Abstract
With the advent of highly active antiretroviral therapy, human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) is becoming a more chronic, manageable disease; nevertheless, the prevalence of neurological complications of AIDS is increasing. In this study, protein levels of tyrosine hydroxylase (TH) and dopamine transporter (DAT) in the substantia nigra of HIV-infected brains and -seronegative controls were determined by immunoblotting. The immunoreactivity of neuronal specific enolase (NSE) was used to assess cell loss. Although there were no changes in levels of immunoreactive DAT or NSE proteins in HIV brains, levels of immunoreactive TH were significantly reduced, relative to controls. These results suggest that decreases in TH, the rate-limiting enzyme of dopamine synthesis, may be a factor in the neurological manifestations of HIV infection.
Keywords: dopamine transporter, HIV-associated dementia, neuro AIDS, tyrosine hydroxylase
Human immunodeficiency virus (HIV) infection may produce a variety of neurological effects, which may progress to HIV-associated dementia (HAD). Following the advent of highly active antiretroviral therapy (HAART), general patient prognosis and survival has improved dramatically; however, increased life span may increase incidence of neurological symptomatology (Arendt, 2005). The nervous system is widely involved in the pathogenesis of acquired immunodeficiency syndrome (AIDS)/HIV. HIV is neuroinvasive, neurovirulent (causing a neuropathy, myopathy, myelopathy, and dementia), but may or may not be especially neurotrophic (Manji and Miller, 2004). Invasion of the central nervous system (CNS) by HIV-1 occurs early in the course of infection (Manji and Miller, 2004). Whether or not HAD is manifested, postmortem examination of brains of AIDS patients reveals neuropathological changes in approximately 75% to 90% of the cases (Koutsiliery et al, 2002; Navia et al, 1986).
The major cell type harboring productive HIV-1 infection in the central nervous system is the perivascular macrophage/microglia. The HIV-1 infection of brain astrocytes is restricted to the expression of regulatory gene products (Epstein, 1998; Nath et al, 2002). Therefore, it has been postulated that widespread neuronal cell loss in HIV-associated brain pathology is caused by soluble products secreted by glial cells infected with the virus. As a result of numerous studies (for example, Nath et al, 2002), it was suggested that neurotoxicity of viral proteins released into the extracellular environment derived either from cytopathic infections, restricted infections, active release from infected cells, formation of defective viral particles, or shedding of the viral coat; and that these viral proteins play a key role in neuropathology associated with HIV. Despite technical problems associated with the detection of HIV-1 proteins in the CNS, evidence for their presence in the brain tissue of patients with HAD has been obtained (Saito et al, 2004; Adamson et al, 1996; Jones et al, 2000; Hudson et al, 2000). The neurotoxic potential of several structural (gp120, gp41) and regulatory (tat, rev, vpr) viral gene products is well established, although the exact cellular mechanisms of neurotoxicity remain unclear. It has been found that HIV-1 neurotoxins may cause neuronal damage through common pathways involving the induction of oxidative stress and excitotoxicity (Aksenov et al, 2003; Dewhurst et al, 1996).
Neurological symptoms in HIV-infected individuals suggest an abnormality of striatal dopaminergic systems (Berger and Arendt, 2000; Koutsiliery et al, 2002). Therefore, recent studies have focused on dopamine systems as possible mechanisms of HIV-mediated neurological symptoms. It has been reported that dopaminergic neurons and dopamine transporters (Wallace et al, 2005) may be vulnerable to the neurotoxic HIV-1 proteins, such as Tat (Nath et al, 2000). Recently published results of dopamine transporter (DAT)-specific magnetic resonance imaging (MRI) of the HAD-affected brain provided evidence for a marked reduction in DAT availability in the putamen and ventral striatum of HAD patients but not HIV-1 patients without dementia (Wang et al, 2004).
Tyrosine hydrolase (TH) enzyme activity, DAT-mediated dopamine (DA) uptake and ligand binding, as well as levels of the expression of DAT and TH proteins, are well-established measures in studies of DA transmission or dysfunction. Although abnormalities in these DA markers have been thoroughly investigated in other subcortical dementias, little is known about changes in components of DA transmission systems in the brain of HIV patients. In the current study, we compared levels of the DA-related proteins, DAT and TH, in extracts prepared from the substantia nigra (SN) of HIV-positive brain tissue and -seronegative controls, in order to obtain evidence for dopamine dysfunction in human HIV-1–infected brains.
All brain samples used in the current study were obtained from symptomatic HIV-seropositive patients with neurological abnormalities. Despite the reported presence of neurological symptoms, no diagnosis of HIV dementia had been confirmed in the HIV-positive donors of the current study. Substantia nigra tissue blocks from seven confirmed HIV-1–infected brains and eight controls were obtained through the National Disease Research Interchange (NDRI) (Philadelphia, PA). HIV-related fatalities were identified and classified as part of ongoing case-control study of neuropathological symptoms, HIV testing, medical treatments, supplemental background information, and autopsy findings (Table 1). All HIV patients were confirmed as HIV-1 seropositive. Additionally, three HIV subjects tested positive for hepatitis C. Because hepatitis C was unlikely to have direct effects on dopaminergic pathways, these patients were included into an experimental group. Neurological symptoms documented in detailed medical histories of HIV patients used in the study include anxiety, depression, increased confusion, headaches with lack of consciousness, seizure disorder, and mental incompetence. Age-matched controls were individuals without a history of neurological diseases, or systemic diseases affecting the brain. Subjects were matched on the basis of postmortem interval (PMI), age, and drug abuse status. Poly-drug abusers were excluded from study. Because of the difficulty of rapidly obtaining autopsy tissue from human brain and confirming HIV diagnosis, average PMI for HIV cases was 13 ± 5 h. Tissue with similar PMI has been used to measure dopamine transporters (Mash et al, 2002) without significant degradation of marker proteins.
Table 1.
Donor data
| Donor | COD | Age | Sex | Race | PMI | Drug abuse |
|---|---|---|---|---|---|---|
| HIV donor | ||||||
| H1 | AIDS related | 38 | M | W | 6 | + |
| H2 | AIDS related | 54 | M | AA | 12 | − |
| H3 | AIDS related | 43 | M | AA | 9 | + |
| H4 | Cardiac arrest | 40 | M | W | 20 | − |
| H5 | Cancer | 57 | M | W | 12 | − |
| H6 | Cardiac arrest | 40 | M | W | 15 | − |
| H7 | Cancer | 42 | M | AA | 18 | + |
| Control donor | ||||||
| C1 | Cardiac arrest | 59 | F | W | 16 | − |
| C2 | Cardiac arrest | 51 | M | W | 9 | + |
| C3 | Cardiac arrest | 49 | M | AA | 15 | + |
| C4 | Cardiac arrest | 62 | M | W | 12 | + |
| C5 | Cancer | 42 | M | W | 13 | − |
| C6 | GI bleeding | 57 | M | W | 8 | − |
| C7 | Cardiac arrest | 58 | M | W | 10 | − |
| C8 | MVA | 42 | F | W | 24 | − |
Note. Background data for the seven HIV1-infected donors and the eight seronegative controls. COD = cause of death; M = male; F = female; AA = African American; W = White; PMI = postmortem interval.
Upon autopsy, brain tissue blocks containing the substantia nigra were removed rapidly, immediately placed in liquid nitrogen, and shipped/stored at −80°C until processed. Brain samples were homogenized in 10 mM HEPES buffer (pH 7.4) containing 0.35 M sucrose 137 mM NaCl, 4.6 mM KCl, 1.1 mM KH2PO4, 0.6 mM MgSO4, and a proteinase inhibitor cocktail. Homogenates were centrifuged at 1000 × g for 10 min to remove debris from the resulting tissue extract. Protein concentration in the supernatant was determined by the Pierce BCA method using the microplate variant of the procedure. Microplates were read in Synergy HT plate reader (Bio-Tek, Winooski, Vermont, USA).
The initial screening of the homogenates prepared from HIV-positive and control substantia nigra tissue blocks was performed by Western blotting. The pattern of immunoreactive protein bands produced by each type of primary antibody (anti-TH, anti-DAT, and anti-NSE [neuronal specific enolase]) was tested for an appropriate molecular weight. To confirm the absence of nonspecific secondary antibody binding, blots were incubated without primary antibodies in presence of only the subsequent secondary antibody. Representative Western blots were prepared using randomly chosen sets of HIV-positive and control extracts. Six HIV and six control extract samples were loaded onto 14-well 8 cm × 7 cm × 1 mm 12.5% Sodium Dodecyl Sulfate (SDS) polyacrylamide minigels. Equal amounts of total protein (10 to 30 µg) were added per well. Duplicate gels were prepared for each set of samples. One was stained with Coomassie blue to confirm equality of protein load through different lanes and another was transferred to nitrocellulose membrane. All Western blot analyses were repeated at least twice.
Following the initial Western blotting screening of the extracts, simultaneous quantitative analysis of all HIV-positive and control substantia nigra extracts was performed using BioDot SF 36-well microfiltration unit (Bio-Rad). All HIV and control samples were blotted onto 0.45-µm nitrocellulose membrane in duplicates.
Western blots and slot blots were blocked for at least 30 min in 3% albumin/Phosphate Buffered Saline with 0.05% Tween-20 (PBST) and then used for immunostaining. After incubation with primary antibodies against TH, DAT, or NSE, blots were washed three times with PBST and incubated with the subsequent secondary alkaline phosphatase (AP)-conjugated antibody. Blots were then washed with PBST and developed with 5 Bromo-4-Chloro-3-Indolyl Phosphate/NitroBlue Tetrazolium (BCIP/NBT) substrate solution. Images of the blots were obtained using CCD video camera (Pixera Corporation, Los Gatos, CA) and analyzed with MCID M7 Gel lane analysis software (Imaging Research, St. Catherines, Ontario).
Data were analyzed using an analysis of variance (ANOVA) of 2 (disease state) × 3 (markers), with markers serving as a within-subject factor. To avoid violation of the homogeneity of variance assumption, data were transformed. Subsequently an unpaired two-sample t test assuming equal variance was used for post hoc analyses. Analysis of slot blots used the mean pixel density (arbitrary units) for the HIV and control group, whereas Western blot analysis used mean percentage of mean control density (pixel density) for the HIV and control group. A significant interaction was detected between disease state and marker in the overall ANOVA (F(2, 26) = 6.02, p < .01).
Tyrosine hydroxylase (TH) catalyzes the rate-limiting step in the biosynthesis of dopamine in the substantia nigra (Kumer and Vrana, 1996). Immunostaining of blots for TH was performed using a mouse monoclonal anti-TH antibody generated against TH that has been isolated and purified from rat PC12 cells. The antibody is believed to have wide species cross-reactivity because it recognizes an epitope in the mid-portion of the TH molecule where extensive species homology exists (Immuno Star, Hudson, WI, USA). The primary antibody was diluted 1:2000 in 0.3% albumin/PBST and incubated with blots overnight at 4°C. Alkaline phosphatase–conjugated anti-mouse immunoglobulin G (IgG) was used as the secondary antibody (Sigma; working dilution 1:5000). Representative Western blot which illustrates the banding pattern for the TH immunoreactivity is shown in Figure 1A. The image of the blot displays TH immunoreactive protein bands of approximately 55 kDa. The intensity of TH-positive bands was significantly (t(13) = −1.812, P < .05) reduced in HIV-1–infected tissue compared to seronegative controls. The preliminary Western blot analyses were confirmed by simultaneous measurements of TH immunoreactivity in all HIV-infected and seronegative control tissue extracts using slot blot (t(13) = 2.817, P < .01). Figure 1B presents results of the slot blot analysis.
Figure 1.
HIV-mediated changes in availability of TH in the substantia nigra. A, Representative Western blot of HIV-1–infected brain tissues compared to seronegative controls using mouse monoclonal anti-TH antibody as the primary antibody and alkaline phosphatase–conjugated anti-mouse IgG as the secondary antibody. The molecular weight of TH is approximately 50 kDa. B, TH immunoreactivity in HIV-1–infected brain tissues compared to controls. This figure represents the slot blot data. Results presented as mean pixel density (arbitrary units) of HIV-1–infected brain tissues versus control ± SEM, n of HIV-1–infected brain tissues = 7, n of controls = 8. *HIV-1–infected brain tissues had significantly lower TH pixel density as compared to seronegative controls, P < .05.
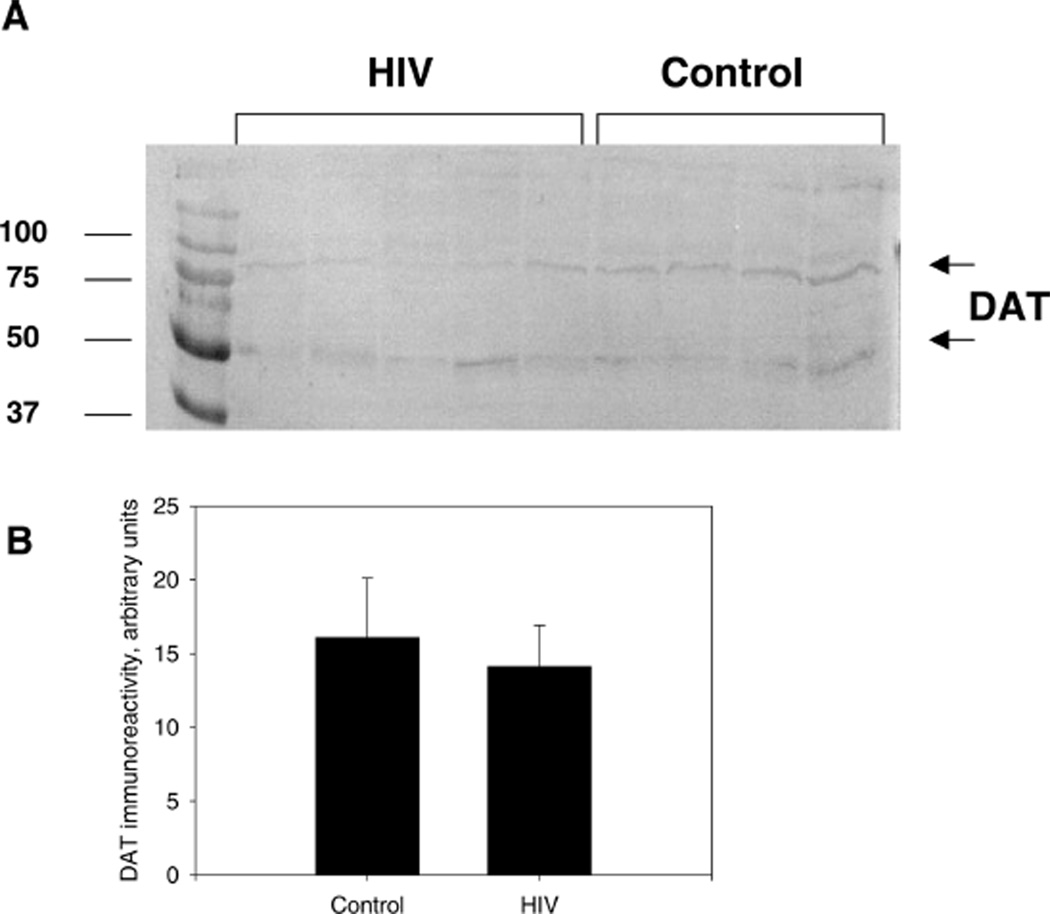
The plasma membrane protein DAT is considered to be a reliable marker of presynaptic dopaminergic terminal loss and immunological analysis of DAT protein provides sensitive means for investigation of molecular mechanisms of neurological disorders involving dopaminergic systems (Miller et al, 1997). The analysis of immunoreactive DAT protein levels in substantia nigra of HIV patients and control subjects was performed using a rabbit polyclonal antibody raised against amino acids 541 ot 620 mapping at the C-terminus of the sodium-dependent dopamine transporter DAT of human origin (Santa-Cruz, Santa Cruz, CA, USA). The primary antibody was diluted 1:200 in 0.3% albumin/PBST. Blots were incubated with the primary antibody solution overnight at 4°C. Alkaline phosphatase–conjugated anti-rabbit IgG were used as the secondary antibody (Sigma; working dilution 1:2500). No significant reduction in DAT immunoreactivity in HIV-1–infected tissue compared to seronegative controls was demonstrated by either the slot blot or Western blot analysis (P > 0.05 in both cases). Figure 2A displays the qualitative banding pattern of DAT immunoreactivity with visible bands at approximately 50 and 70 kDa, whereas Figure 2B displays the quantitative analysis of the DAT slot blot.
Figure 2.
HIV-mediated changes in availability of DAT in the substantia nigra. A, Representative Western blot of HIV-1–infected brains compared to seronegative controls using rabbit polyclonal antibody raised against amino acids 541 to 620, mapping at the C-terminus of the sodium-dependent dopamine transporter DAT of human origin as the primary antibody and alkaline phosphatase–conjugated anti-rabbit IgG as the secondary antibody. The molecular weight of DAT that is fully glycosylated is approximately 80 kDa and the bands at approximately 50 kDa are DAT with at least one less N-linked glycosylation site (see Li et al, 2004). B, DAT immunoreactivity in HIV-1–infected brain tissues compared to controls. This figure represents slot blot data. Results presented as mean pixel density (arbitrary units) of HIV-1–infected brain tissues versus control ± SEM, n of HIV-1–infected brain tissues = 7, n of controls = 8.
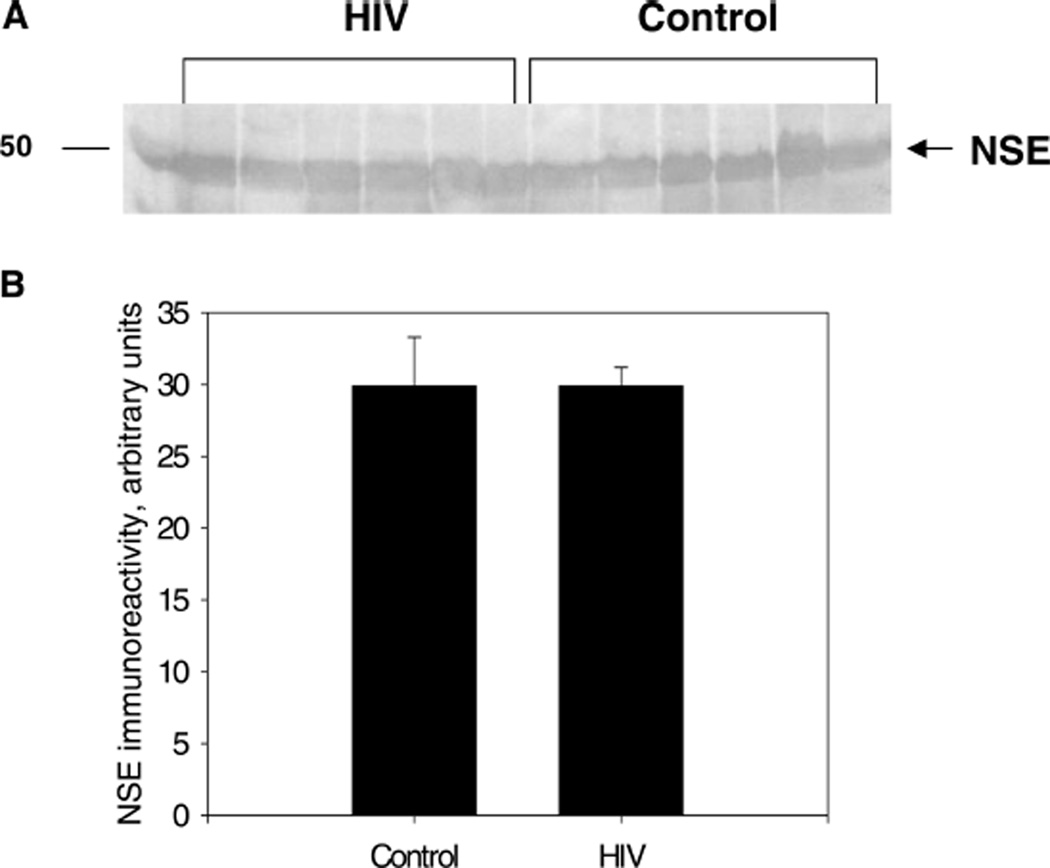
Changes in NSE immunoreactivity are a recognized marker of neuronal cell damage in brain trauma and various neurodegenerative disorders (Blennow et al, 1994; Ding et al, 2000). NSE immunoreactivity levels were determined using a rabbit polyclonal anti-NSE antibody (Santa-Cruz). The primary antibody was diluted 1:200 in 0.3% albumin/PBST. Alkaline phosphatase–conjugated anti-rabbit IgG were used as the secondary antibody (Santa-Cruz; working dilution 1:5000). There was no significant difference in NSE immunoreactivity in HIV-1–infected tissue compared to seronegative controls for either the slot blot or Western blot analysis (P > 0.05 for both assays). Figure 3A displays the qualitative Western blot banding pattern of the NSE immunoreactivity, and Figure 3B represents the Western blot quantitative analysis of the NSE immunoreactivity.
Figure 3.
HIV-mediated changes in NSE density in the substantia nigra. A, Representative Western blot of HIV-1–infected brain tissues compared to seronegative controls using rabbit polyclonal anti-NSE antibody as the primary antibody and alkaline phosphatase–conjugated anti-rabbit IgG as the secondary antibody. The molecular weight of NSE is approximately 47 kDa. B, NSE immunoreactivity in HIV-1–infected brain tissues compared to controls. This figure represents slot blot data. Results presented as mean pixel density (arbitrary units) of HIV-1–infected brain tissues versus control ± SEM, n of HIV-1–infected brain tissues = 7, n of controls = 8.
Although virologic studies indicate possibly that the basal ganglia are a major target of HIV infection, the current study suggests that dopaminergic function located in SN can also be affected. Neuropathological examinations of AIDS patients demonstrate that a subclinical nigral degeneration in AIDS is common and could explain the heightened susceptibility of some patients to drug-induced parkinsonism (Reyes et al, 1991). Our results demonstrate substantial changes in the expression of rate-limiting enzyme of dopamine metabolism, TH, in the substantia nigra of HIV patients with documented neurological abnormalities. This is consistent with previous studies that report marked neurodegenerative changes in the substantia nigra of HIV-1 patients (Itoh et al, 2000). Interestingly, although the immunoreactivity of TH was decreased, there was no concurrent alteration in either DAT protein levels or NSE protein levels in the substantia nigra of HIV-1–infected tissue. The absence of significant changes in DAT and NSE protein levels suggests that the observed decrease in TH protein levels is unlikely to result from death of dopaminergic neurons in the SN of HIV patients.
Several mechanisms could possibly explain decreased TH expression in the SN of HIV brains. First, TH is reported to be a target for reactive oxygen and reactive nitrogen species (ROS and RNS) (Blanchard-Fillion et al, 2001). A sizeable body of evidence suggests that microglial activation and proinflammatory cell responses may be involved in the pathogenesis of HIV-associated brain pathology (Koutsiliery et al, 2002). Activation of macrophages/microglia in HIV-infected brain can lead to increased production of free radical intermediates. Indeed, increased nitrotyrosine formation, a known indicator of peroxynitrite production, has been documented in the HIV brain (Boven et al, 1999). Modification of proteins caused by reactive oxygen ROS and/or RNS is known to increase their degradation by specific proteinases (Butterfield and Stadtman, 1997). Thus, ROS/RNS-induced posttranslational modifications of TH may be the reason for the observed decline of TH immunoreactivity in the SN of HIV patients.
Second, TH activity and expression are modulated by a variety of cellular regulatory mechanisms (Kumer and Vrana, 1996). Of particular interest is the evidence that dopaminergic neural cells can be directly inhibited by the neurotoxic HIV-1 protein Tat (Zauli et al, 2000). Therefore, it is speculated that TH gene expression in dopaminergic neurons may be suppressed by Tat excreted from HIV-infected astrocytes and/or microglia. Thus, post-translational modifications and/or decreased TH gene expression may be involved in the decrease of TH immunoreactive protein in the SN extracts of HIV patients. Future studies are planned to investigate possible mechanisms of TH loss in the brains with documented HIV-associated pathology.
The cell bodies of the nigrostriatal DA neurons are located in the substantia nigra and terminate in the striatum, more specifically the caudate and putamen. In vivo imaging demonstrated that reduction of DAT-specific ligand binding is also seen in the caudate and putamen of HAD patients (Wang et al, 2004). The current study did not address DAT functionality in SN tissue. Our results do not rule out the possibility that although DAT immunoreactive protein levels in the substantia nigra were not decreased, the site-selective binding affinity and/or dopamine transport may be attenuated. Future studies will be needed to test the functionality of DAT in SN samples from HIV and control tissue using a ligand binding paradigm. The previously reported reduction of DAT-specific ligand binding (Wang et al, 2004) was demonstrated in HAD patients, but not HIV patients without dementia. Therefore it is possible that as HIV neurological symptoms progress to dementia, more pronounced dopamine system deficits appear. Additional studies are needed to investigate the time course of dopamine system degradation. With understanding of the progression of the neurological effects of HIV infection, the potential for treating or preventing HAD may increase.
The current studies demonstrate decreased levels of tyrosine hydroxylase in substantia nigra of HIV patients. This decrease is not believed to be cause by selective cell loss of dopaminergic neurons, as there was no decrease in dopamine transporter protein levels or neuronal specific enolase. Decrease in TH may be a precursor to more severe central nervous system alterations which develop with progression of HAD.
Acknowledgments
This work was supported by DA013137 and DA014407 (RMB) HD043680 (CFM) and the National Disease Research Interchange.
References
- Adamson DC, Dawson TM, Zink MC, Clements JE, Dawson VL. Neurovirulent simian immunodeficiency virus infection induces neuronal, endothelial, and glial apoptosis. Mol Med. 1996;2:417–428. [PMC free article] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Temporal relationships between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987:1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Arendt G. Neurological manifestations of HIV-infection in the era of highly active antiretroviral therapy (HAART) Fortschr Neurol Psychiatr. 2005;73:577–586. doi: 10.1055/s-2004-830283. [DOI] [PubMed] [Google Scholar]
- Berger JR, Arendt G. HIV dementia: the role of the basal ganglia and dopaminergic systems. Psychopharmacol. 2000;14:214–221. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- Blanchard-Fillion B, Souza JM, Friel T, Jiang GC, Vrana K, Sharov V, et al. Nitration and inactivation of tyrosine hydroxylase by peroxynitrite. J Biol Chem. 2001;279:46017–46023. doi: 10.1074/jbc.M105564200. [DOI] [PubMed] [Google Scholar]
- Blennow K, Wallin A, Ekman R. Neuron specific enolase in cerebrospinal fluid: a biochemical marker for neuronal degeneration in dementia disorders? J Neural Transm Park Dis Dement Sect. 1994;8:183–191. doi: 10.1007/BF02260939. [DOI] [PubMed] [Google Scholar]
- Boven LA, Gomes L, Hery C, Gray F, Verhoef J, Portegies P, et al. Increased peroxynitrite activity in AIDS dementia complex: implications for the neuropathogenesis of HIV-1 infection. J Immunol. 1999;162:4319–4327. [PubMed] [Google Scholar]
- Butterfield DA, Stadtman ER. Protein oxidation processes in aging brain. Adv Cell Aging Gerontol. 1997;2:161–191. [Google Scholar]
- Dewhurst S, Gelbard HA, Fine SM. Neuropathogenesis of AIDS. Mol Med Today. 1996;2:16–23. doi: 10.1016/1357-4310(96)88754-5. [DOI] [PubMed] [Google Scholar]
- Ding M, Haglid KG, Hamberger A. Quantitative immunochemistry on neuronal loss, reactive gliosis and BBB damage in cortex/striatum and hippocampus/amygdale after systemic kainic acid administration. Neurochem Int. 2000;36:313–318. doi: 10.1016/s0197-0186(99)00139-4. [DOI] [PubMed] [Google Scholar]
- Epstein LG. HIV neuropathogenesis and therapeutic strategies. Acta Paediatr Jpn. 1998;40:107–111. doi: 10.1111/j.1442-200x.1998.tb01892.x. [DOI] [PubMed] [Google Scholar]
- Hudson L, Liu J, Nath A, Jones M, Raghavan R, Narayan O, et al. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J NeuroVirol. 2000;6:145–155. doi: 10.3109/13550280009013158. [DOI] [PubMed] [Google Scholar]
- Itoh K, Mehraein P, Weis S. Neuronal damage of the substantia nigra in HIV-1 infected brains. Acta Neuropathol (Berl) 2000;99:376–384. doi: 10.1007/s004010051139. [DOI] [PubMed] [Google Scholar]
- Jones MV, Bell JE, Nath A. Immunolocalization of HIV envelope gp120 in HIV encephalitis with dementia. AIDS. 2000;14:2709–2713. doi: 10.1097/00002030-200012010-00010. [DOI] [PubMed] [Google Scholar]
- Koutsiliery E, Sopper S, Scheller C, ter Meulen V, Riederer P. Involvement of dopamine in the progression of AIDS dementia complex. J Neural Transm. 2002;109:399–410. doi: 10.1007/s007020200032. [DOI] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Li L-B, Chen N, Ramamoorthy S, Chi L, Cui X-N, Wang LC, et al. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J Biol Chem. 2004;279:21012–21020. doi: 10.1074/jbc.M311972200. [DOI] [PubMed] [Google Scholar]
- Manji H, Miller R. Neurology of HIV infection. J Neurol Neurosurg Psychiatry. 2004;75:29–35. doi: 10.1136/jnnp.2003.034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Pablo J, Ouyang Q, Hearn WL, Izenwasser S. Dopamine transport function is elevated in cocaine users. J Neurochem. 2002;81:292–300. doi: 10.1046/j.1471-4159.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- Miller GW, Staley JK, Heilman CJ, Perez JT, Mash DC, Rye DB, et al. Immunochemical analysis of dopamine transporter protein in Parkinson’s disease. Ann Neurology. 1997;41:530–539. doi: 10.1002/ana.410410417. [DOI] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, et al. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31:S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex. II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Reyes MG, Faraldi F, Senseng CS, Flowers C, Fariello R. Nigral degeneration in acquired immune deficiency syndrome (AIDS) Acta Neuropathol (Berl) 1991;82:39–44. doi: 10.1007/BF00310921. [DOI] [PubMed] [Google Scholar]
- Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, et al. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120- and tat(1–72)-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2005;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Wang J-G, Chang L, Volkow N, Telang F, Logan J, Ernst T, et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Zauli G, Secchierro P, Rodella L, Gibellini D, Mirandola P, Mazzoni M, et al. HIV-1 Tat-mediated inhibition of the tyrosine hydroxylase gene expression in dopaminergic neuronal cells. J Biol Chem. 2000;275:4159–4165. doi: 10.1074/jbc.275.6.4159. [DOI] [PubMed] [Google Scholar]