Abstract
Evidence is accumulating that CD4+ T-helper (Th) responses play a critical role in facilitating effector responses which are capable of controlling and even preventing human immunodeficiency virus (HIV) infection. The present work was undertaken to determine whether immunization with multiple antigens influenced individual Th responses and increased protection relative to a single antigen. Rhesus macaques were primed with DNA and boosted (immune-stimulating complex-formulated protein) with a combination of regulatory and structural antigens (Tat-Env-Gag) or with Tat alone. Immunization with combined antigens reduced the magnitude of the responses to Tat compared to the single-antigen immunization. Interestingly, the Th immune responses to the individual antigens were noticeably different. To determine whether the qualitative differences in vaccine-induced Th responses correlated with vaccine efficacy, animals were challenged intravenously with simian/human immunodeficiency virus (strain SHIV89.6p) 2 months following the final immunization. Animals that developed combined Th1- and Th2-like responses to Gag and Th2 dominant Env-specific responses were protected from disease progression. Interestingly, one animal that was completely protected from infection had the strongest IFN-γ and interleukin-2 (IL-2) responses prior to challenge, in addition to very strong IL-4 responses to Gag and Env. In contrast, animals with only a marked vaccine-induced Tat-specific Th2 response (no IFN-γ) were not protected from infection or disease. These data support the rationale that effective HIV vaccine-induced immunity requires a combination of potent Th1- and Th2-like responses best directed to multiple antigens.
Despite almost 2 decades of research, the immune responses required for robust human immunodeficiency virus (HIV) vaccine-induced immunity are only just beginning to be revealed (44). In early studies with chimpanzees, protection from infection (10) and/or reduction of the virus load (13) correlated with the titer of neutralizing antibodies. Protection by neutralizing antibodies was limited to closely related HIV type 1 (HIV-1) strains (25, 54). This relatively narrow protection could in part be overcome by passive administration of more broadly neutralizing monoclonal antibodies, as demonstrated in both chimpanzees (21, 47) and rhesus monkeys (6, 38, 39). Our early HIV vaccine studies, which revealed the inverse correlation between the neutralizing antibody titer and the virus load, pointed to the role of CD4+ T-helper (Th) and in particular antigen-specific interleukin-2 (IL-2) responses in protection (27, 62). Evidence of the importance of CD4+ T-cell responses in AIDS vaccine-induced immunity has been steadily accumulating (27). The first experiments in rhesus monkey studies were performed with the use of single-antigen Env-based subunit protein vaccines (45), and protection from simian/human immunodeficiency virus (SHIV; strain SHIV89.6p) challenge was subsequently shown (43). Similarly, comparison of three different Env vaccine candidates revealed a correlation with a combination of potent Th1, as well as Th2, responses and vaccine efficacy (67). A more complex HIV subunit vaccine candidate formulated in immune stimulating complexes (ISCOM) was more effective in generating both potent Th1 and Th2 responses and protection from intravenous (i.v.) challenge (29). The importance of these observations is now further underscored by the specific demonstration that the spectrum of cytokines involved in CD4+ Th responses determines the quality of memory CD8+ cytotoxic T-lymphocyte (CTL) responses (12, 33, 34, 57, 61). Furthermore, in the absence of protection from infection by neutralizing antibodies, it has been demonstrated that the CD8+ CTL response to a specific simian immunodeficiency virus (SIV) or HIV epitope inversely correlates with the plasma virus load in the SHIV macaque model of AIDS (4, 7, 9, 56). Indeed, it is likely that not only strong Th1-like gamma interferon (IFN-γ) and IL-2 antigen-specific responses are required to drive qualitative CD8+ CTL effector responses capable of removing infected cells, but also potent Th2 responses are required to synergistically potentiate B-cell responses (28). Our present data suggest that an effective HIV-1 vaccine should be formulated to induce a combination of potent Th1 and Th2 responses (27) capable of driving both potent antibody and CTL effector responses.
The majority of HIV vaccine strategies have focused on the structural antigens of HIV-1, in particular Env. With Env-based vaccines, protection from infection has been achieved but is limited to homologous virus challenge (10, 13, 24, 32, 45). Vaccine-induced protection from heterologous virus challenge has proven to be much more difficult to achieve (43, 60). More recently, HIV-1 vaccine strategies targeting regulatory antigens expressed early in viral replication, such as HIV-1 Tat, have been evaluated, with mixed results (26). Vaccination with biologically active Tat or DNA encoding full-length Tat has been demonstrated to protect cynomolgus monkeys from AIDS (15, 16). Similar results were obtained with Tat toxoid (50) and Tat-Rev (48) in rhesus monkeys. However, these results are debatable as studies in other settings have not shown protection after Tat vaccination (1, 59). Rapid escape from Tat has been observed early in infection (2), raising concerns about its use as a single-antigen-based HIV vaccine.
Previously, we suggested that future HIV vaccine strategies involving multiple antigens including both structural and regulatory antigens should be undertaken to increase the breadth of protection and limit vaccine escape (44). Indeed, evidence that this approach is more effective has recently been demonstrated in that protection from heterologous SHIV challenge was only achieved in animals immunized with a combined multiantigen subunit protein vaccine containing the structural protein Env and the regulatory proteins Tat and Nef (40, 68) but not in animals immunized with the antigens separately. Other studies demonstrated improved protection from CD4+ T-cell loss and AIDS in animals immunized with the combination Env-Gag-Pol, while other animals immunized with only Gag-Pol failed to control the virus following a challenge (3).
To further extend our earlier studies with Env and Gag, we have included the regulatory protein Tat and specifically addressed the question of the quality of Th responses raised to a complex multiantigen vaccine and their potential role in HIV vaccine efficacy in the SHIV model.
MATERIALS AND METHODS
Animals.
Mature outbred rhesus monkeys (Macaca mulatta) of Indian origin bred in captivity were housed at the Biomedical Primate Research Center, Rijswijk, The Netherlands. The animals were negative for antibodies to simian T-cell-tropic virus type I, simian type D retrovirus, and herpesvirus B. During the course of the study, the animals were checked for appetite and general behavior, stool consistency, weight, and rectal temperature. If an animal suffered from opportunistic infections that were not responsive to treatment, had a body weight loss of >10%, or developed persistently low CD4+ T-cell counts and heavy virus loads, then it was euthanized and a full pathologic examination was performed to confirm the diagnosis of AIDS. The Institute's Animal Care and Use Committee approved the study protocols, in accordance with international ethical and scientific standards and guidelines.
Vaccines. (i) DNA plasmids.
Plasmids pc-synTat (HIV-1IIIB), pc-syngp120 (SHIV-189.6p), and pc-synGag (SIVmac239) contain a codon-optimized gene, cloned under transcriptional control of the cytomegalovirus immediate-early promoter-enhancer unit in pcDNA 3.1 (Invitrogen). Protein expression is about four- to fivefold greater than that of the corresponding wild-type construct (R. Wagner, unpublished data).
(ii) Protein-ISCOM.
ISCOM preparations for immunization were obtained as follows. Two hundred micrograms of ISCOM matrix (Isconova, Uppsala, Sweden) was mixed overnight at 4°C with either 25 μg of HIV-189.6 Env gp140 (produced in human 293T cells, containing gp120 and the gp41 ectodomain, and purified by lectin chromatography [University of Pennsylvania, Philadelphia]) or SIVmac239 Gag-Pol (49) in 250 μl of phosphate-buffered saline (PBS). To maintain the biological activity of the Tat protein (HIV-1IIIB) (15), Tat was first administered intramuscularly (i.m.), followed immediately by ISCOM matrix. Thus, an aliquot of 25 μg of Tat protein in 200 μl of physiological saline with 20% autologous serum was first administered with a syringe prerinsed with 20% autologous serum, followed by a 200-μg/200-μl ISCOM matrix injection through the same needle at the same site.
Chimeric SHIV89.6p stock.
SHIV89.6p was constructed with SIVmac239 expressing the HIV-1 Env protein of an unusual macrophage-tropic primary isolate (HIV-189.6) (20) and the associated auxiliary genes tat, vpu, and rev as described previously (53). After in vivo passage, SHIV89.6p became pathogenic (35, 52) and dual-tropic, using both CXCR4 and CCR5 coreceptors for cell entry (14). This virus stock was titrated in vivo and generously provided by N. L. Letvin (Beth Israel Hospital, Boston, Mass.). The final stock contained 3.2 × 104 (104.5) 50% tissue culture infective doses/ml (108 RNA equivalents [eq]/ml) and in vivo was titrated to approximately 5 × 104 50% monkey infective doses (MID50)/ml.
Immunization and SHIV89.6p challenge.
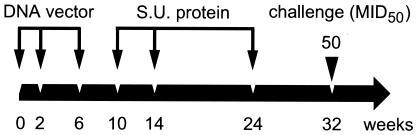
Animals were immunized i.m. three times (weeks 0, 2, and 6) with DNA and then given three boosts (weeks 10, 14, and 24) with protein in ISCOM in accordance with the schedule depicted in Fig. 1. Animals from group A (n = 4) were immunized three times i.m. with a combination of 0.5 mg of HIV-1IIIB pc-syntat DNA (right upper leg), 0.5 mg of SHIV-189.6p pc-synenv DNA (left upper leg), and 0.5 mg of SIVmac239 pc-syngag DNA (right upper arm) each in 250 μl of PBS. Five days before each DNA immunization, the inoculation site was pretreated by giving an i.m. injection of 250 μl of 0.5% bupivacaine (Sigma Chemical, St. Louis, Mo.) and 0.1% p-hydroxybenzoic acid methyl ester (Methyl paraben; Sigma Chemical) to increase DNA uptake and expression by the muscle cells (64). Three i.m. boosts with 25 μg of the HIV-1 Tat and Env and SIV Gag proteins in 200 μg of ISCOM in 250 μl of PBS were administered at the same sites. Animals from group B (n = 4) were immunized three times i.m. (right upper leg) with 0.5 mg of HIV-1IIIB pc-syntat DNA in 250 μl of PBS. Three i.m. boosts with 25 μg of Tat protein and 200 μg of ISCOM in 250 μl of PBS were administered. Control animals (group C, n = 4) were injected three times with the empty pCV-0 DNA plasmid vector in 250 μl of PBS (right upper leg) and three times with 200 μg of ISCOM in 250 μl of PBS. Eight weeks after administration of the last protein boost (at week 32), all animals were challenged i.v. with 50 MID50 of SHIV89.6p (1 ml of a 1:1,000 dilution of the virus stock, kindly provided by N. L. Letvin).
FIG. 1.
Immunization-and-challenge schedule. Animals were immunized i.m. at weeks 0, 2, and 6 with DNA constructs and boosted at weeks 10, 14, and 24 with protein formulated with ISCOM matrix. At weeks 0, 2, and 6, animals from group A received HIV-1IIIB tat-, SHIV-189.6p env-, and SIVmac239 gag-expressing DNA plasmids. At weeks 10, 14, and 24, the same animals were boosted with HIV-1IIIB Tat, HIV-189.6 Env gp140, and SIVmac239 Gag proteins formulated with ISCOM matrix, while animals from group B only received HIV-1IIIB tat-expressing DNA plasmids and HIV-1IIIB Tat protein formulated with ISCOM matrix. Control animals received empty vectors and ISCOM matrix. All animals were subsequently challenged (i.v.) at week 32 (8 weeks after the last immunization) with 50 MID50 of SHIV89.6p (1 ml of a 1:1,000 dilution of the virus stock). S.U., subunit.
Two naive animals were added to the study as additional controls to rule out technical problems at the time of challenge.
Plasma virus load and detection of proviral DNA.
The plasma virus load was determined with a quantitative competitive RNA reverse transcription-PCR assay with plasma from EDTA-treated blood samples (63). The lower detection limit was 40 RNA eq/ml. For detection of proviral DNA in peripheral blood mononuclear cells (PBMC) and lymph node cells, DNA was purified by Triton X-100-proteinase K digestion, followed by ethanol precipitation. Nested PCR was performed for two regions of the chimeric SHIV genome by using SIV gag and HIV-1 env primers as described before (11). The detection limit of both the SIV gag and HIV-1 env PCR assay is 1 copy of proviral DNA per 1.5 × 105 cell eq (43). All assays were performed with samples at multiple time points.
T-cell counts.
Quantitative changes in PBMC subsets were monitored by fluorescence-activated cell sorter analysis. Flow cytometry was performed on a FACsort with the CellQuest software (Becton Dickinson, Etten-Leur, The Netherlands), and 7,500 events in the lymphocyte gate were analyzed per monoclonal antibody mixture as previously described (43). Combinations of CD3FITC, CD16PE, CD8PerCP, and CD4APC, of CD20FITC, HLA-DRPE, CD8PerCP, and CD4APC and of CD62LFITC, CD45RAPE, CD8PerCP, and CD4APC were made to quantify the percentage of CD4+ and CD4+, CD62L−, CD45RA− memory T cells. Anti-mouse immunoglobulin coupled with fluorescein isothiocyanate, phycoerythrin, peridinin chlorophyll and allophycocyanin (IgFITC, IgPE, IgPerCP, and IgAPC, respectively) were used as control antibodies. White blood cell counts were performed to allow calculation of the absolute number of circulating blood cell subsets.
Cellular immune responses.
Freshly isolated PBMC from each monkey were assayed for HIV-1 Tat protein (15)-specific T-cell responses by a technique that has been previously reported (66). PBMC from animals that received additional immunizations with HIV-1 gp120 and SIV Gag-Pol were also assayed for specific T-cell responses to HIV-1 gp120W6.1D protein (ARP-648; Medical Research Council [MRC] AIDS Reagent Programme, Potters Bar, United Kingdom) and SIVmac239 Gag virus-like particles (49). Evaluation of Th responses to 5 μg of protein per ml was based on the enumeration of antigen-specific cytokine (IFN-γ, IL-2, and IL-4)-secreting cells performed by ELIspot assay with the Ucytech kit (Ucytech, Utrecht, The Netherlands). The negative control was medium alone, while the positive control was phorbol myristate acetate (50 ng/ml)-ionomycin (1 μg/ml). The number of specific T cells was adjusted by subtracting the negative control values and was expressed as the number of cytokine-secreting cells per 106 PBMC. Since CD4+ T-cell depletion abolished the protein-induced responses and not the peptide-induced responses (data not shown), we, in agreement with others (18, 36), considered protein-specific cytokine responses to be CD4 mediated and peptide-specific cytokine responses to be predominantly CD8 mediated.
Since early IFN-γ release upon peptide stimulation is indicative of potential CD8+ CTL responses (5, 36), we performed peptide-based ELIspot assays. Two weeks after the last immunization, the numbers of cells producing IFN-γ in response to peptide stimulation were quantified. The cells were exposed to peptides (2 μg of peptide per ml, 20-mers, 10-amino-acid [aa] overlap) in the following pools: SIVmac251 Gag (MRC, ARP-714.1-22), SHIV89.6p Env (National Institutes of Health [NIH], 4702-4725 and 4726-4749), and HIV-1LAI Tat (MRC, EVA-779.1-8). To confirm peptide-specific IFN-γ ELIspot responses prior to challenge, functional CTL activity after challenge was measured in all animals that controlled the virus load by performing classical 51Cr release assays with peptide-pulsed autologous B cells as targets, as previously described (30, 43). Pools of 20-mers with a 10-aa overlap of SIVmac251 Gag (MRC, ARP-714.1-22) and SHIV89.6p Env (NIH, 4702-47025 and 4726-4749) or individual peptides of SIVmac251 Gag (10-mers with an 8-aa overlap, aa 246 to 265; LUMC, Leiden, The Netherlands) were used. CTL activity was tested at effector-to-target cell ratios ranging from 50 to 2.5. Percent specific lysis was calculated as (experimental lysis − spontaneous lysis)/maximal lysis − spontaneous lysis) × 100. A CTL response was considered positive if more than 10% specific lysis of peptide-pulsed target cells was measured compared to that of controls (unpulsed targets).
Proliferative responses in PBMC samples taken during the immunization period were measured with concanavalin A (5 μg/ml, positive mitogen control); 5 μg of HIV-1 Tat protein, HIV-1 gp120W6.1D protein, or SIVmac239 Gag virus-like particles (antigen-specific response) per ml; or medium alone (negative control, background). Proliferation was determined at the end of the culture period (72 h) by the incorporation of [3H]thymidine (2 μCi/25 μl per well, TRK758, 86 mCi/mmol) for 16 h. The cells were harvested on glass fiber filters with a MicroMate 196 cell harvester, and the incorporated [3H]thymidine was measured with a Matrix 96 Direct beta counter (Packard Instrument Company, Meriden, Conn.). The mean counts per minute of triplicate cultures are presented as stimulation indices (antigen-specific counts per minute/background counts per minute).
Humoral immune responses.
Serum samples were tested for antibodies to the Tat HIV-1IIIB (15) Env gp120 HIV-1IIIB (MRC, EVA-607) or Gag P27 SIVmac251 (MRC, ARP-643) protein by enzyme-linked immunosorbent assay as previously described (15, 43). The titers reported here represent the reciprocal of the serum dilution giving an optical density of more than the mean plus twice the standard deviation of the optical density of a control serum (uninfected rhesus monkey) at the same dilution.
To determine the neutralizing activity of the sera from immunized rhesus macaques, the “GHOST cell” assay was used (17, 65). The infectivity and neutralization assays were performed as described previously (17). The number of virus-infected cells was determined by measuring the green fluorescent protein signal with a FACScan flow cytometer with a scattergram of fluorescence versus forward scatter after setting the gates with uninfected cells. A total of 15,000 to 25,000 events were scored.
Statistical analysis.
The best fit of the decrease in CD4+ T-cell levels following infection was estimated with a nonlinear mixed-effects model in the statistical software program S-PLUS (51). A four-parameter logistic function was used to estimate CD4+ T-cell levels before and after infection (challenge). The statistical significance of the treatment effect was assessed by analysis of variance (ANOVA), while effects of treatment were estimated by treatment contrasts. Virus load reduction was analyzed with linear mixed-effects models in S-PLUS (51). The fits of the models were compared by ANOVA. The best-fitting model was selected on the basis of maximum likelihood, and estimates of differences between groups were calculated on the basis of restricted maximum likelihood.
RESULTS
Vaccine-induced cellular immune responses.
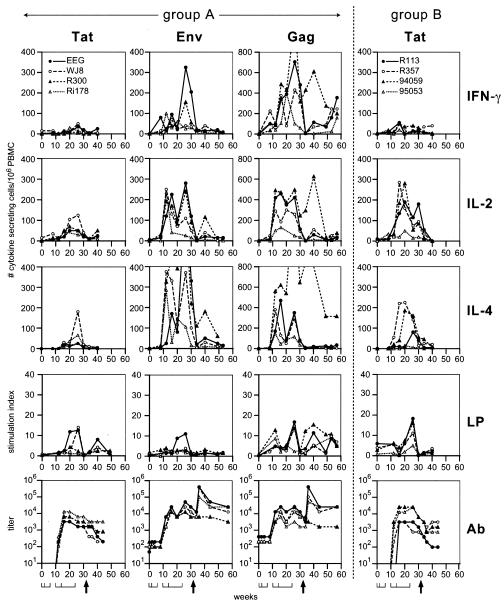
Control animals, which received empty DNA vector and ISCOM, did not show any Env-, Gag-, or Tat-specific cellular response at any time point. As shown in Fig. 2, DNA priming in animals immunized with Tat-Env-Gag (group A) resulted predominantly in a Gag-specific Th1-type response characterized by Gag-specific IFN-γ production. In one animal (EEG) of group A, Env-specific IFN-γ production was also observed 2 weeks following the third DNA immunization. Priming with tat DNA did not result in detectable Tat-specific Th responses in either group A or group B (immunized with Tat alone) animals (Fig. 2).
FIG. 2.
Protein-specific immune responses of immunized macaques. Tat-, Env-, and Gag-specific immune responses of Tat-Env-Gag-immunized animals (group A [EEG, WJ8, R300, and Ri178]) are displayed on the left site of the dashed line. Tat-specific immune responses of Tat-immunized animals (group B [R113, R357, 94059, and 95053]) are presented on the right site of the dashed line. From top to bottom, the number of antigen-specific IFN-γ-, IL-2-, and IL-4-secreting cells; antigen-specific lymphoproliferation (LP); and antibody (Ab) titers are depicted. The Env- and Gag-specific cell-mediated immune responses of Tat-immunized group B animals were negative and are not shown. At the bottom, the first horizontal line represents the time points of DNA immunization (weeks 0, 2, and 6), the second horizontal line represents the times of protein boosts (weeks 10, 14, and 24), and the arrow indicates the time of challenge (week 32).
ISCOM-formulated subunit protein immunizations strongly boosted immune responses to all antigens. Interestingly, depending on the antigen, different Th profiles were observed. Tat-specific cytokine responses were stronger in animals immunized with Tat alone (Fig. 2, group B, right panel) compared to the responses in animals immunized with the Tat-Env-Gag antigen combination (Fig. 2, group A, left panels). In group A animals, Tat-specific responses were weak and were observed only in animals EEG and WJ8. Tat-specific immune responses in group B were Th2 like, with moderate IL-4 and IL-2 and no IFN-γ production, but substantial Tat-specific proliferation (Fig. 2, right panel). Only animal 95053 showed a reduced response in this group, developing very weak Tat-specific cellular responses.
Immunization with multiple antigens boosted a Th0-Th1-oriented immune response to the Gag protein. The low number of Gag-specific IFN-γ-producing cells primed by DNA immunization was markedly increased by protein boosts. Following boosts, also the number of IL-2-producing cells dramatically increased, while the increase in IL-4 production was somewhat smaller. Gag-specific proliferation was also boosted by protein immunizations. Gag-specific immune responses were very weak in group A animal Ri178, while in animal R300 very high numbers of Gag-specific IL-4-producing cells were induced following protein immunization. Interestingly, this particular animal also showed the highest number of Gag-specific IFN-γ- and IL-2-producing cells (Fig. 2, left panels).
Immunization with multiple antigens in group A animals resulted in a Th2-oriented response to Env, with strong IL-4 production, good IL-2 production, and low or absent IFN-γ. Env-specific proliferation was only observed in one animal, EEG. This animal also showed marked Env-specific IFN-γ, IL-2, and IL-4 production (Fig. 2, left panels).
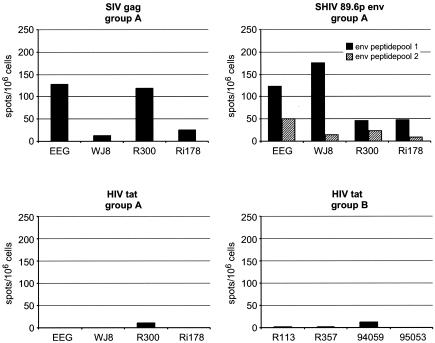
IFN-γ peptide-based ELIspot assays were performed to assess potential CD8+ CTL responses (5, 36). Two weeks after the last immunization, peptide-specific IFN-γ production in response to pools of peptides from SIVmac251 Gag, SHIV89.6p Env (NIH, 4702-4725 and 4726-4749) and HIV-1LAI Tat (MRC, EVA-779.1-8) was measured. Marked Gag peptide-specific IFN-γ production was observed in animals EEG and R300 (Fig. 3). These animals also developed the highest number of Gag protein-specific IFN-γ-producing cells (Fig. 2). Env peptide-specific IFN-γ responses were also observed in animals EEG and WJ8, but not in animal R300. Peptide-specific IFN-γ production in monkey Ri178 was very low in response to all of the antigens tested. With this immunization schedule, Tat peptide-specific IFN-γ production could not be detected in any of the group A animals or in animals immunized with Tat alone (group B).
FIG. 3.
Peptide-specific IFN-γ responses. The number of antigen-specific IFN-γ-secreting cells generated in immunized animals, indicative of potential CD8+ CTL responses, as measured by ELIspot assay, 2 weeks after the last immunization. Peptide-specific (20-mers, 10-aa overlap) IFN-γ production to pools of peptides (2 μg of peptide per ml) from SIVmac251 Gag, SHIV89.6p Env, and HIV-1LAI Tat of each individual monkey per million PBMC is represented.
Antibody responses.
In control animals, Tat, Env, or Gag antibody responses were not detected prior to challenge. In vaccinees, DNA priming did not result in detectable levels of Tat, Env, or Gag antibodies (Fig. 2, lower panels). The initial protein boost resulted in moderate Tat antibody titers in the vaccinees from both groups, which were boosted by the second protein immunization (Fig. 2). The third protein immunization did not boost the Tat antibody titers further, but levels were sustained. Env and Gag antibody responses were induced after the initial protein immunization in vaccinated animals, while the second and third protein immunizations continued to boost the Env and Gag antibody titers substantially, with the exception of monkey Ri178 from group A. Neutralizing antibody titers were below detection at the time of challenge in all animals. However, given that the Env antigen available for immunization was derived from the parental HIV-189.6 isolate and not the in vivo-modified SHIV89.6p Env (22), this result is not surprising.
Evaluation of vaccine efficacy.
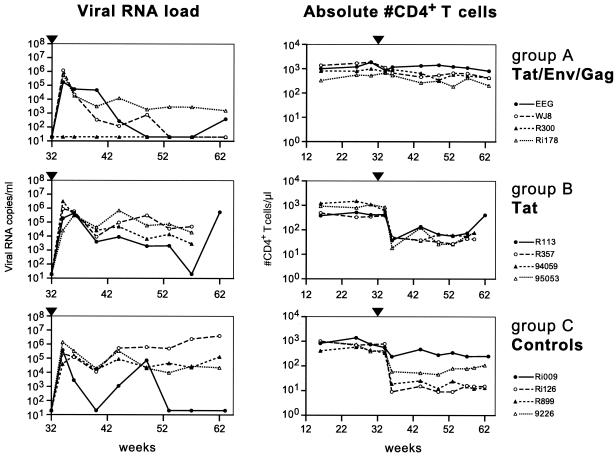
Group A animals, immunized with the Tat-Env-Gag antigen combination, showed a reduced virus RNA load following challenge. Statistical analysis showed that a model incorporating a vaccine effect fit the data significantly better than one with no vaccine effect (P = 0.0142), indicating that vaccination influences the virus RNA load over time. The virus set point level (at 8 weeks postchallenge) in group A animals was estimated to be log10 2.9, which was lower (P = 0.0382, ANOVA) than the estimated set point level in control animals (log10 4.1). Furthermore, in group A animals, the virus load declined from 8 weeks postchallenge onward, with a slope of −0.07 log10 RNA eq/week, while the virus RNA load in control animals did not decline (slope of 0.01 log10 RNA eq/week). One animal from group A (R300) was completely protected against virus infection (Fig. 4, top left panel). Viral RNA was not detected at any time point tested after challenge. At 12 weeks after challenge, proviral DNA was detected in PBMC from all animals, except in those from protected monkey R300. Animal EEG from group A reduced its viral RNA load to undetectable levels 18 weeks after infection, while animal WJ8 did so after 22 weeks. Animal Ri178 was able to reduce its viral RNA load below the pathogenic threshold of 105 RNA copies/ml. After 62 weeks, the viral RNA load was also reduced to undetectable levels in this animal (data not shown). Group A animals showed significantly higher numbers of CD4+ T cells (P = 0.0009, ANOVA) after challenge compared to control animals (Fig. 4, top right panel). At 82 weeks after challenge (data not shown), all group A animals were still completely healthy, with normal CD4+ T-cell counts, and controlled viral RNA to undetectable levels.
FIG. 4.
Viral RNA loads and absolute number of CD4+ T cells in peripheral blood. Viral RNA loads (RNA equivalents per milliliter of plasma; left panels) and absolute numbers of CD4+ T cells (per microliter of blood; right panels) are given for animals immunized with Tat-Env-Gag (top), Tat (middle), and vector controls (bottom). At week 32, animals were exposed to 50 MID50 of SHIV89.6p (arrowhead).
All group B animals, immunized with Tat alone, became infected after challenge. In contrast to that in group A, however, the virus RNA set point level at week 8 postchallenge in group B animals was not significantly different from the viral RNA set point level in control animals (log10 4.6 versus log10 4.1), although the virus load declined, with a slope of −0.05 log10 RNA eq/week (Fig. 4, middle left panel). CD4+ T-cell levels declined dramatically 4 weeks after challenge, but this was not significantly different from the decline observed in control monkeys (P = 0.82) (Fig. 4, middle right panel). The decline was most marked in the CD4+ CD62L+ CD45RA+ naive T-cell subset (data not shown). Eventually, 25 weeks after infection, animal R113 from group B was able to control viral replication, only for its RNA load to dramatically increase again at 30 weeks postchallenge. At the end of the study period (week 77, 45 weeks after infection), no signs of disease were detected in any of the group B animals, despite low CD4+ T-cell counts.
Following challenge with 50 MID50 (1:1,000 dilution) of the SHIV89.6p stock, two challenge control monkeys became infected, with a peak virus load of approximately 106 RNA eq/ml at 2 weeks postinfection, indicating that this challenge dose was sufficient to infect naive rhesus monkeys (data not shown). Also, all four vector control animals became infected, with a peak virus load between 105 and 106 RNA eq/ml 2 to 4 weeks after challenge (Fig. 4, bottom left panel). In three control animals (Ri126, R899, and 9226), the virus load remained high (slope of 0.01 log10 RNA eq/week). Monkey Ri126 developed AIDS after 38 weeks, while the other two (R899 and 9226) remained symptom free until 45 weeks after infection despite persistently low CD4+ T-cell counts (Fig. 4, bottom right panel). One vector control (Ri009, Fig. 4) appeared to control the virus load at 20 weeks after infection and remained symptom free during the study period.
Correlation between vaccine-induced immune responses and protection.
All group A animals were able to either completely reduce their viral RNA loads to undetectable levels (animals EEG, WJ8, and Ri178) or were protected from persistent infection (animal R300). All of the animals in this group developed potent vaccine-induced Th responses to Env and Gag before challenge, while their Tat-specific cellular immune responses were relatively weak. Env induced mainly a Th2-like response with moderate IL-4 and weak IL-2 responses but almost no IFN-γ. A Th1 profile in response to the Gag antigen was observed in three out of four animals. Gag-stimulated PBMC produced primarily IFN-γ and IL-2, while IL-4 production was lower. Notably, the one immunized animal that resisted infection (R300) had developed an additional and marked Gag-specific IL-4 response prior to challenge. Of further relevance, at the time of challenge, this monkey had developed the most marked Gag-specific IFN-γ response while being the only one to retain a strong Gag-specific IL-4 response.
Two weeks after challenge (week 34), all cellular immune responses were observed to dramatically decrease in each animal except monkey R300, in which Gag-specific IFN-γ, IL-2, and IL-4 production and proliferation were boosted after challenge. The immune responses developed in R300 correlated with prevention of viral RNA replication and establishment of proviral DNA (not detected in PBMC from this animal). Indeed, evidence of protection from infection in R300 was further substantiated by the absence of an anamnestic antibody response following challenge. Taken together, the virological and immunological data suggest that monkey R300 was protected from infection before virus replication and dissemination could take place. Indeed, in contrast to all of the other study animals, Tat-, Env-, and Gag-specific antibody responses gradually declined in this animal, while in all other monkeys, Env and Gag antibody levels initially increased 4 weeks after challenge and only then gradually declined in cases in which viremia was controlled and CD4+ T-cell levels were maintained. Also in group A, animal EEG was able to reduce the virus load to undetectable levels by 18 weeks after challenge, while WJ8 was able to do so after 22 weeks. Env-specific cellular responses before challenge were stronger in animal EEG than were those in WJ8, while the Gag-specific responses before challenge were similar in the two animals. Following challenge, Gag-specific IFN-γ production and proliferation and Env- and Gag-specific antibody responses were stronger in EEG than were those in WJ8. Thus, strong CD4+ Th responses to multiple antigens correlated with control and reduction of the virus load. In this group, the animal that developed a very slow virus load reduction (Ri178) 62 weeks after challenge also showed relatively weaker Tat-, Env-, and Gag-specific Th cytokine responses, as well as antigen-specific proliferation before and after challenge.
Animals that initially showed viremia but were able to reduce their virus loads (EEG, WJ8, and Ri178) also developed CTL responses to Gag and/or Env after challenge, as was demonstrated by the classical and functional 51Cr release assay (Table 1). Also, in control monkey Ri009, which reduced its virus load after infection, Gag-specific CTL were detected. This animal showed CTL responses to the Gag epitope QNPIPVGNIY (data not shown), which was previously described (2, 46). Group B animals showed Tat-specific IL-2 and IL-4 responses, proliferation, and antibody titers before challenge. However, these immune responses were not sufficient to prevent infection, reduce the virus load, or sustain CD4+ T-cell counts. Interestingly, after challenge, Tat-specific immune responses were not boosted, likely owing to the marked loss of CD4+ T cells and an impaired ability to muster recall responses. Env- and Gag-specific cellular immune responses were slow to develop and remained very weak in these animals (data not shown).
TABLE 1.
Confirmatory functional CTL responses following challenge in animals capable of controlling their virus loads and preserving CD4+ T-cell levelsa
| Monkey | Groupc | Wk 40b | Wk 53 | Wk 63 | Wk 73 | Wk 86 | Wk 94 |
|---|---|---|---|---|---|---|---|
| EEG | A | Gag (12)d | Gag (20) | Gag (45) | |||
| WJ8 | A | Gag (50), Env (80,e 50f) | Gag (30), Env (20e, 25f) | Env (17f) | |||
| R300 | A | None | None | None | |||
| Ri178 | A | Gag (70), Env (30)e | |||||
| Ri009 | C | Gag (35) | Gag (30) | Gag (80) |
Functional CTL responses were measured (51Cr release assay) at the time of virus load decline following challenge in all animals that controlled the virus load. Positive responses are indicated by listing the peptide pools spanning the protein (i.e., Gag, Env) to which lytic responses were directed.
Weeks after first immunization.
Immunization group.
Percent specific lysis minus background lysis of unpulsed targets is given in parentheses.
Env peptide pool 1 (NIH, 4702-4725) specific lysis.
Env peptide pool 2 (NIH, 4726-4749) specific lysis.
DISCUSSION
This study revealed the complexity of different Th cytokine responses to individual antigens in a multiantigen-based HIV-1 vaccine. Each individual antigen induced a different characteristic cytokine profile, and when one antigen (Tat) was combined with Env and Gag, the magnitude of the response was reduced considerably. Furthermore, the nature of the route and delivery (adjuvant and vector) had an impact on the ultimate immune response. In this context, the characteristics of both the prime and boost are important to consider. Following DNA immunization, a typical Th1-like response was induced, as has been described before (31, 67). Only Gag-specific IFN-γ responses were measured following DNA priming, while antibody responses could not be detected. The conclusion that DNA immunization induces mainly a Th1 type of response is further supported by reports from numerous groups indicating that DNA immunization elicits CTL responses in nonhuman primates (7, 9, 55). Subunit protein in classical alum adjuvant has been reported to result in mainly a Th2-biased response, while we have reported that protein in ISCOM induced both Th1 and Th2 types of immune responses (29, 67). Also in this study, we were able to show that boosting with protein formulated with ISCOM resulted in strong antibody and cellular responses, characterized by IFN-γ, IL-2, and IL-4 secretion by antigen-specific cells.
Here we report that the nature of the Th responses induced by immunization of naive animals is in part dependent on the nature of the antigen itself. Immunization with Tat or Env induced mainly a Th2 type of response, with marked IL-4 and IL-2 responses but low or absent IFN-γ. Immunization with Gag induced a Th1 type of profile with marked IFN-γ and IL-2 responses and lower IL-4 production. This study further revealed that immunization with a combination of antigens reduced the magnitude of the Tat-specific immune response. Most probably, the Env and Gag antigens were more immunodominant in relation to the Tat antigen. However, competition at the level of antigen presentation, which can be a factor for immunodominance of certain epitopes over others (69), is unlikely since immunization with the different antigens was performed at different sites.
In those animals that became viremic, protection from disease progression (reduction of virus load and protection from CD4+ T-cell loss) correlated with marked Env and Gag Th1 and Th2 responses before challenge and CTL responses after challenge. These findings are in agreement with the hypothesis that preexisting CD4+ Th responses facilitated the recruitment and rapid expansion of potent CD8+ effector CTL responses (33). In HIV-infected individuals, in whom the number of CD4+ T cells in the blood is diminished, maturation of CD8+ memory CTL is impaired (18). Furthermore, our results support the observations that multicomponent Env-, Gag-, and Tat-based vaccines may preempt virus escape, thus avoiding CD4+ T-cell destruction (41) and prophylactically avoiding CTL dysfunction by facilitating rapid expansion of mature CTL, resulting in effective control of the virus (42).
In animals EEG and WJ8, in which Gag-specific IFN-γ production increased again after challenge (after a strong initial drop), Gag-specific CTL responses were detected. In addition, animal WJ8 also showed Env-specific CTL (Table 1). It is of note that in animal WJ8, the virus load dropped to undetectable levels more rapidly than in animal EEG, confirming our initial hypothesis that responses to multiple antigens induce better protection. This is in agreement with several other studies showing protection after vaccination with a combination of SHIV antigens (3, 40, 68). This is further supported by the observations in group A animal Ri178, which developed the poorest immune responses before (and after) challenge and also had the slowest RNA virus load reduction of any animal in its group. Eventually, virus RNA levels declined to undetectable levels at 62 weeks after challenge, when CTL responses to Gag and Env could be detected (Table 1). In naive control animal Ri009, the virus load reduction correlated with strong IFN-γ and CTL response to a Gag epitope (QNPIPVGNIY) after challenge (Table 1).
Animal R300 was completely protected from plasma viremia. Proviral DNA could not be detected in PBMC from this animal, indicating that protection from infection was achieved. It is possible that the highly stimulated immune system at the time of challenge of R300 eliminated virus or virus-infected cells before the virus replicated to sufficient levels to be disseminated systemically. We do feel that monkey R300 had a limited infection following challenge, as evidenced by the rapid boosting of Env- and Gag-specific cell-mediated immune responses directly after exposure of this animal to virus (Fig. 2). Since there was no detectable SHIV replication in animal R300, there was no possibility of antigenic boosting so that further increases in antibody and Th responses were not expected, resulting in a gradual decline in these responses following challenge. Functional CTL responses to Gag and Env (measured with the classical 51Cr release assay at 8, 21, and 31 weeks after challenge; Table 1) could not be detected in this animal, in contrast to those that became infected. It remains to be determined whether functional SHIV-specific CTL effector responses were fully mature following immunization and present in sufficient numbers at the time of challenge to be responsible for directly eliminating virus-producing cells. Importantly, this animal did develop Gag peptide-specific IFN-γ-producing T cells before challenge (Fig. 3), indicating the presence of potential CD8+ CTL responses (36). In addition to cellular immune responses, neutralizing antibodies can protect from virus infection and disease progression (10, 13, 24, 62), even in the absence of CD8+ T cells (19). Although anti-Env and -Gag antibody titers were high in animal R300 at the time of challenge and a very high IL-4 production level was also observed in this animal, SHIV89.6p neutralizing antibodies could not be detected with the assay we used. Although it has been reported that SHIV89.6p has become a purely CXCR4-tropic virus following sequential in vivo passage (23, 70), other data indicate that SHIV89.6p also maintains the use of CCR5 as a coreceptor for cell entry (14). Also, in our neutralization assay, SHIV89.6p infected the same numbers of CXCR4-expressing and CCR5-expressing GHOST cells. This suggests that our assay is suitable for measuring SHIV89.6p neutralizing antibodies. Indeed, potent Th1-like, as well as Th2-like, responses to Tat, Env, and Gag were generated, but the quality of the effector response, especially neutralizing antibodies, is dependent on the nature of the antigen. It is possible that the Env antigen from the parental HIV-189.6 strain was incapable of inducing neutralizing antibodies to SHIV89.6p because of substantial differences in the Env antigen of SHIV (37), further underscoring the difficulty of inducing cross-reactive neutralizing antibodies.
In summary, this study revealed that immunization with a Tat-Env-Gag antigen combination resulted in strong and diverse Th immunity, facilitating CTL responses, containment of the virus load, maintenance of CD4+ T-cell levels, and protection from progression to AIDS. Immunization with only the Tat antigen did not elicit protective responses. Despite the strong induced anti-Tat responses observed in this study, protection from disease progression could not be induced by immunization with Tat only. This is in contrast to earlier findings obtained with cynomolgus monkeys, in which control of viral infection was observed after Tat protein (15) or CpG-rich tat DNA vaccination alone (16). We emphasize caution in attempting to focus the immune system toward only one viral antigen, as it has been described that Tat-specific CTL select for SIV escape variants in SIV-infected macaques (2). Furthermore, although immunization with SIV Gag as a single immunogen was sufficient to protect macaques from disease progression after SHIV89.6p challenge (58), viral escape from Gag-specific CTL has been described in a vaccinated rhesus monkey previously capable of controlling viral replication (8).
We conclude that strong Th responses to multiple antigens are beneficial in facilitating effector responses such as CTL responses and/or neutralizing antibodies capable of effectively controlling or ultimately preventing HIV-1 infection (44). An effective HIV-1 vaccine candidate should not only contain a combination of immune targets distributed over both regulatory and structural viral antigens, but ideally they should elicit a diverse array of immune effector mechanisms to most effectively limit and even prevent virus replication.
Acknowledgments
This work was supported by the European Vaccine Effort Against HIV/AIDS (EuroVacc) project (grant QLK2-CT-1999-01321) from the European Commission and NIH project 1PO1 AI48225-01A2. Reagents were purchased in part through the EU Programme EVA Centralised Facility and the UK MRC.
We thank E. Remarque for assisting with statistical analysis. We thank C. Rollier and D. Davis for critical reading of the manuscript and N. L. Letvin (Beth Israel Hospital, Harvard University, Boston, Mass.) for kindly providing the SHIV89.6p challenge stock.
REFERENCES
- 1.Allen, T. M., L. Mortara, B. R. Mothé, M. Liebl, P. Jing, B. Calore, M. Piekarczyk, R. Ruddersdorf, D. H. O'Connor, X. Wang, C. Wang, D. B. Allison, J. D. Altman, A. Sette, R. C. Desrosiers, G. Sutter, and D. I. Watkins. 2002. Tat-vaccinated macaques do not control simian immunodeficiency virus SIVmac239 replication. J. Virol. 76:4108-4112. [DOI] [PMC free article] [PubMed]
- 2.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothé, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 3.Amara, R. R., J. M. Smith, S. I. Staprans, D. C. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. S. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol in control of a simian-human immunodeficiency virus 89.6P challenge by a DNA prime/recombinant modified vaccinia virus Ankara vaccine. J. Virol. 76:6138-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 5.Appay, V., L. Papagno, C. A. Spina, P. Hansasuta, A. King, L. Jones, G. S. Ogg, S. Little, A. J. McMichael, D. D. Richman, and S. L. Rowland-Jones. 2002. Dynamics of T cell responses in HIV infection. J. Immunol. 168:3660-3666. [DOI] [PubMed] [Google Scholar]
- 6.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 7.Barouch, D. H., A. Craiu, S. Santra, M. A. Egan, J. E. Schmitz, M. J. Kuroda, T. M. Fu, J. H. Nam, L. S. Wyatt, M. A. Lifton, G. R. Krivulka, C. E. Nickerson, C. I. Lord, B. Moss, M. G. Lewis, V. M. Hirsch, J. W. Shiver, and N. L. Letvin. 2001. Elicitation of high-frequency cytotoxic T-lymphocyte responses against both dominant and subdominant simian-human immunodeficiency virus epitopes by DNA vaccination of rhesus monkeys. J. Virol. 75:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 9.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 10.Berman, P. W., T. J. Gregory, L. Riddle, G. R. Nakamura, M. A. Champe, J. P. Porter, F. M. Wurm, R. D. Hershberg, E. K. Cobb, and J. W. Eichberg. 1990. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature 345:622-625. [DOI] [PubMed] [Google Scholar]
- 11.Bogers, W. M., H. Niphuis, P. ten Haaft, J. D. Laman, W. Koornstra, and J. L. Heeney. 1995. Protection from HIV-1 envelope-bearing chimeric simian immunodeficiency virus (SHIV) in rhesus macaques infected with attenuated SIV: consequences of challenge. AIDS 9:F13-F18. [PubMed] [Google Scholar]
- 12.Bourgeois, C., B. Rocha, and C. Tanchot. 2002. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science 297:2060-2063. [DOI] [PubMed] [Google Scholar]
- 13.Bruck, C., C. Thiriart, L. Fabry, M. Francotte, P. Pala, O. Van Opstal, J. Culp, M. Rosenberg, M. De Wilde, P. Heidt, and J. L. Heeney. 1994. HIV-1 envelope-elicited neutralizing antibody titres correlate with protection and virus load in chimpanzees. Vaccine 12:1141-1148. [DOI] [PubMed] [Google Scholar]
- 14.Buch, S., D. Pinson, Y. Hou, I. Adany, Z. Li, S. Mukherjee, F. Jia, G. Mackay, P. Silverstein, A. Kumar, and O. Narayan. 2000. Neuropathogenesis of chimeric simian human immunodeficiency virus infection in rhesus macaques. J. Med. Primatol. 29:96-106. [DOI] [PubMed] [Google Scholar]
- 15.Cafaro, A., A. Caputo, C. Fracasso, M. T. Maggiorella, D. Goletti, S. Baroncelli, M. Pace, L. Sernicola, M. L. Koanga-Mogtomo, M. Betti, A. Borsetti, R. Belli, L. Akerblom, F. Corrias, S. Butto, J. Heeney, P. Verani, F. Titti, and B. Ensoli. 1999. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 5:643-650. [DOI] [PubMed] [Google Scholar]
- 16.Cafaro, A., F. Titti, C. Fracasso, M. T. Maggiorella, S. Baroncelli, A. Caputo, D. Goletti, A. Borsetti, M. Pace, E. Fanales-Belasio, B. Ridolfi, D. R. Negri, L. Sernicola, R. Belli, F. Corrias, I. Macchia, P. Leone, Z. Michelini, P. ten Haaft, S. Butto, P. Verani, and B. Ensoli. 2001. Vaccination with DNA containing tat coding sequences and unmethylated CpG motifs protects cynomolgus monkeys upon infection with simian/human immunodeficiency virus (SHIV89.6P). Vaccine 19:2862-2877. [DOI] [PubMed] [Google Scholar]
- 17.Cecilia, D., V. N. KewalRamani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 72:6988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Champagne, P., G. S. Ogg, A. S. King, C. Knabenhans, K. Ellefsen, M. Nobile, V. Appay, G. P. Rizzardi, S. Fleury, M. Lipp, R. Forster, S. Rowland-Jones, R. P. Sekaly, A. J. McMichael, and G. Pantaleo. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature 410:106-111. [DOI] [PubMed] [Google Scholar]
- 19.Cherpelis, S., X. Jin, A. Gettie, D. D. Ho, S. W. Barnett, I. Shrivastava, and L. Stamatatos. 2001. DNA-immunization with a V2 deleted HIV-1 envelope elicits protective antibodies in macaques. Immunol. Lett. 79:47-55. [DOI] [PubMed] [Google Scholar]
- 20.Collman, R., J. W. Balliet, S. A. Gregory, H. Friedman, D. L. Kolson, N. Nathanson, and A. Srinivasan. 1992. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J. Virol. 66:7517-7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conley, A. J., J. A. Kessler II, L. J. Boots, P. M. McKenna, W. A. Schleif, E. A. Emini, G. E. Mark III, H. Katinger, E. K. Cobb, S. M. Lunceford, S. R. Rouse, and K. K. Murthy. 1996. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J. Virol. 70:6751-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crawford, J. M., P. L. Earl, B. Moss, K. A. Reimann, M. S. Wyand, K. H. Manson, M. Bilska, J. T. Zhou, C. D. Pauza, P. W. H. I. Parren, D. R. Burton, J. G. Sodroski, N. L. Letvin, and D. C. Montefiori. 1999. Characterization of primary isolate-like variants of simian-human immunodeficiency virus. J. Virol. 73:10199-10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feinberg, M. B., and J. P. Moore. 2002. AIDS vaccine models: challenging challenge viruses. Nat. Med. 8:207-210. [DOI] [PubMed] [Google Scholar]
- 24.Girard, M., M. P. Kieny, A. Pinter, F. Barre-Sinoussi, P. Nara, H. Kolbe, K. Kusumi, A. Chaput, T. Reinhart, E. Muchmore, and P. Fultz. 1991. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 88:542-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girard, M., B. Meignier, F. Barre-Sinoussi, M. P. Kieny, T. Matthews, E. Muchmore, P. L. Nara, Q. Wei, L. Rimsky, K. Weinhold, et al. 1995. Vaccine-induced protection of chimpanzees against infection by a heterologous human immunodeficiency virus type 1. J. Virol. 69:6239-6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldstein, G., K. Manson, G. Tribbick, and R. Smith. 2000. Minimization of chronic plasma viremia in rhesus macaques immunized with synthetic HIV-1 Tat peptides and infected with a chimeric simian/human immunodeficiency virus (SHIV33). Vaccine 18:2789-2795. [DOI] [PubMed] [Google Scholar]
- 27.Heeney, J. L. 2002. The critical role of CD4+ T-cell help in immunity to HIV. Vaccine 20:1961-1963. [DOI] [PubMed] [Google Scholar]
- 28.Heeney, J. L., P. Beverley, A. McMichael, G. Shearer, J. Strominger, B. Wahren, J. Weber, and F. Gotch. 1999. Understanding the immune correlates of protection from HIV infection and disease progression to AIDS: more answers but yet more questions. Immunol. Today 20:247-251. [DOI] [PubMed]
- 29.Heeney, J. L., V. J. Teeuwsen, M. van Gils, W. M. Bogers, C. De Giuli Morghen, A. Radaelli, S. Barnett, B. Morein, L. Akerblom, Y. Wang, T. Lehner, and D. Davis. 1998. Beta-chemokines and neutralizing antibody titers correlate with sterilizing immunity generated in HIV-1 vaccinated macaques. Proc. Natl. Acad. Sci. USA 95:10803-10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heeney, J. L., C. van Els, P. de Vries, P. ten Haaft, N. Otting, W. Koornstra, J. Boes, R. Dubbes, H. Niphuis, M. Dings, et al. 1994. Major histocompatibility complex class I-associated vaccine protection from simian immunodeficiency virus-infected peripheral blood cells. J. Exp. Med. 180:769-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horner, A. A., S. K. Datta, K. Takabayashi, I. M. Belyakov, T. Hayashi, N. Cinman, M. D. Nguyen, J. H. Van Uden, J. A. Berzofsky, D. D. Richman, and E. Raz. 2001. Immunostimulatory DNA-based vaccines elicit multifaceted immune responses against HIV at systemic and mucosal sites. J. Immunol. 167:1584-1591. [DOI] [PubMed] [Google Scholar]
- 32.Hu, S. L., K. Abrams, G. N. Barber, P. Moran, J. M. Zarling, A. J. Langlois, L. Kuller, W. R. Morton, and R. E. Benveniste. 1992. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science 255:456-459. [DOI] [PubMed] [Google Scholar]
- 33.Janssen, E. M., E. E. Lemmens, T. Wolfe, U. Christen, M. G. von Herrath, and S. P. Schoenberger. 2003. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421:852-856. [DOI] [PubMed] [Google Scholar]
- 34.Kaech, S. M., and R. Ahmed. 2003. Immunology: CD8 T cells remember with a little help. Science 300:263-265. [DOI] [PubMed] [Google Scholar]
- 35.Karlsson, G. B., M. Halloran, J. Li, I. W. Park, R. Gomila, K. A. Reimann, M. K. Axthelm, S. A. Iliff, N. L. Letvin, and J. Sodroski. 1997. Characterization of molecularly cloned simian-human immunodeficiency viruses causing rapid CD4+ lymphocyte depletion in rhesus monkeys. J. Virol. 71:4218-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lalvani, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liao, H.-X., B. Etemad-Moghadam, D. C. Montefiori, Y. Sun, J. Sodroski, R. M. Scearce, R. W. Doms, J. R. Thomasch, S. Robinson, N. L. Letvin, and B. F. Haynes. 2000. Induction of antibodies in guinea pigs and rhesus monkeys against the human immunodeficiency virus type 1 envelope: neutralization of nonpathogenic and pathogenic primary isolate simian/human immunodeficiency virus strains. J. Virol. 74:254-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 40.Mathy, N. L., P. Mooij, K. Manson, D. I. Watkins, D. Wyand, J. L. Heeney, and G. Voss. 2003. Functional characterization of Tat-containing AIDS vaccines and their efficacy against SHIV-induced disease in rhesus monkeys, p. 139-143. In M. Vicari, B. Dodet, and M. Girard (ed.), Retroviruses of human AIDS and related animal diseases, XIIIth Cent Gardes Symposium—2002. Elsevier, Paris, France.
- 41.McKay, P. F., D. H. Barouch, J. E. Schmitz, R. S. Veazey, D. A. Gorgone, M. A. Lifton, K. C. Williams, and N. L. Letvin. 2003. Global dysfunction of CD4 T-lymphocyte cytokine expression in simian-human immunodeficiency virus/SIV-infected monkeys is prevented by vaccination. J. Virol. 77:4695-4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKay, P. F., J. E. Schmitz, D. H. Barouch, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, D. A. Gorgone, and N. L. Letvin. 2002. Vaccine protection against functional CTL abnormalities in simian human immunodeficiency virus-infected rhesus monkeys. J. Immunol. 168:332-337. [DOI] [PubMed] [Google Scholar]
- 43.Mooij, P., W. M. J. M. Bogers, H. Oostermeijer, W. Koornstra, P. J. F. Ten Haaft, B. E. Verstrepen, G. Van Der Auwera, and J. L. Heeney. 2000. Evidence for viral virulence as a predominant factor limiting human immunodeficiency virus vaccine efficacy. J. Virol. 74:4017-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mooij, P., and J. L. Heeney. 2001. Rational development of prophylactic HIV vaccines based on structural and regulatory proteins. Vaccine 20:304-321. [DOI] [PubMed] [Google Scholar]
- 45.Mooij, P., M. van der Kolk, W. M. Bogers, P. J. ten Haaft, P. Van Der Meide, N. Almond, J. Stott, M. Deschamps, D. Labbe, P. Momin, G. Voss, P. Von Hoegen, C. Bruck, and J. L. Heeney. 1998. A clinically relevant HIV-1 subunit vaccine protects rhesus macaques from in vivo passaged simian-human immunodeficiency virus infection. AIDS 12:F15-F22. [DOI] [PubMed] [Google Scholar]
- 46.Mothé, B. R., J. Weinfurter, C. Wang, W. Rehrauer, N. Wilson, T. M. Allen, D. B. Allison, and D. I. Watkins. 2003. Expression of the major histocompatibility complex class I molecule Mamu-A*01 is associated with control of simian immunodeficiency virus SIVmac239 replication. J. Virol. 77:2736-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murthy, K. K., E. K. Cobb, S. R. Rouse, S. M. Lunceford, D. E. Johnson, and A. R. Galvan. 1996. Correlates of protective immunity against HIV-1 infection in immunized chimpanzees. Immunol. Lett. 51:121-124. [DOI] [PubMed] [Google Scholar]
- 48.Osterhaus, A. D., C. A. van Baalen, R. A. Gruters, M. Schutten, C. H. Siebelink, E. G. Hulskotte, E. J. Tijhaar, R. E. Randall, G. van Amerongen, A. Fleuchaus, V. Erfle, and G. Sutter. 1999. Vaccination with Rev and Tat against AIDS. Vaccine 17:2713-2714. [DOI] [PubMed] [Google Scholar]
- 49.Paliard, X., Y. Liu, R. Wagner, H. Wolf, J. Baenziger, and C. M. Walker. 2000. Priming of strong, broad, and long-lived HIV type 1 p55gag-specific CD8+ cytotoxic T cells after administration of a virus-like particle vaccine in rhesus macaques. AIDS Res. Hum. Retrovir. 16:273-282. [DOI] [PubMed] [Google Scholar]
- 50.Pauza, C. D., P. Trivedi, M. Wallace, T. J. Ruckwardt, H. Le Buanec, W. Lu, B. Bizzini, A. Burny, D. Zagury, and R. C. Gallo. 2000. Vaccination with tat toxoid attenuates disease in simian/HIV-challenged macaques. Proc. Natl. Acad. Sci. USA 97:3515-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinheiro, J. C., and D. M. Bates. 2000. Mixed-effects models in S and S-PLUS. Springer Verlag, New York, N.Y.
- 52.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I.-W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reimann, K. A., J. T. Li, G. Voss, C. Lekutis, K. Tenner-Racz, P. Racz, W. Lin, D. C. Montefiori, D. E. Lee-Parritz, Y. Lu, R. G. Collman, J. Sodroski, and N. L. Letvin. 1996. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J. Virol. 70:3198-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robert-Guroff, M. 2000. IgG surfaces as an important component in mucosal protection. Nat. Med. 6:129-130. [DOI] [PubMed] [Google Scholar]
- 55.Robinson, H. L. 1997. Nucleic acid vaccines: an overview. Vaccine 15:785-787. [DOI] [PubMed] [Google Scholar]
- 56.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 57.Shedlock, D. J., and H. Shen. 2003. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science 300:337-339. [DOI] [PubMed] [Google Scholar]
- 58.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 59.Silvera, P., M. W. Richardson, J. Greenhouse, J. Yalley-Ogunro, N. Shaw, J. Mirchandani, K. Khalili, J. F. Zagury, M. G. Lewis, and J. Rappaport. 2002. Outcome of simian-human immunodeficiency virus strain 89.6p challenge following vaccination of rhesus macaques with human immunodeficiency virus Tat protein. J. Virol. 76:3800-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stott, E. J., N. Almond, K. Kent, B. Walker, R. Hull, J. Rose, P. Silvera, R. Sangster, T. Corcoran, J. Lines, K. Silvera, P. Luciw, M. Murphy-Corb, P. Momin, and C. Bruck. 1998. Evaluation of a candidate human immunodeficiency virus type 1 (HIV-1) vaccine in macaques: effect of vaccination with HIV-1 gp120 on subsequent challenge with heterologous simian immunodeficiency virus-HIV-1 chimeric virus. J. Gen. Virol. 79:423-432. [DOI] [PubMed] [Google Scholar]
- 61.Sun, J. C., and M. J. Bevan. 2003. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science 300:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ten Haaft, P., M. Cornelissen, J. Goudsmit, W. Koornstra, R. Dubbes, H. Niphuis, M. Peeters, C. Thiriart, C. Bruck, and J. L. Heeney. 1995. Virus load in chimpanzees infected with human immunodeficiency virus type 1: effect of pre-exposure vaccination. J. Gen. Virol. 76:1015-1020. [DOI] [PubMed] [Google Scholar]
- 63.Ten Haaft, P., B. Verstrepen, K. Überla, B. Rosenwirth, and J. Heeney. 1998. A pathogenic threshold of virus load defined in simian immunodeficiency virus- or simian-human immunodeficiency virus-infected macaques. J. Virol. 72:10281-10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomason, D. B., and F. W. Booth. 1990. Stable incorporation of a bacterial gene into adult rat skeletal muscle in vivo. Am. J. Physiol. 258:C578-C581. [DOI] [PubMed] [Google Scholar]
- 65.Trkola, A., T. Ketas, V. N. KewalRamani, F. Endorf, J. M. Binley, H. Katinger, J. Robinson, D. R. Littman, and J. P. Moore. 1998. Neutralization sensitivity of human immunodeficiency virus type 1 primary isolates to antibodies and CD4-based reagents is independent of coreceptor usage. J. Virol. 72:1876-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van der Meide, P. H., R. J. Groenestein, M. C. de Labie, J. Heeney, P. Pala, and M. Slaoui. 1995. Enumeration of lymphokine-secreting cells as a quantitative measure for cellular immune responses in rhesus macaques. J. Med. Primatol. 24:271-281. [DOI] [PubMed] [Google Scholar]
- 67.Verschoor, E. J., P. Mooij, H. Oostermeijer, M. van der Kolk, P. ten Haaft, B. Verstrepen, Y. Sun, B. Morein, L. Akerblom, D. H. Fuller, S. W. Barnett, and J. L. Heeney. 1999. Comparison of immunity generated by nucleic acid-, MF59-, and ISCOM-formulated human immunodeficiency virus type 1 vaccines in rhesus macaques: evidence for viral clearance. J. Virol. 73:3292-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Voss, G., K. Manson, D. Montefiori, D. I. Watkins, J. Heeney, M. Wyand, J. Cohen, and C. Bruck. 2003. Prevention of disease induced by a partially heterologous AIDS virus in rhesus monkeys by using an adjuvanted multicomponent protein vaccine. J. Virol. 77:1049-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yewdell, J. W., and J. R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51-88. [DOI] [PubMed] [Google Scholar]
- 70.Zhang, Y.-I., B. Lou, R. B. Lal, A. Gettie, P. A. Marx, and J. P. Moore. 2000. Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J. Virol. 74:6893-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]