Abstract
Chronic kidney disease (CKD) is common, but under-recognized, among patients in the health care system, where improving patient safety is a high priority. Poor disease recognition and several other features of CKD make it a high risk condition for adverse safety events. In this review, we will discuss the unique attributes of CKD, which make it a high risk condition for patient safety mishaps. We will point out that adverse safety events in this disease have the potential to contribute to disease progression; namely, accelerated loss of kidney function and an increased incidence of end-stage renal disease (ESRD). We will also propose a framework in which to consider patient safety in CKD, highlighting the need for disease-specific safety indicators that reflect unsafe practices in this disease. Finally, we will discuss the hypothesis that increased recognition of CKD will reduce disease-specific safety events, and in this way decrease the likelihood of adverse outcomes including an accelerated rate of kidney function loss and an increased incidence of ESRD.
Keywords: patient safety, chronic kidney disease, disease recognition, medical errors
Patient safety has been identified as a high priority area for improvement in health care. In 1999, the Institute of Medicine (IOM) issued a report entitled “To Err is Human: Building a Safer Health System”, which included the widely cited statistic that between 44,000 to 98,000 in-hospital deaths occurring each year were due to medical errors1. The Agency for Healthcare Research and Quality (AHRQ) has taken the lead in guiding efforts in improvement of patient safety and reducing medical errors 2-5; however, despite their efforts these improvements have been slow in coming across the health system 6. Definitions of safety incidents vary and in an effort to apply standards in measurement of patient safety across hospitals and health systems, the AHRQ established a set of patient safety indicators (PSIs)2. Using these PSIs, several investigators have identified variations in patient safety events across hospitals 7;8, by payer status 9, and with varying demographic factors including race and ethnicity 10.
Despite the acceptance of AHRQ PSIs as a set of tools to evaluate patient safety, these measures have many shortcomings 11. First, the AHRQ PSIs only capture a portion of the type of events that might be included in the domain of patient safety. Others use alternative definitions of patient safety and place a greater emphasis on medication errors12, which are absent from the AHRQ PSIs. Moreover, the AHRQ PSIs are optimally used to evaluate in-hospital incidents, which are only a sub-set of all patient safety events. Also, the AHRQ PSIs generally have low incidence rates in the hospital population, and often require large sample surveys to assess trends and to test hypotheses. This could partly be due to the fact that the AHRQ PSIs are heavily weighted to surgical and obstetric misadventures. However, it is plausible, and quite likely, that the majority of patient safety problems are in the growing population of Americans who suffer from chronic medical ailments – including chronic kidney disease (CKD).
In this review, we will discuss the various aspects of CKD that make it a uniquely high risk condition for both general, and disease-specific, adverse safety events. We assert that new efforts are needed to formally define PSIs, which are specific to CKD and are derived from the unique disease attributes described. We will propose the use of the Donabedian structure-process-outcome paradigm as a foundation for the consideration of disease-specific patient safety in CKD11;13. We will also discuss the potential role of increased disease recognition as a key structural intervention necessary to reduce the incidence of CKD-specific safety events, and in turn reduce the incidence of adverse disease outcomes including hastened kidney function loss and incidence of ESRD.
Chronic kidney disease is a high risk condition for adverse safety events
CKD is becoming increasingly prevalent in the US 14;15, and with its complexity and preponderance of co-morbidities, this disease often requires frequent hospitalizations, prolonged length of hospital stay, and has an increased cost of care 16-18. The diagnosis of CKD is frequently under-recognized19-21, and the failure to recognize CKD in patients, who are frequent users of the health system, is a lost opportunity to initiate recommended treatments for the disease, as well as to minimize threats to patient safety.
In a national cohort of veterans receiving care at the Veterans’ Health Administration (VHA), with at least one hospitalization during fiscal year 04′-05′, and an outpatient creatinine for determination of estimated GFR, we examined whether CKD was a risk factor for the incidence of AHRQ-established PSIs. We demonstrated that CKD was a significant risk factor for several of the AHRQ PSIs and revealed a stepwise increase in risk for a composite of all the PSIs with declining kidney function. We concluded that CKD was a risk factor for the general AHRQ-derived PSIs but noted that these indicators tend to be uncommon in a general population and do not have a high degree of relevance in a chronic medical condition like CKD. It was clear from the study that a set of disease-specific safety indicators were needed for CKD22.
The manifestations of CKD that relate to patient safety can range from sequela of the disease, which are improperly managed, to consequences of misguided therapeutic interventions employed in this highly co-morbid disease population, and are listed in Table 1. Many of these are not mutually exclusive and have the potential to relate to others on the list. These clinical events are not included in the AHRQ-derived set of PSIs but comprise the basis for a disease-specific set of safety events in CKD. While there is a substantial body of literature describing these events, there has been minimal consideration of them within the context of patient safety or to what extent they fit on the spectrum ranging from inadequately managed disease sequela to unanticipated iatrogenic complications of a treatment or intervention.
Table 1. Features of CKD and its management which relate to patient safety.
RAAS: renin angiotensin aldosterone system, CHF: congestive heart failure
Adverse safety outcome in chronic kidney disease: acceleration of kidney function loss
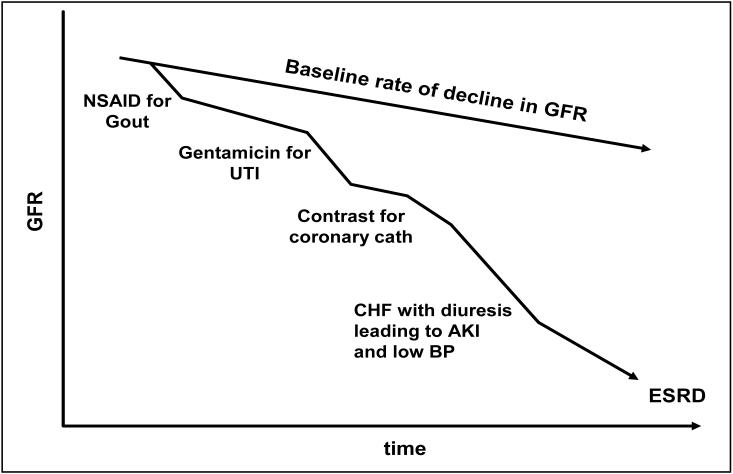
A patient safety event is typically defined as an unintended incident which usually results in a hospitalization, prolonged length of hospital stay, unexpected injury, or death. With CKD, the unintended consequences of a patient safety event must be broadened to include progression of disease. The rate of kidney function decline associated with CKD is variable with the most significant risk factors including proteinuria, uncontrolled hypertension, and lack of diabetes control. While beneficial therapies are available such as renin angiotensin aldosterone system (RAAS) blockers, these are limited in their ability to successfully arrest kidney disease progression in most patients. With the absence of curative therapies, an important tactic to slow progression in CKD is to minimize nephrotoxic exposures, which may accelerate the rate of kidney function loss. In fact, the National Kidney Foundation (NKF) Kidney Disease Outcome Quality Initiative (K/DOQI) Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification places an emphasis on practices to be avoided because they confer unreasonable risks to the CKD patient49. Guidelines 2 and 13 of that report, discuss minimizing exposure to medications which contribute to disease progression and identifying several potential factors which might lead to acute episodes of kidney injury and contribute to the net loss of kidney function over time. Figure 1 depicts the clinical course of a theoretical, but typical, patient with CKD who has been subject to such exposures. These common exposures have the potential to accelerate disease beyond the baseline rate of kidney function decay. The importance of identifying these safety events is underscored by the fact that they are often preventable. The identification and prevention of CKD-related safety events offers an opportunity to reduce the adverse outcomes associated with CKD; namely, the accelerated decline in kidney function and the incidence of ESRD.
Figure 1.
A theoretical patient with chronic kidney disease is subject to several events which might be classified as preventable and related to patient safety. These events contribute to an accelerated rate of kidney function decay.
Establishing a robust framework to survey patient safety in CKD
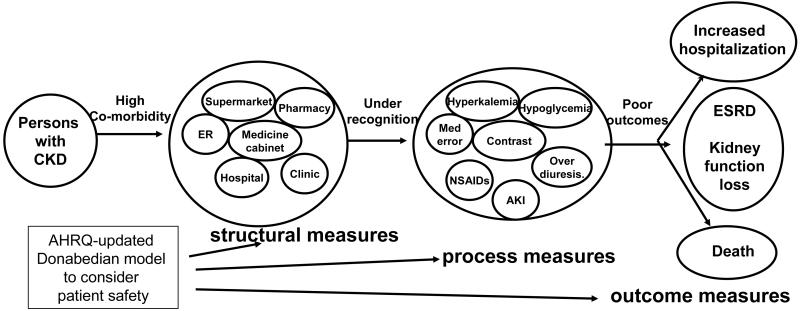
Given the shortcoming of the current tools to evaluate patient safety, investigators at AHRQ have proposed a framework for future efforts in this area and targeted at different populations13. This framework is basic in its elements but intended to be acceptable across multiple disease domains, and flexible in its ability to incorporate the disparate types of events which can fit into the rubric of patient safety. The conceptual scheme is based on the Donabedian’s classic structure-process-outcome model of quality measures 4;13. Figure 2 offers a schematic demonstrating how the Donabedian model can be applied to the unique circumstances of CKD. As outlined below, important steps are necessary to tailor the Donabedian model to address patient safety in CKD.
Figure 2.
The Donabedian structure-process-outcome framework13 applied to the chronic kidney disease model. The model proposes a causal link between the disease-specific structural deficit of poor disease recognition and adverse disease outcomes, mediated through several candidate disease-specific safety measures.
Structural measures: includes dimensions of infrastructure, process, or systems which contribute to the environment that affects patient safety. These are not directly attributable to individuals or care providers although structural conditions may create circumstances that have the potential to lead to unintended consequences for a patient or an error by a provider. The pivotal structural aspect of the health care system that is likely to contribute to patient safety in CKD is lack of disease recognition. This includes the absence of cues that alert providers to the unique specifications for care in a patient with CKD. As described above, it is well-documented that the majority of patients with CKD and their associated impaired kidney function are often not recognized. Improving disease recognition might reduce medical errors; however, overcoming the problem of poor disease recognition may be more challenging than first realized given the broad environment in which CKD patients dwell. As demonstrated in Figure 2, patients with CKD may encounter health agents and receive interventions in a wide variety of venues including the hospital, clinic, pharmacy, nursing home, ambulance, or medicine cabinet. Therefore strategies to increase disease recognition must attempt to go beyond those center-based systems already established to improve disease recognition.
Process measures: include practices, lifestyles, interventions, or treatments which are ill-advised, improper, misused, overused or omitted. These actions may be implemented by providers or patients, and include medication errors or unsafe medical practices. However, the classification of a medical action as one related to safety is based on the clinical context. For instance, a treatment may be indicated for one disease state, but not appropriate for another. Moreover, what makes a practice or treatment unsafe, or use of a medication as an error, is not necessarily based on the occurrence of an unintended (iatrogenic) event, but rather the potential for such an occurrence. The unique aspects of CKD described above lead to a condition with the rich potential for unsafe practices, injudicious treatments, and medical errors. The byproducts of such actions can include serious adverse outcomes. Although candidate safety measures are highlighted in Table 1 and illustrated in Figure 2, a validated list of such CKD-specific patient safety process measures have not been established. A prerequisite to any intervention related to safety will be the consensus acceptance of a set of such safety indicators specific to CKD. However, any patient safety indicator which is specific to CKD must meet certain criteria to be useful. The safety indicators must be relatively common, substantiating their significance as a health problem. They must be relevant to the disease process with a link to adverse outcomes that are significant in the disease. Finally, and perhaps most importantly, the safety measure must be preventable.
Outcome measures: includes adverse safety events and the realization of the potential consequences of high risk structural and process measures. These would include a medical injury, manifest toxicity from a medication error, iatrogenic illness, nosocomial infections, disease progression related to an inappropriate medical action, and death related to medical treatment or accident. It is well-known that patients with CKD are at an increased risk of adverse disease outcomes including loss of kidney function, ESRD, hospitalization, and death; however, it is not known to what extent these adverse outcomes are related to patient safety events. The confirmation of a relationship between safety process measures and disease-specific outcomes, as outlined in Figure 2, is critical since prevention of the former may play a significant role in reducing the incidence of the latter.
Improving CKD-specific patient safety: a new approach to slowing disease progression
Establishing an association between CKD recognition and patient safety is an important first step to developing new approaches to treating this disease; where added emphasis is placed on health services in addition to the use of medical therapies. With a clear definition of CKD-specific safety indicators – developed within the structure-process-outcome paradigm outline above in Figure 2 – investigators can move forward with studies and demonstration projects designed to improve patient safety and assess to what extent this reduces adverse disease outcomes. Such efforts should include interventions intended to alter the key structural deficiency affecting patients with CKD –poor disease recognition. Previous work has demonstrated the benefit in reducing medication errors of computer-based systems which alert providers to impaired kidney function when prescribing in-hospital medications 23;50. However, efforts to increase the recognition of CKD does not necessarily ensure that appropriate therapy is given 51-53.The most recent surveys of practice patterns in large practice or health network settings have demonstrated either persistent shortfalls in recognition of moderate CKD or marginal improvement in adherence to consensus-based practice guidelines for CKD54;55. Moreover, innovative strategies which transmit alerts about a CKD patient’s condition across all dimensions of the health care landscape are required since many of the exposures which relate to patient safety are present not only in the hospital but also in the community and home.
While efforts to increase recognition of CKD across a patient’s domain may be a challenge there are still opportunities to improve patient safety in this disease population, which is frequently hospitalized. The preponderance of cardiovascular disease (CVD) in patients with CKD who present to the hospital represents a target for efforts to reduced safety-related adverse events. Several aspects of prudent CVD management when implemented in patients with CKD may lead to preventable adverse safety events. Exposure to iodinated contrast agents with coronary catheterization can lead to acute kidney injury34;35; however, kidney-sparing tactics can be employed as means to reduce acute episodes of kidney function loss in patients with CKD and a clear indication for coronary catheterization 56;57. Additionally, patients with CKD often present with atypical cardiac symptoms, which may mislead providers away from indicated CVD treatments, but with increased awareness of the atypical manifestations of CVD in CKD adverse outcomes may be prevented 37. Moreover, patients with recognized coronary artery disease are often treated with thrombolysis, percutaneous angioplasty, or stents, which may lead to iatrogenic safety events in CKD such as early restenosis or hemorrhage which can be minimized with attention to the high rate of complications in CKD 38;39.
The hospital setting is also a place for frequent misuse of medications including those that contribute to accelerated loss of kidney function or other complications. Increased recognition of CKD would be an effective means to ensure avoidance of NSAIDs, aminoglycosides, or other agents which are ill-advised in this disease24;25. Additionally, patients might receive electrolyte-based preparations which are poorly handled in CKD such as or magnesium containing antacids or laxatives32 or phosphate containing purgatives33. Many hospitals have computer-based alerts to guide proper use of drugs in the setting of CKD, and these have been shown to be an effective means to reduce medical errors 23;50. Alternatively, pharmacist may pay a key role in team rounds to ensure proper use of drugs among hospitalized patients who might have CKD58. This is especially helpful for physicians who have a significant challenge in accessing critical information on a broad set of disease processes such as CKD, which are active in the complex hospitalized patient59. However, the effectiveness of these interventions is predicated on screening for CKD which may not necessarily be universal in hospitalized patients.
Another potential source of CKD-specific safety events among hospitalized patients is the under-managed patient with congestive heart failure who receives diuretics or treatment with a RAAS blocker. Patients who have un-recognized CKD and who receive such treatment are at high risk for symptomatic hypotension, azotemia or acute kidney injury41;42. Recognition of impaired kidney function and appropriate longitudinal management either by the primary provider or by an appropriate nephrology consultant may avert many of these safety events which often contribute to accelerated loss of kidney function.
Since patients with CKD are at risk for safety events in many settings, not the least of which is the hospital, it remains unclear how to increase recognition of CKD beyond the boundaries of the hospital. Certainly hospitalized patients are a captive audience and can be provided with increased education to enhance their understanding of the relationship between recognition of CKD and patient safety events. The educated patient is the most committed stakeholder in their own medical care, and can carry this message to other encounters out of the hospital, and in this way play a role in improving their safety.
Interventions that increase CKD recognition, may play an important role in reducing patient safety events, and in turn, alter disease progression and reduce the incidence of ESRD. While there is good reason to consider CKD is an important disease modifier in the realm of patient safety there is also likely to be a unique and perhaps pivotal role of patient safety and medical errors in the epidemic of kidney disease. The best expectation of current therapies for this disease is slowing the rate of kidney function loss in most patients. Additional interventions, such as those directed at patient safety, that provide incremental benefit in treatment should be considered and sought out. Improving patient safety in CKD may offer an important opportunity to alter the progression of disease in a significant portion of the affected population and in this way reduce the incidence of ESRD.
Acknowledgments
Research support: NIDDK 1R21DK075675
Footnotes
Financial disclosures: None
REFERENCES
- 1.Kohn KT, Corrigan JM, Donaldson MS. To Err is Human: Building a Safer Health System. National Academy Press; Washington DC: 1999. [PubMed] [Google Scholar]
- 2.Agency for Healthcare Resarch and Quality . AHRQ quality indicators-guide to patient safety indicators. Agency for Healthcare Quality and Research; Rockville, MD: 2003. [Google Scholar]
- 3.Zhan C, Miller MR. Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA. 2003;290:1868–1874. doi: 10.1001/jama.290.14.1868. [DOI] [PubMed] [Google Scholar]
- 4.Zhan C, Kelley E, Yang HP, et al. Assessing patient safety in the United States: challenges and opportunities. Med Care. 2005;43(suppl 3):I42–I47. doi: 10.1097/00005650-200503001-00007. [DOI] [PubMed] [Google Scholar]
- 5.Leape LL, Berwick DM. Five years after to Err is Human: what have we learned. JAMA. 2005;293:2384–2390. doi: 10.1001/jama.293.19.2384. [DOI] [PubMed] [Google Scholar]
- 6.HealthGrades . The Fifth Annual HealthGrades Patient Safety in American Hospitals Study. 2008. [Google Scholar]
- 7.Encinosa WE, Bernard DM. Hospital finances and patient safety outcomes. Inquiry. 2005;42:60–72. doi: 10.5034/inquiryjrnl_42.1.60. [DOI] [PubMed] [Google Scholar]
- 8.Weiner BJ, Alexander JA, Baker LC, Shortell SM, Becker M. Quality improvement implementation and hospital performance on patient safety indicators. Med Care Res Rev. 2006;63:29–57. doi: 10.1177/1077558705283122. [DOI] [PubMed] [Google Scholar]
- 9.Clement JP, Lindrooth RC, Chukmaitov AS, Chen HF. Does the patient’s payer matter in hospital safety? Med Care. 2007;45:131–138. doi: 10.1097/01.mlr.0000244636.54588.2b. [DOI] [PubMed] [Google Scholar]
- 10.Coffey RM, Andrews RM, Moy E. Racial, ethnic, and socioeconomic disparities in estimates of AHRQ Patient Safety Indicators. Med Care. 2005;43:I48–I57. doi: 10.1097/00005650-200503001-00008. [DOI] [PubMed] [Google Scholar]
- 11.Zhan C, Kelley E, Yang HP, et al. Assessing patient safety in the United States: challenges and opportunities. Med Care. 2005;43(3 Suppl):I42–I47. doi: 10.1097/00005650-200503001-00007. [DOI] [PubMed] [Google Scholar]
- 12.Baker GR, Norton PG, Flintoft V, et al. The Canadian Adverse Events Study: the incidence of adverse events among hospital patients in Canada. CMAJ. 2004;170:1678–1686. doi: 10.1503/cmaj.1040498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donabedian A. Explorations in quality assessment and monitoring: the definition of quality and approaches to its assessment. Health Administration Press; Ann Arbor MI: 1980. [Google Scholar]
- 14.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 15.Saydah S, Eberhardt M, Rios-Burrows N, Williams D, Geiss L, Dorsey R. Prevalence of chronic kidney disease and associated risk factors-US, 1999-2004. MMWR. 2007;56:161–165. [PubMed] [Google Scholar]
- 16.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 18.Nissenson AR, Collins AJ, Hurley J, Petersen H, Pereira BJG, Steinberg EP. Opportunities for improving the care of patients with chronic renal insufficiency: current practice patterns. J Am Soc Nephrol. 2001;12:1713–1720. doi: 10.1681/ASN.V1281713. [DOI] [PubMed] [Google Scholar]
- 19.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic disease awareness, prevalence, and trends among US among US adults. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]
- 20.Stevens LA, Fares G, Fleming J, et al. Low rates of testing and diagnostic codes usage in a commercial clinical laboratory: evidence for lack of physician awareness of chronic kidney disease. J Am Soc Nephrol. 2005;16:2439–2448. doi: 10.1681/ASN.2005020192. [DOI] [PubMed] [Google Scholar]
- 21.Akbari A, Swedko PJ, Clark HD, et al. Detection of chronic kidney disease with laboratory reporting of estimated glomerular filtration rate and an education program. Arch Intern Med. 2004;164:1788–1792. doi: 10.1001/archinte.164.16.1788. [DOI] [PubMed] [Google Scholar]
- 22.Seliger SL, Zhan M, Hsu VD, Walker LD, Fink JC. Chronic kidney disease adversely influences patient safety. J Am Soc Nephrol. 2008 Sep; doi: 10.1681/ASN.2008010022. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chertow GM, Lee J, Kuperman GJ, et al. Guided medication dosing for inpatients with renal insufficiency. JAMA. 2001;286:2839–2844. doi: 10.1001/jama.286.22.2839. [DOI] [PubMed] [Google Scholar]
- 24.Blix HS, Viktil KK, Moger TA, Reikvam A. Use of renal risk drugs in hospitalized patients with impaired renal function--an underestimated problem? Nephrol Dial Transplant. 2006;21:3164–3171. doi: 10.1093/ndt/gfl399. [DOI] [PubMed] [Google Scholar]
- 25.Corsonello A, Pedone C, Corica F, et al. Concealed renal insufficiency and adverse drug reactions in elderly hospitalized patients. Arch Intern Med. 2005;165:790–795. doi: 10.1001/archinte.165.7.790. [DOI] [PubMed] [Google Scholar]
- 26.Salomon L, Deray G, Sandon MC, et al. Medication misuse in hospitalized patients with renal impairment. Int J Qual Health Care. 2003;15:331–335. doi: 10.1093/intqhc/mzg046. [DOI] [PubMed] [Google Scholar]
- 27.Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585–592. doi: 10.1056/NEJMra035279. [DOI] [PubMed] [Google Scholar]
- 28.Weir MR. Are drugs that block the renin-angiotensin system effective and safe in patients with renal insufficiency? Am J Hypertens. 1999;12(12 Pt3):195S–203S. doi: 10.1016/s0895-7061(99)00104-1. [DOI] [PubMed] [Google Scholar]
- 29.Snyder KW, Berns JS. Use of insulin and oral hypoglycemic medications in patients with diabetes and advanced kidney disease. Semin Dial. 2004;17:365–370. doi: 10.1111/j.0894-0959.2004.17346.x. [DOI] [PubMed] [Google Scholar]
- 30.Biesenbach G, Raml A, Schemkal B, Eichbauer-Sturm G. Decreased insulin requirement in relationship to GFR in nephropathic Type 1 and insulin-treated Type 2 diabetic patients. Diabet Med. 2003;20:642–645. doi: 10.1046/j.1464-5491.2003.01025.x. [DOI] [PubMed] [Google Scholar]
- 31.Jung GJ, Gil HW, Yang JO, Lee EY, Hong SY. Severe hypermagnesemia causing quadriparesis in a CAPD patient. Perit Dial Int. 2008;28:206. [PubMed] [Google Scholar]
- 32.Zaman F, Abreo K. Severe hypermagnesemia as a result of laxative use in renal insufficiency. South Med J. 2003;96:102–103. doi: 10.1097/01.SMJ.0000049844.49028.1D. [DOI] [PubMed] [Google Scholar]
- 33.Hurst FP, Bohen EM, Osgard EM, et al. Association of oral sodium phosphate purgative use with acute kidney injury. J Am Soc Nephrol. 2007;18:3192–3198. doi: 10.1681/ASN.2007030349. [DOI] [PubMed] [Google Scholar]
- 34.Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both: a prospective controlled study. N Engl J Med. 1989;320:143–149. doi: 10.1056/NEJM198901193200303. [DOI] [PubMed] [Google Scholar]
- 35.Solomon R. Contrast-medium-induced acute renal failure. Kidney Int. 1998;53:230–242. doi: 10.1038/sj.ki.4495510. [DOI] [PubMed] [Google Scholar]
- 36.Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007;243:148–157. doi: 10.1148/radiol.2431062144. [DOI] [PubMed] [Google Scholar]
- 37.Sosnov J, Lessard D, Goldberg RJ, Yarzebski J, Gore JM. Differential symptoms of acute myocardial infarction in patients with kidney disease: a community-wide perspective. Am J Kidney Dis. 2006;47:378–384. doi: 10.1053/j.ajkd.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Gibson CM, Dumaine RL, Gelfand EV, et al. Association of glomerular filtration rate on presentation with subsequent mortality in non-ST-segment elevation acute coronary syndrome: observations in 13,307 patients in five TIMI trials. Eur Heart J. 2004;25:1998–2005. doi: 10.1016/j.ehj.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Sadeghi HM, Stone GW, Grines CL, et al. Impact of renal insufficiency in patients undergoing primary angioplasty for acute myocardial infarction. Circulation. 2003;108:2769–2775. doi: 10.1161/01.CIR.0000103623.63687.21. [DOI] [PubMed] [Google Scholar]
- 40.Wilcox CS. Metabolic and adverse effects of diuretics. Semin Nephrol. 1999;19:557–568. [PubMed] [Google Scholar]
- 41.Kittleson M, Hurwitz S, Shah MR, et al. Development of circulatory-renal limitations to angiotensin-converting enzyme inhibitors identifies patiens with severe heart failure and early mortality. J Am Coll Cardiol. 2003;41:2029–2035. doi: 10.1016/s0735-1097(03)00417-0. [DOI] [PubMed] [Google Scholar]
- 42.Oster JR, Materson BJ. Renal and electrolyte complications of congestive heart failure and effects of therapy with angiotensin-converting enzyme inhibitors. Arch Intern Med. 1992;152:704–710. [PubMed] [Google Scholar]
- 43.Hsu CY, McCulloch CE, Fan D, Ordonez JD, Chertow GM. Community-based incidence of acute renal failure. Kidney Int. 2007;72:208–212. doi: 10.1038/sj.ki.5002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74:101–107. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liangos O, Wald R, O’Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 46.Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol. 2007;18:282–286. doi: 10.1681/ASN.2006050546. [DOI] [PubMed] [Google Scholar]
- 47.Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008;19:135–140. doi: 10.1681/ASN.2007030308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson LB, Venugopal AA, Pawlak J, Saravolatz LD. Emergence of community-associated methicillin-resistant Staphylococcus aureus infection among patients with end-stage renal disease. Infect Control Hosp Epidemiol. 2006;27:1057–1062. doi: 10.1086/507958. [DOI] [PubMed] [Google Scholar]
- 49.Kidney Disease Outcome Quality Initiative (K/DOQI) Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(Feb suppl):S1–S242. [PubMed] [Google Scholar]
- 50.Rind DM, Safran C, Phillips RS, et al. Effect of computer-based alerts on the treatment and outcomes of hospitalized patients. Arch Intern Med. 1994;154:1511–1517. [PubMed] [Google Scholar]
- 51.Kausz AT, Khan SS, Abichandani R, et al. Management of patients with chronic renal insufficiency in Northeastern United States. J Am Soc Nephrol. 2001;12:1501–1507. doi: 10.1681/ASN.V1271501. [DOI] [PubMed] [Google Scholar]
- 52.McClellan WM, Knight DF, Karp H, Brown WW. Early detection and treatment of renal disease in hospitalized diabetic and hypertensive patients: important differences between practice and published guidelines. Am J Kidney Dis. 1997;29:368–375. doi: 10.1016/s0272-6386(97)90197-9. [DOI] [PubMed] [Google Scholar]
- 53.Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS. Prevalence of high blood pressure and elevated serum creatinine level in the United States: Findings from the Third National Health and Nutrition Examination Survey (1988-1994) Arch Intern Med. 2001;161:1207–1216. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 54.Wyatt C, Konduri V, Eng J, Rohatgi R. Reporting of estimated GFR in the primary care clinic. Am J Kidney Dis. 2007;49:634–641. doi: 10.1053/j.ajkd.2007.02.258. [DOI] [PubMed] [Google Scholar]
- 55.Rothberg MB, Kehoe ED, Courtemanche AL, et al. Recognition and management of chronic kidney disease in an elderly ambulatory population. J Gen Intern Med. 2008;23:1125–1130. doi: 10.1007/s11606-008-0607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solomon R, Werner C, Mann D, D’Elia J, Silva P. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416–1420. doi: 10.1056/NEJM199411243312104. [DOI] [PubMed] [Google Scholar]
- 57.Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-induced reductions in renal function with acetylcysteine. N Engl J Med. 2000;343:180–194. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 58.Kucukarsian SN, Peters M, Mlynarek M, Nafziger DA. Pharmacists on rounding teams reduce preventable adverse drug events in hospital general units. Arch Intern Med. 2003;163:2014–2018. doi: 10.1001/archinte.163.17.2014. [DOI] [PubMed] [Google Scholar]
- 59.Tinetti ME, Bogardus ST, Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351:2870–2874. doi: 10.1056/NEJMsb042458. [DOI] [PubMed] [Google Scholar]