Abstract
The Epstein-Barr virus (EBV) nuclear antigen 2 (EBNA-2) is a key gene expressed in EBV type III latent infection that can transactivate numerous promoters, including those for all the other type III viral latency genes as well as cellular genes responsible for cell proliferation. EBNA-2 is essential for EBV-mediated immortalization of primary B lymphocytes. We now report that EBNA-2, a phosphoprotein, is hyperphosphorylated specifically in mitosis. Evidence that the cyclin-dependent kinase p34cdc2 may be involved in this hyperphosphorylation includes (i) coimmunoprecipitation of EBNA-2 and p34cdc2, suggesting physical association; (ii) temporal correlation between hyperphosphorylation of EBNA-2 and an increase in p34cdc2 kinase activity; and (iii) ability of purified p34cdc2/cyclin B1 kinase to phosphorylate EBNA-2 in vitro. Hyperphosphorylation of EBNA-2 appears to suppress its ability to transactivate the latent membrane protein 1 (LMP-1) promoter by about 50%. The association between EBNA-2 and PU.1 is also decreased by about 50% in M-phase-arrested cells, as shown by coimmunoprecipitation from cell lysates, suggesting that hyperphosphorylation of EBNA-2 impairs its affinity for PU.1. Finally, endogenous LMP-1 mRNA levels in M phase are around 55% of those in asynchronously growing cells. These results suggest that regulation of gene expression during type III latency may be regulated in a cell-cycle-related manner.
The biologic hallmark of Epstein-Barr virus (EBV) and its interaction with B lymphocytes is latency. Gene expression in latent EBV infection is strictly regulated in a stereotypic fashion. Four types of latency, each having a distinct pattern of gene expression, have been described. Type I latency is associated with Burkitt's lymphoma (BL) and BL cell lines; in this form of latency, viral antigen expression is generally restricted to EBV nuclear antigen 1 (EBNA-1) (13, 19). In immunocompetent persons, the EBV genome persists in a pool of resting G0 B lymphocytes (5, 47) in which EBNA-1 transcripts and protein are not detected, termed type 0 latency (47). Type II latency is found in nasopharyngeal carcinoma and in Hodgkin's disease (9, 87). EBNA-1, latent membrane protein 1 (LMP-1), LMP-2A, and LMP-2B are expressed in type II latency, which can also be established in vitro by fusing lymphoblastoid cell line cells with certain EBV-negative human epithelial, fibroblast, or hemopoietic cell lines (3, 32). Type III latency is represented by lymphoblastoid cell lines established after EBV infection of primary B cells in vitro and by group III BL lines (33, 86). Nine viral proteins are expressed, including six nuclear proteins (EBNA-1, EBNA-2, EBNA-3A, EBNA-3B, EBNA-3C, and EBNA-LP) and the three integral membrane proteins (LMP-1, LMP-2A, and LMP-2B) (reviewed in references 7 and 9).
EBNA-2, a key gene expressed in type III latency, is a phosphoprotein (17, 18) that localizes to various compartments of the nucleus, including the nucleoplasm, the chromatin fraction, and the nuclear matrix (54, 58). EBNA-2 is essential for EBV transformation, since EBV with a region including EBNA-2 deleted is unable to transform B lymphocytes (45). In addition, EBNA-2 regulates promoters for both viral and cellular genes that are important for transformation of B lymphocytes, including LMP-1, which is the principal EBV oncoprotein (75, 76) and is also essential for transformation of B lymphocytes and other cell types. EBNA-2 is the best-characterized viral factor that transactivates the LMP-1 promoter (LMP-1p) (30, 62, 63, 74, 78). EBNA-LP, which is also a phosphoprotein, can activate LMP-1p in cooperation with EBNA-2 (24, 52). In addition to LMP-1, it is well established that EBNA-2 can transactivate a number of other genes, including the cellular CD21, CD23, bcl-2, and c-fgr and the EBV LMP-2A and LMP-2B genes as well as the BamHI C promoter (Cp), which gives rise to all the viral nuclear antigens, EBNA-1, -2, -3A, -3B, -3C, and -LP (39, 63, 66). EBNA-2 transactivates Cp and the other promoters through EBNA-2 response elements within each promoter. EBNA-2 does not bind directly to DNA but exerts its function by interacting with the cellular proteins recombination signal-binding protein (RBP-Jκ) (20, 26) and PU.1 (30) and by recruiting TFIIB, TFIIH, TAF40, p100, and p300/CBP via EBNA-2's acidic activating domain (70-72, 79). All EBNA-2-responsive promoters described to date have DNA-binding sequences for RBP-Jκ, while interaction between EBNA-2 and PU.1 is specific to the LMP-1 promoter, which contains a binding sequence for PU.1 (30).
Although there is much information on how expression of individual genes is regulated in EBV latent infection, the possibility that there is a general mechanism for regulating the latency genes has received little attention. We have provided evidence that in type I latency control of latent gene expression is regulated by the cell cycle (4). Both EBNA-1 and a host factor, E2F, are involved in the transcriptional regulation of Qp, from which EBNA-1, the only EBV nuclear antigen expressed in type I latency, is transcribed. EBNA-1 acts in an autoregulatory manner to repress Qp transcription by binding to sites in the Q locus located downstream of the Qp start site (57, 68). However, in vitro, E2F can overcome EBNA-1-mediated repression of Qp in transient-transfection assays by competing with and displacing EBNA-1 from the Q locus, thereby activating Qp (67). Since E2F transcriptional activity is cell cycle regulated with higher activity in the S phase, transcription of endogenous EBNA-1 mRNA in vivo is regulated in a cell-cycle-dependent manner, resulting in increased EBNA-1 mRNA levels in S phase (4).
In the more complex type III latency, during which all the latency genes are expressed, whether or how they are regulated in relation to the cell cycle is unexplored. The only report germane to this idea is that the phosphorylation pattern of EBNA-LP is regulated during the cell cycle, being hyperphosphorylated specifically in the G2/M phase (34). It has been reported that the ability of EBNA-LP to induce LMP-1 expression cooperatively with EBNA-2 is regulated by phosphorylation, that mutation of the major phosphorylation sites of EBNA-LP reduce its coactivation ability (85), and that p34cdc2 kinase is involved in phosphorylation of this site in vitro (31). However this study was carried out in asynchronously growing cells, in which EBNA-LP is hypophosphorylated (34). Thus, the effect of G2/M-phase-specific hyperphosphorylation on EBNA-LP's function remains unknown, leaving the question of whether and how cell cycle might influence the function of EBV latent genes in type III latency essentially unaddressed. Since EBNA-LP by itself does not activate specific promoters (24, 52), whereas EBNA-2 is the major transactivator for all the other viral genes expressed in type III latency (35, 43, 63, 73, 78), we therefore chose EBNA-2 as a target for study during the cell cycle.
In this paper we report that EBNA-2 is hyperphosphorylated in a mitosis-specific manner and that this modification appears to suppress the ability of the protein to transactivate the LMP-1 promoter. We found also that the cyclin-dependent kinase, p34cdc2, which is a master regulator of the G2-to-M transition in eukaryotic cells (12, 41), is likely involved in EBNA-2 hyperphosphorylation.
MATERIALS AND METHODS
Cell culture.
HeLa cells were obtained from the American Type Culture and grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. The X50-7 cell line was derived from human cord blood lymphocytes immortalized in vitro by infection of B95-8 EBV; it expresses the genes for EBNAs and LMPs (type III latency) (81). DG75 is an EBV-negative BL cell line (2). The stable BJAB cell line expressing EBNA-2 was a gift from Fred Wang (77). All these cell lines were maintained in RPMI 1640 plus 10% fetal bovine serum.
Enrichment for mitotic cells.
Cells were enriched for mitosis by treatment with nocodazole for 12 to 18 h at a final concentration of 60 ng/ml for X50-7 cells and DG75 cells and 250 ng/ml for HeLa cells. Both treated and untreated HeLa cells in mitosis were collected by vigorous shaking off into the medium as described earlier (69); the interphase cells remain attached. To arrest transfected cells in mitosis, nocodazole was applied 20 h after transfection of HeLa cells (50) and 36 to 48 h after transfection of DG75. The mitotic index was determined by 4′,6′-diamidino-2-phenylindole (DAPI) staining and microscopic examination (14, 11).
Propidium iodide staining and FACS analysis.
To determine DNA content and cell-cycle status at each time point, 2 × 106 cells from each fraction fixed in 70% ethanol were collected by centrifugation and washed once with 1× phosphate-buffered saline (PBS) solution and resuspended in PBS containing 0.1% Triton X-100, 2 mg of RNase A/ml, and 50 mg of propidium iodine (Sigma)/ml. Data were acquired on a Becton Dickinson FACSStarPlus and analyzed by use of ModFit (Verity Software House). To sort cells in different phases, asynchronously growing cells were labeled with 0.01 mM Hoechst 33342 (Sigma) at 37°C for 45 min and sorted with a DAKO Cytomation fluorescence-activated cell sorting (FACS) Moflo sorter. Cells within the G0/G1, S, or G2/M phases were collected in sterile PBS at 4°C (56).
Plasmids.
The LMP1 promoter construct pGL2 (−512/+72)-luciferase, which has two tandem copies of the LMP1 −512/+72 promoter element, was provided by Jeffery Lin and Elliott Kieff (37). The pBS-LMP1 plasmid for RNase protection assay (RPA) was provided by Paul Farrell (65). The β-galactosidase (β-Gal) expression plasmid pCMVβ-Gal (6177-1) was purchased from Clontech. pSG5-EBNA-2 contains the entire EBNA-2 open reading frame of EBV strain W91 under the control of the simian virus 40 (SV40) early promoter in the vector pSG5 (Stratagene) and was provided by Elliott Kieff (37). The pGL2 promoter construct, which contains the SV40 early promoter upstream of the luciferase gene, was purchased from Promega.
In vitro transcription and translation.
In vitro transcription and translation of EBNA-2 was carried out in rabbit reticulocytes with the TNT system (Promega) according to the manufacturer's instructions with 1 μg of plasmid pSG5-EBNA-2.
Immunoprecipitation.
Cells were washed once with PBS and then resuspended in lysis buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, 5 mM dithiothreitol, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride [Complete; Roche]). After freezing and thawing three times, debris was removed by centrifugation at 4°C for 15 min at 14,000 × g. Protein concentration was determined by the Bradford assay (Bio-Rad). Whole-cell lysates (1 mg) from asynchronous and nocodazole-synchronized G2/M-phase X50-7 cells were first incubated with 10 μg of EBNA-2 antibody, p34cdc2 antibody, or normal mouse immunoglobulin G (IgG) in 500 μl of protein lysis buffer at 4°C for 1 h; immune complexes were incubated with protein A/G-Sepharose beads (Santa Cruz) at 4°C overnight, washed four times with protein lysis buffer, and then eluted from the protein G-Sepharose with 2× Laemmli's buffer by boiling for 3 min. Denatured immune complexes were separated by electrophoresis and analyzed by immunoblotting.
Immunoblotting.
Whole-cell lysates (100 μg) were separated on sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE) gels for detection of PU.1, p34cdc2, and cyclin B1 and on 8% gels for detection of EBNA-2. The proteins were then transferred to nitrocellulose membrane. Membranes were blocked in 5% milk for 30 min at room temperature (RT), rinsed in Tris-buffered saline with Tween (TBST) three times for 5 min, and then incubated at RT for 1 h or overnight at 4°C with the following antibodies: EBNA-2 monoclonal antibody (PE2; 1:500; DAKO), LMP-1 antibody (CS1-4; 1:100; DAKO), p34cdc2 monoclonal antibody (clone 17; 1:200; Santa Cruz), cyclin B1 monoclonal antibody (GNS-1; 1:250; BD Pharmingen), PU.1 polyclonal antibody (D19; 1:100; Santa Cruz), β-actin monoclonal antibody (AC-15; 1:5,000; Sigma), and antitubulin monoclonal antibody (clone B7; 1:500; Santa Cruz). After rinsing three times for 5 min in TBST, the membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (1:5,000; Amersham Biosciences) for 1 h at RT and subsequently washed with TBST three times for 5 min. Specific signals were detected by using the Supersignal detection reagent following the manufacturer's instructions (Pierce) and exposed to Kodak XAR-5 film.
Phosphatase treatment.
EBNA-2 immunoprecipitated from whole-cell lysates (500 μg) was treated for 1 h at 30°C with 2,000 U of lambda phosphatase (New England Biolabs, Inc.). Samples were then diluted with 2× Laemmli's buffer and electrophoresed on an SDS-8% polyacrylamide gel. EBNA-2 was detected by immunoblot analysis.
Kinase assay.
A histone H1 kinase assay was carried out as described previously (34). Briefly, p34cdc2 and cyclin B1 were immunoprecipitated from whole-cell lysates (500 μg) at indicated time points with 10 μg of monoclonal p34cdc2 and cyclin B1 antibodies. After washing three times with lysis buffer and once with reaction buffer, the immunoprecipitate complex was collected and incubated at 30°C in 50 μl of kinase reaction mixture containing 50 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 1 mM dithiothreitol, 10 μM ATP, 5 μCi of [γ-32P]ATP (6,000 Ci/mmol; Amersham), and 0.5 mg of histone H1/ml for 15 min. The reaction was terminated by the addition of 10 μl of 6× Laemmli's sample buffer and boiling for 5 min. The 32P-phosphorylated histone H1 was separated on 10% polyacrylamide gels and analyzed on a PhosphorImager (Molecular Dynamics).
To assay EBNA-2 phosphorylation by purified p34cdc2/cyclin B1 kinase (New England Biolabs), EBNA-2 was immunoprecipitated from in vitro-transcribed and -translated reticulocyte lysates or from whole-cell lysates (1 mg) of asynchronously growing X50-7 cells with PE2 antibody. EBNA-2 phosphorylation was carried out in a 25-μl reaction mixture containing 50 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 1 mM EGTA, 2 mM dithiothreitol, 0.01% Brij 35, 80 μM ATP, and 40 μCi of [γ-32P]ATP (6,000 Ci/mmol; Amersham) in the presence of 40 U of purified p34cdc2/cyclin B1 kinase (New England Biolabs). After incubation for 30 min at 30°C, the immune complex was washed three times with kinase reaction buffer. The reaction was terminated by the addition of 2× Laemmli's sample buffer. After boiling for 5 min, the samples were subjected to SDS-8% PAGE. Proteins were transferred to a polyvinylidene difluoride membrane and subjected to immunoblotting of EBNA-2 as described above. The 32P-incorporated EBNA-2 protein was analyzed by autoradiography.
Transfections.
Approximately 106 log-phase DG75 cells were transfected in 0.4 ml of RPMI 1640-10% fetal bovine serum with a Bio-Rad Gene-Pulser at 220 V and 975 μF. For reporter assays, each transfection mixture contained 10 μg of the LMP-1 promoter construct pGL2 (−512/+72)-luciferase plasmid, the indicated amounts of EBNA-2 plasmid, and 1 μg of pCMVβ-Gal to permit normalization for transfection efficiency. Total plasmid DNA was made constant by the addition of pSG5 vector DNA (Stratagene). In some circumstances, 10 μg of pGL2-promoter plasmid and 1 μg of pCMVβ-Gal were cotransfected. After transfection, cells were incubated in 10 ml of complete medium and subjected to M-phase enrichment by nocodazole treatment as described above. HeLa cells were transiently transfected with the use of FuGENE6 according to the manufacturer's instructions.
Reporter assay.
Sixty-six hours posttransfection, cells were rinsed in PBS and lysed in reporter lysis buffer (Promega) with one freeze-thaw cycle. For luciferase reporter assays, cell lysates (20 μl each) were combined with luciferase assay reagent (Promega), and the relative light units were measured in an Lmax luminometer (Molecular Devices Corp.). β-Gal assays were performed as described elsewhere (15). β-Gal values were used to normalize all results for transfection efficiency. All reporter assay results are averages derived from three independent repetitions done in triplicate.
RNA isolation and RPA.
Total RNAs were isolated with a Qiagen RNeasy mini kit. RPAs were performed with total RNA with the RNase protection kit II (Ambion). The hybridization temperature was 37°C. The human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was supplied by U.S. Biochemicals, Inc. The LMP-1 probe for RPA, which was from the pBS-LMP1 construct and labeled with [α-32P]UTP by in vitro transcription, corresponds to nucleotides 169033 to 169423 in the B95-8 EBV genome (65). The protected fragments are 220 and 90 bp (65). The EBNA-2 probe was generated by PCR with the Extend high-fidelity PCR system (Boehringer Mannheim) with pSG5-EBNA-2 as template and specific primers. The sequence of primer A was 5′-GCTCTAGATAATACGACTCACTATAGGGCGACAGACCCAAGCTTGGTACCGAGCTCGGATCCGATGGAGGATACCAATCATCGG-3′ and that of primer B was 5′-GCTCTAGACTAAGTCCAGTCCTCGGTCTTC-3′. Primer A contained the T7 RNA polymerase promoter sequence to allow the transcription of the antisense EBNA-2 RNA probe and a spacer to provide separation between the unprotected and protected regions of the probe in the RPA. The protected region of EBNA-2 corresponds to nucleotides 49656 to 49846 of the EBV B95-8 strain. The protected fragments are 190 bp. The purified PCR product was confirmed by enzymatic digestion and used directly for RNA probe synthesis by use of T7 RNA polymerase (Promega) and [γ32P]UTP (Amersham).
RESULTS
Mitosis-specific hyperphosphorylation of EBNA-2.
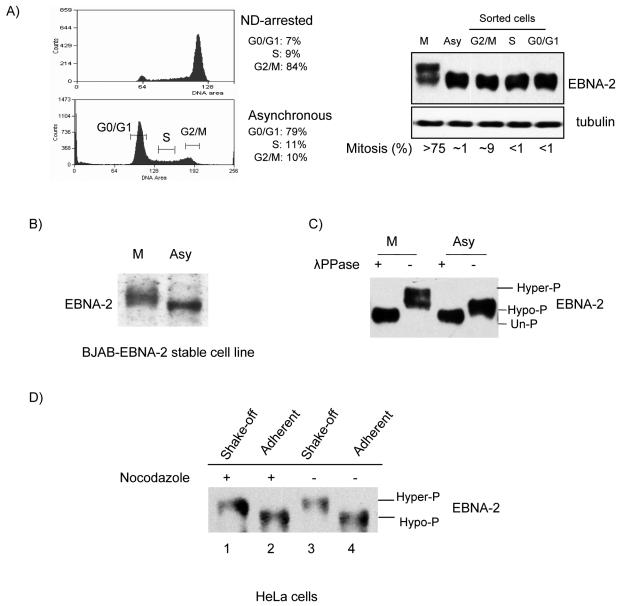
To study EBNA-2 expression during the cell cycle, X50-7 cells were arrested by nocodazole, or asynchronously growing cells were sorted in G0/G1, S, and G2/M phases by FACS (Fig. 1A, left panel). In the sorted G2/M fraction, the mitotic index was 9% (range, 8 to 10%) as determined by DAPI staining (11, 14) (Fig. 1A, right panel), so most of the cells in this fraction were in G2 phase, whereas in nocodazole-arrested cells more than 75% were in mitosis. Western blotting showed that the protein level of EBNA-2 did not vary appreciably during the cell cycle. However, in nocodazole-arrested cells (M), a component of EBNA-2 migrated more slowly (Fig. 1A, right panel). This shift was not detected in the FACS-analyzed cells in G2/M, in which there were only 9% in mitosis. Similar slower electrophoretic mobility of EBNA-2 was also detected in nocodazole-arrested EBV-negative BJAB cells stably expressing EBNA-2 (77) (Fig. 1B).
FIG. 1.
EBNA-2 is hyperphosphorylated specifically in mitosis. (A) Left top panel, X50-7 cells arrested by nocodazole (ND); left bottom panel, G0/G1, S and G2/M phases sorted from asynchronously growing cells by FACS (brackets indicate collected fractions). Percentages in each phase are indicated. Right panel, EBNA-2 protein analyzed by immunoblotting with PE2 antibody; tubulin was the loading control. The mitotic index was determined by DAPI staining. (B) Immunoblots of EBNA-2 stably expressed in EBV-negative BJAB cells in nocodazole-arrested M phase (M) or in asynchronous cells (Asy) in whole-cell lysates. (C) Hyperphosphorylation of EBNA-2 in nocodazole-arrested M-phase cells. Immunoprecipitated EBNA-2 from M-phase and asynchronous cells was subjected to immunoblotting directly (−) or after treatment with 2,000 U of λ phosphatase (λPPase) (+). (D) EBNA-2 is hyperphosphorylated in both nocodazole-arrested and untreated mitotic cells. HeLa cells were transfected with EBNA-2 and treated with nocodazole (250 ng/ml) for 18 h, at 20 h after transfection, or were left untreated. M-phase cells were separated from other phases by vigorous shaking. Immunoblots for EBNA-2 of extracts from mitotic cells collected by shake-off from nocodazole-arrested (lane 1) and untreated (lane 3) cell monolayers and from cells remaining adherent (lanes 2 and 4) were analyzed.
The most likely explanation for the shift in migration detected in the EBNA-2 protein is modification by phosphorylation. We therefore treated EBNA-2 immunoprecipitated from asynchronously growing and nocodazole-arrested cells with λ phosphatase to remove any potential phosphates. Treatment of EBNA-2 from nocodazole-arrested cells caused an increase in mobility of EBNA-2 as detected by Western blotting (Fig. 1C); λ phosphatase treatment also affected migration of EBNA-2 obtained from asynchronous cells (Fig. 1C). This result is consistent with an earlier report that EBNA-2 is phosphorylated in asynchronously growing cells and is dephosphorylated by calf intestinal alkaline phosphatase or potato acid phosphatase in vitro (18). The results indicate, therefore, that EBNA-2 is hyperphosphorylated in nocodazole-arrested cells. Phospho-amino acid analysis of EBNA-2 disclosed that the protein is phosphorylated on serine and threonine and not on tyrosine residues in both hyper- and hypophosphorylated forms of EBNA-2 (unpublished data).
Further, we wanted to establish whether EBNA-2 hyperphosphorylation observed in nocodazole-arrested cells reflected a mitosis-specific modification, since in cells in suspension it is physically impossible to separate the M phase from G2. We therefore transfected EBNA-2 into HeLa cells and separated cells in mitosis from other phases by vigorous shaking (49, 53, 55, 69). As shown in Fig. 1D, hyperphosphorylation of EBNA-2 was confined to mitotic cells obtained by shaking off from the monolayer. Most importantly, EBNA-2 hyperphosphorylation was observed in mitotic cells collected by shake-off from an asynchronously growing population of untreated HeLa cells. Above 90% of the shake-off fraction of cells were in M phase, as determined by DAPI staining, and hyperphosphorylation of EBNA-2 was confirmed by a λ phosphatase assay (data not shown). Together these results indicate that (i) EBNA-2 is subjected to mitosis-specific hyperphosphorylation, both during physiological mitotic division and during mitotic arrest induced by a microtubule-targeting drug; (ii) hyperphosphorylation of EBNA-2 does not require any other EBV gene; and (iii) hyperphosphorylation of EBNA-2 is independent of cell type. Thus, hyperphosphorylation of EBNA-2 is most likely regulated by a cellular kinase which is active in the M phase of the cell cycle.
EBNA-2 physically associates with p34cdc2, and hyperphosphorylation of EBNA-2 coincides with the increase in p34cdc2/cyclin B1 kinase activity.
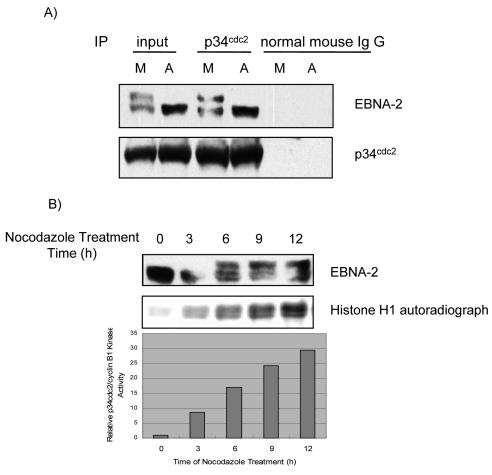
p34cdc2/cyclin B1 is the kinase complex responsible for the G2-to-M transition, and it has maximal activity in mitosis (55). Therefore, we asked whether EBNA-2 and p34cdc2 kinase are physically associated in type III latency. Study of the physical association of EBNA-2 with p34cdc2 by coimmunoprecipitation in X50-7 cells disclosed that EBNA-2 could be coimmunoprecipitated with p34cdc2 antibody in both asynchronously growing and M-phase-arrested cells (Fig. 2A), indicating that both hypophosphorylated and hyperphosphorylated EBNA-2 associates with p34cdc2. Thus, these results suggest that EBNA-2 physically associates with p34cdc2 kinase. Then we determined if M-specific hyperphosphorylation of EBNA-2 correlates with p34cdc2/cyclin B1 kinase activity. As shown in Fig. 2B, an increase of hyperphosphorylation of EBNA-2 temporally coincides with increased p34cdc2/cyclin B1 kinase activity, as evaluated by histone H1 kinase assay (40).
FIG. 2.
(A) Physical association of EBNA-2 with p34cdc2 kinase. Whole cell X50-7 lysates from asynchronous or nocodazole-arrested M-phase cells (A and M, respectively) were immunoprecipitated with p34cdc2 antibody or normal mouse IgG; immune complexes were separated by electrophoresis. EBNA-2 and p34cdc2 were detected by immunoblotting. (B) Temporal correlation between EBNA-2 hyperphosphorylation and p34cdc2 kinase activity. X50-7 cells were continuously treated with nocodazole for the indicated times, and whole-cell lysates were separated by electrophoresis and immunoblotted with EBNA-2 antibody (upper panel). For the kinase assay, p34cdc2/cyclin B1-kinase complex was immunoprecipitated from the same cell lysates, and kinase activity was determined by [γ-32p]ATP incorporation into histone H1 (middle panel) and measured with a PhosphorImager (lower panel).
Role of p34cdc2 kinase in EBNA-2 phosphorylation.
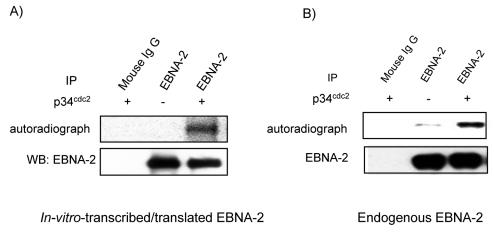
A number of proteins are subjected to mitosis-specific phosphorylation, which in most cases is involved with p34cdc2 kinase (22, 36, 40). To test whether the p34cdc2 kinase might be involved in phosphorylation of EBNA-2, we carried out in vitro kinase assays by using purified p34cdc2 kinase. EBNA-2 immunoprecipitated from in vitro-transcribed and -translated rabbit reticulocyte lysates was first used as substrate to test whether p34cdc2 kinase might phosphorylate EBNA-2 in vitro. As shown in Fig. 3A, EBNA-2 can be phosphorylated efficiently by addition of p34cdc2 kinase. Next we investigated whether endogenous EBNA-2 could be phosphorylated by p34cdc2 kinase. As shown in Fig. 3B, the endogenous EBNA-2 protein immunoprecipitated from asynchronously growing cells was weakly phosphorylated without the addition of p34cdc2 kinase, whereas it was dramatically phosphorylated with the addition of the purified p34cdc2 kinase. A control Western analysis for EBNA-2 showed that essentially equal amounts of EBNA-2 protein were used in the kinase assays (Fig. 3). These results suggest that p34cdc2 kinase may be involved in EBNA-2 mitotic hyperphosphorylation, at least in part.
FIG. 3.
Involvement of p34cdc2 kinase in EBNA-2 phosphorylation. In vitro-transcribed-translated EBNA-2 from rabbit reticulocyte lysates (A) or endogenous EBNA-2 from total lysates of asynchronously growing cells (B) were immunoprecipitated from cells and used as substrates for kinase assays in the presence (+) or absence (−) of purified p34cdc2/cyclin B1 kinase. Immunoprecipitation with normal mouse IgG served as the negative control. After resolving by electrophoresis and transfer onto membrane, phosphorylation of EBNA-2 was detected by autoradiography (top panel). Equal levels of EBNA-2 were confirmed by immunoblotting (bottom panel).
Transactivation of the LMP-1 promoter by EBNA-2 is decreased in M phase.
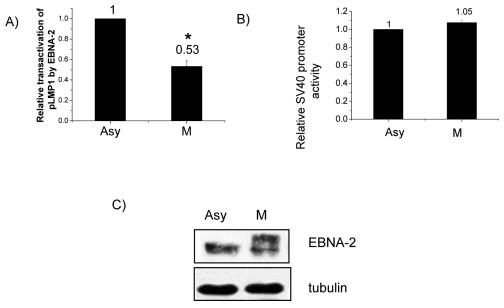
We next turned to study functional consequences of hyperphosphorylation of EBNA-2. EBNA-2 can transactivate the LMP-1 promoter, as shown by cotransfection with LMP-1 promoter reporter constructs (30, 63, 78). However, all such studies have been done in asynchronously growing cells, when EBNA-2 is hypophosphorylated. We therefore compared transactivation of the LMP-1 promoter by EBNA-2 in asynchronous and M-phase-arrested cells to test whether hyperphosphorylation of EBNA-2 might affect its functional ability. EBNA-2 can transactivate the LMP-1 promoter in both asynchronously growing and M-phase-arrested cells; however, transactivation of the LMP-1 promoter by EBNA-2 in M phase decreased by about 50% (P < 0.01) (Fig. 4A). Western blotting showed essentially equal amounts of EBNA-2 in the asynchronous and M-phase cells as well as hyperphosphorylation of EBNA-2 in M phase (Fig. 4C). Therefore, the decreased transactivation of the LMP-1 promoter by EBNA-2 in M phase is related to hyperphosphorylation of EBNA-2, not to variation in EBNA-2 expression level. To rule out a cell-cycle-independent effect of nocodazole, we used an irrelevant promoter, the SV40 early promoter, as a control and found that its activity was not affected by nocodazole (Fig. 4B).
FIG. 4.
Decreased transactivation of the LMP-1 promoter by EBNA-2 in M-phase-arrested cells. DG75 cells were transfected with reporter plasmid p(−512/+72)LMP1p-luc in addition to 5 μg of pSG5-EBNA-2 expression vector. After transfection, half the cells were arrested at M phase by nocodazole (M); the other half served as the asynchronous control (Asy). Luciferase assays were performed to evaluate promoter activity. In each phase, transactivation of the LMP-1 promoter by EBNA-2 was normalized to the vector control. (A) Comparison of transactivation of the LMP-1 promoter by EBNA-2 in M-phase-arrested and asynchronous control cells. (B) Comparison of SV40 early promoter activity in G2/M-arrested and asynchronous control cells. Each data point in panels A and B represents the average of three independent experiments done in triplicate. Error bars represent the means ± standard errors of the means. *, P < 0.01. (C) Immunoblot of EBNA-2 with PE2 antibody in asynchronously growing (Asy) and M-phase-arrested (M) cells in a representative experiment; tubulin was used as a loading control.
Association of EBNA-2 with PU.1 is decreased in M phase when EBNA-2 is hyperphosphorylated.
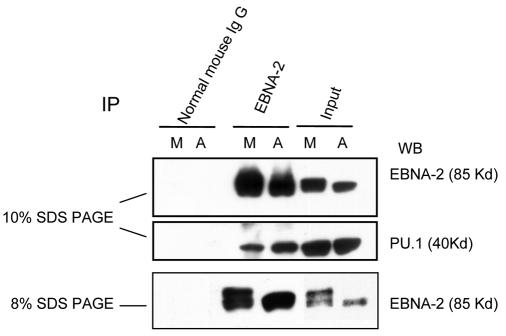
EBNA-2 transactivates downstream promoters by interacting with the cellular sequence-specific DNA-binding proteins RBP-Jκ/CBF-1 (20, 26) and PU.1 (30); PU.1 is specific for the LMP-1 promoter (30). To examine why there was decreased transactivation of the LMP-1 promoter in the M phase, we compared the association of EBNA-2 with PU.1 in asynchronously growing and M-phase-arrested cells by coimmunoprecipitation. Glutathione S-transferase (GST)-EBNA-2 fusion proteins can interact directly with PU.1 in vitro; however, association of EBNA-2 with PU.1 has not been detected in vivo (30). As shown in Fig. 5, PU.1 can be coimmunoprecipitated by EBNA-2 antibody in both asynchronous and M-phase X50-7 cells. Interestingly, we consistently observed that less PU.1 was coimmunoprecipitated by EBNA-2 in M phase, although the input protein level of PU.1 was constant (Fig. 5). Thus, the association of EBNA-2 and PU.1 is decreased when EBNA-2 is hyperphosphorylated. These data help to explain why LMP-1 promoter activity decreases in M-phase-arrested cells (Fig. 4A).
FIG. 5.
Decreased association of PU.1 and EBNA-2 in M-phase-arrested cells when EBNA-2 is hyperphosphorylated. Immunoprecipitation of EBNA-2 was performed with whole-cell lysates from asynchronously growing (A) and nocodazole-arrested M-phase cells (M). A portion of the immunoprecipitated lysates was separated on a 10% gel, and immunoblotting of EBNA-2 and PU.1 was carried out on the same membrane (top and middle panels). The same samples were separated on an 8% gel to show more clearly the EBNA-2 hyperphosphorylation (bottom panel).
Decreased levels of endogenous LMP-1 mRNA and protein in M phase.
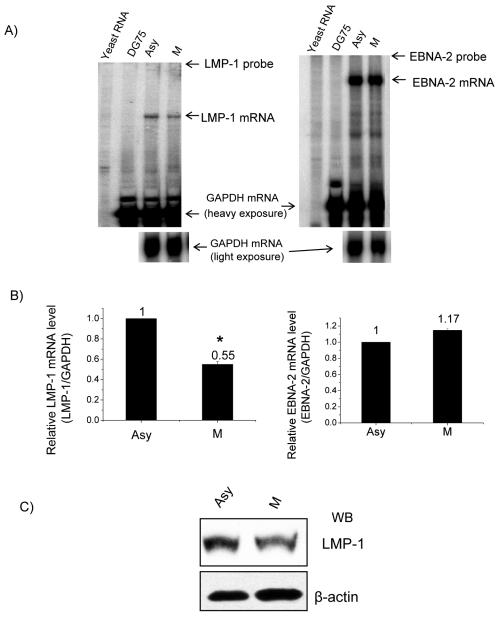
Finally, to determine whether the level of endogenous LMP-1 mRNA is associated with EBNA-2 hyperphosphorylation, we compared EBNA-2 and LMP-1 steady-state mRNA levels in asynchronously growing and nocodazole-arrested M-phase X50-7 cells by RPA. As shown in Fig. 6A and B, in the M phase the LMP-1 steady-state level of mRNA was around 50% of that detected in asynchronously growing cells, which indicated a decreased abundance of LMP-1 mRNA, whereas the EBNA-2 steady-state level of mRNA did not show an obvious change. Also, the LMP-1 protein level decreased in M phase (Fig. 6C). These results confirm in vivo the LMP-1 promoter reporter assay results and demonstrate that hyperphosphorylation of EBNA-2 correlates with decreased steady-state levels of endogenous LMP-1 mRNA and protein in the M phase of the cell cycle.
FIG. 6.
Decreased endogenous LMP-1 mRNA and protein levels in M-phase-arrested cells. (A) RPA was performed on total RNA from asynchronous (Asy) and M-phase-arrested X50-7 cells (M) with GAPDH and LMP-1 probes (left panel) or GAPDH and EBNA-2 probe (right panel). Yeast RNA and total RNA from DG75 cells were used as negative controls. A representative result from three independent experiments is shown. (B) Relative mRNA levels of LMP-1 and EBNA-2 were analyzed by normalizing LMP-1 (left panel) or EBNA-2 mRNA (right panel) levels to the GAPDH mRNA level with a PhosphorImager. Each data point represents the average of three independent experiments. Error bars represent the means ± standard errors of the means. *, P < 0.01. (C) Western blot of LMP-1 from total lysates of asynchronous (Asy) and M-phase-arrested X50-7 cells (M). β-actin levels were used as a loading control.
DISCUSSION
As a major gene in type III latency, EBNA-2's function in transactivation and transformation has been extensively addressed (30, 62, 63, 74, 78), and it is known to be a phosphoprotein (17, 18). However, modification of phosphorylation of the protein during the cell cycle has not been reported. In this work, we showed that EBNA-2 phosphorylation status is clearly regulated during the cell cycle, being hyperphosphorylated specifically in mitosis, which suggests involvement of the p34cdc2 kinase. Further, cell-cycle-related hyperphosphorylation of EBNA-2 appears to affect its function, resulting in decreased transactivation of one of its responsive promoters, LMP-1.
In addition to X50-7 cells, we detected the M-phase-specific hyperphosphorylation of EBNA-2 in three other type III latency cell lines (W91 [44], BL41-B95-8 [21], and CB0 B95-8 [a gift of N. Raab-Traub]) (data not shown), indicating that the mitosis-specific hyperphosphorylation of EBNA-2 is a general phenomenon in type III latency. Since it can also be detected in the EBV-negative cell lines BJAB, DG75, and even HeLa cells expressing EBNA-2 stably or transiently (Fig. 1B and D and 4C), the mitotic hyperphosphorylation of EBNA-2 is independent of any other EBV gene product and must be regulated by a cellular kinase or kinases. We suggest a possible role for p34cdc2/cyclin B1 kinase, which has its highest level of activity in mitosis (55) and regulates the G2-to-M transition (51). Hyperphosphorylated EBNA-2 contains phosphoserines and phosphothreonines but no phosphotyrosine (unpublished results), which indicates the involvement of a Ser/Thr kinase. Cyclin-dependent kinase p34cdc2 (CDK1) is a Ser/Thr kinase (48), and we have presented evidence that p34cdc2 kinase might be involved in hyperphosphorylation of EBNA-2. First, both hypo- and hyperphosphorylated EBNA-2 could be coimmunoprecipitated with p34cdc2 antibody (Fig. 2A), indicating physical association with p34cdc2. An attempt to coimmunoprecipitate p34cdc2 with EBNA-2 antibody (PE2 [86]) was unsuccessful, perhaps because the binding site was masked by the PE2 antibody. In fact, EBNA-2 has been reported to associate with a cellular protein with a molecular mass of ∼32 kDa, which is similar to that of p34cdc2 (34 kDa), by use of another EBNA-2 antibody (6, 7). Second, there was a temporal correlation between hyperphosphorylation of EBNA-2 and an increase in p34cdc2/cyclin B1 kinase activity (Fig. 2B). Third, p34cdc2 kinase could dramatically increase EBNA-2 phosphorylation in vitro (Fig. 3). In mitosis, a number of proteins involved in transcriptional regulation and some sequence-specific transcription factors (28, 36, 42, 59) are subjected to mitosis-specific phosphorylation, which in most cases is performed by a mitotic protein kinase, either p34cdc2 kinase and/or a kinase activated by it (25, 36). Whether other kinases may also be involved in hyperphosphorylation of EBNA-2 is unknown. The efficient phosphorylation of EBNA-2 in the presence of p34cdc2 kinase suggests that p34cdc2 may well be involved in mitotic hyperphosphorylation of EBNA-2 directly or indirectly.
Actually, EBNA-2 is not the only viral gene product that is associated with p34cdc2 and whose phosphorylation is involved with p34cdc2. EBNA-LP was reported to be hyperphosphorylated in nocodazole-arrested X50-7 cells, and it can be phosphorylated by p34cdc2 kinase in vitro (31, 34). Also, both Ser-89 and Ser-219, the major adenovirus E1A phosphorylation sites, have been phosphorylated in vitro by p34cdc2 purified from HeLa cells (8). In another case, the herpes simplex virus type 1 protein product of UL42 can bind and be phosphorylated by p34cdc2 kinase in vitro (1). Thus, there may be common mechanisms whereby the host cell regulates phosphorylation of viral proteins. On the other hand, the association of viral proteins with CDKs may influence the kinase function. The Tax oncoprotein of human T-cell leukemia virus type 1 (HTLV-1) induces leukemia in transgenic mice and permanent T-cell growth in vitro. Tax directly interacts with CDK4 in vitro and in vivo. The Tax/CDK4 complex represents an active holoenzyme that phosphorylates the retinoblastoma (Rb) protein in vitro; binding-deficient Tax mutants failed to activate CDK4 (23), indicating that direct association with Tax is required for enhanced kinase activity. Tax also increased the association of CDK4 with its positive cyclin regulatory subunit. The EBNA-2-p34cdc2 interaction warrants further study, since the association of EBNA-2 with p34cdc2 may be required to ensure proper regulation of EBNA-2 function; moreover, the association may also affect the function of p34cdc2 similarly to the effect of the Tax-CDK4 interaction.
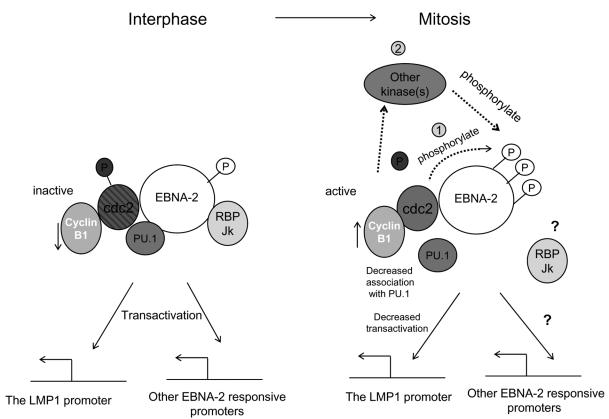
All studies of EBNA-2 transactivation of the LMP-1 promoter have been done in asynchronously growing cells when EBNA-2 was hypophosphorylated (30, 63, 78). Here we report that in M-phase-arrested cells, when EBNA-2 was hyperphosphorylated its interaction with PU.1 was suppressed and the transactivation of the LMP-1 promoter by EBNA-2 decreased by about 50%. There are numerous examples of protein phosphorylation regulating functions of transcription factors or coregulators, including modulating protein-protein interactions (reviewed in reference 80). A well-known example of the influence of cell-cycle-related phosphorylation on protein association that causes functional change is the Rb protein. Rb associates with and represses the transactivation ability of E2F, and the variation of phosphorylation status of Rb during the cell cycle influences its association with E2F; as a result, E2F-responsive promoters are regulated during the cell cycle (27, 46). Thus, the decreased association of EBNA-2 with PU.1 in M phase (Fig. 6), which is likely a result of the conformational changes caused by hyperphosphorylation (10, 29) of EBNA-2, might help explain its decreased transactivation of the LMP-1 promoter in M phase. Interestingly, mutation of the PU.1-binding site in the LMP-1 promoter abolishes approximately 50% of its responsiveness to EBNA-2 (30). It has been reported that purified GST-EBNA-2(310-376) protein can bind to in vitro-translated PU.1 (30), but attempts to detect the association by immunoprecipitation with EBNA-2 antibody were unsuccessful (30). Here, however, we have detected the association of EBNA-2 with PU.1 by coimmunoprecipitation in X50-7 cells, providing evidence of an association of these two proteins in vivo for the first time. Our current ideas about how EBNA-2 may be hyperphosphorylated in mitosis and the functional consequences are summarized in Fig. 7.
FIG. 7.
Mitotic hyperphosphorylation of EBNA-2 and its possible functional consequences. In the interphase, p34cdc2 kinase is inactive due to the low level of cyclin B1, its regulatory subunit, and phosphorylation of p34cdc2. EBNA-2 is hypophosphorylated in the interphase and transcriptionally active. Upon entering the M phase of the cell cycle, p34cdc2 kinase becomes activated by accumulation of cyclin B1 and dephosphorylation of p34cdc2. EBNA-2 is hyperphosphorylated during mitosis by p34cdc2 kinase directly (1) and probably by other kinases as well (2). Association of EBNA-2 with PU.1 decreases, which may be one of the mechanisms whereby transactivation of the LMP-1 promoter by EBNA-2 is impaired. In general, the suppression of EBNA-2 transcriptional activity by hyperphosphorylation may repress all the other EBNA-2-responsive genes during the M phase of the cell cycle.
Based on these results, it is tempting to conclude that hypophosphorylated EBNA-2 is active and hyperphosphorylated EBNA-2 is inactive. So it is then reasonable to expect that in type III latency cell lines, when EBNA-2 is hyperphosphorylated, endogenous LMP-1 mRNA steady-state levels should decrease, and in fact the LMP-1 mRNA level did decrease in M phase (Fig. 6). The decreased LMP-1 mRNA level was not due to decreased EBNA-2 expression, as indicated by the RPA showing that the EBNA-2 mRNA level did not change in M phase (Fig. 6). Transcription of all the EBNA genes is driven by one of two promoters, Cp or Wp, and the activity of these promoters is mutually exclusive (64, 82, 83). Wp is used during the initial stages of B-cell immortalization, followed by a switch to Cp usage (64, 82, 83). In X50-7 cells, in which Cp is deleted from the EBV genome, all the EBNAs are transcribed from Wp (84). There is no report that EBNA-2 can transactivate Wp. Thus, the constant levels of EBNA-2 mRNA suggest that Wp activity appears not to be affected by hyperphosphorylation of EBNA-2.
In eukaryotic cells, transcription in mitosis is generally repressed, and this transcriptional repression is thought to be required for the accurate division of chromosomes at mitosis. Transcriptional repression in mitosis is regulated at different levels, among which phosphorylation of a series of transcriptional factors by a mitotic kinase, in most cases involved with p34cdc2 kinase, is one of the important mechanisms (reviewed in reference 16). Here we found that EBNA-2, the EBV key transcriptional transactivator, experiences similar mitosis-specific phosphorylation that might involve p34cdc2, which is associated with its decreased transactivation of the LMP-1 promoter in M phase. Besides the LMP-1 promoter, EBNA-2 can also transactivate all the other viral promoters, including the BamHI C (66) and LMP-2A (43) (also called TP1) promoters. That these promoters are similarly linked to the cell cycle by hyperphosphorylation of EBNA-2 seems likely, because hyperphosphorylation of EBNA-2 might also influence its interaction with RBP-Jκ, which tethers EBNA-2 to Cp and TP1p in a manner similar to EBNA-2 and PU.1. Hyperphosphorylation of EBNA-2 might also influence the interaction of EBNA-2 with basal transcription factors TFII B (72) and TFII H (70) and therefore influence transcription in mitosis. It has been known for decades that the latent EBV episomes are located in chromosomes (60, 61), and the copy number of EBV episomes remains essentially constant in each cell line (38). Thus, transcription from the EBV episome may be similarly repressed during mitosis in type III latency, which might be important for the accurate division of EBV episomes into daughter cells. Definitive proof of this hypothesis requires study of other type III latency gene products in the M phase of the cell cycle and identification of the phosphorylation sites of EBNA-2; this work is in progress. In conclusion, our finding that EBNA-2 is specifically hyperphosphorylated during mitosis allows us to place the significance of the suppression of EBNA-2 transcriptional function into the general context of the cell in which mitosis is coupled with repression of general transcriptional activity.
Acknowledgments
We thank Jeffery Lin and E. Kieff for the pGL2(−512/+72)LMP1p-Luc plasmid, L. C. Spender and P. J. Farrell for pBS-LMP1 plasmid, N. Raab-Traub for the CB0 B95-8 cell line, and F. Wang for BJAB cells stably expressing EBNA-2. We thank L. Arnold for his help with FACS. We thank E. Gershburg, L. E. Huye, S. Ning, A. M. Hahn, and Yue Xiong for reading the manuscript and helpful discussions.
This work was supported by a grant from the National Cancer Institute (CA 19014).
REFERENCES
- 1.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2001. cdc2 cyclin-dependent kinase binds and phosphorylates herpes simplex virus 1 UL42 DNA synthesis processivity factor. J. Virol. 75:10326-10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben-Bassat, H., N. Goldblum, S. Mitrani, T. Goldblum, J. M. Yoffey, M. M. Cohen, Z. Bentwith, B. Ramot, E. Klein, and G. Klein. 1977. Establishment in continuous culture of a new type of lymphocyte from a “Burkitt-like” malignant lymphoma (line D.G.-75). Int. J. Cancer 19:27-33. [DOI] [PubMed] [Google Scholar]
- 3.Conteras-Brodin, B. A., M. Anvret, S. Imreh, E. Altiok, G. Klein, and M. G. Masucci. 1991. B cell phenotype-dependent expression of the Epstein-Barr virus nuclear antigens EBNA-2 to EBNA-6: studies with somatic cell hybrids. J. Gen. Virol. 72:3025-3033. [DOI] [PubMed] [Google Scholar]
- 4.Davenport, M. G., and J. S. Pagano. 1999. Expression of EBNA-1 mRNA is regulated by cell cycle during Epstein-Barr virus type I latency. J. Virol. 73:3154-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decker, L. L., L. D. Klaman, and D. A. Thorley-Lawson. 1996. Detection of the latent form of Epstein-Barr virus DNA in the peripheral blood of healthy individuals. J. Virol. 70:3286-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dillner, J., B. Kallin, G. Klein, H. Jornvall, H. Alexander, and R. Lerner. 1985. Antibodies against synthetic peptides react with the second Epstein-Barr virus-associated nuclear antigen. EMBO J. 4:1813-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dillner, J., V. Wendel-Hansen, G. Kjellstrom, B. Kallin, and A. Rosen. 1988. Purification and characterization of the Epstein-Barr virus nuclear antigen 2 using monoclonal antipeptide antibody. Int. J. Cancer 42:721-727. [DOI] [PubMed] [Google Scholar]
- 8.Dumont, D. J., and P. E. Branton. 1992. Phosphorylation of adenovirus E1A proteins by the p34cdc2 protein kinase. Virology 189:111-120. [DOI] [PubMed] [Google Scholar]
- 9.Fahraeus, R., H. Li-Fu, and I. Ernberg. 1988. Expression of Epstein-Barr virus-encoded proteins in nasopharyngeal carcinoma. Int. J. Cancer 42:329-338. [DOI] [PubMed] [Google Scholar]
- 10.Fujitani, N., M. Kanagawa, T. Aizawa, T. Ohkubo, S. Kaya, M. Demura, K. Kawano, S. i. Nishimura, K. Taniguchi, and K. Nitta. 2003. Structure determination and conformational change induced by tyrosine phosphorylation of the N-terminal domain of the α-chain of pig gastric H+/K+-ATPase. Biochem. Biophys. Res. Commun. 300:223-229. [DOI] [PubMed] [Google Scholar]
- 11.Fulco, M., A. Costanzo, P. Merlo, R. Mangiacasale, S. Strano, G. Blandino, C. Balsano, P. Lavia, and M. Levrero. 2003. p73 is regulated by phosphorylation at the G2/M transition. J. Biol. Chem. 278:49196-49202. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa, Y., S. Iwase, Y. Terui, J. Kikuchi, T. Sakai, M. Nakamura, S. Kitagawa, and M. Kitagawa. 1996. Transcriptional activation of the cdc2 gene is associated with Fas-induced apoptosis of human hematopoietic cells. J. Biol. Chem. 271:28469-28477. [DOI] [PubMed] [Google Scholar]
- 13.Gage, J. R., C. Meyers, and F. O. Wettstein. 1990. The E7 proteins of the nononcogenic human papillomavirus type 6b (HPV-6b) and of the oncogenic HPV-16 differ in retinoblastoma protein binding and other properties. J. Virol. 64:723-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillian, A. P., T. Robert, and J. A. Martin. 2000. Epstein-Barr virus EBNA3C can disrupt multiple cell cycle checkpoints and induce nuclear division divorced from cytokinesis. Oncogene 19:700-709. [DOI] [PubMed] [Google Scholar]
- 15.Gorman, C. M., L. F. Moffat, and B. H. Howard. 1982. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol. Cell. Biol. 2:1044-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottesfeld, J. M., and D. J. Forbes. 1997. Mitotic repression of the transcriptional machinery. Trends Biochem. Sci. 22:197-202. [DOI] [PubMed] [Google Scholar]
- 17.Grasser, F. A., S. Gottel, P. Haiss, B. Boldyreff, O. G. Issinger, and N. Mueller-Lantzsch. 1992. Phosphorylation of the Epstein-Barr virus nuclear antigen 2. J. Virol. 186:1694-1710. [DOI] [PubMed] [Google Scholar]
- 18.Grasser, F. A., P. Haiss, and N. Mueller-Lantzsch. 1991. Biochemical characterization of Epstein-Barr virus nuclear antigen 2A. J. Virol. 65:3779-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregory, C. D., M. Rowe, and A. B. Richinson. 1990. Different Epstein-Barr virus (EBV)-B cell interactions in phenotypically distinct clones of a Burkitt lymphoma cell line. J. Gen. Virol. 71:1481-1495. [DOI] [PubMed] [Google Scholar]
- 20.Grossman, S. R., E. Johannsen, X. Tong, R. Yalamanchili, and E. Kieff. 1994. The Epstein-Barr virus nuclear antigen 2 transactivator is directed to response elements by the Jκ recombination signal binding protein. Proc. Natl. Acad. Sci. USA 91:7568-7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulley, M. L., M. Raphael, C. T. Lutz, D. W. Ross, and N. Raab-Traub. 1992. Epstein-Barr virus integration in human lymphomas and lymphoid cell lines. Cancer 70:185-191. [DOI] [PubMed] [Google Scholar]
- 22.Gurley, L. R., J. G. Valdez, and J. S. Buchanan. 1995. Characterization of the mitotic specific phosphorylation site of histone H1. J. Biol. Chem. 270:27653-27660. [DOI] [PubMed] [Google Scholar]
- 23.Haller, K., Y. Wu, E. Derow, I. Schmitt, K.-T. Jeang, and R. Grassmann. 2002. Physical interaction of human T-cell leukemia virus type 1 Tax with cyclin-dependent kinase 4 stimulates the phosphorylation of retinoblastoma protein. Mol. Cell. Biol. 22:3327-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada, S., and E. Kieff. 1997. Epstein-Barr virus nuclear protein LP stimulates EBNA-2 acidic domain-mediated transcriptional activation. J. Virol. 71:6611-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartl, P., J. Gottesfeld, and D. J. Forbes. 1993. Mitotic repression of transcription in vitro. J. Cell Biol. 120:613-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henkel, T., P. D. Ling, S. D. Hayward, and M. G. Peterson. 1994. Mediation of Epstein-Barr virus EBNA2 transactivation by recombination signal-binding protein Jκ. Science 265:92-95. [DOI] [PubMed] [Google Scholar]
- 27.Herwig, S., and M. Strauss. 1997. The retinoblastoma protein: a master regulator of cell cycle, differentiation and apoptosis. Eur. J. Biochem. 246:581-601. [DOI] [PubMed] [Google Scholar]
- 28.Hunter, T., and M. Karin. 1992. The regulation of transcription by phosphorylation. Cell 70:375-387. [DOI] [PubMed] [Google Scholar]
- 29.Jiro, U., N. Yuji, S. Atsushi, K. Kazuto, N. Toshiharu, and H. Masatoshi. 2000. Direct imaging of phosphorylation-dependent conformational change and DNA binding of CREB by electron microscopy. Genes Cells 5:515-522. [DOI] [PubMed] [Google Scholar]
- 30.Johannsen, E., E. Koh, G. Mosialos, X. Tong, E. Kieff, and S. R. Grossman. 1995. Epstein-Barr virus nuclear protein 2 transactivation of the latent membrane protein 1 promoter is mediated by Jκ and PU.1. J. Virol. 69:253-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kato, K., A. Yokoyama, Y. Tohya, H. Akashi, Y. Nishiyama, and Y. Kawaguchi. 2003. Identification of protein kinases responsible for phosphorylation of Epstein-Barr virus nuclear antigen leader protein at serine-35, which regulates its coactivator function. J. Gen. Virol. 84:3381-3392. [DOI] [PubMed] [Google Scholar]
- 32.Kerr, B. M., A. L. Lear, M. Rowe, D. Croom-Carter, L. S. Young, S. M. Rookes, P. H. Gallimore, and A. B. Rickinson. 1992. Three transcriptionally distinct forms of Epstein-Barr virus latency in somatic cell hybrids: cell phenotype dependence of virus promoter usage. Virology 187:189-201. [DOI] [PubMed] [Google Scholar]
- 33.Kieff, E. 1995. Epstein-Barr virus and its replication, p. 2343-2396. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology. Lippincott-Raven Publishers, Philadelphia, Pa.
- 34.Kitay, M. K., and D. T. Rowe. 1996. Cell cycle stage-specific phosphorylation of the Epstein-Barr virus immortalization protein EBNA-LP. J. Virol. 70:7885-7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laux, G., F. Dugrillon, C. Eckert, B. Adam, U. Zimber-Strobl, and G. W. Bornkamm. 1994. Identification and characterization of an Epstein-Barr virus nuclear antigen 2-responsive cis element in the bidirectional promoter region of latent membrane protein and terminal protein 2 genes. J. Virol. 68:6947-6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leresche, A., V. J. Wolf, and J. M. Gottesfeld. 1996. Repression of RNA polymerase II and III transcription during M phase of the cell cycle. Exp. Cell Res. 229:282-288. [DOI] [PubMed] [Google Scholar]
- 37.Lin, J., E. Johannsen, E. Robertson, and E. Kieff. 2002. Epstein-Barr virus nuclear antigen 3C putative repression domain mediates coactivation of the LMP1 promoter with EBNA-2. J. Virol. 76:232-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindahl, T., A. Adams, G. Bjursell, G. W. Bornkamm, C. Kaschka-Dierich, and U. Jehn. 1976. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J. Mol. Biol. 102:511-530. [DOI] [PubMed] [Google Scholar]
- 39.Ling, P. D., J. J. Hsieh, I. K. Ruf, D. R. Rawlins, and S. D. Hayward. 1994. EBNA-2 upregulation of Epstein-Barr virus latency promoters and the cellular CD23 promoter utilizes a common targeting intermediate, CBF1. J. Virol. 68:5375-5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ling, Y. H., C. Tornos, and R. Perez-Soler. 1998. Phosphorylation of Bcl-2 is a marker of M phase events and not a determinant of apoptosis. J. Biol. Chem. 273:18984-18991. [DOI] [PubMed] [Google Scholar]
- 41.Lowe, M., C. Rabouille, N. Nakamura, R. Watson, M. Jackman, E. Jamsa, D. Rahman, D. J. C. Pappin, and G. Warren. 1998. Cdc2 kinase directly phosphorylates the cis-Golgi matrix protein GM130 and is required for Golgi fragmentation in mitosis. Cell 94:783-793. [DOI] [PubMed] [Google Scholar]
- 42.Luscher, B., and R. N. Eisenman. 1992. Mitosis-specific phosphorylation of the nuclear oncoproteins Myc and Myb. J. Cell Biol. 118:775-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meitinger, C., L. J. Strobl, G. Marschall, G. W. Bornkamm, and U. Zimber-Strobl. 1994. Crucial sequences within the Epstein-Barr virus TP1 promoter for EBNA2-mediated transactivation and interaction of EBNA2 with its responsive element. J. Virol. 68:7497-7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, G., L. Heston, and G. Hoffman. 1982. Neutralization of lymphocyte immortalization by different strains of Epstein-Barr virus with a murine monoclonal antibody. Infect. Immun. 37:1028-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller, G., J. Robinson, L. Heston, and M. Lipman. 1974. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc. Natl. Acad. Sci. USA 71:4006-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mittnacht, S. 1998. Control of pRB phosphorylation. Curr. Opin. Genet. Dev. 8:21-27. [DOI] [PubMed] [Google Scholar]
- 47.Miyashita, E. M., B. Yang, G. J. Babcock, and D. A. Thorley-Lawson. 1997. Identification of the site of Epstein-Barr virus persistence in vivo as a resting B cell. J. Virol. 71:4882-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno, S., and P. Nurse. 1990. Substrates for p34 cdc2: in vivo veritas? Cell 61:549-551. [DOI] [PubMed] [Google Scholar]
- 49.Morla, A. O., G. Draetta, D. Beach, and J. Y. J. Wang. 1989. Reversible tyrosine phosphorylation of cdc2: dephosphorylation accompanies activation during entry into mitosis. Cell 58:193-203. [DOI] [PubMed] [Google Scholar]
- 50.Nakajima, H., F. Toyoshima-Morimoto, E. Taniguchi, and E. Nishida. 2003. Identification of a consensus motif for Plk (Polo-like kinase) phosphorylation reveals Myt1 as a Plk1 substrate. J. Biol. Chem. 278:25277-25280. [DOI] [PubMed] [Google Scholar]
- 51.Nigg, E. A. 1995. Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays 17:471-480. [DOI] [PubMed] [Google Scholar]
- 52.Nitsche, F., A. Bell, and A. Rickinson. 1997. Epstein-Barr virus leader protein enhances EBNA-2-mediated transactivation of latent membrane protein 1 expression: a role for the W1W2 repeat domain. J. Virol. 71:6619-6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pagano, M., R. Pepperkok, F. Verde, W. Ansorge, and G. Draetta. 1992. Cyclin A is required at two points in the human cell cycle. EMBO J. 11:961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petti, L., C. Sample, and E. Kieff. 1989. Subnuclear localization and phosphorylation of Epstein-Barr virus latent infection nuclear proteins. Virology 176:563-574. [DOI] [PubMed] [Google Scholar]
- 55.Pines, J., and T. Hunter. 1989. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell 58:833-846. [DOI] [PubMed] [Google Scholar]
- 56.Ruf, I. K., and J. Sample. 1999. Repression of Epstein-Barr virus EBNA-1 gene transcription by pRb during restricted latency. J. Virol. 73:7943-7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sample, J., E. Henson, and C. Sample. 1992. The Epstein-Barr virus nuclear protein 1 promoter active in type I latency is autoregulated. J. Virol. 66:4654-4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sauter, M., and N. Mueller-Lantzsch. 2003. Characterization of an Epstein-Barr virus nuclear antigen 2 variant (EBNA2B) by specific sera. Virus Res. 8:152. [DOI] [PubMed] [Google Scholar]
- 59.Segil, N., M. Guermah, A. Hoffmann, R. G. Roeder, and N. Heintz. 1996. Mitotic regulation of TFIID: inhibition of activator-dependent transcription and changes in subcellular localization. Genes Dev. 10:2389-2400. [DOI] [PubMed] [Google Scholar]
- 60.Sexton, C. J., and J. S. Pagano. 1989. Analysis of the Epstein-Barr virus origin of plasmid replication (oriP) reveals an area of nucleosome sparing that spans the 3′ dyad. J. Virol. 63:5505-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaw, J. E., L. F. Levinger, and C. W. Carter, Jr. 1979. Nucleosomal structure of Epstein-Barr virus DNA in transformed cell lines. J. Virol. 29:657-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sjoblom, A., A. Jansson, W. Yang, S. Lain, T. Nilsson, and L. Rymo. 1995. PU box-binding transcription factors and a POU domain protein cooperate in the Epstein-Barr virus (EBV) nuclear antigen 2-induced transactivation of the EBV latent membrane protein 1 promoter. J. Gen. Virol. 76:2679-2692. [DOI] [PubMed] [Google Scholar]
- 63.Sjoblom, A., A. Nerstedt, A. Jansson, and L. Rymo. 1995. Domains of the Epstein-Barr virus nuclear antigen 2 (EBNA2) involved in the transactivation of the latent membrane protein 1 and the EBNA Cp promoters. J. Gen. Virol. 76:2669-2678. [DOI] [PubMed] [Google Scholar]
- 64.Speck, S. H., and J. L. Strominger. 1989. Transcription of Epstein-Barr virus in latently infected growth-transformed lymphocytes. Adv. Vir. Oncol. 8:133-150. [Google Scholar]
- 65.Spender, L. C., G. H. Cornish, B. Rowland, B. Kempkes, and P. J. Farrell. 2001. Direct and indirect regulation of cytokine and cell cycle proteins by EBNA-2 during Epstein-Barr virus infection. J. Virol. 75:3537-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sung, N. S., S. Kenney, D. Gutsch, and J. S. Pagano. 1991. EBNA-2 transactivates a lymphoid-specific enhancer in the BamHI C promoter of Epstein-Barr virus. J. Virol. 65:2164-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sung, N. S., G. Wilson, M. Davenport, N. D. Sista, and J. S. Pagano. 1994. Reciprocal regulation of the Epstein-Barr virus BamHI-F promoter by EBNA-1 and an E2F transcription factor. Mol. Cell. Biol. 14:7144-7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sung, N. S., J. Wilson, and J. S. Pagano. 1993. Characterization of cis-acting elements of the BamHI-F promoter of EBV, p. 239-242. In T. Tursz, J. S. Pagano, D. V. Ablashi, G. de The, G. Lenoir, and G. R. Pearso (ed.), The Epstein-Barr virus and associated diseases. INSERM/John Libbey Eurotext Limited, London, United Kingdom.
- 69.Terasima, T., and L. J. Tolmach. 1963. Growth and nucleic acid synthesis in synchronously dividing populations of HeLa cells. Exp. Cell Res. 30:344-362. [DOI] [PubMed] [Google Scholar]
- 70.Tong, X., R. Drapkin, D. Reinberg, and E. Kieff. 1995. The 62- and 80-kDa subunits of transcription factor IIH mediate the interaction with Epstein-Barr virus nuclear protein 2. Proc. Natl. Acad. Sci. USA 92:3259-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tong, X., R. Drapkin, R. Yalamanchili, G. Mosialos, and E. Kieff. 1995. The Epstein-Barr virus nuclear protein 2 acidic domain forms a complex with a novel cellular coactivator that can interact with TFIIE. Mol. Cell. Biol. 15:4735-4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tong, X., F. Wang, C. J. Thut, and E. Kieff. 1995. The Epstein-Barr virus nuclear protein 2 acidic domain can interact with TFIIB, TAF40, and RPA70 but not with TATA-binding protein. J. Virol. 69:585-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsang, S. F., F. Wang, K. M. Izumi, and E. Kieff. 1991. Delineation of the cis-acting element mediating EBNA-2 transactivation of latent infection membrane protein expression. J. Virol. 65:6765-6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voss, M. D., A. Hille, S. Barth, A. Spurk, F. Hennrich, D. Holzer, N. Nueller-Lantzsch, E. Kremmer, and F. A. Grasser. 2001. Functional cooperation of Epstein-Barr virus nuclear protein 2 and the survival motor neuron protein in transactivation of the viral LMP1 promoter. J. Virol. 75:11781-11790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang, D., D. Liebowitz, and E. Kieff. 1985. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 43:831-840. [DOI] [PubMed] [Google Scholar]
- 76.Wang, D., D. Liebowitz, F. Wang, C. Gregory, A. Rickinson, R. Larson, T. Springer, and E. Kieff. 2003. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J. Virol. 62:4173-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang, F., C. Gregory, C. Sample, M. Rowe, D. Liebowitz, R. Murray, A. Rickinson, and E. Kieff. 1990. Epstein-Barr virus latent membrane protein (LMP-1) and nuclear protein 2 and 3C are effectors of phenotypic changes in B lymphocytes: EBNA-2 and LMP-1 cooperatively induce CD23. J. Virol. 64:2309-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang, F., S. F. Tsang, M. G. Kurilla, J. I. Cohen, and E. Kieff. 1990. Epstein-Bar virus nuclear antigen 2 transactivates latent membrane protein LMP-1. J. Virol. 64:3407-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang, L., S. R. Grossman, and E. Kieff. 2000. Epstein-Barr virus nuclear protein 2 interacts with p300, CBP, and PCAF histone acetyltransferases in activation of the LMP1 promoter. Proc. Natl. Acad. Sci. USA 97:430-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whitmarsh, A. J., and R. J. Davis. 2000. Regulation of transcription factor function by phosphorylation. Cell. Mol. Life Sci. 57:1172-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson, G., and G. Miller. 1979. Recovery of Epstein-Barr virus from nonproducer neonatal human lymphoid cell transformants. Virology 95:351-358. [DOI] [PubMed] [Google Scholar]
- 82.Woisetschlaeger, M., J. L. Strominger, and S. H. Speck. 1989. Mutually exclusive use of viral promoters in Epstein-Barr virus latently infected lymphocytes. Proc. Natl. Acad. Sci. USA 86:6498-6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Woisetschlaeger, M., C. N. Yandava, L. A. Furmanski, J. L. Strominger, and S. H. Speck. 1990. Promoter switching in Epstein-Barr virus during the initial stages of infection of B lymphocytes. Proc. Natl. Acad. Sci. USA 87:1725-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yandava, C. N., and S. H. Speck. 1992. Characterization of the deletion and rearrangement in the BamHI C region of the X50-7 Epstein-Barr virus genome, a mutant viral strain with exhibits constitutive BamHI W promoter activity. J. Virol. 66:5646-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yokoyama, A., M. Tanaka, G. Matsuda, K. Kato, M. Kanamori, H. Kawasaki, H. Hirano, I. Kitabayashi, M. Ohki, K. Hirai, and Y. Kawaguchi. 2001. Identification of major phosphorylation sites of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP): ability of EBNA-LP to induce latent membrane protein 1 cooperatively with EBNA-2 is regulated by phosphorylation. J. Virol. 75:5119-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Young, L., C. Alfieri, K. Hennessy, H. Evans, C. O'Hara, K. C. Anderson, J. Ritz, R. S. Shapiro, A. B. Rickinson, E. Kieff, and J. I. Cohen. 1989. Expression of Epstein-Barr virus transformation-associated genes in tissues of patients with EBV lymphoproliferative disease. N. Engl. J. Med. 321:1080-1085. [DOI] [PubMed] [Google Scholar]
- 87.Young, L. S., E. M. Deacon, M. Rowe, J. Crocker, H. Herbst, G. Niedobitek, S. J. Hamilton-Dutoit, and G. Pallesen. 1991. Epstein-Barr virus latent genes in tumour cells of Hodgkin's disease. Lancet 337:1617. [DOI] [PubMed] [Google Scholar]