4 (133) | 44.5 – 62.1 | 49.4 | 59.3 | 0.291† |
| NHIF‡ Participant, % | 53.7 (123) | 44.4 – 62.7 | 53.9 | 53.2 | 1.000† |
| Age In Months, mean | 41 (133) | 36.7 – 45.3 | 40.0 | 42.4 | 0.601§ |
| Months of Symptoms Prior to Diagnosis, mean | 3.1 (66) | 2.2 – 3.9 | 2.97 | 3.2 | 0.800§ |
| Days From Presentation To Diagnosis, mean | 15.5 (133) | 9.6 – 21.4 | 20.8 | 7.8 | 0.033§ |
| Days From Presentation To First Therapeutic Intervention, mean | 41.2 (118) | 27.6 – 54.8 | 41.4 | 41.0 | 0.973§ |
| Days of Follow-Up, mean | 263.3 (133) | 210.4 – 316.2 | 270.4 | 253.0 | 0.750§ |
| Number Mobile Numbers, mean | 1.3 (132) | 1.2 – 1.4 | 1.3 | 1.3 | 0.910§ |
| Patient HIV** positive, % | 2.4 (42) | 00.0 – 12.6 | 3.7 | 0.0 | 1.000† |
| Parent HIV positive, % | 7.4 (27) | 00.9 – 24.2 | 5.9 | 10.0 | 1.000† |
| Up Front Resection††, % | 27.6 (127) | 20.0 – 36.1 | 21.6 | 35.9 | 0.107† |
| Preoperative Chemotherapy††, % | 74.3 (82) | 63.6 – 83.4 | 64.7 | 90.3 | 0.010† |
| Resection††, % | 69.9 (133) | 61.4 – 77.6 | 58.2 | 87.0 | 0.080† |
| Postoperative Chemotherapy††, % | 51.8 (83) | 40.6 – 62.9 | 41.5 | 61.9 | 0.002† |
| Radiotherapy††, % | 16.8 (131) | 10.8 – 24.3 | 12.7 | 23.1 | 0.153† |
| Completed all Therapy, % | 24.8 (133) | 17.7 – 33.0 | 13.9 | 40.7 | 0.001† |
| Currently Receving Treatment, % | 18.8 (133) | 12.5 – 26.5 | 31.7 | 0.0 | < 0.001† |
| Stage Estimates, % (n = 125) | |||||
| I | 3.2 (4) | 2.6 | 4.1 | ||
| II | 42.4 (53) | 47.4 | 34.7 | ||
| III | 36 (45) | 26.3 | 51.0 | ||
| IV | 16.8 (21) | 21.1 | 10.2 | ||
| V | 1.6 (2) | 2.6 | 0.0 | 0.034† | |
| Laterality % (n = 131) | |||||
| Right | 48.1 (63) | 56.4 | 41.5 | ||
| Left | 50.4 (66) | 41.0 | 58.5 | ||
| Bilateral Synchronous | 1.5 (2) | 2.6 | 0.0 | 0.085† | |
p compares NOT lost to follow up to lost to follow up.
Fishers Exact
National Hospital Insurance Fund
T-Test
Human Immunodeficiency Virus
Patient completed the given therapy
2.2 Loss To Follow Up (LTFU)
LTFU was 50%. Because LTFU can cause inaccuracy in study outcomes, we reviewed demographic and staging information with respect to patients LTFU (1). The LTFU group differed in that they had been diagnosed in a shorter period of time, had completed resection in greater proportion, and had completed radiotherapy in greater proportion than patients not LTFU. As would be expected, fewer patients in the LTFU group completed therapy in any given phase.
2.3 Survival and the Effect of National Health Insurance Fund Enrollment
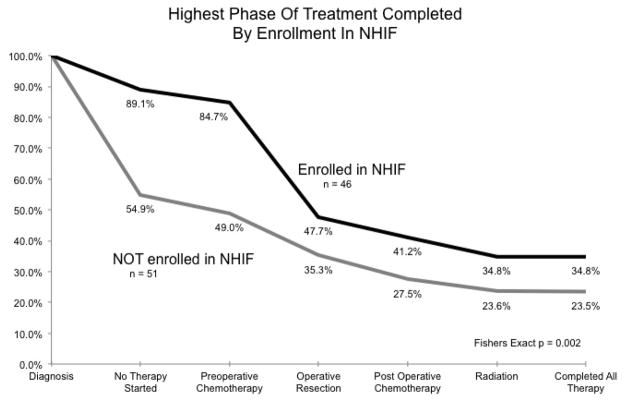
Two-year event-free survival (EFS) from WT was 52.7% for all patients. Among the 33 patients who completed all therapy, 2-year EFS was 94%. (Fig. 1) Cox proportional hazard of death was 0.046 for those who completed all therapy (Table 3; p = 0.004). As predicted, patients enrolled in the NHIF tended to complete more or all therapy. (Fig. 2) 34.8% of NHIF enrollees completed all therapy, but only 23.5% of those without NHIF completed all therapy (Fig. 2; Fishers exact, p = 0.002). Cox proportional hazard of death was 0.184 for those enrolled in NHIF (Table 3; Fig. 3; p < 0.001).
Fig 1.
Percentage of patients completing each phase of Wilms Tumor therapy for those enrolled and not enrolled in the Kenya National Hospital Insurance Fund and included in the Kenyan Wilms Tumor Registry.
Table 3.
Tribal affiliation for children in the Kenyan Wilms Tumor Registry compared with Kenya tribal distribution reported by the 2012 World Factbook(14).
| Tribes*(n=101) | n | % | % Kenya Population (14)† | p‡ |
|---|---|---|---|---|
| Kikuyu | 36 | 35.6 | 22 | 0.001 |
| Luhya | 16 | 15.8 | 14 | 0.7 |
| Kamba | 13 | 12.9 | 11 | 0.658 |
| Kalenjin | 10 | 9.9 | 12 | 0.62 |
| Kisii | 5 | 5.0 | 6 | 0.814 |
| Luo | 4 | 4.0 | 13 | 0.01 |
| Meru | 4 | 4.0 | 6 | 0.513 |
Tribes with less than four cases not shown.
Percentage of each tribe represented in the Kenyan population.
One sample test of proportion with Yates’s correction for continuity.
Fig 2.
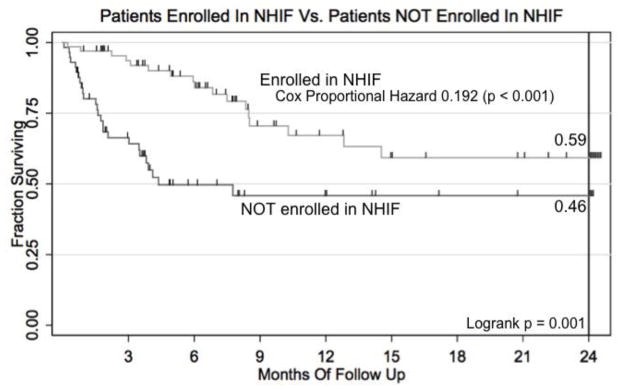
Kaplan-Meier survival curve for patients in the Kenyan Wilms Tumor Registry enrolled vs. NOT enrolled in the Kenya National Hospital Insurance Fund.
Fig 3.
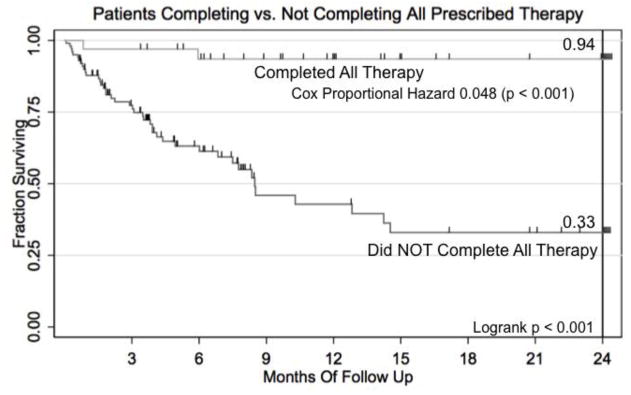
Kaplan-Meier Survival curve for patients in the Kenyan Wilms Tumor Registry completing vs. not completing all prescribed therapy.
2.4 Tribe of Origin
To assess for hereditary predisposition, tribal affiliation was evaluated. Overall, most tribes were distributed within our WT cohort at the expected rate(14), but Kikuyu tribe members were over-represented. Although the Kikuyu tribe comprises 22% of the Kenyan population, it comprised 35.6% of WT patients within this study (p = 0.001, One sample test of proportion). Conversely, the Luo tribe represents 13% of the Kenyan population, but only 4% of WT patients originated from this tribal subgroup (p = 0.007, One sample test of proportion).
2.5 Staging
In Kenya, imaging to establish a WT diagnosis can include abdominal ultrasound, computed tomography, intravenous urography, and chest x-ray. Staging imaging was not performed uniformly; furthermore, many patients receive imaging at sites outside the treating hospital. When imaging is obtained outside the hospital system, results may not be recorded in the patient chart or may be on a loose piece of paper that is easily lost. For these reasons, information adequate for minimum initial staging according to COG criteria was available for only 125 patients. Where possible, this staging information was updated using operative findings and pathology results. We report a minimum COG stage for this Kenyan cohort of WT patients, recognizing that some may be under staged. Of those patients having sufficient data to estimate a minimum stage, 4 (3.2%) were stage 1, 53 (42.4%) stage 2, 45 (36%) stage 3, 21 (16.8%) stage 4, and 2 (1.6%) stage 5. Staging did differ by LTFU and is displayed in Table 1.
2.6 Complications
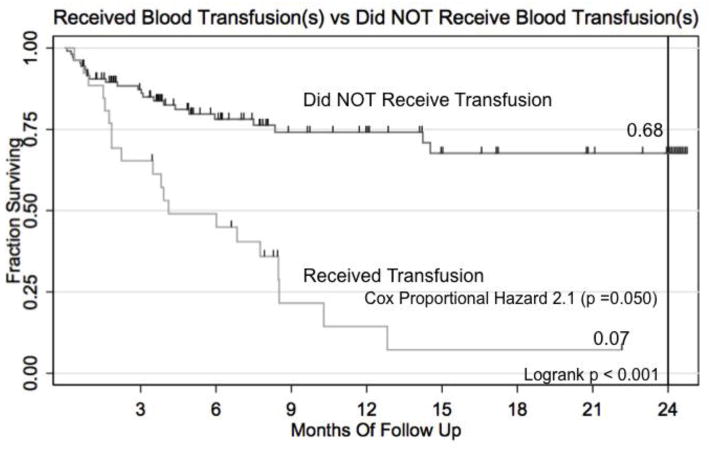
One third (33.8%) of patients experienced some type of soft tissue infection while receiving therapy, including surgical wound infection. Interestingly in this unvaccinated population, 8.3% of patients contracted varicella zoster during their therapy. At least two patients experienced doxorubicin-related cardiac dysfunction. Nearly 20% required blood transfusions outside the operating theater. These transfused patients had a hazard ratio of death of 2.13 (95% CI 1.01 – 4.49, p = 0.048) on Cox Multiple Hazard regression analysis (Table 4; Fig. 4).
Table 4.
Multiple Cox Hazard Regression for hazard of death for patients in the Kenyan Wilms Tumor Registry.
| Hazard of Death | 95% CI | p | |
|---|---|---|---|
| Male | 1.6 | 0.76 – 3.51 | 0.214 |
| Age | 1.0 | 0.96 – 1.02 | 0.757 |
| NHIF* Member | 0.19 | 0.06 – 0.45 | 0.000 |
| Completed All Therapy | 0.05 | 0.01 – 0.39 | 0.004 |
| Transfusion | 2.13 | 1.01 – 4.49 | 0.048 |
| Member of Kikuyu Tribe | 0.73 | 0.33 – 1.57 | 0.414 |
National Hospital Insurance Fund
Fig 4.
Kaplan-Meier survival curve for those in the Kenyan Wilms Tumor Registry receiving vs. NOT receiving a blood transfusion.
3. Discussion
Overall, we observed a two-year EFS from WT in Kenya of 52.7%, which represents a small improvement since the last report in 2001(6). More significantly, those who completed all standard therapy achieved a survival rate of 94% at two years. NHIF enrollment increased the number of patients completing successive phases of therapy and greatly decreased the hazard of death. An unexpected finding was that the Kikuyu tribe was over-represented among those diagnosed with WT. Tribal origin was not associated with a higher risk of death. Finally, blood transfusions received outside of the operating theater were associated with a greater hazard of death.
Death and morbidity from cancer in developing countries are increased for all 27 cancers evaluated by Soerjomatoram in 2008(15). This is again demonstrated in this study. Cancer care inequalities are vast. Large opportunities exist for improving cancer care and correcting health care disparities.
Of particular note, we learned from this study that enrollment in the NHIF was associated with greater survival. Moreover, membership in NHIF also increased the percent of WT patients completing all standard therapy from 23.5 to 34.8%. Although health insurance can be costly in developed nations, membership in the Kenyan NHIF for the entire family costs 320 Kenya Shillings (KES) per month for those employed and 160 KES per month for those who are self-employed, ($3.75 U.S. and $1.88 U.S, respectively) (16). KNH now requires newly diagnosed patients to apply for enrollment in NHIF. There is a 90-day waiting period for new claims, and pre-existing diseases do not preclude membership. Because of this beneficial effect of enrollment in NHIF, in the next phase of our ongoing studies, we will finance enrollment of patients in the NHIF in order to determine whether the relative survival advantage can be realized on a prospective basis. Every effort should be made to enroll children diagnosed with WT (or any malignancy) in the NHIF.
A discouraging finding from this study was that half of the patients were LTFU. Many patients stopped follow-up after definitive tumor resection was performed and before adjuvant chemotherapy was completed. In many cases, preoperative chemotherapy was the only medicinal adjunct that many patients received. It is unclear if this abandonment of care reflects social misconception regarding the treatability of WT beyond definitive surgery or reflects a strain on family resources to complete all levels of treatment. In Kenya, some families indeed believe that cancer is never curable or only occasionally curable by surgery, after which further chemotherapy and radiation is perceived as futile or unnecessary (personal communication, Abdallah, F.).
This current study is limited by the realities of research in the developing world. For example, files for 37 patients could not be located, follow-up was not uniform, and LTFU was considerable. WT treatment was not standardized and varied from institution to institution. Also, we could not measure the true incidence of WT in Kenya, because estimates of the population served by institutions vary widely and overlap. Tribal population distributions of WT also appeared to vary considerably. Regarding staging, regional and distant metastatic data were limited in the detail of description, and not sufficiently reliable to permit a comparative stage-for-stage survival analysis with developed nations. Gathering the same data in a prospective manner should be more accurate to gage responses to therapy and to associate molecular profiles with outcome variables. This retrospective analysis study design cannot determine causation in the NHIF impact on survival or barriers that prevent enrollment of all patients. It may be that NHIF membership is a surrogate marker of those of higher socio-economic status, who are more capable of paying medical costs, which would translate into improved outcomes. Despite these acknowledged limitations, we believe this study makes a significant contribution to our understanding of factors affecting survival from WT in Kenya, and importantly, it lays the sustainable foundation, in the form of a registry and tissue repository, for future investigation, both epidemiologic and molecular in nature.
In summary, we observed limited progress in the outcomes from WT in Kenya since the last publication of data compiled from the mid-1990’s(6). This study further highlights a cooperative effort between American and Kenyan investigators to improve care through a comprehensive Kenyan WT Registry and Tissue Repository. Most notably, NHIF enrollment enhanced therapy completion and was associated with a large decrease in hazard of death. Patients who completed all phases of therapy showed 2-year event-free survival approaching standards of developed nations. Yet Kenyan WT patients continued to experience high in-therapy death rates, which may represent advanced-stage or treatment-resistant disease, delay in presentation, malnutrition, other undefined co-morbid disease, or susceptibility to treatment toxicity. Efforts should be made to enhance compliance with WT treatment protocols through interventions such as NHIF enrollment. The KWTR can be used to coordinate patient care and to facilitate patient entry into NHIF membership. The initiation of this registry shows a snapshot of the current situation for children diagnosed with this burdensome disease and highlights many opportunities for improvement. Discussions have already begun to create a standardized nationwide WT treatment protocol based on COG and SIOP protocols but specific to Kenyan epidemiologic and biologic features. These efforts will ensure that more patients complete all phases of treatment. Only then can our advanced proteomic analysis, which is currently underway, accurately assign a molecular fingerprint to specific outcome parameters and risk groups, and only then can we also determine whether a unique biology contributes to the described poor outcomes. We anticipate that the next report from Kenya will show great improvement in WT patient survival.
Acknowledgments
This work was supported in part by the Vanderbilt CTSA grant, UL1 RR024975, from the National Center for Research Resources of the National Institutes of Health, by the National Cancer Institute grant, 5T32CA106183-06A1 (JA), and by NCI grant 1R21CA155946-01 (HNL). All research was conducted in accordance with the ethical standards of, and with prior approval from, the Vanderbilt University Institutional Review Board (IRB study numbers 020888 and 100734) and the combined ethics review committee (ERC) for AIC Kijabe Hospital and Tenwek Hospital, the ERCs of University of Nairobi/Kenyatta National Hospital, and Moi Teaching and Referral Hospital. The authors would like to express gratitude to the Vanderbilt Institute for Global Health membership, especially Dr. Sten Vermund and Cathryn Rolfe, for their advice, guidance, and unwavering support of these efforts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Breslow NE, Beckwith JB. Epidemiological features of Wilms“ tumor: results of the National Wilms” Tumor Study. J Natl Cancer Inst. 1982 Mar;68(3):429–36. [PubMed] [Google Scholar]
- 2.Hadley LGP, Rouma BS, Saad-Eldin Y. Challenge of pediatric oncology in Africa. Semin Pediatr Surg. 2012 May;21(2):136–41. doi: 10.1053/j.sempedsurg.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Mostert S, Njuguna F, Kemps L, Strother M, Aluoch L, Buziba G, et al. Epidemiology of diagnosed childhood cancer in western kenya. Arch Dis Child. 2012 Apr 25; doi: 10.1136/archdischild-2011-300829. [DOI] [PubMed] [Google Scholar]
- 4.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S (1992–2004) Cancer. 2008;112(2):416–32. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 5.Kyambi JM, Kasili EG, Onyango JN, Kitonyi GW. The management of Wilms’ tumour in Kenya. East Afr Med J. 1981 Jun;58(6):424–30. [PubMed] [Google Scholar]
- 6.Abdallah FK, Macharia WM. Clinical presentation and treatment outcome in children with nephroblastoma in Kenya. East Afr Med J. 2001 Jul;78(7 Suppl):S43–7. [PubMed] [Google Scholar]
- 7.Angio DGJ. The National Wilms Tumor Study: a 40 year perspective. Lifetime Data Anal. 2007 Nov 20;13(4):463–70. doi: 10.1007/s10985-007-9062-0. [DOI] [PubMed] [Google Scholar]
- 8.Davidoff AM. Wilms’ tumor. Curr Opin Pediatr. 2009 Jun;21(3):357–64. doi: 10.1097/MOP.0b013e32832b323a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stiller CA, Parkin DM. Geographic and ethnic variations in the incidence of childhood cancer. Br Med Bull. 1996 Oct;52(4):682–703. doi: 10.1093/oxfordjournals.bmb.a011577. [DOI] [PubMed] [Google Scholar]
- 10.Axt J, Murphy AJ, Seeley EH, Martin CA, Taylor C, Pierce J, et al. Race Disparities in Wilms Tumor Incidence and Biology. Journal of Surgical Research. 2011 Mar;170(1):112–9. doi: 10.1016/j.jss.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy AJ, Axt JR, de Caestecker C, Pierce J, Correa H, Seeley EH, et al. Molecular characterization of wilms tumor from a resource-constrained region of sub-Saharan Africa. Int J Cancer. 2012 Mar 22; doi: 10.1002/ijc.27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009 Apr;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perlman EJ. Pediatric renal tumors: practical updates for the pathologist. Pediatr Dev Pathol. 2005 May;8(3):320–38. doi: 10.1007/s10024-005-1156-7. [DOI] [PubMed] [Google Scholar]
- 14.Agency CI. The World Factbook 2012. 2011. Washington DC: 2012. Apr 7, pp. 1–5. Available from: http://www.indexmundi.com/kenya/demographics_profile.html. [Google Scholar]
- 15.Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, et al. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012 Oct 15; doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 16.Forexpros. USD/KES Historical Data. 2009 Jan 29; Available from: http://www.forexpros.com/currencies/usd-kes-historical-data.