Abstract
A human antibody library constructed by utilizing a phage display system was used for the isolation of human antibodies with neutralizing activity specific for human rotavirus. In the library, the Fab form of an antibody fused to truncated cp3 is expressed on the phage surface. Purified virions of strain KU (G1 serotype and P[8] genotype) were used as antigen. Twelve different clones were isolated. Based on their amino acid sequences, they were classified into three groups. Three representative clones—1-2H, 2-3E, and 2-11G—were characterized. Enzyme-linked immunosorbent assay with virus-like particles (VLP-VP2/6 and VLP-VP2/6/7) and recombinant VP4 protein produced from baculovirus recombinants indicated that 1-2H and 2-3E bind to VP4 and that 2-11G binds to VP7. The neutralization epitope recognized by each of the three human antibodies might be human specific, since all of the antigenic mutants resistant to mouse monoclonal neutralizing antibodies previously prepared were neutralized by the human antibodies obtained here. After conversion from the Fab form of an antibody into immunoglobulin G1, the neutralizing activities of these three clones toward various human rotavirus strains were examined. The 1-2H antibody exhibited neutralizing activity toward human rotaviruses with either the P[4] or P[8] genotype. Similarly, the 2-3E antibody showed cross-reactivity against HRVs with the P[6], as well as the P[8] genotype. In contrast, the 2-11G antibody neutralized only human rotaviruses with the G1 serotype. The concentration of antibodies required for 50% neutralization ranged from 0.8 to 20 μg/ml.
Rotavirus is the major cause of severe acute gastroenteritis among infants and young children. Rotavirus infection is life-threatening in developing countries, resulting in 500,000 to 600,000 deaths annually (33). In developed countries, rotavirus infections lead to a high disease burden with considerable medical expense due to the high morbidity. Furthermore, adults, particularly the elderly, are also affected by rotavirus infection (34, 39), and immunocompromised children and adults develop persistent rotavirus diarrhea (12, 42, 43). Thus, vaccination is thought to be the best way to reduce severe rotavirus gastroenteritis worldwide. Tetravalent rhesus rotavirus (RRV) human reassortant vaccine comprising RRV and three RRV-based monoreassortants carrying the VP7 genes from G1, G2, and G4 human rotaviruses (HRVs) was developed (25), and 1.5 million doses of this vaccine had been administered to infants by the end of May 1999 in the United States. However, the vaccine was withdrawn due to the occurrence of gut intussusception, which appeared to be epidemiologically linked to vaccine application (5, 38). Moreover, even if a safe and effective rotavirus vaccine is developed, vaccination would be less effective in immunocompromised patients.
Rotaviruses have two outer capsid proteins, viral protein 4 (VP4) and VP7, encoded on RNA segment 4 and RNA segment 7, 8, or 9, depending on the strain, respectively (19). VP4 and VP7 are known to induce neutralizing antibodies (Abs) in the sera and stools of infected patients, and they are relevant to protection against rotavirus infection (14, 18, 41, 45-47, 51). It is well known that the rotavirus G serotypes and P genotypes defined by VP7 and VP4, respectively, exhibit diversity. A total of 15 G serotypes and 22 P genotypes have been described (11). Although the majority of HRVs prevailing worldwide have G1, G2, G3, or G4 as the G serotype, and P[4] or P[8] as the P genotype, at least 10 G and 10 P types have been reported on HRVs (8). Recently, a number of HRV strains with unusual G or P types and rare combinations of G and P types have been detected worldwide. For example, G9 is increasing rapidly. In contrast, both VP4 and VP7 carry heterotypic (cross-reactive) neutralization epitopes, which are thought to be related to heterotypic protection (29, 30, 45-47, 49). An individual can be repeatedly infected with various strains of HRVs, suggesting that he or she has broadly and strongly effective Abs to HRVs. Although the validity of passive immunization remains unclear (17), oral administration of cross-reactive human immunoglobulins could be one of the measures for both prophylaxis and therapy for HRV diseases.
The natural repertoire formed in the human body should be composed of two different types of Abs. One type, which forms a naive repertoire, should show a wide range of antigen (Ag) specificity, the Ab binding avidity of each Ab being low in general. The other type, which is raised against specific Ags by immunization, should show a narrow range of specificity, the Ab binding avidity of each Ab being strong. In the present study, we used the Ab library called AIMS4 constructed from the B lymphocyte-rich tissues of a few dozen patients. Since this library is human-derived and rotavirus infection is considered to be very common worldwide, we expected that the Ab repertoire formed in AIMS4 should reflect a variety of rotavirus-specific Abs acquired through natural exposure. It would be interesting to directly explore the Ab repertoire of humans. In particular, comparison of the neutralization epitopes recognized by humans and mice would be useful for understanding the immune response against rotavirus infection in humans.
We describe here the successful isolation of anti-VP4 cross-reactive Abs and an anti-VP7 G1-specific Ab with neutralizing activities toward rotaviruses.
MATERIALS AND METHODS
Viruses.
The following HRV strains and reassortants were used for the present study: KU (G1P[8]), Wa (G1P[8]), M37 (G1P[6]), K8 (G1P[9]), S2 (G2P[4]), 1076 (G2P[6]), YO (G3P[8]), McN13 (G3P[6]), AU-1 (G3P[9]), Hosokawa (G4P[8]), 69M (G8P[10]), WI61 (G9P[8]), L26 (G12P[4]), and two bovine strain UK-based single gene-reassortants, UK/Wa (G1P[5]) and UK/DS1 (G2P[5]), carrying the VP7 gene from HRV strains Wa and DS1, respectively (32). Eleven antigenic KU mutants resistant to each of 11 neutralizing mouse monoclonal Abs (MAbs) were also used in the present study: six (V-YO-1E6, V-ST-1F2, V-YO-1S3, V-YO-2C2, V-KU-4D7, and V-KU-6B11) and five (V-KU-3C7, V-YO-4C2, V-KU-5H1, V-KU-6A11, and V-KU4) mutants have been prepared by cultivating strain KU in the presence of anti-VP4 cross-reactive neutralizing MAbs and anti-VP7 G1-specific neutralizing MAbs, respectively (45, 47). Virus propagation and purification were carried out as described previously (49). Unless otherwise stated, the culture fluids of MA104 cells infected with rotaviruses were used for the assays.
Preparation of virus-like particles (VLPs) and recombinant VP4.
Construction of the artificial VLP of HRV KU origin is described elsewhere (K. Taniguchi et al., unpublished data). Briefly, the reverse transcription-PCR products of the VP2, VP4, VP6, and VP7 genes of human strain KU were cloned into a TA cloning vector, pCRII (Invitrogen, San Diego, Calif.), to generate pKU-VP2, pKU-VP4, pKU-VP6, and pKU-VP7. After digestion with restriction enzymes, the fragments were ligated into transfer vector pVL1392 to yield pVL1392/KU-VP2, -VP4, -VP6, and -VP7. Sf9 cells were coinfected with linearized wild-type Autographa californica nuclear polyhedrosis virus DNA (Pharmingen) and either pVL1392/KU-VP2, -VP4, -VP6, or -VP7 by the Lipofectin-mediated method. The baculovirus recombinants thus obtained were used for preparation of recombinant VP4, VLP-VP2/6, and VLP-VP2/6/7 in Tn5 cells.
Ab library.
Abs were isolated from the Abs library called AIMS4, which was constructed in Y. Kurosawa's laboratory. In brief, B lymphocyte-rich fractions of human tissues such as tonsils, umbilical cord blood, peripheral blood, and bone marrow were used as gene sources of Abs (35; Y. Akahori et al., unpublished data). Using a phage-display system, the Fab form of an Ab fused to a truncated cp3 (Fab-cp3) was expressed on the phage surface. The library is composed of 1011 independently established clones, and it has been shown that >70% of the phages express Abs.
Screening of the library.
Selection of phages exhibiting rotavirus (strain KU)-binding activity was performed by a panning method that was essentially the same as that described previously (20, 27). The immunotubes (Nunc-Immunomodules Polysorp) were coated with 200 μg of a purified KU virion/ml in phosphate-buffered saline containing 100 μg of Ca2+ and Mg2+/ml [PBS(+)] overnight at 4°C. After a blocking step with 2% skim milk, a solution of phages (1014 CFU) was added to each tube, followed by incubation at room temperature for 2 h. The unbound clones were washed out four times with PBS(+). Bound phages were eluted with 0.1 M triethylamine (pH 12.3), and the eluent was then immediately neutralized with 1 M Tris-HCl (pH 6.8). E. coli DH12S cells cultured in 2xYT medium were infected with the eluted phages, precipitated by centrifugation, and then resuspended in 2xYT containing 1% glucose and 100 μg of ampicillin/ml, followed by superinfection with helper phages and further cultivation under kanamycin-selective conditions (70 μg/ml) in order to replicate phage clones harboring KU-reactive Abs. The phage clones obtained through this process were used for the next round of panning. The input titers of the phages and the number of washings with PBS(+) were 1.06 × 1013 and 8 for the second panning and 3.46 × 1013 and 16 for the third panning, respectively. After the third round of panning, DH12S cells infected with the selected phages were spread on LB plates containing 1% glucose and 100 μg of ampicillin/ml and incubated at 30°C overnight.
Preparation of various forms of Abs.
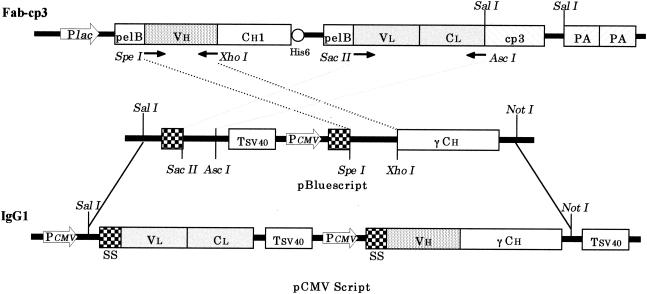
The individual clones of E. coli infected with phages were grown in 2xYT medium containing 0.1% glucose and 100 μg of ampicillin/ml. After the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), the Fab-cp3 molecules were initially accumulated in the periplasm of E. coli and then gradually secreted and/or released into the culture medium (crude Fab-cp3). On average, 1 μg of Fab-cp3 molecules/ml is present in the culture fluid. The Fab-cp3 molecules can be purified with anti-cp3 MAb-conjugated Sepharose beads. After isolation of phage particles, the gene encoding an Fab-cp3 molecule can be easily converted into another gene encoding an Fab-PP (P denotes a single Fc-binding domain of protein A) form of Ab by digestion with SalI followed by self-ligation (Fig. 1) (22). The Fab-PP molecules can be purified with an immunoglobulin G (IgG)-conjugated column. For conversion of an Fab to a human IgG1, the variable heavy-chain region (VH) and the variable and constant light-chain region (VL CL) of an Fab fragment were amplified by PCR (15 cycles of amplification at 94°C for 2 min, 55°C for 2 min, and 72°C for 2 min) with primers designed for each clone (Table 1). After digestion with SpeI and XhoI for the VH region and SacII and AscI for the VL CL region, the PCR products were subcloned into an IgG1 construction vector. The constructed IgG1 cassette was further cloned into the pCMV-Script expression vector (Fig. 1) (Akahori et al., unpublished). Expression of IgG1 molecules was performed by transfection of the IgG1 expression vectors into CHO-K1 cells by using GenePORTER (Gene Therapy Systems), which allows production and/or secretion of a whole human IgG1 type of Ab in culture fluid.
FIG. 1.
Scheme for the conversion of a phage Ab (Fab-cp3) to an human IgG1. The VH and the VL CL regions were amplified by PCR with the proper primers listed in Table 1, followed by subcloning into the proper restriction enzyme sites to construct an IgG1 cassette. The IgG1 cassette was then cloned into expression vector pCMVScript. Plac, lac promoter; pelB, pelB leader sequence; CH 1, the first H-chain constant domain of human IgG1; His6, His tag-encoding part; cp3, truncated cp3; PA, Fc-binding domain of protein A; Tsv40, simian virus 40 terminator; PCMV, cytomegalovirus promoter; γCH, human γCH domain; SS, signal sequence.
TABLE 1.
Oligonucleotide primers used for the conversion of phage antibodies (Fab-cp3) to human IgG1
| Primer | Orientationa | Sequence |
|---|---|---|
| 1-2HVH | F | 5′-TTCCTCCTACTAGTGGCAGCTCCCAGATGGGTCCTGTCCCAGGTGCAGCTGGTGCAGTCTGG-3′ |
| R | 5′-GGTGGAGGCACTCGAGACGGTGACCAGGGTTC-3′ | |
| 1-2HVLCL | F | 5′-CTACTCTGGCTCCGCGGTGCCAGACAGTCTGTGTTGACGCAGCCG-3′ |
| R | 5′-TCGACTGGCGCGCCCTATGAACATTCTGTAGGGGCCACTGTCTTC-3′ | |
| 2-3EVH | F | 5′-TTCCTCCTACTAGTGGCAGCTCCCAGATGGGTCCTGTCCGAGGTGCAGCTGGTGGAGTCTGG-3′ |
| R | 5′-GGTGGAGGCACTCGAGACGGTGACCATTGTCC-3′ | |
| 2-3EVLCL | F | 5′-CTACTCTGGCTCCGCGGTGCCAGAGAAACGACACTCACGCAGTCT-3′ |
| R | 5′-TCGACTGGCGCGCCCTAACACTCTCCCCTGTTGAAGCTCTTTGTG-3′ | |
| 2-11GVH | F | 5′-TTCCTCCTACTAGTGGCAGCTCCCAGATGGGTCCTGTCCGAGGTGCAGCTGGTGGAGTCTGG-3′ |
| R | 5′-GGTGGAGGCACTCGAGACGGTGACCGTGGTCC-3′ | |
| 2-11GVLCL | F | 5′-CTACTCTGGCTCCGCGGTGCCAGAGAAACGACACTCACGCAGTCT-3′ |
| R | 5′-TCGACTGGCGCGCCCTAACACTCTCCCCTGTTGAAGCTCTTTGTG-3′ |
F, forward; R, reverse. SpeI, XhoI, SacII, and AscI sites are underlined.
Enzyme-linked immunosorbent assay (ELISA).
To determine the reactivity to or titer of the Abs for strain KU, immunoplates (96-well; Nalgen Nunc International) were coated with highly purified virions (500 ng/well) suspended in PBS(+) for 1 day at 4°C. After a blocking treatment with 5% bovine serum albumin in PBS(+), the plates were washed with PBS(+). For the reactivity study, the plates were then incubated with 50 ng of purified Fab-cp3 in PBS(+) at 4°C overnight. For the titration study, the plates were then incubated with serial dilutions of purified IgG1-formed Abs ranging from 0.01 to 5,000 ng/well in PBS(+) at 4°C overnight. In both cases, after the plates had been washed with PBS(+), a 1:2,500 dilution of peroxidase-conjugated goat anti-human IgG (H+L chain; MBL) was added to each well. One optical density at 492 nm (OD492) unit was defined as the Ab concentration at which the OD492 reading was 1.0. To identify virus proteins recognized by the isolated Abs, the immunoplates were coated with 500 ng of purified Fab-cp3 at 4°C overnight, followed by blocking with 5% BSA in PBS(+). Then, unpurified VLPs or recombinant VP4 in the culture supernatant were added. After incubation at 4°C overnight and additional washing with PBS(+), 50 μl of 1:4,500 diluted rabbit anti-HRV antiserum (a mixture of 1:1,500 diluted rabbit anti-KU, anti-AK13, and anti-YO antiserum) was added to each well, followed by incubation at 4°C for 1 day. After the plates had been washed with PBS(+), a 1:2,500 dilution of peroxidase-conjugated goat anti-rabbit IgG (H+L chain; MBL) was added to each well. In all cases, the reactivity of the Abs to Ags was assessed after addition of the substrate.
Assay for virus-neutralizing activity.
Screening of crude Fab-cp3 Abs for preliminary selection as to neutralizing activity and determination of the titers of the purified Abs against HRVs or antigenic mutants were determined by the fluorescent focus reduction (FF) method. A total of 25 μl of crude Fab-cp3 or purified Abs in PBS(+) at various concentrations (0.4 to 20 μg/ml) was mixed with an equal volume of virus suspension containing 3.6 × 104 to 14.4 × 104 focus-forming units in Eagle minimum essential medium, followed by reaction at 37°C for 1 h. Aliquots (50 μl) of the mixtures of Abs and viruses were inoculated onto MA104 cells in 96-well culture plates and, after an additional 1 h of incubation at 37°C, 100 μl of fresh Eagle minimum essential medium was added, followed by 16 to 18 h cultivation at 37°C. Fixation in the cold (−80°C), and reaction with the first and second Abs were performed as described previously (49). The neutralization assays were performed in duplicate and at least twice.
Sequence analysis.
The nucleotide sequences of VL and VH regions were determined with an ABI Prism 320 genetic analyzer by using a BigDye terminator cycle sequencing kit (Applied Biosystems). The T7 primer (5′-TGTAATACGACTCACTATAG-3′) and the huCH1J primer (5′-ATTAATAAGAGCTATCCCGG-3′) were used for VH and VL sequencing, respectively.
Nucleotide sequence accession numbers.
The nucleotide sequence data for the HRV neutralizing Abs reported in the present study have been submitted to the DDBJ database and were assigned the following accession numbers: AB114449 (for 1-2H H chain), AB114450 (2-1D H chain), AB114451 (2-2D H chain), AB114452 (2-3E H chain), AB114453 (2-4F H chain), AB114455 (2-7G H chain), AB114456 (2-9B H chain), AB114457 (2-9D H chain), AB114458 (2-11G H chain), AB114459 (2-12B H chain), AB114460 (3-1G H chain), AB114461 (4-3-C H chain), AB114461 (1-2H L chain), AB114462 (2-1D L chain), AB114463 (2-2D L chain), AB114464 (2-3E L chain), AB114465 (2-4F L chain), AB114466 (2-7G L chain), AB114467 (2-9B L chain), AB114468 (2-9D L chain), AB114469 (2-11G L chain), AB114470 (2-12B L chain), AB114471 (3-1G L chain), and AB114472 (4-3C L chain).
RESULTS
Isolation of Fab forms of Abs with neutralizing activities toward HRVs.
After three rounds of panning, the recovered phages were used to infect E. coli, which was spread on plates containing ampicillin without infection with helper phages. We picked up 321 colonies and cultured them in 96-well plates. The supernatants, crude Fab-cp3, were directly subjected to analysis of neutralizing activities by means of the FF assay. Among the 321 clones analyzed, 24 appeared to exhibit neutralizing activities toward rotavirus strain KU.
Amino acid sequences of Fab H and L chains.
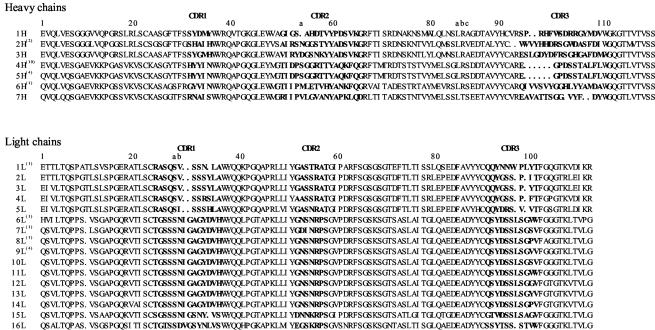
In order to confirm the successful selection of phages with Fab-cp3 specific to strain KU and also to classify the 24 clones, we determined the sequences of variable regions of both their H and L chains. Some redundants were included in the 24 clones (Fig. 2), indicating the specific and successful selection and enrichment of KU-reactive phage Abs. The amino acid sequences of the H chains could be divided into 7 clones and the L chains could be divided into 16 clones (Fig. 2). As a result, 16 of the 24 clones were found to be independent.
FIG. 2.
Amino acid sequences of variable regions of the H and L chains of Abs that appeared to exhibit neutralizing activities toward strain KU. The sequences of CDRs are shown in boldface. Numbers in parentheses indicate number of redundant clones found among the 24 Abs analyzed, with 16 Abs being independent. The combinations of H and L chains in the independent Abs are indicated in Table 2. The numbering of amino acid positions is according to the method of Kabat et al. (23).
Neutralizing activity of the purified Fab fragments.
Since the use of crude Fab-cp3 in the FF assay gave ambiguous results due to contaminants in the E. coli culture supernatant, the Fab-cp3 coding phagemid DNAs of the 16 independent clones (2-7G, 3-1G, 2-9D, 2-4F, 2-9B, 1-2H, 4-3C, 2-12B, 2-2D, 2-3E, 2-1D, 2-11G, 1-8A, 2-2G, 2-5G, and 1-4D) were reconstructed to produce Fab-PP fragments (Fig. 1) to facilitate purification. After purification by means of affinity selection, their neutralizing activities toward strain KU were assessed by FF assay. Except for 1-4D, 1-8A, 2-2G, and 2-5G, we could detect the neutralizing activity against strain KU (Table 2). Although the Ag-binding site of each Ab is formed by amino acid residues in the six complementarity-determining regions (CDRs) of the H and L chains, the contribution of CDR3 of the H chain to Ag specificity is greatest among them in a usual case, especially that to protein Ags (21). Judging from this, the two H chains, 4H and 5H, are essentially the same (Fig. 2), and the 12 clones exhibiting the neutralizing activities toward KU could be classified into three groups (Table 2). We finally selected 1-2H, 2-3E, and 2-11G as representative clones of the three group, and further investigations were carried out on these three.
TABLE 2.
Neutralization activity of Fab-PP forms of Abs to strain KU
| Clone (n)a | H chain | L chain | Antibody concn (μg/ml)b |
|---|---|---|---|
| 2-7G (1) | 4H | 6L | 1.6 |
| 3-1G (1) | 7L | 8.0 | |
| 2-9D (4) | 9L | 1.6 | |
| 2-4F | 10L | 1.6 | |
| 2-9B | 12L | 1.6 | |
| 1-2H | 5H | 13L | 1.6 |
| 4-3C (1) | 8L | 1.6 | |
| 2-12B | 11L | 1.6 | |
| 2-2D | 14L | 1.6 | |
| 2-3E (1) | 2H | 1L | 1.6 |
| 2-1D | 15L | 1.6 | |
| 2-11G | 1H | 2L | 1.6 |
| 1-8A | 3H | 5L | >40.0 |
| 2-2G | 6H | 4L | >40.0 |
| 2-5G | 3L | >40.0 | |
| 1-4D | 7H | 16L | >40.0 |
n = number of redundant clones found among the 24 clones analyzed.
Concentration of antibodies required for a 50% reduction of the fluorescent focus in the FF assay.
Virus proteins recognized by Abs.
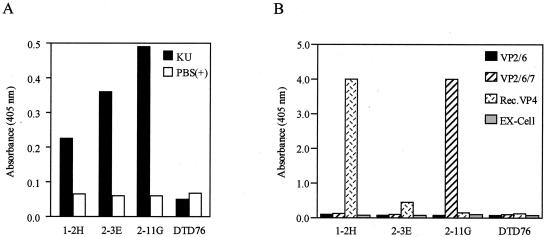
The reactivity of the three representative Fab fragments was examined by means of ELISA, their specific reactivity with strain KU being shown (Fig. 3A). Although an isolated VP4 molecule could expose its neutralizing epitope on the surface, VP7 exposed its epitope only when the molecule was embedded in inner proteins VP2 and VP6 (49). Therefore, we prepared two kinds of VLP, VLP-V2/6 and VLP-2/6/7, and recombinant VP4. As shown in Fig. 3B, the targeted virus protein of 1-2H and 2-3E was found to be VP4, and that of 2-11G was found to be VP7. A Western blot analysis to confirm the ELISA results was unsuccessful (data not shown).
FIG. 3.
ELISA of the three Abs (purified Fab-cp3 forms). (A) Reactivities of the three Abs to strain KU. Purified virions suspended in PBS(+) were used as Ags. (B) Virus proteins directed by the three Abs. The culture fluids of Tn5 cells containing VLP-VP2/6, VLP-VP2/6/7, or recombinant VP4 were used as Ags. EX-Cell was the culture medium of Tn5. DTD76 is anti-diphtheria toxin Ab isolated from AIMS4, which was used as a negative control. VP2/6, VLPs constructed with VP2 and VP6; VP2/6/7. VLPs constructed with VP2, VP6, and VP7; Rec.VP4, recombinant VP4.
Neutralizing activities toward various HRV strains.
After conversion of the Fab form into an IgG1 Ab, we analyzed the neutralizing activities of the three Abs against 13 HRVs by means of the FF assay (Table 3). The 1-2H Ab neutralized 7 strains—S2, L26, KU, Wa, YO, Hosokawa, and WI61—all of which exhibited either P[4] or P[8] type specificity on VP4. The 2-3E Ab showed neutralizing activities toward 8 strains—M37, 1076, McN13, KU, Wa, YO, Hosokawa, and WI61—all of which are classified as either a P[6] or P[8] virus strain. The 2-11G Ab reacted with four strains—M37, KU, Wa, and K8—all of which are G1 strains. The conclusion that the 2-11G Ab reacts with VP7 with the G1 serotype was further supported by using two VP7 single gene reassortants, UK/Wa and UK/DS1. The former has VP7 of G1 and VP4 of P[5] specificity, and the latter has VP7 of G2 and VP4 of P[5] specificity. The 2-11G Ab neutralized only the former strain. Of the 13 HRVs examined, 11 were neutralized by any of the three Abs. The concentration of Abs required for 50% neutralization ranged from 0.8 to 20 μg/ml, which corresponds to 5.3 to 133 nM.
TABLE 3.
Neutralization of HRVs and reassortants by purified human IgG1s
| Strain | P genotype | G serotype | Antibody concn (μg/ml)a
|
||
|---|---|---|---|---|---|
| 1-2H | 2-3E | 2-11G | |||
| S2 | P[4] | G2 | 4-20 | >20 | >20 |
| L26 | P[4] | G12 | 0.8 | >20 | >20 |
| M37 | P[6] | G1 | >20 | 4 | 0.8 |
| 1076 | P[6] | G2 | >20 | 4 | >20 |
| McN13 | P[6] | G3 | >20 | 4 | >20 |
| KU | P[8] | G1 | 8 | 1.6 | 0.4 |
| Wa | P[8] | G1 | 4 | 4 | 4-20 |
| YO | P[8] | G3 | 8 | 1.6 | >8 |
| Hosokawa | P[8] | G4 | 4 | 0.8 | >20 |
| WI61 | P[8] | G9 | 4-20 | 0.8-4 | >20 |
| AU-1 | P[9] | G3 | >20 | >20 | >20 |
| K8 | P[9] | G1 | >20 | >20 | 4 |
| 69M | P[10] | G8 | >20 | >20 | >20 |
| UK/Wa | P[5] | G1 | >20 | >20 | 4 |
| UK/DS-1 | P[5] | G2 | >20 | >20 | >20 |
Antibody concentration that reduced the fluorescent focus count by >50% in the neutralization test (FF assay).
Neutralizing activities toward antigenic mutants.
We also examined the neutralizing activities of the three representative Abs isolated in the present study with the mouse MAb-resistant mutants prepared in our previous studies (45-47). All of the three Abs of the Fab-cp3 form turned out to neutralize all of the mutants examined (Table 4). The concentration of the each Ab required for 50% reduction of the fluorescent focus in the FF assay was <1.6 μg/ml.
TABLE 4.
Neutralization activity of purified antibodies (cp3 form) to antigenic variants
| Strain | Mutant protein | No. of virus-infected cells with:a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-2H at:
|
2-3E at:
|
2-11G at:
|
DTD76 at:
|
||||||||||
| 1.6 μg/ml | 8 μg/ml | 40 μg/ml | 1.6 μg/ml | 8 μg/ml | 40 μg/ml | 1.6 μg/ml | 8 μg/ml | 40 μg/ml | 1.6 μg/ml | 8 μg/ml | 40 μg/ml | ||
| V-1E6 | VP4 | 63 | 69 | 60 | 4 | 2 | 1 | 14 | 2 | 14 | 296 | 378 | 358 |
| V-1F2 | VP4 | 64 | 76 | 43 | 45 | 108 | 21 | 10 | 5 | 1 | 350 | 462 | 368 |
| V-1S3 | VP4 | 31 | 32 | 19 | 127 | 91 | 18 | 26 | 9 | 4 | 312 | 302 | 304 |
| V-2C2 | VP4 | 87 | 46 | 56 | 9 | 3 | 4 | 16 | 0 | 2 | 296 | 342 | 344 |
| V-4D7 | VP4 | 68 | 13 | 12 | 6 | 1 | 0 | 35 | 3 | 4 | 396 | 222 | 354 |
| V-6B11 | VP4 | 21 | 17 | 11 | 8 | 63 | 4 | 3 | 1 | 0 | 212 | 324 | 240 |
| V-3C7 | VP7 | 121 | 123 | 81 | 14 | 17 | 11 | 19 | 3 | 2 | 482 | 532 | 554 |
| V-4C2 | VP7 | 96 | 87 | 120 | 20 | 9 | 5 | 23 | 2 | 3 | 596 | 660 | 656 |
| V-5H1 | VP7 | 146 | 156 | 90 | 13 | 4 | 4 | 46 | 1 | 1 | 372 | 395 | 355 |
| V-6A11 | VP7 | 57 | 84 | 79 | 57 | 23 | 27 | 24 | 8 | 6 | 425 | 554 | 564 |
| V-KU4 | VP7 | 47 | 22 | 14 | 3 | 4 | 0 | 6 | 0 | 3 | 206 | 186 | 206 |
| KU | - | 26 | 15 | 13 | 3 | 3 | 3 | 52 | 3 | 5 | 282 | 300 | 305 |
That is, the numbers of virus-infected cells detected by the FF method (see Materials and Methods). Virus-positive cells in a one-ninth area of one well of a 96-well tissue culture plate were counted. Results are presented as the means for two independent experiments performed in duplicate.
Titers of the Abs to strain KU.
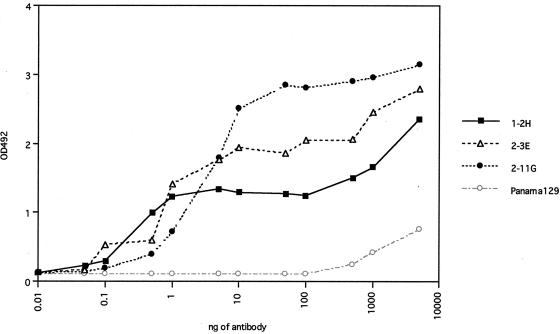
We determined the titers of three Abs (IgG1 form) to strain KU by ELISA (Fig. 4). All three Abs showed dose-dependent binding. There was no correlation between the absorbance density and the neutralizing activity of each Ab. The values for one OD492 unit for the 1-2H, 2-3E, and 2-11G Abs were 0.70, 0.75, and 1.8 μg/ml, respectively.
FIG. 4.
Abs titration curves on ELISA. The reactivities between purified IgG1-formed Abs and purified virions of strain KU were assessed. The assay was performed in duplicate, and the mean data are plotted. Panama129 is the IgG1-formed anti-influenza virus Ab isolated from the AIMS4 library and converted to the IgG1 form as described in Materials and Methods.
DISCUSSION
Ab libraries constructed with a means of a phage-display system are convenient for the rapid isolation of Abs specific for various Ags (2, 27, 31, 44, 52). Recently, several recombinant human Fab fragments exhibiting neutralizing activity toward viruses such as human immunodeficiency virus types 1 and 2, Ebola virus, measles virus, Puumala virus, and respiratory syncytial virus were prepared by means of a phage display system (1, 3, 6, 7, 28, 37, 50). Since the amount of surface proteins with neutralization epitopes on viruses is small and the immunogenicity of inner proteins is quite high, it is generally much more difficult to obtain MAbs with neutralizing capacity than nonneutralizing MAbs. This requires some modifications of the panning and/or screening processes. For example, blockade of a common, nonneutralizing epitope with a representative Fab has been used in panning assays to isolate respiratory syncytial virus-neutralizing human MAbs (50). In the present study, we used highly purified virion for panning and directly screened numerous clones by means of rapid microneutralization FF tests, which have been found to be very efficient for screening neutralizing MAbs with common mouse hybridoma technology (49).
We used HRV strain KU as the Ag for panning, since strain KU exhibits representative G1 and P[8] specificity, which is the most prevalent HRV serotype worldwide (24), and since the neutralization epitopes on VP4 and VP7 of the strain have well been characterized by using mouse MAbs (36, 45, 47, 49). The three human Abs characterized (1-2H, 2-3E, and 2-11G) are specific to P[8], P[4]; P[8], P[6]; and G1 HRVs, respectively. In particular, the former two are broadly reactive with a wide spectrum of HRVs. Since a total of 15 G serotypes have been defined for rotavirus and at least 10 G serotypes have been isolated from humans, it is desirable to prepare such broadly reactive heterotypic Abs for therapeutic purposes. The reactivity of the three Abs covered most HRV strains, and they indeed neutralized 11 of the 13 HRVs examined. In previous studies, an Ab response to cross-reactive neutralization epitopes (YO-2C2 epitopes) was observed much more frequently in schoolchildren and adults than in infants (13, 48). Since the library was constructed from the tissues of adults, who would have been repeatedly infected with HRVs with distinct serotypes and would have immunological memory for cross-reactive neutralization epitopes, cross-reactive Abs may have been readily selected in the present study. In other words, a cross-reactive immune response should be common in the immune system in humans, particularly in adults, infected with rotaviruses.
A number of murine MAbs have been prepared for rotaviruses by means of conventional hybridoma technology. Although many of them were directed to the inner protein VP6, some were directed to VP7 and VP4. However, the number of neutralizing MAbs with hetetrotypic specificity was limited. In particular, ones directed to HRVs were few; i.e., there was only one to VP7 and seven to VP4 (26, 36, 45-47, 49). They have been useful for the analysis of heterotypic neutralization epitopes on VP7 and VP4. By analyzing the mutants resistant to each of the neutralizing mouse MAbs directed to VP4 or VP7 of HRVs, critical amino acids in the neutralization epitopes have been identified (29, 45, 46). It has been shown that the cross-reactive neutralization epitopes on VP4 are the 305th, 385th, 392nd, 428th, 433rd, and 439th amino acid residues (26, 46, 47) and that the G1-specific epitopes on VP7 are the 94th and 96th residues (45). Furthermore, by means of neutralization tests on various combinations of MAb-resistant mutants and MAbs, operational maps of the neutralization epitopes have been constructed (26, 36). We examined the reactivities of the three human Abs isolated in the present study with the MAb-resistant mutants prepared in our previous studies (45-47): mutants resistant to anti-VP4 MAbs (YO-2C2, KU-6B11, YO-1S3, ST-1F2, KU-4D7, and YO-1E6) and mutants resistant to anti-VP7 MAbs (KU-2, KU-4, KU-3C7, KU5H1, KU-6A11, and YO-4C2). These three Abs turned out to neutralize all of the mutants examined (Table 4). This finding strongly suggests that the human Abs isolated in the present study recognize neutralization epitopes distinct from those recognized by mouse MAbs obtained to date. These results could have been predicted, since the specificity showed by the human Abs, such as 1-2H Ab to P[4] and P[8] and 2-3E Ab to P[6] and P[8], had not been shown by mouse MAbs isolated to date. These results imply that the cross-reactive neutralization epitopes recognized by humans, in particular adults, infected with rotaviruses and by mice immunized with rotaviruses are quite distinct. We are now attempting to prepare mutants resistant to each human Ab for analysis of the neutralization epitopes recognized by them.
The mechanism of protective immunity against rotavirus infection has not been well elucidated. Both humoral and cellular immunity are likely to be involved in the protection from rotavirus infection (11, 24, 40). The mucosal Ab response has been believed to be effective for such protection. Furthermore, passive immunity has also been found to be effective (40). Maternal transfer of anti-rotavirus immunoglobulins protects babies from rotavirus infection. Oral administration of bovine immunoglobulins, mouse MAbs, and human immunoglobulins has been found to be effective for protecting suckling mice from rotavirus infection (9, 30, 41). In addition, therapeutic reports on the passive immunity of children with rotavirus diarrhea have also been published. Guarino et al. reported that the oral administration of human serum immunoglobulins to children with rotavirus-induced diarrhea resulted in a faster recovery from the disease (15), even though the children were immunocompromised due to human immunodeficiency virus infection (16). In contrast, there have also been reports showing no clinical effect of oral administration of bovine immunoglobulins for prophylaxis or therapy for HRV infection (4, 10). We are now examining a mouse model to determine whether the human MAbs prepared in the present study are effective and practically relevant to immunotherapy and/or prophylaxis for diseases caused by rotavirus.
Acknowledgments
We thank Yukari Hirono, Mai Kakita, and Kazuhiro Suzuki for technical suggestions.
This study was supported in part by a grant for Research on Pharmaceutical and Medical Safety from the Ministry of Health, Labor, and Welfare of Japan to Y.K. and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan to K.T.
REFERENCES
- 1.Barbas, C. F., III, E. Bjorling, F. Chiodi, N. Dunlop, D. Cababa, T. M. Jones, S. L. Zebedee, M. A. A. Persson, P. L. Nara, E. Norrby, and D. R. Burton. 1992. Recombinant human Fab fragments neutralize human type 1 immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. USA 89:9339-9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbas, C. F., III, A. S. Kang, R. A. Lerner, and S. J. Benkovic. 1991. Assembly of combinational antibody libraries on phage surfaces: the gene III site. Proc. Natl. Acad. Sci. USA 88:7978-7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Björling, E., E. von Garrelts, A. Morner, M. Ehnlund, and M. A. A. Persson. 1999. Human neutralizing immunodeficiency virus type 2-specific Fab molecules generated by phage display. J. Gen. Virol. 80:1987-1993. [DOI] [PubMed] [Google Scholar]
- 4.Brunser, O., J. Espinoza, G. Figueroa, M. Araya, E. Spencer, H. Hilpert, H. Link-Amster, and H. Brussow. 1992. Field trial of an infant formula containing anti-rotavirus and anti-Escherichia coli milk antibodies from hyperimmunized cows. J. Pediatr. Gastroenterol. Nutr. 15:63-72. [DOI] [PubMed] [Google Scholar]
- 5.Cale, C. M., and N. J. Klein. 2002. The link between rotavirus vaccination and intussusception: implications for vaccine strategies. Gut 50:11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Carvalho Nicacio, C., Å. Lundkvist, K. B. Sjölander, A. Plyusnin, E. M. Salonen, and E. Björling. 2000. A neutralizing recombinant human antibody Fab fragment against Puumala hantavirus. J. Med. Virol. 60:446-454. [DOI] [PubMed] [Google Scholar]
- 7.de Carvalho Nicacio, C., R. A. Williamson, P. W. H. I. Parren, Å. Lundkvist, D. R. Burton, and E. Björling. 2002. Neutralizing human Fab fragments against measles virus recovered by phage display. J. Virol. 76:251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desselberger, U., M. Iturriza-Gómara, and J. J. Gray. 2000. Rotavorus epidemiology and surveillance. Novartis Found. Symp. 238:125-152. [DOI] [PubMed] [Google Scholar]
- 9.Ebina, T., A. Sato, K. Umezu, N. Ishida, S. Ohyama, A. Ohizumi, K. Aikawa, S. Katagiri, N. Katsushima, and A. Imai. 1983. Prevention of rotavirus infection by cow colostrum antibody against human rotaviruses. Lancet ii:1029-1030. [DOI] [PubMed] [Google Scholar]
- 10.Ebina, T., A. Sato, K. Umezu, N. Ishida, S. Ohyama, A. Oizumi, K. Aikawa, S. Katagiri, N. Katsushima, and A. Imai. 1985. Prevention of rotavirus infection by oral administration of cow colostrum containing anti-human rotavirus antibody. Med. Microbiol. Immunol. 174:177-185. [DOI] [PubMed] [Google Scholar]
- 11.Estes, M. K. 2001. Rotaviruses and their replication, p. 1747-1786. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 12.Gilger, M. A., D. O. Matson, M. E. Conner, H. M. Rosenblatt, M. J. Finegold, and M. K. Estes. 1992. Extraintestinal rotavirus infections in children with immunodeficiency. J. Pediatr. 120:912-917. [DOI] [PubMed] [Google Scholar]
- 13.Green, K. Y., K. Taniguchi, E. R. Mackow, and A. Z. Kapikian. 1990. Homotypic and heterotypic epitope-specific antibody responses in adult and infant rotavirus vaccines: implications for vaccine development. J. Infect. Dis. 161:667-679. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg, H. B., J. Valdesuso, K. van Wyke, K. Midthun, M. Walsh, V. McAuliffe, R. G. Wyatt, A. R. Kalica, J. Flores, and Y. Hoshino. 1983. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J. Virol. 47:267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guarino, A., R. B. Canani, S. Russo, F. Albano, M. B. Canani, F. M. Ruggeri, G. Donelli, and A. Rubino. 1994. Oral immunoglobulins for treatment of acute rotaviral gastroenteritis. Pediatrics 93:12-16. [PubMed] [Google Scholar]
- 16.Guarino, A., S. Russo, A. Castaldo, M. I. Spagnuolo, L. Tarallo, and A. Rubino. 1996. Passive immunotherapy for rotavirus-induced diarrhoea in children with HIV infection. AIDS 10:1176-1178. [PubMed] [Google Scholar]
- 17.Hammarström, L. 1999. Passive immunity against rotavirus in infants. Acta Pediatr. Suppl. 430:127-132. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino, Y., L. J. Saif, M. M. Sereno, R. M. Chanock, and A. Z. Kapikian. 1988. Infection immunity of piglets to either VP3 or VP7 outer capsid protein confers resistance to challenge with a virulent rotavirus bearing the corresponding antigen. J. Virol. 62:744-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino, Y., M. M. Sereno, K. Midthun, J. Flores, A. Z. Kapikian, and R. M. Chanock. 1985. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc. Natl. Acad. Sci. USA 82:8701-8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iba, Y., W. Ito, and Y. Kurosawa. 1997. Expression vectors for the introduction of highly diverged sequences into the six complementarity-determining regions of an antibody. Gene 194:35-46. [DOI] [PubMed] [Google Scholar]
- 21.Ichihara, Y., H. Matsuoka, and Y. Kurosawa. 1988. Organization of human immunoglobulin heavy chain diversity gene loci. EMBO J. 7:4141-4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito, W., and Y. Kurosawa. 1993. Development of an artificial antibody system with multiple valency using an Fv fragment fused to a fragment of protein A. J. Biol. Chem. 268:20668-20675. [PubMed] [Google Scholar]
- 23.Kabat, E. A., T. T. Wu, H. M. Perry, K. S. Gottesman, and C. Foeller. 1991. Sequences of proteins of immunological interest. National Institutes of Health, Bethesda, Md.
- 24.Kapikian, A. Z., Y. Hoshino, and R. M. Chanock. 2001. Rotaviruses, p. 1787-1833. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 25.Kapikian, A. Z., Y. Hoshino, R. M. Chanock, and I. Pérez-Schael. 1996. Efficacy of a quadrivalent rhesus rotavirus-based human rotavirus vaccine aimed at preventing severe rotavirus diarrhea in infants and young children. J. Infect. Dis. 174(Suppl. 1):S65-S72. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi, N., K. Taniguchi, and S. Urasawa. 1990. Identification of operationally overlapping and independent cross-reactive neutralization regions on human rotavirus VP4. J. Gen. Virol. 71:2615-2623. [DOI] [PubMed] [Google Scholar]
- 27.Marks, J. D., H. R. Hoogenboom, T. P. Bonnert, J. McCafferty, A. D. Griffiths, and G. Winter. 1991. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J. Mol. Biol. 222:581-597. [DOI] [PubMed] [Google Scholar]
- 28.Maruyama, T., L. L. Rodroguez, P. B. Jahring, A. Sanchez, A. S. Khan, S. T. Nichol, C. J. Peters, P. W. H. I. Parren, and D. R. Burton. 1999. Ebola virus can be effectively neutralized by antibody produced in natural human infection. J. Virol. 73:6024-6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsui, S. M., E. R. Mackow, and H. B. Greenberg. 1989. Molecular determinant of rotavirus neutralization and protection. Adv. Virus Res. 36:181-214. [DOI] [PubMed] [Google Scholar]
- 30.Matsui, S. M., P. A. Offit, P. T. Vo, E. R. Mackow, D. A. Benfield, R. D. Shaw, L. Padilla-Noriega, and H. B. Greenberg. 1989. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to the heterotypic neutralization domain of VP7 and the VP8 fragment of VP4. J. Clin. Microbiol. 27:780-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCafferty, J., A. D. Griffiths, G. Winter, and D. J. Chiswell. 1990. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348:552-554. [DOI] [PubMed] [Google Scholar]
- 32.Midthun, K., Y. Hoshino, A. Z. Kapikian, and R. M. Chanock. 1986. Single gene substitution rotavirus reassortants containing the major neutralization protein (VP7) of human rotavirus serotype 4. J. Clin. Microbiol. 24:822-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, M. A., and L. McCann. 2000. Policy analysis of the use of hepatitis B, Haemophilus influenzae type B-, Streptococcus pneumoniae-conjugate, and rotavirus vaccines in national immunization schedules. Health Econ. 9:19-35. [DOI] [PubMed] [Google Scholar]
- 34.Mori, I., K. Matsumoto, K. Sugimoto, M. Kimura, N. Daimon, T. Yokochi, and Y. Kimura. 2002. Prolonged shedding of rotavirus in a geriatric inpatient. J. Med. Virol. 67:613-615. [DOI] [PubMed] [Google Scholar]
- 35.Morino, K., H. Katsumi, Y. Akahori, Y. Iba, M. Shinohara, Y. Ukai, Y. Kohara, and Y. Kurosawa. 2001. Antibody fusions with fluorescent proteins: a versatile reagent for profiling protein expression. J. Immunol. Methods 257:175-184. [DOI] [PubMed] [Google Scholar]
- 36.Morita, Y., K. Taniguchi, T. Urasawa, and S. Urasawa. 1988. Analysis of serotype-specific neutralization epitopes on VP7 of human rotavirus by the use of neutralizing monoclonal antibodies and antigenic variants. J. Gen. Virol. 69:451-458. [DOI] [PubMed] [Google Scholar]
- 37.Moulard, M., S. K. Phogat, Y. Shu, A. F. Labrijn, X. Xiao, J. M. Binley, M. Zhang, I. A. Sidorov, C. C. Broder, J. Robinson, P. W. H. I. Parren, D. R. Burton, and D. S. Dimitrov. 2002. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc. Natl. Acad. Sci. USA 99:6913-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy, T. V., P. M. Gargiullo, M. S. Massoudi, D. B. Nelson, A. O. Jumaan, C. A. Okoro, L. R. Zanardi, S. Setia, E. Fair, C. W. LeBaron, M. Wharton, and J. R. Livingood. 2001. For the rotavirus intussusception investigation team: intussusception among infants given an oral rotavirus vaccine. N. Engl. J. Med. 344:564-572. [DOI] [PubMed] [Google Scholar]
- 39.Nakajima, H., T. Nakagomi, T. Kamisawa, N. Sakaki, K. Muramoto, T. Mikami, H. Nara, and O. Nakagomi. 2001. Winter seasonality and rotavirus diarrhoea in adults. Lancet 357:1950. [DOI] [PubMed] [Google Scholar]
- 40.Offit, P. A. 1996. Host factors associated with protection against rotavirus disease: the skies are clearing. J. Infect. Dis. 174(Suppl. 1):S59-S64. [DOI] [PubMed] [Google Scholar]
- 41.Offit, P. A., R. D. Shaw, and H. B. Greenberg. 1986. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins VP3 and VP7. J. Virol. 58:700-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pedley, S., F. Hundley, I. Chrystie, M. A. McCrae, and U. Desselberger. 1984. The genomes of rotaviruses isolated from chronically infected immunodeficient children. J. Gen. Virol. 65:1141-1150. [DOI] [PubMed] [Google Scholar]
- 43.Peigue-Lafeuille, H., C. Henquell, M. Chambon, N. Gazuy, C. De Champs, and R. Cluzel. 1991. Nosocomial rotavirus infections in adult renal transplant recipients. J. Hosp. Infect. 18:67-70. [DOI] [PubMed] [Google Scholar]
- 44.Sheets, M. D., P. Amersdorfer, R. Finnern, P. Sargent, E. Lindquist, R. Schier, G. Hemingsen, C. Wong, J. C. Gerhart, and J. D. Marks. 1998. Efficient construction of a large nonimmune phage antibody library: the production of high-affinity human single-chain antibodies to protein antigens. Proc. Natl. Acad. Sci. USA 95:6157-6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taniguchi, K., Y. Hoshino, K. Nishikawa, K. Y. Green, W. L. Maloy, Y. Morita, S. Urasawa, A. Z. Kapikian, R. M. Chanock, and M. Gorziglia. 1988. Cross-reactive and serotype-specific neutralization epitopes on VP7 of human rotavirus: nucleotide sequence analysis of antigenic mutants selected with monoclonal antibodies. J. Virol. 62:1870-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taniguchi, K., W. L. Maloy, K. Nishikawa, K. Y. Green, Y. Hoshino, S. Urasawa, A. Z. Kapikian, R. M. Chanock, and M. Gorziglia. 1988. Identification of cross-reactive and serotype 2-specific neutralization epitopes on VP3 of human rotavirus. J. Virol. 62:2421-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taniguchi, K., Y. Morita, T. Urasawa, and S. Urasawa. 1987. Cross-reactive neutralization epitopes on VP3 of human rotavirus: analysis with monoclonal antibodies and antigenic variants. J. Virol. 61:1726-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taniguchi, K., T. Urasawa, N. Kobayashi, M. U. Ahmed, N. Adachi, S. Chiba, and S. Urasawa. 1991. Antibody response to serotype-specific and cross-reactive neutralization epitopes on VP4 and VP7 after rotavirus infection or vaccination. J. Clin. Microbiol. 29:483-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taniguchi, K., S. Urasawa, and T. Urasawa. 1985. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. J. Gen. Virol. 66:1045-1053. [DOI] [PubMed] [Google Scholar]
- 50.Tsui, P., M. A. Tornetta, R. S. Ames, B. C. Bankosky, S. Griego, C. Silverman, T. Porter, G. Moore, and R. W. Sweet. 1996. Isolation of a neutralizing human RSV antibody from a dominant, non-neutralizing immune repertoire by epitope-blocked panning. J. Immunol. 157:772-780. [PubMed] [Google Scholar]
- 51.Ward, R. L., D. R. Knowlton, G. M. Schiff, Y. Hoshino, and H. B. Greenberg. 1988. Relative concentrations of serum neutralizing antibody to VP3 and VP7 proteins in adults infected with a human rotavirus. J. Virol. 62:1543-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winter, G., A. D. Griffiths, R. E. Hawkins, and H. R. Hoogenboom. 1994. Making antibodies by phage display technology. Annu. Rev. Immunol. 12:433-455. [DOI] [PubMed] [Google Scholar]