SUMMARY
Notch2, but not Notch1, plays indispensable roles in kidney organogenesis and Notch2 haploinsufficiency is associated with Alagille syndrome. We proposed that proximal nephron fates are regulated by a threshold that requires nearly all available free Notch intracellular domains (ICDs), but we could not identify the mechanism explaining why Notch2 (N2) is more important than Notch1 (N1). By generating mice that swap their ICDs, we establish that overall protein concentration, expression domain, or ICD amino acid composition does not account for the differential requirement for these receptors. Instead, we find that the N2 extracellular domain (ECD) increases Notch protein localized to the cell surface during kidney development and is cleaved more efficiently upon ligand binding. This context-specific asymmetry in NICD release efficiency is further enhanced by Fringe. Our results indicate that elevating N1 surface level could compensate for the loss of N2 signal in specific cell contexts.
Keywords: Kidney, Alagille Syndrome, Notch, Paralog Dominance, Domain Swap
INTRODUCTION
The kidney is an essential organ with growing clinical importance in the aging western population. It regulates excretion of soluble waste, maintains pH and electrolyte balance, and controls blood pressure and vitamin D levels. Its functional unit, the nephron, consists of a filtration apparatus called the glomerulus, followed by renal tubules made up of specialized epithelial cells that modify the filtrate, which eventually flows into the collecting duct system and drains into the bladder.
During development, nephrons form as the outcome of reciprocal interactions between the metanephric mesenchyme (MM) and the ureteric bud (UB) (Costantini and Kopan, 2010). GDNF, secreted by the MM, induces UB branching; Wnt9b, secreted by the UB, induces a few MM cells to undergo mesenchymal to epithelial transition (MET) and form a renal vesicle (RV), which grows into the S-shaped body (SSB) after fusing with the ureteric stalk (Figure 1A; (Georgas et al., 2009)). Together with endothelial and mesangial cells, the proximal third of the SSB forms the glomerulus; the rest of the SSB gives rise to the various segments and cell types that link the glomerulus to the collecting duct.
Figure 1.
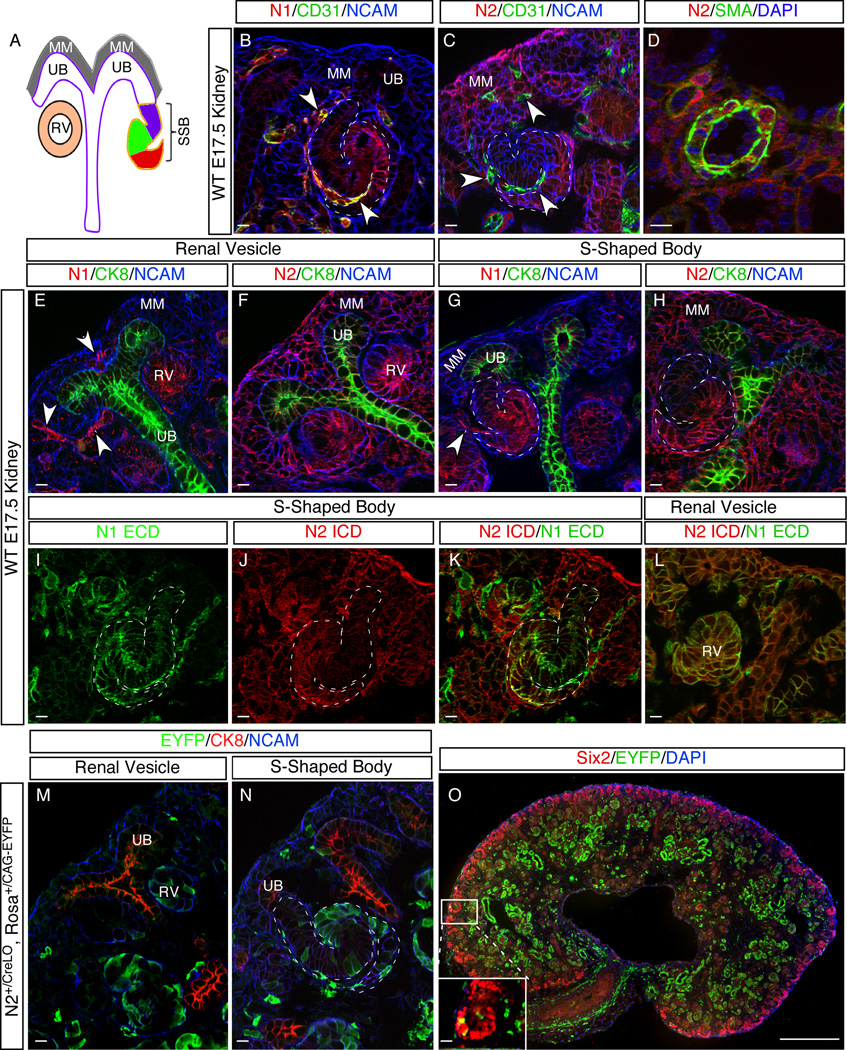
Variation in the expression pattern of N1 and N2 does not explain their functional difference. (A) Diagram showing major structures of developing nephrons. MM, metanephric mesenchyme; RV, renal vesicle; SSB, S-shaped body; UB, ureteric bud. The presumptive distal and proximal tubules, as well as podocyte precursor cells, are denoted in purple, green and red, respectively. (B–L) Comparison of N1 and N2 expression in different structures of an E17.5 kidney. CD31 marks endothelial cells; SMA (smooth muscle actin) marks vascular smooth muscle cells; CK8 (Cytokeratin 8) marks UB and its derivatives; NCAM marks all epithelial cells. Arrowheads denote endothelial cell precursors. (I–L) show the double staining with N1 ICD and N2 ECD antibodies. (M–O) Labeling pattern of N2∷Cre reporter in E17.5 kidney. All scale bars are 10µm except for O, which is 500µm. See also Figure S1.
In both man and mouse, proper renal organogenesis requires the Notch signaling pathway. This pathway is comprised of four Notch receptors, N1–4, and five canonical ligands, Dll1, Dll3, Dll4, Jag1 and Jag2 (Kopan and Ilagan, 2009). As all receptors and ligands are type I transmembrane proteins, the Notch pathway mediates communications between adjacent cells. Binding of the ligand to the Notch extracellular domain (NECD) exposes the S2 cleavage site that is, by default, masked by the negative regulation region (NRR). Cleavage at the S2 site is followed by intramembrane proteolysis at the S3 site by γ-secretase, which releases the Notch intracellular domain (NICD) from the cell membrane. Subsequently, NICD translocates to the nucleus and forms a transcriptional activation complex with RBP and Mastermind on specific DNA sites to turn on the expression of target genes, including Hes/Hey family members. In addition to these core pathway components, various other factors can modulate the strength of the Notch signaling pathway (Kopan and Ilagan, 2009).
Despite the presence of N1, N2, Dll1 and Jag1 in the developing nephron, only haploinsufficiency in either JAG1 or N2 causes Alagille syndrome in humans, a disease characterized by craniofacial abnormalities, and heart, liver and kidney malformations (Penton et al., 2012). To model Alagille syndrome in mice, simultaneous reduction in both N2 and Jag1 is required (McCright et al., 2002). Moreover, whereas removal of N1 from the nephron progenitors is well tolerated, conditionally removing N2 alone from nephron progenitors in the intermediate mesoderm (with Pax3-Cre) results in complete loss of the proximal nephron and death of newborn pups within 48 hours (Cheng et al., 2007). The contribution of N1 could only be revealed in a sensitized genetic background in which N2 levels were reduced (Surendran et al., 2010). Thus far, a molecular explanation for the unequal role of N2 (vs N1) and JAG1 (vs other ligands) in human and mouse kidney development and disease has remained elusive.
To address this question, we used multiple approaches to determine if differences in the spatial expression domains, the expression level or the amino acid composition accounted for the unequal contributions of N1 and N2 to nephron development. We demonstrated that expression levels of N1 and N2 proteins are equivalent within the renal epithelia, and that differential expression outside of this domain did not contribute to the functional differences. To address the role of amino acid composition, we seamlessly swapped the entire N1ICD and N2ICD genomic coding regions to create two new strains of mice harboring genes we call N12 and N21. These mice provide a unique platform in which to distinguish NICD dose-dependent phenomena from NICD composition-dependent ones in various tissues and disease models (Chu et al., 2011; Fan et al., 2004; Graziani et al., 2008; Parr et al., 2004; Rangarajan et al., 2001). Using these new tools, we demonstrated that N1ICD and N2ICD are fully interchangeable during kidney development; nephrogenesis occurs normally in each of the 10,000 nephrons as long as the N2 ECD controls ICD release, but fails to complete any nephrons when the N1 ECD controls ICD release. This confirmed the existence of a threshold, a developmental switch that is controlled by the concentration, but not the composition, of Notch ICDs. The switch determines if an individual nephron will develop its proximal elements (Cheng et al., 2007).
To gain more insight into how the ECD controls the free NICD concentration, we determined whether N1 and N2 ECDs differed in efficiency of ICD release in vitro using the Notch luciferase complementation imaging assay (Notch LCI; (Ilagan et al., 2011)). We show that when present at similar levels on the surface of HEK293 cells, the N2 ECD is consistently, but only marginally (~2 fold), better than the N1 ECD at releasing ICD in response to either Jag1 or Dll1. We further found that in RVs and SSB cells, N2 is more abundant on the cell surface than N1. Using N12 and N21 strains we could demonstrate that this uneven distribution is determined by ECD. Finally, a series of ligand loss-of-function alleles revealed a dose–dependent effect for ligands and a dominant requirement for Jag1 in the kidney context relative to that of Dll1. This may be amplified by ECD glycosylation by one of the three Fringe genes, Lunatic Fringe (Lfng), whose expression overlaps with N1 in the developing nephron. We propose that the combined effects of these factors make the N2 contribution critical to kidney development. The importance of ECD in the kidney epithelial cell is also reflected in the labeling frequencies of N1∷CreLO and N2∷CreLO reporter mice (Liu et al., 2011; Morimoto et al., 2010; Vooijs et al., 2007), in which the release of Cre recombinase is solely determined by the Notch ECD. In summary, these data imply that the number of NICD molecules in the nucleus of RV cells is near the amount needed to promote proximal nephron development, thereby explaining why loss of one N2 or Jag1 allele causes a developmental syndrome in humans. Because the ICDs are interchangeable, investigating N1 trafficking in organs affected by Alagille syndrome may lead to therapeutic benefit without the risk associated with agonist use.
RESULTS
N1 and N2 have similar expression patterns in developing renal epithelia
We reasoned that the functional difference between the two Notch paralogs during metanephric kidney development could be explained by one or a combination of several possible mechanisms: (1) differences in promoters/enhancers, which give rise to differential temporal or spatial expression domains by controlling mRNA levels; (2) differences in the 3’UTRs, which may affect the stability/translation of mRNAs of Notch paralogs and therefore protein abundance; (3) differences in ECD composition, which lead to differential responses to ligands and, consequently, different numbers of NICD molecules released, resulting in different signal “strength”; (4) differences in ICD composition, which lead to differential associations with distinct binding partners and activation of unique downstream targets (Spitz and Furlong, 2012).
A careful examination of the N1 and N2 expression patterns in the developing kidney has not been possible before due to the lack of appropriate antibodies. After confirming the specificity of newly developed antibodies against the N1ICD and the N2ICD (see below), we analyzed the expression patterns of these receptors at E17.5 using immunofluorescence on wild type kidneys (Figure 1). Both receptors are expressed in an overlapping cell population in the RV and the SSB that is thought to give rise to proximal tubules and podocytes (Figure 1E–L). In addition to the renal epithelia, N1 is expressed in endothelial precursor cells within the kidney anlagen (Figure 1B, arrowheads). In contrast, N2 is broadly expressed in the MM, vascular smooth muscle cells (VSMC), and the UB, but is absent from endothelial cells (Figure 1C,D).
The exclusive expression of N2 in the MM may explain why this protein is indispensible (Fujimura et al., 2010). However, because progenitor maintenance and MET proceed normally in the absence of N2 (Cheng et al., 2007; McCright et al., 2002), N2 activation in MM is unlikely to perform a significant function there (Boyle et al., 2011). To directly test whether N2 is activated in MM cells, we examined the labeling pattern of a N2 activation-dependent reporter line, N2∷CreLO (Figure S1) (Liu et al., 2011; Vooijs et al., 2007). In this reporter line, one copy of the N2 ICD is replaced with Cre recombinase, which is released upon N2 activation. In the presence of the reporter allele RosaCAG-EYFP (Madisen et al., 2010), the released Cre will excise the floxed “stop” cassette between the Rosa/CAG promoter and EYFP reporter and activate EYFP expression, indelibly marking cells that have experienced N2 activation (and their progeny) (Vooijs et al., 2007). The N2∷CreLO labeling pattern in E17.5 kidneys revealed only a few EYFP-positive cells in Six2-positive MM cells (Figure 1M,O). The few MM cells experiencing N2 activation will most likely exit the stem cell niche (Boyle et al., 2011; Cheng et al., 2007; Fujimura et al., 2010). If N2 receptors were activated in cells as they underwent MET, most RV cells would be labeled. Instead, only a few EYFP-positive cells are detected in RVs. Consistent with Notch activation in the RV, many labeled cells are seen in the SSBs, proximal tubules and podocytes. N2 activation thus occurs in the epithelial cells and not in their mesenchymal precursors (Figure 1M–O). In summary, both receptors are expressed in the domain where Notch proteins impact the decision to make proximal nephron cells and the differential expression of N2 in the MM does not explain why N2 is essential for kidney development while N1 is not.
ICD swap between N1 and N2 creates N12 and N21 chimeric receptors
All NICD paralogs form transcriptional activation complexes with RBPjk and Mastermind on target promoters. Although Notch proteins can activate similar targets and can act redundantly in vivo (Riccio et al., 2008), in vitro and in vivo studies suggest that in some contexts, these complexes are distinct as NICD paralogs can have different or even opposite functions (Chu et al., 2011; Fan et al., 2004; Graziani et al., 2008; Parr et al., 2004; Rangarajan et al., 2001). Amino acids not conserved between N1 and N2 ICD are located at the solvent-accessible surface of the Ankyrin domain and could therefore participate in unique interactions with putative co-activators or co-repressors, contributing to their functional differences (Spitz and Furlong, 2012). To investigate this, we used galK-selection-based BAC recombineering (Warming et al., 2005) to swap the entire genomic regions coding the ICDs between the N1 and N2 loci in B6-derived ES cells (Figure 2A; Figure S2) . The swapped region ranged from Exon 28, coding for the transmembrane domain (TMD), to the stop codon in Exon 34. We did not swap the 3’UTRs in order to retain transcript-specific regulation of mRNA stability and translation. We designated the new alleles N12 (N2ICD in the N1 locus) and N21 (N1ICD in the N2 locus) (Figure 2A). To facilitate ES cell screening and post-recombination analysis with pyrosequencing based methods (Liu et al., 2009; Liu et al., 2010), we introduced silent single nucleotide variations (SNVs) into the TMD coding regions (G38066C for N12 and G125011C for N21), as well as an SNV in the ICD coding region of N12 (G38129A) (Figure 2A). After germline transmission was obtained, the frt-flanked neomycin/G418 selection cassette was removed by mating with flippase deleter mice (Rodriguez et al., 2000). This left a 34bp frt sequence between the stop codon and the 3’UTR in mice with N12 and N21 chromosomes (Figure 2A; Figure S2).
Figure 2.
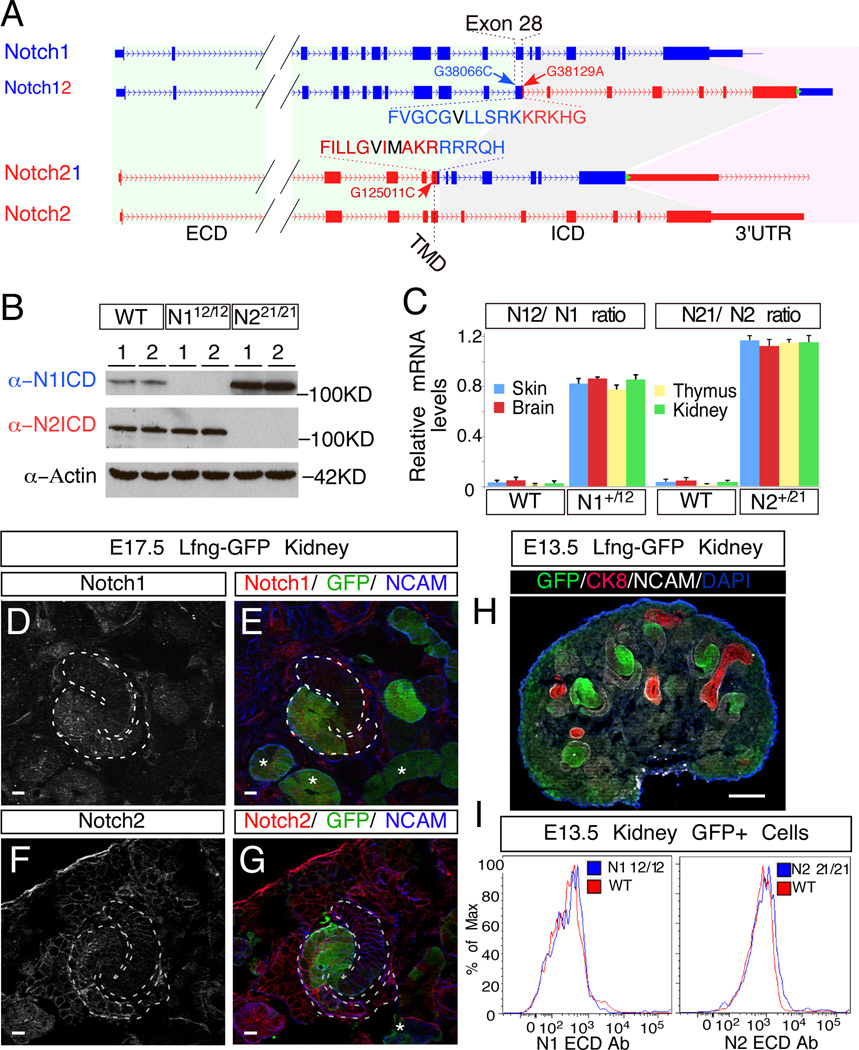
Generation of the N12 and N21 alleles. (A) Schematic illustration of N1 (blue) and N2 (red) loci before and after the ICD swap. The N1 ICD encompasses 5,926bp on chromosome 2, ranging from nucleotide +38,103 to +44,028 (A in ATG is +1) and encoding amino acid 1,750 to 2,531; for N2, the ICD encompasses 8,699bp on chromosome 3, ranging from nucleotide +125,048 to +133,746 and encoding amino acid 1,705 to 2,473. Amino acids in black denote the S3 cleavage sites. Green triangle denotes FRT site. (B) Western blot analyses with ICD-specific antibodies of kidney extracts from newborn pups with designated genotypes (WT, N112/12 and N221/21; two different individuals per genotype). (C) mRNA level comparisons between chimeric N12 and N21 and their corresponding endogenous alleles in various tissues of wild type (WT), N1+/12 and N2+/21 newborn pups. Allele ratios were calculated by determining the G/C ratio at SNVs G38066C and G125011C introduced into the targeting constructs with pyrosequencing. Error bars represent standard deviation. (D–G) Double staining of EGFP and N1 (D and E) or N2 (F and G) on E17.5 Lfng-GFP kidneys. Asterisks (*) denote EGFP+ tubules. (H) EGFP labeling patterns in E13.5 Lfng-GFP kidney. (I) E13.5 Lfng-GFP kidneys with wild type (WT) or single homozygous (N112/12 or N221/21) Notch alleles were dissociated into single cells, stained with PE-conjugated N1 or N2 ECD-specific antibodies (eBioscience) and analyzed with flow cytometry. The cell surface levels of wild type (N1, N2) and chimeric (N12, N21) were compared in EGFP+ cells. Scale bars in D-G are 10µm and the one in H is 100µm. See also Figure S2, S3.
PCR amplification confirmed the presence of the hybrid exon 28 in the genome (Figure S3A, B). Loss of sequences from N1 exon 30 or N2 exon 34 respectively identified N112/12 and N221/21 homozygous mice, which are both viable (Figure S3A, B; Detailed phenotypic analysis of other organs will be described elsewhere). The loss of N1ICD in N112/12 or N2ICD in N221/21 mice was also confirmed by Western blot with N1ICD- and N2ICD-specific antibodies, respectively (Figure 2B). The introduced SNVs allowed us to compare the mRNA levels transcribed from the N12 chromosome to N1 and N21 chromosome to N2 in various heterozygous tissues of N1+/12 and N2+/21 mice, respectively, with pyrosequencing (Figure 2C). This analysis revealed that the shorter transcript was slightly more abundant in all tissues examined (Figure 2C). Western blot and immunostaining with either anti-Notch ICD or anti-Notch ECD antibody confirms the expression of chimeric proteins (Figure. 2B, Figure S3C, D). To assess whether the chimeric Notch receptors could reach the cell surface as efficiently as the endogenous receptors, we isolated RV and SSB cells from Lfng-GFP mice, in which EGFP is expressed under the control of LFringe regulatory sequences. Double staining of E17.5 kidneys with either N1 or N2 ICD antibodies shows extensive overlap with EGFP (Figure 2D–G; Figure S3E). To exclude the epithelial cells from differentiated tubules, we isolated GFP+ cells from E13.5, Lfng-GFP; N1+/+; N2+/+ (denoted as WT), Lfng-GFP; N112/12; N2+/+ (denoted as N112/12) and Lfng-GFP; N1+/+; N221/21 (denoted as N221/21) kidneys before tubule formation (Figure 3H), and stained them with anti-N1 ECD or anti-N2 ECD specific antibodies (Fiorini et al., 2009). Flow cytometry analysis confirmed that the cell surface distribution of N12 and N21 is similar to N1 and N2, respectively (Figure 3I).
Figure 3.
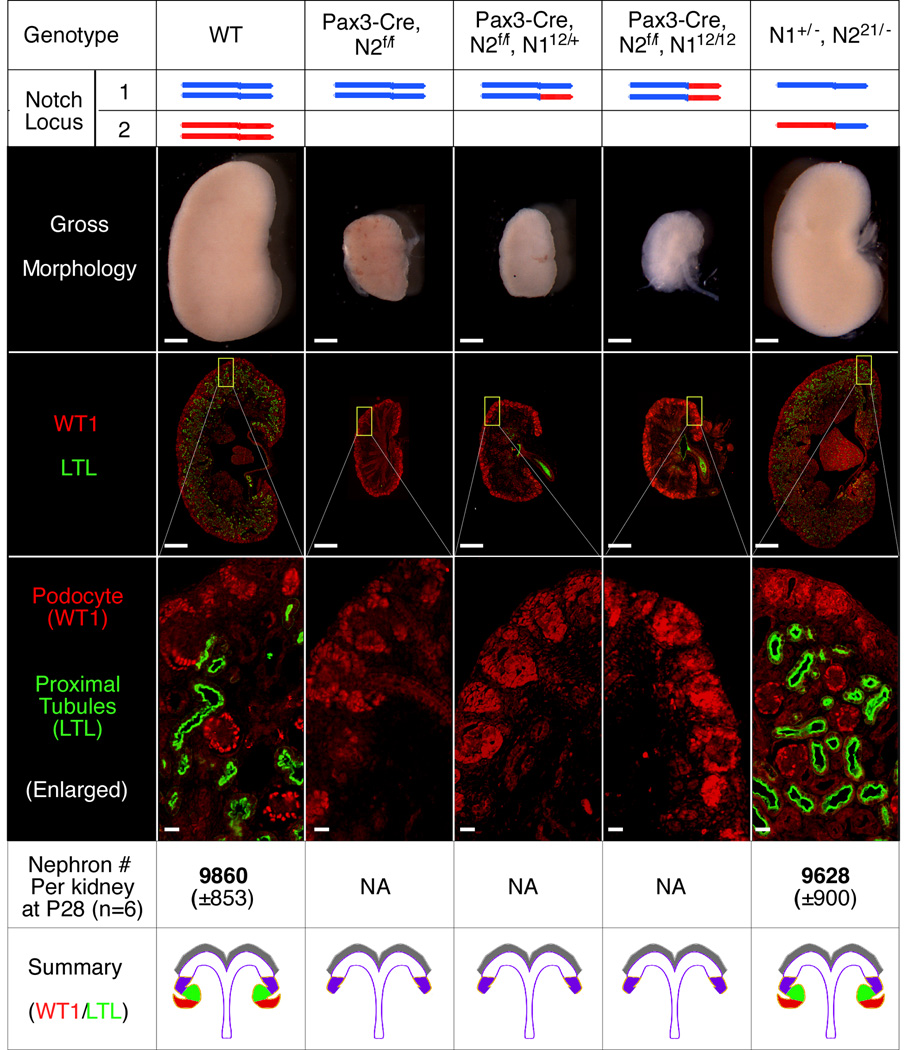
Notch ICDs can functionally replace each other and thus do not contribute to the functional difference of N1 and N2 in kidney development. Kidney phenotypes were characterized in newborn mice with the indicated genotypes. Scale bars: 500µm for whole kidneys; 20µm for the magnified windows showing WT1 and LTL staining. Standard deviations of nephron number are shown in the parentheses.
N1ICD and N2ICD are interchangeable in the kidney
We have shown previously that conditional deletion of N2 from the intermediate mesoderm (with Pax3-Cre) produced mice with non-functional, hypoplastic kidneys lacking podocytes and proximal tubules (Cheng et al., 2007). We found that N221/21 and compound heterozygous N1+/−; N221/− mice (both lacking N2ICD) formed functional nephrons in normal numbers (Figure 3). This result demonstrates that even a single copy of N1ICD can fully rescue the loss of N2ICD when expressed from the N2 locus. In contrast, when the endogenous N2 alleles are conditionally deleted, even the presence of two N2ICD expressed from the N1 locus (Pax3-Cre; N2f/f; N112/12) cannot rescue a single nephron (Figure 3). These mice were indistinguishable from Pax3-Cre; N2f/f mice in their kidney morphology and died within 24 hours of birth (Figure 3). These data demonstrate that N1ICD and N2ICD are fully interchangeable, and that the functional differences between N2 and N1 are determined by differences in their ECDs and/or their corresponding protein levels during kidney development.
N2 and N1 promoters/3’UTRs deliver similar levels of protein in the RV and SSB cells
We next sought to determine if N1 is less abundant than N2 protein within RVs and/or SSBs, thereby explaining the differences in their function during kidney development. This is a technically challenging question to answer: First, it proved impractical to isolate enough RV and SSB cells for Western blot analysis. Second, different antibodies recognizing unique epitopes in Notch paralogs may have different affinities, making the comparison difficult. Fortunately, the domain swap offered us the opportunity to examine N2ICD protein levels by immunostaining in wild type (where N2ICD production is under the control of the endogenous N2 locus) and in N12; N21 double-homozygous (N112/12; N221/21) mice where N2ICD production is under the control of the N1 locus.
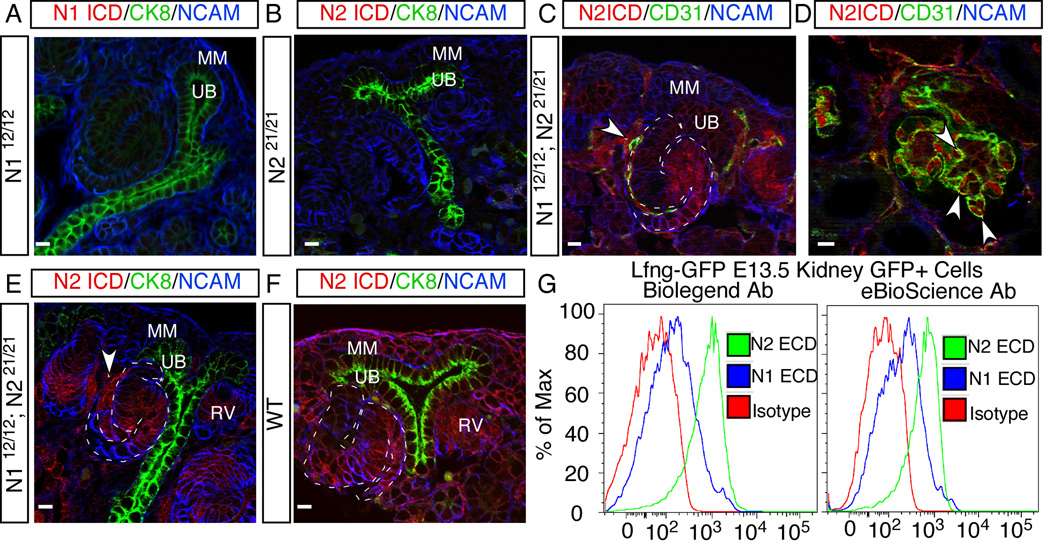
To perform this experiment, we first confirmed the specificity of the anti-N1ICD and anti-N2ICD antibodies on kidney sections from either N112/12 or N221/21 mice (Figure 4A, B). Next, we used the anti-N2ICD antibody and analyzed immunostained kidneys from N2+/−, wild type, and N112/12; N2+/+ mice, which have 1, 2 and 4 copies of the N2ICD antigen, respectively (Figure S4A–C). Pixel intensity correlated well with gene dose (Figure S4D), confirming that this assay is sensitive enough to quantitatively compare N2ICD levels in wild type and N112/12; N221/21 mice. Finally, we compared the abundance of N2ICD protein levels in kidneys from wild type and N112/12; N221/21 by immunostaining (Figure 4). Anti-N2ICD antibody staining of N112/12; N221/21 kidneys confirmed that N2ICD recapitulated the N1 expression pattern, including its strong expression in the developing RVs, SSBs and all endothelial precursor cells, and its absence from the MM (Figure 4C,D). Importantly, the expression levels within RVs and SSBs are comparable in the two samples (Figure 4E,F). Therefore, the functional differences between N1 and N2 could not be attributed to differential expression levels, miRNA targeting of their 3’UTRs, or their ICD composition.
Figure 4.
Although the total amount of N1 and N2 protein is similar in the epithelial cells of developing nephrons, N2 is more abundant at the cell surface. (A, B) Confirmation of the specificity of anti-N1 and -N2 ICD antibodies on kidney sections from N112/12 (A) and N221/21 (B) mice. (C, D) Anti-N2 ICD antibody staining on kidney sections from N112/12; N221/21 double-homozygous mice, in which all N2ICD is expressed from the N1 locus. (E, F) The levels of protein expressed from the N1 and N2 loci in developing RVs and SSBs were compared by immunostaining with N2 ICD specific antibodies on N112/12; N221/21 mice (the N1 locus, E) and wild type (the N2 locus, F). The secondary antibody was used without signal amplification and exposure times were identical for the red channel to allow quantitative comparisons. Arrowhead denotes endothelial cells. MM, metanephric mesenchyme; RV, renal vesicle; UB, ureteric bud. (G) Flow cytometry analysis on EGFP+ live cells from E13.5 Lfng-GFP kidneys with two different sets of anti-Notch ECD antibodies. All scale bars: 10µm. See also Figure S4.
Receptors containing the N2 ECD are more abundant on the plasma membrane of RV and SSB cells
Since only receptors on the cell surface could engage with ligands for activation, we determined whether the cell surface level of N1 and N2 are comparable by flow cytometry, using LFng-GFP kidneys (Figure 2H). To rule out antibody-based artifacts, we employed two different sets of monoclonal anti-N1 and anti-N2 ECD antibodies: one set was raised in Armenian hamster (Moriyama et al., 2008); the other set in rat (Fiorini et al., 2009). The geometric mean fluorescence intensity (GMFI) of antibodies against N2 in EGFP+ epithelial cells isolated from E13.5 Lfng-GFP kidneys is about four fold (4.5±1.0) higher than that generated by anti N1 antibodies (Figure 4G). To ensure that this result did not reflect differential affinity, we sorted stable HEK293 cell lines in which surface biotinylation assays confirmed that the amounts of N1 ECD and N2 ECD on the cell surface are similar (described in the next section and in Figure S5A–C). The results show that the differences in affinity between N1 and N2 antibodies (Figure S5D) could account for only a fraction of distribution difference seen in RV and SSB. Therefore, N2 is more abundant than N1on the surface of renal epithelial cells in the developing nephron. Most importantly, because N21 has the same surface abundance as N2 (Figure 2I), the ECD, but not the ICD, determines the surface level of N2 and N1.
The N2 ECD releases more N1ICD than the N1 ECD does in response to ligands
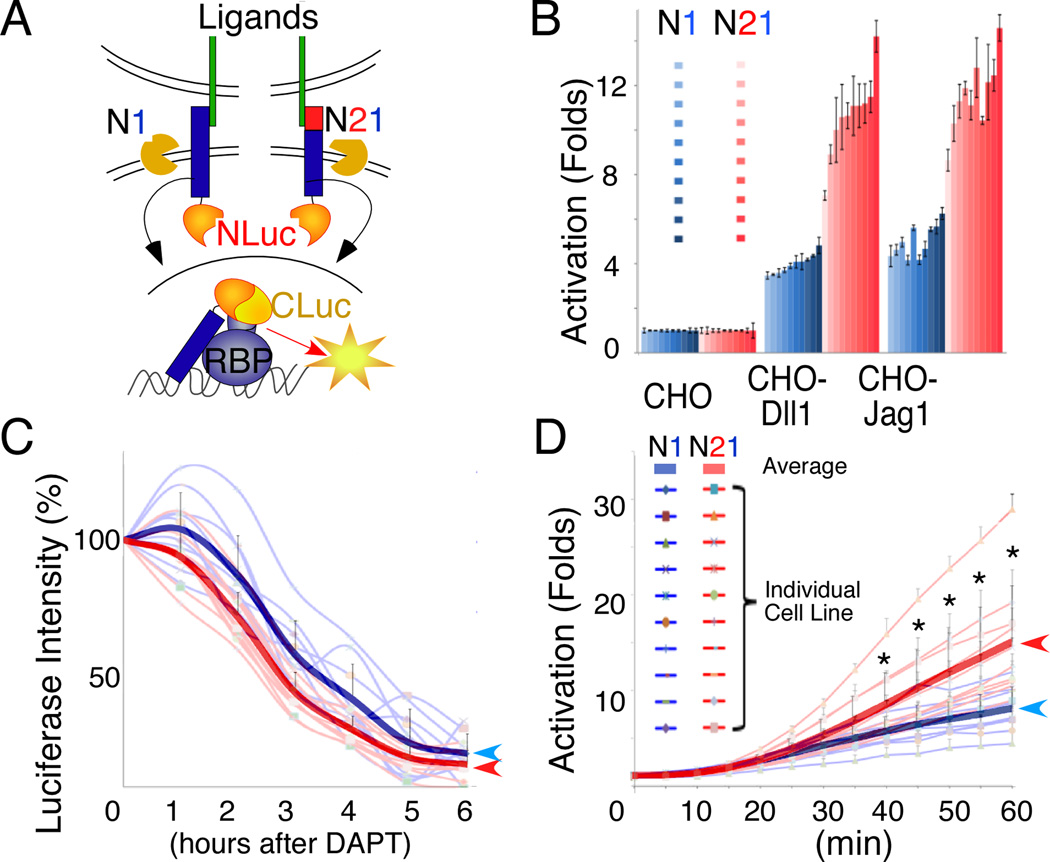
Considering that only one allele of N2 is sufficient for normal kidney development whereas two alleles of N1 could not (Figure. 3), a small difference in surface distribution alone may not explain the functional dominance of N2. Therefore, we asked whether differences in ECD composition could also impact the amount of ICDs released in response to ligand. To address this, we used a quantitative in vitro assay based on luciferase complementation imaging (LCI) system in kidney-derived HEK293 cells (Figure 5A; (Ilagan et al., 2011)). We first fused the carboxy-terminal half of luciferase (CLuc) to the N-terminus of RBP and generated two parental CLuc-RBP-expressing HEK293 Flp-In™ cell lines by random integration. Then we fused the N-terminal half of luciferase (NLuc) to the C-terminus of full length N1 and N21 respectively, and targeted them into the same genomic locus in the two parental cell lines using the Flp-In system (Figure 5A). In these Notch Flp-In cells, the isogenic expression of N1-NLuc and N21-NLuc minimizes positional effects and ensures similar expression levels (Figure S5A–C). In the absence of ligand binding, N1-NLuc and N21-NLuc fusion proteins are anchored to the cell membrane, whereas CLuc-RBP fusion protein is segregated into the nucleus and no luciferase activity is detected. The binding of ligands to the ECD (or unfolding of the NRR by calcium chelation with EGTA) triggers receptor proteolysis and the release of the N1ICD-NLuc fragment, which then translocates into the nucleus and interacts with CLuc-RBP to reconstitute a quantifiable luciferase activity. The amount of light emitted is directly proportional to the amount of N1ICD released and is therefore a measure of signal strength (Ilagan et al., 2011). To control for cell-line specific variations, we tested a total of 10 N1-NLuc sub-clones and 10 N21-NLuc sub-clones for each of the two CLuc-RBP parental cell lines.
Figure 5.
N2 ECD is more potent than N1 ECD in mediating ligand-induced ICD release. (A) The Notch LCI strategy for comparing the potency of N1 and N2 ECDs: NLuc is fused to the C-terminus of N1 or N21. These two constructs were expressed from the same genomic locus in parental cell lines that stably express CLuc-RBP. For both N1 and N21, activation releases N1ICD-NLuc. The subsequent interaction of N1ICD-NLuc with CLuc-RBP reconstitutes luciferase. The amount of NICD released is proportional to the light produced. (B) and (D) show LCI results for 10 independent cell lines in the presence of either co-cultured ligand-expressing cells (CHO-Dll1 and CHO-Jag1) (B) or 100µM EGTA (D) (* denotes p<10−6, Student T-test). (C) The stability of N1ICD-Nluc fragments released from N1 and N21 fusion proteins, which differ by 6 amino acids at N-terminus (VLLSRK and VIMAKR, respectively), was determined by the luminescence lifetime measurements after blocking NICD-NLuc release with the γ-secretase inhibitor DAPT. Thick lines in (C) and (D) represent the average of N1 and N21 cell lines. All scale bars represent standard deviation. See also Figure S5.
To compare signal strength of N1 and N21 in these cells, we co-cultured the Notch Flp-In cells with either ligand-presenting cells (CHO-Dll1 or -Jag1) or control CHO cells. After 24hrs of co-culturing, significantly more light is emitted from N21-NLuc than N1-NLuc cells (p<10–6, Student T-test, Figure 5B; similar results were obtained with subclones derived from the other CLuc-RBP-expressing parental cell line, not shown). Considering that the released N1ICD-NLuc fragments from N21-NLuc and N1-NLuc differ by six amino acids at their N-termini (VLLSRK for N1-NLuc vs. VIMAKR for N21-NLuc), we tested whether differential stability accounted for the apparent difference in bioluminescence between N1-Nluc and N21-NLuc. After activating the reporter cells overnight on immobilized ligand, we added a γ-secretase inhibitor (DAPT) to block the release of additional N1ICD-NLuc fragments and followed the decay of bioluminescence as a function of time (Figure 5C). The N1ICD-NLucVLLSRK proved to be as stable as the N1ICD-NLucVIMAKR, allaying the concern that we were detecting differences in protein stability. Finally, as mentioned above, the amounts of N1 and N21 on the cell surface are similar (Figure S5), suggesting that the difference in luminescence is not simply due to unequal amounts of surface receptors. Collectively, these experiments suggest that N2ECD is more efficient in eliciting ligand-mediated receptor activation in kidney cells.
The activation of Notch receptors requires the unfolding of the NRR domain within the ECD to expose the S2 cleavage site (Kopan and Ilagan, 2009). We therefore tested if differences in the dynamics of NRR unfolding may contribute to the differences between the two ECDs. We monitored the kinetics of N1 and N21 activation in our Flp-In lines in the presence of the calcium chelator EGTA for one hour. After 30 minutes of EGTA treatment, more bioluminescence was detected with N21-NLuc than with N1-NLuc (Figure 5D), suggesting that subtle differences in NRR unfolding may contribute to the higher activation probability of N21.
Dll1 and Jag1 contribute differentially to nephron segmentation
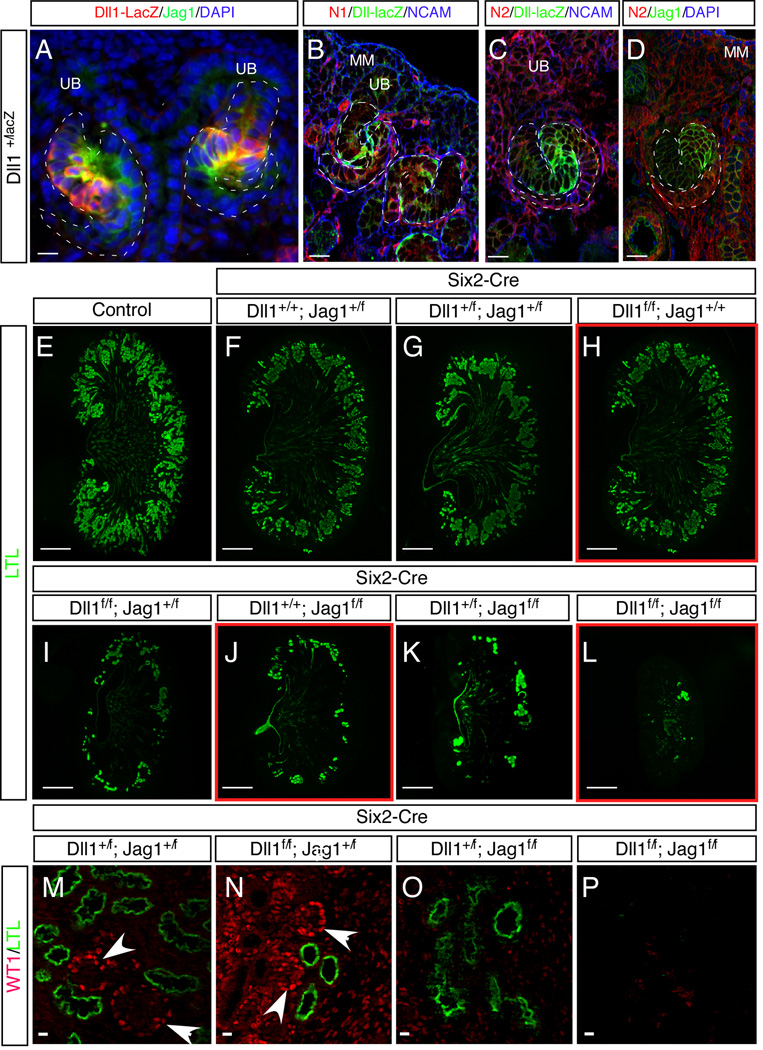
Two major Notch ligands, Dll1 and Jag1, are expressed in the developing renal epithelia (Chen and Al-Awqati, 2005; Leimeister et al., 2003); co-immunostaining of SSBs shows that their expression domains largely overlap with each other (Figure 6A), with LFng (Figure 2 E, G and Figure S3E) and Notch receptors in the middle part of the SSBs (Figure 6B–D). To assess the contribution of each ligand to nephron development, we created an allelic series of conditionally deleted ligands in the MM using Six2-Cretg/+ (Kobayashi et al., 2008) (Figure 6E–P). Because Six2-Cre is strongly expressed in metanephric mesenchymal cells from which all renal epithelial cells are derived, near complete deletion of floxed Jag1 and Dll1 is achieved at the genomic DNA level in these cells (Fig. S6A–D). Staining with the proximal tubule marker LTL revealed mildly disrupted nephron development in Dll1 mutants (Figure 6H) but a drastic reduction in the number of nephrons in Jag1 mutants (Figure 6J). Interestingly, in the presence of one Jag1 allele (Six2-Cretg/+; Dll1f/f; Jag1+/f), nephron number was severely compromised, but some WT1+ podocytes formed (compare Figure 6N to 6M); the presence of one Dll1 allele could not support production of podocytes, despite the presence of some proximal tubules (Figure 6O). Simultaneous deletion of both ligands led to near complete loss of nephrons (Figure 6L, P), approaching the drastic phenotype seen in N2 mutants where both glomeruli and proximal tubules are missing (Cheng et al 2007). These data collectively demonstrate that although both ligands contribute to the normal development of the nephrons, Jag1 plays a dominant role in general and in the development of podocytes in particular.
Figure 6.
Jag1 is the dominant ligand of N2 in the kidney. (A–D) Comparison of N1, N2, Dll1 and Jag1 expression in the developing nephron. (E–P) Phenotypes of newborn kidneys after ligand deletion. Scale bars: (A-D, M-P), 10µm; (E–L), 500µm. See also Figure S6.
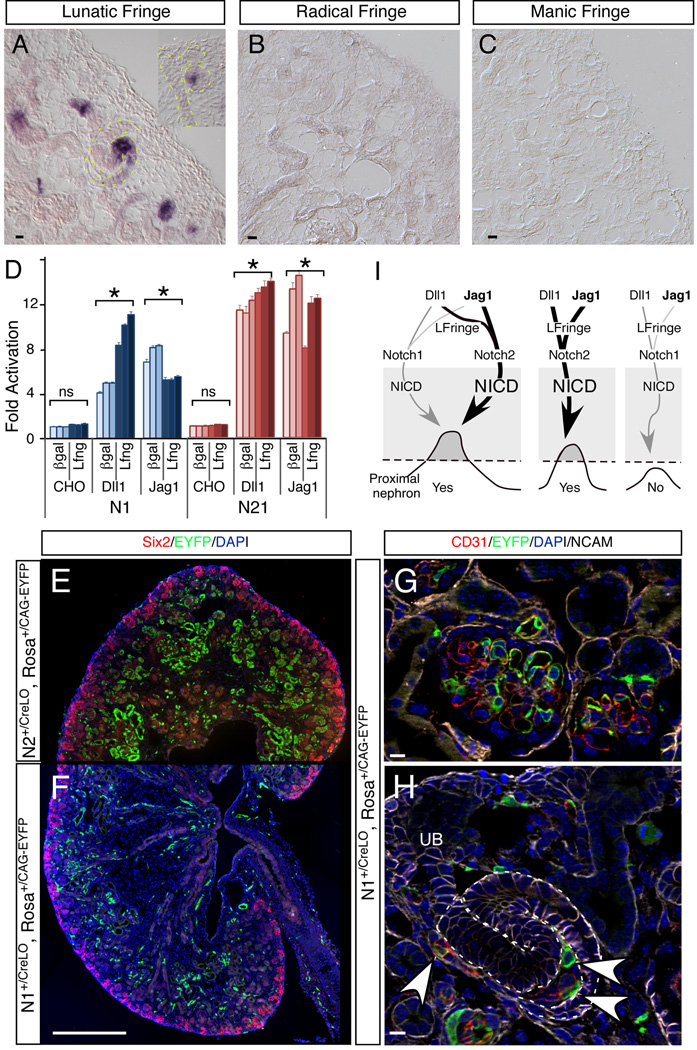
Fringe family members can modulate the response of N1 and N2 to Dll1 and Jag1 ligands. In most contexts, fringe modification renders N1 more responsive to Dll1 ligand and less responsive to Jag1. In contrast, fringe modification of N2 could potentiate, reduce or have no effect on ligand-mediated signaling, depending on the context (Stanley and Okajima, 2010). We therefore examined the expression pattern of the three fringe family members by in situ hybridization in E17.5 kidneys (Figure 7A–C). Only Lfng was detected; it was expressed in a pattern similar to EGFP from Lfng-GFP mice with strong signal in some epithelial cells of RVs and the middle segment of SSBs and weak signal in differentiated tubules (Figure 7A). We next tested NICD release from N1-NLuc and N21-NLuc cell lines co-cultured with Dll1 or Jag1-expressing CHO cells in the presence and absence of LFng. Overexpression of Lfng significantly enhanced the N1-NLuc response to Dll1 and suppressed its response to Jag1. In contrast, its effects on N21-NLuc were minimal (Figure 7D; only 3 line of 20 shown). Although the net loss in response to Jag1 may be offset by the gain in response to Dll1 in vitro (Figure 7D), these data suggest that fringe could contribute to the unequal contribution of N1 and N2 in vivo.
Figure 7.
The dominance of N2 in the developing kidney can be explained by a combination of factors that are mediated by the ECD: higher cell surface expression, greater responsiveness to ligand and selective modulation by Lfng. (A–C) In situ hybridization of three fringe genes in the developing kidney. (D) Effects of Lfng modification on Notch1 and Notch21 activation in HEK293 cells (* denotes p<0.05, Student T-test). All scale bars represent standard deviation. (E–H) Comparison of the labeling pattern between N2∷CreLO (E) and N1∷CreLO (F–H) in vivo in developing nephrons. Arrowheads denote endothelial cells. (I) A model proposing a NICD-dependent switch that regulates proximal nephron development and explaining how N2 achieves its dominant roles over N1. See discussion for details. The weight of lines indicates the weight of effects. Scale bars: (A–C), (G–H) 10µm; (E, F) 500µm.
N2 ECD released Cre more efficiently than N1 ECD in developing nephrons of N∷Cre mice
All the data presented thus far ascribes the difference between N1 and N2 to their ECDs. Unfortunately, we have not yet identified antibodies that specifically recognize activated N2ICD. Therefore, we used a surrogate assay to compare N1 and N2 cleavage in vivo by comparing the effectiveness of Cre release in two activation-dependent Notch reporter mice, N1∷CreLo (Liu et al., 2011; Vooijs et al., 2007) and N2∷CreLo (Figure S1). The release of Cre in both lines is under the control of a Notch ECD, and Cre activity will provide an estimate of the efficiency of its release. Many labeled epithelial cells were seen in RVs and SSBs of N2∷CreLo; RosaCAG-EYFP mice (Figure 1M–O, Figure 7E). In contrast, we could not find any labeled epithelial cells in RVs or SSBs of N1∷CreLo; RosaCAG-EYFP mice, although endothelial cells are very efficiently labeled (Figure 7F–H), as are cells in many other tissues (Vooijs et al., 2007). Because we obtained immunohistological and genetic evidence that N1ICD complements N2 activity in a sensitized background (Cheng et al., 2007; Surendran et al., 2010), these observations are consistent with a model that in renal epithelia N2 is more abundant at the cell surface, where it undergoes proteolysis more efficiently than N1 in response to available ligands. Like Notch ICDs, only Cre6MT released by the N2 ECD, but not the N1 ECD, reached a concentration threshold needed to excise the floxed stop allele in renal epithelial cells.
DISCUSSION
Human patients and mouse models of Alagille syndrome support the idea that kidney development is particularly sensitive to N2 dosage even in the presence of N1. We investigated several possible mechanisms that could explain the dominant contribution of N2 over N1 to nephrogenesis (Cheng et al., 2007; Surendran et al., 2010). A precise mechanistic understanding will not only enhance our knowledge of how Notch signaling contributes to kidney organogenesis, but more importantly, could offer insights into therapeutic options for kidney defects seen in Alagille syndrome (Penton et al., 2012) and perhaps other Notch-related congenital disorders. Furthermore, such understanding may prove generally applicable to many other organs and signaling pathways.
Although N2 is expressed in the MM, none of the known Notch ligands or targets are expressed there (Boyle et al., 2011; Chen and Al-Awqati, 2005; Leimeister et al., 2003; Ong et al., 2006). Consistent with a ligand-poor environment, N2 activation is an infrequent event in the MM. Genetic analyses confirmed that Notch proteins function in nascent renal epithelial cells (this study, (Cheng et al., 2007; Wang et al., 2003)), where the N1 and N2 expression domains are indistinguishable (Chen and Al-Awqati, 2005; Leimeister et al., 2003). These results rule out enhancer evolution as the mechanistic explanation for the functional importance of N2.
We therefore focused on two alternative hypotheses: either N1ICD is a weak activator of key target(s) regulated normally by N2ICD due to its amino acid composition, or N1ICD concentration is insufficient to functionally compensate for N2 deficiency. To differentiate between these possibilities, we generated two new alleles of Notch (N12 and N21) in which we swapped the entire genomic sequences coding for Notch ICDs, in contrast to a previous study that established the equivalence of the domain C-terminal to the ankyrin repeats of Notch (Kraman and McCright, 2005). The availability of mice in which the same epitope is transcribed and translated from different loci enabled comparison of protein abundance with the same ICD-specific antibody. This analysis demonstrated that the two paralogs are expressed at similar levels in the developing renal epithelium and, therefore, differences in overall protein concentration cannot explain the dominant role of N2.
We next addressed the role of Notch amino acid composition. We demonstrated that N1ICD and N2ICD are fully interchangeable during kidney development: even one copy of N1ICD expressed under the N2 ECD control is sufficient to produce a normal kidney. If neither overall protein concentration nor NICD composition could explain the unequal roles of N1 and N2 in the developing kidney, ECD control over NICD nuclear concentration is likely the differentiating factor between N2 and N1. We discovered that the N2 ECD indeed generates more NICD than the N1 ECD does in the renal epithelial cell context by a combination of two mechanisms. First, N2 is more abundant at the cell surface than N1. Accounting for the differences in affinity between anti-N1 and anti-N2 antibodies, the difference is between 2 and 3 fold. Importantly, N21 and N2 are equally abundant at the cell surface indicating that surface distribution is determined by sequences in the Notch ECD, not ICD. Although other transmembrane proteins may contain trafficking signals in their ECD (Albu and Constantinescu, 2011; Steiner et al., 2008; VandenBussche et al., 2009), the only indication that the Notch2 ECD may play a role in its trafficking comes from a study on the importance of S1 cleavage to the exocytosis of N1, but not N2 (Gordon et al., 2009). Second, in luciferase complementation imaging assays (Ilagan et al., 2011) that quantified the amount of NICD released from N21 and N1 in cultured human embryonic kidney cells in response to ligand or EGTA, N21 consistently released more NICD. In the EGTA paradigm, all surface receptors are activated; given that surface biotinylation confirmed that N1 and N21 are present at equal amounts, and the NICDs have the same half-life, we conclude that the N2 NRR must be easier to activate than N1 NRR in kidney epithelia, and since it contains the S1 site, may be regulating exocytosis as well. However, where the trafficking signals reside within the ECD, and whether they only functions in the developing kidney, remains to be investigated. Supporting the conclusion that the ICD passively reflects the advantages provided by a specific ECD, the N2 ECD is more potent than the N1 ECD in Notch∷Cre reporter mice, where the amount of Cre released directly reflects the activation frequency by the respective Notch ECD.
These two factors (surface density and ease of activation) may work in synergy or simply be additive, but other factors may serve to further amplify the effectiveness of the N2 ECD in vivo. As reported before (Hicks et al., 2000), the response of N1 to Jag1 is significantly inhibited by Lfng modification, whereas that of N2 is not; considering that Lfng is co-expressed with N1 and N2 in the epithelial cells of developing nephrons, it may be promoting a potent N2-JAG1 signaling axis during nephrogenesis. Indeed, analysis of ligand loss-of-function alleles showed that, although Dll1 and Jag1 are co-expressed with N1 and N2, Jag1 plays a dominant role. This is consistent with reports that only mutations in JAG1, but not DLL1, cause Alagille syndrome (Piccoli and Spinner, 2001).
The data herein indicates that a NICD nuclear concentration threshold must be met to define the identity of the proximal renal epithelia, and this threshold acts as a digital (all or nothing) switch (Figure 7I). This is very reminiscent of the intestinal differentiation program in C. elegans (Raj et al., 2010). In that system, mRNA levels must rise above a threshold to activate expression of a master regulatory gene. The relative strength, or penetrance, of various alleles reflects the number of cells reaching the ON decision. In the Notch system, variability in the number of NICDs released to the nucleus might regulate an ON/OFF decision to form the proximal nephron. The advantage of the N2 ECD/NRR and the large impact of these differences suggest that the overall numbers of NICD in the nucleus are just above the ON state in RV cells and thus highly prone to perturbations. Whether a master regulator lies downstream of NICD and the identity/number of targets that must be activated to promote proximal development remain unknown.
In summary, our experiments produce strong evidence of functional equivalence between the N1 and N2 ICDs in vivo, despite apparent differences in multiple assays based on overexpression, including our own (Ong et al., 2006). A higher surface level coupled with greater responsiveness of the N2 ECD to ligand translates into a higher probability of NICD release during a critical step in kidney development. These data illustrate the binary nature of a critical step in nephron segmentation, where nephrons with NICD concentration falling below a threshold fail completely to produce proximal structures, and highlight an underappreciated importance for the ECD/NRR in controlling surface distribution. In contrast to binary response to NICD levels, the outcome of ligand reduction is graded: nephrons can form proximal tubules without podocytes when only Dll1 is present, suggestive of a second Notch-dependent decision. Finally, investigation into Notch paralogs trafficking to cell surface may provide leads for treating the renal (and perhaps all) manifestations of Alagille syndrome.
EXPERIMENTAL PROCEDURES
Mice
Generation, genotyping strategy and PCR primers related to N12 and N21 mice and the source of other mouse lines are described in detail in Supplemental Experimental Procedures. All mice were housed in the Washington University animal facility and all experimental procedures were approved by Washington University Animals Studies Committee.
Pyrosequencing
Total RNA was purified, reverse transcribed and used for pyrosequencing as described (Liu et al., 2010).
Immunohistochemistry
Kidneys were dissected and fixed in 4% PFA overnight, washed extensively with 1XPBS, soaked overnight in 30% sucrose and embedded in Optimal Cutting Medium for frozen sections, or dehydrated through a 30%, 50%, 70% ethanol series and embedded for paraffin sections. For frozen sections, antigen retrieval was achieved by permeabilization in 1XPBS, 0.1% Triton X-100 for 20 minutes at room temperature. For paraffin sections, this was achieved by boiling in 10 mM Sodium Citrate (pH 6.0) for 20 minutes. For both frozen and paraffin sections, 1XPBS containing 3%BSA and 0.1% Tween was used for blocking. Detailed information on primary antibodies and their dilution were described in Supplemental Experimental Procedures. FITC-, Cy3-, and Cy5-conjugated secondary antibodies or streptavidin (Jackson ImmunoResearch) was used for visualization.
Flow Cytometry
Flow cytometry were performed as described (Liu et al., 2011). Briefly, E13.5 embryonic kidneys were dissected, mechanically disrupted and digested with 1mg/ml collagenase at 37°C for 15 minutes to get single cell suspensions. Cells were further washed and stained with PE- or APC-conjugated anti-N1 or -N2 ECD antibodies (eBioscience and Biolegend) in staining buffer (1XPBS +3% BSA) on ice for 20–30 minutes, followed by flow cytometry analysis. Cultured 293 cells were mechanically removed from culture plates and analyzed in a similar manner. Data were collected on BD FACScan with FlowJo Collectors’ Edition and analyzed with FlowJo software (TreeStar).
In situ Hybridization, Western blot and Nephron Number Quantification
Conventional methods were used for in situ hybridization. Probes for lunatic, radical and manic fringe were labeled with Digoxigenin and detected with alkaline phosphatase-conjugated anti-Digoxigenin antibody (Roche). Kidneys from newborn pups or cultured cells were used for Western blot. Nephron number was determined as described (Godley et al., 1996). Details are described in Supplemental Experimental Procedures.
Generation and maintenance of isogenic N1 and N21 LCI reporter lines
Flp-In™TRex™293 host cells (Invitrogen, R780–07), which were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1% Pen-Strep (henceforth, media) and 100 µg/ml Zeocin™, were cotransfected with pcDNA3-(Click beetle green) CBG CLuc-RBP and a Puromycin expression construct using FuGENE6 (Roche). Puromycin-resistant clones were selected in media containing 0.6 µg/ml puromycin to generate Flp-In™TRex™ 293 CBG CLuc-RBP parental cell lines. LCI imaging was performed on CBG CLuc-RBP expressing clones by transiently transfecting a constitutively active N1ΔE-NLuc plasmid to verify luciferase complementation. Two LCI positive clones, D10 and D6, were selected as the CLuc-RBP parental cell lines and expanded in media containing 0.4µg/ml puromycin and 100µg/ml Zeocin™. Stable expression of CLuc-RBP after different passages was confirmed by Western blot. To generate N1-NLuc and N21-NLuc Flp-In cells, D10 and D6 clones were co-transfected with pcDNA5/FRT expression vector (Invitrogen, V6010–20) containing either N1-CBG NLuc or N21-CBG NLuc and pOG44 vector at a 1:9 ratio. Positive clones, identified by selection for hygromycin-resistance (150µg/ml) and gain of Zeocin™ sensitivity, were tested for their ability to reconstitute luciferase activity upon EGTA treatment. 20 sub-clones were subsequently maintained in media containing 0.4µg/ml puromycin (to maintain CBG CLuc-RBP) and 100 µg/ml hygromycin (to maintain Notch CBG NLuc).
Luciferase Complementation Imaging (LCI) Assays
LCI assays are described in detail in (Ilagan et al., 2011). For ligand-dependent activation, 104 ligand-presenting cells (CHO-Dll, CHO-Jag or CHO control cells) were seeded into each well of uncoated 96-well black plates 24 hours prior to the seeding of 4×104 N1-NLuc or N21-NLuc cells. After another 24 hours, co-cultured cells were imaged in phenol red-free culture medium containing 150 µg/ml D-luciferin. For the ligand-independent activation assays, black 96-well plates were coated with 0.1mg/ml poly-lysine at room temperature overnight, washed twice with PBS and air-dried for 30 minutes before 4×104 cells were seeded into each well. Twenty-four hours later, an initial image (t=0) was obtained of the cells using Hank’s Balanced Salt Solution (HBSS) containing 150 µg/ml D-luciferin (100µ/well). Then, another 100µl of HBSS/D-luciferin solution containing 2X EGTA (200µM) was added per well and images were obtained every 5 minutes for 1 hour.
To monitor the rate of NICD degradation, black 96-well plates were coated with 5µg/ml anti-Fc antibody (Jackson Immunoresearch) for 6 hours at 4°C. Unbound antibodies were washed off twice with PBS and conditioned media containing Fc control or Dll1-Fc/Jag-Fc were added (50µl/well) and incubated overnight at 4°C. Excess ligands were washed off twice with PBS before cells were seeded. Reporter cells were plated on the immobilized ligands as described in (Ilagan et al., 2011). After 24 hours, an initial image was obtained (t=0), after which DMSO or DAPT (5µM) was added to the wells to stop NICD production, and images were taken every hour for 6 hours. Additional, detailed methods for ligand conditioned media preparation, IVIS imaging and photon flux quantification can be found in (Ilagan et al, 2011).
Surface Biotinylation Assay
Surface biotinylation was used to compare the surface level of N1-NLuc and N21-NLuc in Flp-In cells. Details are described in Supplemental Experimental Procedures.
Supplementary Material
Highlights.
Notch2 dominance in the kidney is not determined by expression pattern or level
Knock-in mice show that Notch1/2 intracellular domains are interchangeable
Notch2 extracellular domain controls cell-surface presentation and ligand response
Tubules and podocytes display greater sensitivity to the dose of Jag1 over Dll1
ACKNOWLEDGEMENTS
We thank the Washington University Siteman Cancer Center Murine Embryonic Stem Cell Core, Mouse Genetics Core, Siteman Flow Cytometry Core. We thank Dr. MacDonald for un-conjugated N1 and N2 antibodies, Dr. Groves for Lfng-GFP mice and Dr. Blacklow for communicating unpublished results. We thank Kopan lab members for stimulating discussions, Mary Fulbright and Hila Barak for technical assistance. ZL, SC, SCB, AZ and RK were supported by National Institutes of Diabetes and Digestive and Kidney Disease (DK066408). MXGI and DPW were supported in part by the national Cancer Institute (CA094056)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Albu RI, Constantinescu SN. Extracellular domain N-glycosylation controls human thrombopoietin receptor cell surface levels. Frontiers in endocrinology. 2011;2:71. doi: 10.3389/fendo.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle SC, Kim M, Valerius MT, McMahon AP, Kopan R. Notch pathway activation can replace the requirement for Wnt4 and Wnt9b in mesenchymal-to-epithelial transition of nephron stem cells. Development. 2011;138:4245–4254. doi: 10.1242/dev.070433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Al-Awqati Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am J Physiol Renal Physiol. 2005;288:F939–F952. doi: 10.1152/ajprenal.00369.2004. [DOI] [PubMed] [Google Scholar]
- Cheng HT, Kim M, Valerius MT, Surendran K, Schuster-Gossler K, Gossler A, McMahon AP, Kopan R. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development. 2007;134:801–811. doi: 10.1242/dev.02773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D, Zhang Z, Zhou Y, Wang W, Li Y, Zhang H, Dong G, Zhao Q, Ji G. Notch1 and Notch2 have opposite prognostic effects on patients with colorectal cancer. Ann Oncol. 2011;22:2440–2447. doi: 10.1093/annonc/mdq776. [DOI] [PubMed] [Google Scholar]
- Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, Ball D, Brat DJ, Perry A, Eberhart CG. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- Fiorini E, Merck E, Wilson A, Ferrero I, Jiang W, Koch U, Auderset F, Laurenti E, Tacchini-Cottier F, Pierres M, et al. Dynamic regulation of notch 1 and notch 2 surface expression during T cell development and activation revealed by novel monoclonal antibodies. J Immunol. 2009;183:7212–7222. doi: 10.4049/jimmunol.0902432. [DOI] [PubMed] [Google Scholar]
- Fujimura S, Jiang Q, Kobayashi C, Nishinakamura R. Notch2 Activation in the Embryonic Kidney Depletes Nephron Progenitors. J Am Soc Nephrol. 2010 doi: 10.1681/ASN.2009040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgas K, Rumballe B, Valerius MT, Chiu HS, Thiagarajan RD, Lesieur E, Aronow BJ, Brunskill EW, Combes AN, Tang D, et al. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol. 2009;332:273–286. doi: 10.1016/j.ydbio.2009.05.578. [DOI] [PubMed] [Google Scholar]
- Godley LA, Kopp JB, Eckhaus M, Paglino JJ, Owens J, Varmus HE. Wild-type p53 transgenic mice exhibit altered differentiation of the ureteric bud and possess small kidneys. Genes & development. 1996;10:836–850. doi: 10.1101/gad.10.7.836. [DOI] [PubMed] [Google Scholar]
- Gordon WR, Vardar-Ulu D, L'Heureux S, Ashworth T, Malecki MJ, Sanchez-Irizarry C, McArthur DG, Histen G, Mitchell JL, Aster JC, et al. Effects of S1 cleavage on the structure, surface export, and signaling activity of human Notch1 and Notch2. PLoS One. 2009;4:e6613. doi: 10.1371/journal.pone.0006613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani I, Eliasz S, De Marco MA, Chen Y, Pass HI, De May RM, Strack PR, Miele L, Bocchetta M. Opposite effects of Notch-1 and Notch-2 on mesothelioma cell survival under hypoxia are exerted through the Akt pathway. Cancer Res. 2008;68:9678–9685. doi: 10.1158/0008-5472.CAN-08-0969. [DOI] [PubMed] [Google Scholar]
- Hicks C, Johnston SH, diSibio G, Collazo A, Vogt TF, Weinmaster G. Fringe differentially modulates Jagged1 and Delta1 signalling through Notch1 and Notch2. Nature Cell Biology. 2000;2:515–520. doi: 10.1038/35019553. [DOI] [PubMed] [Google Scholar]
- Ilagan MX, Lim S, Fulbright M, Piwnica-Worms D, Kopan R. Real-time imaging of notch activation with a luciferase complementation-based reporter. Sci Signal. 2011;4 doi: 10.1126/scisignal.2001656. rs7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell stem cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraman M, McCright B. Functional conservation of Notch1 and Notch2 intracellular domains. FASEB J. 2005;19:1311–1313. doi: 10.1096/fj.04-3407fje. [DOI] [PubMed] [Google Scholar]
- Leimeister C, Schumacher N, Gessler M. Expression of Notch pathway genes in the embryonic mouse metanephros suggests a role in proximal tubule development. Gene Expr Patterns. 2003;3:595–598. doi: 10.1016/s1567-133x(03)00114-5. [DOI] [PubMed] [Google Scholar]
- Liu Z, Obenauf AC, Speicher MR, Kopan R. Rapid identification of homologous recombinants and determination of gene copy number with reference/query pyrosequencing (RQPS) Genome Res. 2009;19:2081–2089. doi: 10.1101/gr.093856.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Schneider DL, Kornfeld K, Kopan R. Simple copy number determination with reference query pyrosequencing (RQPS) Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5491. pdb prot5491. [DOI] [PubMed] [Google Scholar]
- Liu Z, Turkoz A, Jackson EN, Corbo JC, Engelbach JA, Garbow J, Piwnica-Worms D, Kopan R. Notch1 loss of heterozygosity causes vascular tumors and lethal hemorrhage in mice. J Clin Invest. 2011;121:800–808. doi: 10.1172/JCI43114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature neuroscience. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCright B, Lozier J, Gridley T. A mouse model of Alagille syndrome: Notch2 as a genetic modifier of Jag1 haploinsufficiency. Development. 2002;129:1075–1082. doi: 10.1242/dev.129.4.1075. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. Journal of cell science. 2010;123:213–224. doi: 10.1242/jcs.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama Y, Sekine C, Koyanagi A, Koyama N, Ogata H, Chiba S, Hirose S, Okumura K, Yagita H. Delta-like 1 is essential for the maintenance of marginal zone B cells in normal mice but not in autoimmune mice. International immunology. 2008;20:763–773. doi: 10.1093/intimm/dxn034. [DOI] [PubMed] [Google Scholar]
- Ong C, Cheng H, Chang LW, Ohtsuka T, Kageyama R, Stormo DG, Kopan R. Target selectivity of vertebrate Notch proteins: collaboration between discrete domains and CSL binding site architecture determine activation probability. J Biol Chem. 2006;281:5106–5119. doi: 10.1074/jbc.M506108200. [DOI] [PubMed] [Google Scholar]
- Parr C, Watkins G, Jiang WG. The possible correlation of Notch-1 and Notch-2 with clinical outcome and tumour clinicopathological parameters in human breast cancer. Int J Mol Med. 2004;14:779–786. doi: 10.3892/ijmm.14.5.779. [DOI] [PubMed] [Google Scholar]
- Penton AL, Leonard LD, Spinner NB. Notch signaling in human development and disease. Semin Cell Dev Biol. 2012;23:450–457. doi: 10.1016/j.semcdb.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccoli DA, Spinner NB. Alagille syndrome and the Jagged1 gene [Review] Seminars in Liver Disease. 2001;21:525–534. doi: 10.1055/s-2001-19036. [DOI] [PubMed] [Google Scholar]
- Raj A, Rifkin SA, Andersen E, van Oudenaarden A. Variability in gene expression underlies incomplete penetrance. Nature. 2010;463:913–918. doi: 10.1038/nature08781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan A, Talora C, Okuyama R, Nicolas M, Mammucari C, Oh H, Aster JC, Krishna S, Metzger D, Chambon P, et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. The EMBO journal. 2001;20:3427–3436. doi: 10.1093/emboj/20.13.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LJ, Honjo T, Clevers H, Radtke F. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27(Kip1) and p57(Kip2) EMBO Rep. 2008;9:377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez CI, Buchholz F, Galloway J, Sequerra R, Kasper J, Ayala R, Stewart AF, Dymecki SM. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25:139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- Spitz F, Furlong EE. Transcription factors: from enhancer binding to developmental control. Nat Rev Genet. 2012;13:613–626. doi: 10.1038/nrg3207. [DOI] [PubMed] [Google Scholar]
- Stanley P, Okajima T. Roles of glycosylation in Notch signaling. Current topics in developmental biology. 2010;92:131–164. doi: 10.1016/S0070-2153(10)92004-8. [DOI] [PubMed] [Google Scholar]
- Steiner NK, Dakshanamurthy S, VandenBussche CJ, Hurley CK. Extracellular domain alterations impact surface expression of stimulatory natural killer cell receptor KIR2DS5. Immunogenetics. 2008;60:655–667. doi: 10.1007/s00251-008-0322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran K, Boyle S, Barak H, Kim M, Stromberski C, McCright B, Kopan R. The contribution of Notch1 to nephron segmentation in the developing kidney is revealed in a sensitized Notch2 background and can be augmented by reducing Mint dosage. Dev Biol. 2010;337:386–395. doi: 10.1016/j.ydbio.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandenBussche CJ, Mulrooney TJ, Frazier WR, Dakshanamurthy S, Hurley CK. Dramatically reduced surface expression of NK cell receptor KIR2DS3 is attributed to multiple residues throughout the molecule. Genes and immunity. 2009;10:162–173. doi: 10.1038/gene.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vooijs M, Ong CT, Hadland B, Huppert S, Liu Z, Korving J, van den Born M, Stappenbeck T, Wu Y, Clevers H, et al. Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development. 2007;134:535–544. doi: 10.1242/dev.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Pereira FA, Beasley D, Zheng H. Presenilins are required for the formation of comma- and S-shaped bodies during nephrogenesis. Development. 2003;130:5019–5029. doi: 10.1242/dev.00682. [DOI] [PubMed] [Google Scholar]
- Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.