Abstract
Purpose
Surgical treatment options for renal masses include radical versus partial nephrectomy and the open versus laparoscopic approach. Using American Board of Urology case log data, we investigated contemporary trends in these treatment options and how surgeon and practice characteristics may influence these trends.
Materials and Methods
Annualized case log data for nephrectomies were obtained from the American Board of Urology for all urologists certifying or recertifying, from 2002 to 2010. We evaluated the trends in nephrectomy use. Logistic regressions were used to evaluate surgeon and practice characteristics as predictors for partial and laparoscopic procedures.
Results
From the 3,852 case logs submitted by non-pediatric urologists, 48,384 nephrectomies were analyzed. From 2002 to 2010, the proportion of annual nephrectomies that were performed as open radical nephrectomies gradually decreased from 54% to 29%. During the same period, there was a moderate gradual increase of laparoscopic radical nephrectomy usage, from 30% to 39%. The proportion of open partial nephrectomy remained stable at 15% while laparoscopic partial nephrectomy increased from 2% to 17%. On multivariable analysis, usage of partial nephrectomy and laparoscopy was predicted by a urologist’s annual nephrectomy volume, initial or recertification status, subspecialty, practice area size, and geographic region.
Conclusions
Since 2002, usage of laparoscopic nephrectomy and partial nephrectomy has increased. However, the diffusion of these techniques is not uniform. Initial certification, higher surgical volume, and practicing in areas over 1,000,000 and northeast region were associated with higher usage of laparoscopy and partial nephrectomy. Factors that affect the adoption of these techniques require further research.
Keywords: kidney neoplasms, laparoscopy, nephrectomy, physician’s practice patterns
INTRODUCTION
Kidney cancer incidence has been steadily increasing, with an estimated 64,770 new cases diagnosed in the United States in 2012.1 The five-fold increase observed since the 1970s is largely due to modern widespread use of imaging: greater than 70% of renal tumors are now incidentally detected and are often less than 4 cm.2 Given the more indolent course of these small renal masses, attention is being given to minimizing treatment morbidity. The once gold standard ORN is increasingly being challenged by surgical techniques aimed at sparing renal parenchyma and reducing incisional morbidity.
During the past two decades, two significant treatment advances have been made. The first was the expanded use of the PN. It was initially utilized only in patients with absolute indications for renal-sparing surgery. However, given equivalent oncologic outcomes for PN and RN and increasing evidence that surgically-induced renal failure results in a host of negative cardiovascular and metabolic consequences,3 PN is now considered the treatment of choice for appropriately selected patients with tumors up to 7cm.4, 5 The other major treatment change came with the minimally invasive era when the first LRN was described in 1991.6 The advantages of laparoscopy are shorter convalescence, decreased postoperative pain, and improved cosmesis.7 More recently, the LPN has also been utilized successfully, but due to its technical complexity, its usage has not been widespread.
Each of these newer operations (OPN, LPN, LRN) is associated with a differing degree of technical complexity, varying complications, and a learning curve that must be mastered. Several recent studies have suggested that ORN continues to be overused,8, 9 and this may be in part because the choices of surgical approach are more reflective of surgeon characteristics than of patients’ disease characteristics.10 Furthermore, the concurrent introduction of LRN and PN within a short time period may be inhibiting the complete diffusion of these techniques, with surgeons opting for one innovation or the other.11 We investigated these concepts by analyzing contemporary case logs from American urologists and evaluating surgeon and practice characteristics as predictors for partial and laparoscopic nephrectomy.
PATIENTS AND METHODS
Study Cohort and Data Source
Since 1985, urologists who seek initial certification by the ABU must submit case logs containing CPT codes for procedures done within a consecutive 6-month period.12 This process is then repeated every ten years to maintain certification.13 Thus, each year the ABU receives case log data representing the surgical volume of roughly 10% of the estimated 6,000 urologists who have certified since 1985.
For our study, de-identified, annualized electronic case log data for nephrectomy between October 2002 and August 2010 were obtained from the ABU. Queried CPT codes are listed in the Appendix. Given the limits in coding, we were unable to identify robotic or hand-assisted nephrectomies among traditional laparoscopic procedures. Patient and tumor characteristics were unknown. Urologists provided self-descriptive information including age, gender, initial versus recertification status, self-identified sub-specialization, and practice details (see Table 1). Practice type was classified as private, non-private, or both based on self-selection of up to three of 14 practice options provided by the ABU. The first two zip code digits were available and were used to categorize the urologist’s geographic region. Urologists who identified themselves as pediatric urologists were excluded.
Table 1.
Characteristics of certifying urologists
| N=3852 | |
|---|---|
| Median age (IQR) | 47 (41–54) |
|
Median annual volume of partial or radical nephrectomies (IQR) |
8 (4–16) |
| No. male (%) | 3599 (93%) |
|
No. year of Initial certification (%) (N=3629) |
|
| 1976–1990 | 675 (19%) |
| 1991–1995 | 399 (11%) |
| 1996–2000 | 851 (23%) |
| 2001–2005 | 746 (21%) |
| 2006–2010 | 958 (26%) |
| No. type of certification (%) | |
| Initial certification | 1501 (39%) |
| First recertification | 1379 (36%) |
| Second recertification | 972 (25%) |
| No. specialty (%) | |
| Andrology | 29 (1%) |
| Endourology | 212 (6%) |
| Female urology | 71 (2%) |
| General urology | 3168 (82%) |
| Infertility | 2 (0%) |
| Oncology | 310 (8%) |
| Urolithiasis | 60 (2%) |
| No. practice type (%)* | |
| Private | 2445 (63%) |
| Non-private | 1117 (29%) |
| Both private and non-private | 290 (8%) |
| No. population practice area size (%) | |
| Less than 100,000 | 356 (9%) |
| 100,000–250,000 | 484 (13%) |
| 250,001–500,000 | 407 (11%) |
| 500,001–1,000,000 | 397 (10%) |
| Over 1,000,000 | 900 (23%) |
| Unknown | 1308 (34%) |
| No. region of United States (%) | |
| Northeast | 782 (20%) |
| South | 1377 (36%) |
| Midwest | 867 (23%) |
| West | 816 (21%) |
| Foreign/Other | 10 (0%) |
Private practice included urologists in group, solo, or managed care; non-private practice included urologists employed by military/government including Veterans Affairs, academic faculty, medical administration, hospital/clinic salaried employees, urologists working in industry or those in state/local government.
Statistical Methods
We described trends in the use of PN and laparoscopic technique among urologists submitting case logs for board certification. We hypothesized that younger urologists (those initially certifying) would be more likely to perform laparoscopic and nephron-sparing procedures than older (recertifying) urologists because of the recent adoption of NSS and MIS techniques. Furthermore, based on increasing data showing a survival benefit for PN14 and changes in AUA guidelines for the management of the small renal mass,4 we further hypothesized that the proportion of NSS performed would have increased over time. Lastly, we assessed surgeon and practice characteristics associated with NSS and laparoscopy.
We used multivariable logistic regression to evaluate the surgeon factors (age, type of certification, annual nephrectomy volume, and specialty) and practice factors (practice type, practice area size, and region) associated with partial and laparoscopic nephrectomy. Separate models were built for the procedure type and approach. Age of surgeon was found to be collinear with type of certification and subsequently removed from the models. Type of certification was used as a surrogate for surgeon experience. All statistical analyses were conducted using STATA 12.0 (StataCorp, College Station, TX).
RESULTS
In total, 3,852 non-pediatric urologists submitted case logs to the ABU that included 48,384 procedures for RN or PN between 2002 and 2010. Nephrectomies steadily increased from 4,110 cases in 2003 to 7,676 in 2010. Table 1 shows characteristics of urologists in our study cohort. Most were male (93%), in general urology rather than a subspecialty (82%), and practiced solely in a private setting (63%). The median number of RN or PN performed in a year was 8, with 25% of urologists performing fewer than 4.
From 2002 to 2010, 2,912 urologists performed a total of 17,640 ORNs, 1,558 urologists performed 7,104 OPNs, 2,340 urologists performed 18,852 LRNs, and 853 urologists performed 4,788 LPNs. The annual proportion of ORN decreased by half, from 54% of all nephrectomies in 2003 to 29% in 2010 (Figure 1). At the same time, we observed an eight-fold increase in the annual proportion of LPN, from 2% in 2003 to 17% in 2010. There was no noticeable change in the annual proportion of OPN performed, averaging 15% between 2003 and 2010. LRN usage remained stable following a moderate increase between 2003 and 2004 (from 30% to 39%).
Figure 1.
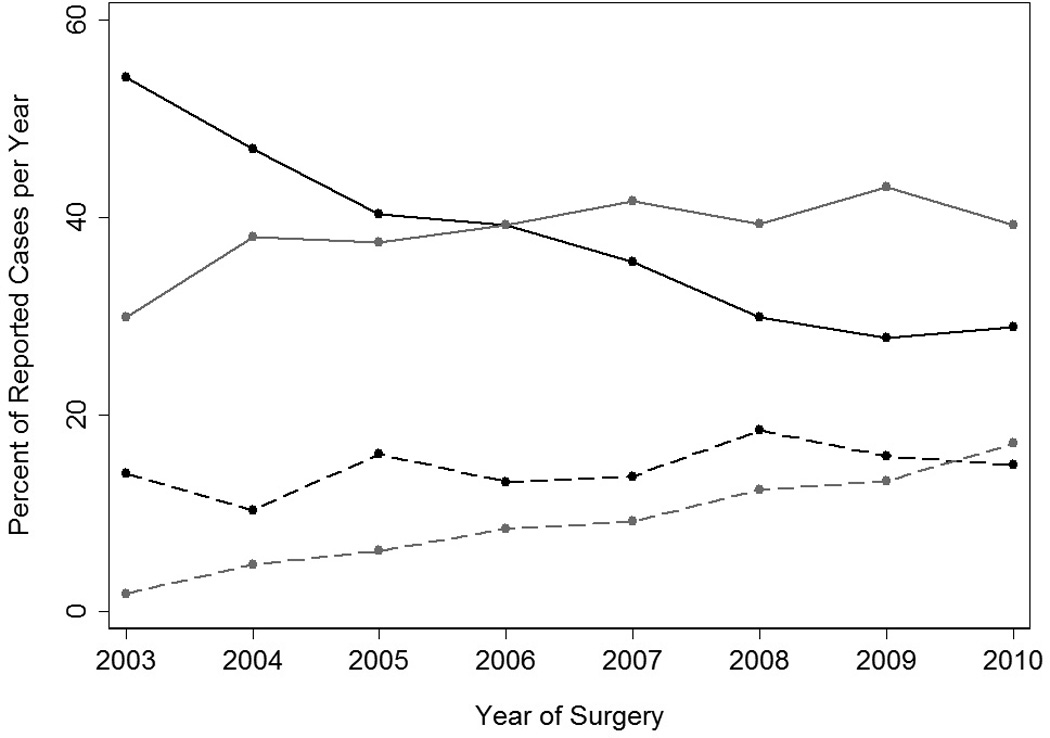
Percentage of total number of nephrectomy procedures performed as open radical nephrectomy (black line), laparoscopic radical nephrectomy (grey line), open partial nephrectomy (black dashed line), or laparoscopic partial nephrectomy (grey dashed line). 2002 log cases were excluded due to the small number of cases submitted (n=84).
Figure 2 depicts the increase in individual proportion of PN as volume of annual nephrectomies increases; this was true of all certification types. However, initial certifiers performed, on average, a larger proportion of PN than first-time and second-time recertifying urologists regardless of their annual volume. We did not observe a corresponding difference between first- and second-time recertifying urologists. Lowess methods were also used to address the non-normality in surgeon volume, and the results were similar. For example, in our main analysis, the percentage of PNs for first certification increased in an approximately linear fashion from 21%–25% as volume increased from 1–40; in the sensitivity analysis, we again saw a linear relationship with an increase from 26% to 30%.
Figure 2.
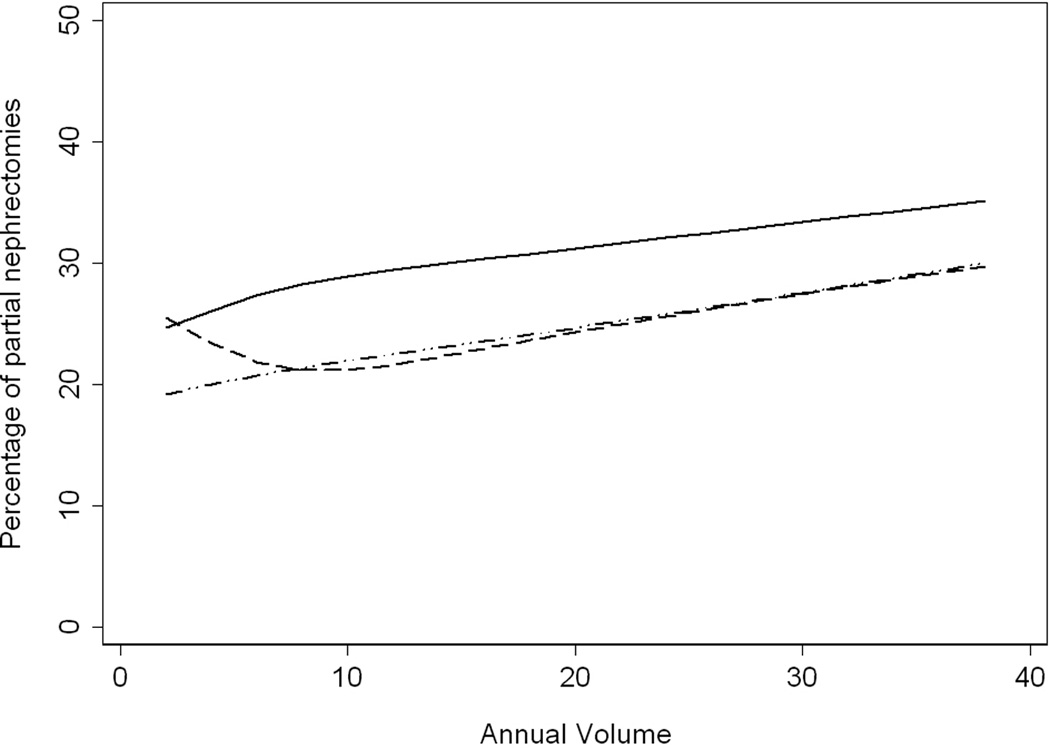
Percentage of nephrectomy procedures performed as partial nephrectomy (open or laparoscopic) by urologist’s annual volume of nephrectomies, stratified by certification type. (Initial certification = solid line, first recertification = dashed line, second recertification = dotted dash line.)
On multivariable analyses, the urologist’s annual volume was associated with performing PN (OR 1.06, p <0.0001, see Table 2). Newly certified urologists had significantly higher odds of performing PN (OR 1.15, p <0.0001) but there was no significant difference in the odds of performing PN between first and second-time recertifiers after adjusting for surgeon and practice characteristics (p = 0.2).
Table 2.
Multivariable analyses of characteristics associated with partial nephrectomy, laparoscopic nephrectomy, or laparoscopic partial nephrectomy
| Partial Nephrectomy | Laparoscopic Nephrectomy | Laparoscopic Partial Nephrectomy |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P-value | OR | 95%CI | P-value | OR | 95%CI | P-value | |
| Volume per 10 cases | 1.06 | 1.05, 1.07 | <0.0001 | 1.08 | 1.07, 1.09 | <0.0001 | 1.09 | 1.07, 1.10 | <0.0001 |
| Specialty | |||||||||
| General urology | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Andrology | 1.35 | 0.89, 2.02 | 0.2 | 0.88 | 0.60, 1.28 | 0.5 | 0.62 | 0.29, 1.31 | 0.2 |
| Endourology | 1.00 | 0.91, 1.09 | 0.9 | 2.12 | 1.93, 2.32 | <0.0001 | 1.13 | 1.01, 1.27 | 0.031 |
| Female urology | 0.64 | 0.43, 0.95 | 0.026 | 0.60 | 0.44, 0.82 | 0.001 | 0.65 | 0.33, 1.27 | 0.2 |
| Infertility | 2.19 | 0.76, 6.33 | 0.15 | 1.76 | 0.55, 5.61 | 0.3 | 0.95 | 0.20, 4.49 | 0.9 |
| Oncology | 1.34 | 1.24, 1.45 | <0.0001 | 0.52 | 0.48, 0.56 | <0.0001 | 1.11 | 1.00, 1.25 | 0.059 |
| Urolithiasis | 0.71 | 0.58, 0.87 | 0.001 | 1.37 | 1.15, 1.64 | 0.0004 | 0.62 | 0.47, 0.82 | 0.001 |
| Certification | |||||||||
| Initial certification | 1.15 | 1.07–1.24 | <0.0001 | 1.94 | 1.82, 2.07 | <0.0001 | 1.14 | 1.02, 1.28 | 0.016 |
| 1st recertification | 0.95 | 0.88, 1.02 | 0.2 | 1.32 | 1.24, 1.41 | <0.0001 | 0.93 | 0.83, 1.04 | 0.2 |
| 2nd recertification | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Practice Area Size | |||||||||
| <500,000 population | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| 500,001 – 1,000,000 | 1.14 | 1.06, 1.23 | 0.001 | 1.00 | 0.94, 1.07 | 1 | 1.30 | 1.17, 1.45 | <0.0001 |
| Over 1,000,000 | 1.22 | 1.15, 1.30 | <0.0001 | 1.13 | 1.07, 1.20 | <0.0001 | 1.34 | 1.23, 1.47 | <0.0001 |
| Practice Type | |||||||||
| Non-private practice | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Private practice | 0.79 | 0.73, 0.84 | <0.0001 | 1.05 | 0.98, 1.12 | 0.14 | 0.74 | 0.67, 0.81 | <0.0001 |
| Both private and non-private practice |
0.98 | 0.88, 1.08 | 0.6 | 1.09 | 1.00, 1.20 | 0.064 | 0.88 | 0.76, 1.01 | 0.071 |
| Region | |||||||||
| Regions other than Northeast | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Northeast | 1.09 | 1.02, 1.17 | 0.010 | 1.14 | 1.07, 1.21 | <0.0001 | 1.07 | 0.97, 1.18 | 0.2 |
Certain subspecialties, practice area size, practice type, and region were also associated with PN (Table 2). After adjusting for surgeon and practice characteristics, the odds of performing PN were significantly higher if the urologist specialized in oncology (OR 1.34, p <0.0001). However, urologists had significantly lower odds of performing PN if they subspecialized in female urology (OR 0.64, p = 0.026) or urolithiasis (OR 0.71, p = 0.001). Having a practice in areas with a population larger than 500,000 (500,000–1,000,000: OR 1.14, p = 0.001; over 1,000,000: OR 1.22, p <0.0001) or in the Northeast (OR 1.09, p = 0.010) was also associated with higher odds of performing PN on multivariable analyses. Conversely, the odds were significantly lower after controlling for the covariates if the urologist was in a private practice (OR 0.79, p <0.0001).
Similar to the trend we observed for PN, the individual proportion of laparoscopic procedures increased with the surgeon’s volume of annual nephrectomies (see Figure 3). Additionally, initial certifiers performed on average a larger proportion of laparoscopic nephrectomies than first and second recertifiers; this remained true regardless of annual volume. Both annual volume and certification type were associated with using a laparoscopic approach after controlling for surgeon and practice characteristics (all p <0.0001, see Table 2). Recently trained urologists had double the odds of using a laparoscopic approach and first recertifiers had one-third higher odds, compared to second recertifiers. Multivariable analyses revealed that endourology (OR 2.12, p <0.0001) and urolithiasis (OR 1.37, p =0.0004) subspecialties were associated with higher odds of using laparoscopy, while female (OR 0.60, p = 0.001) and oncology (OR 0.52, p <0.0001) subspecialties were associated with lower odds of using laparoscopy (Table 2). Urologists who practiced in areas larger than 1,000,000 population or within the Northeast had slightly higher odds of using laparoscopy (OR 1.13, p <0.0001 and OR 1.14, p <0.0001, respectively).
Figure 3.
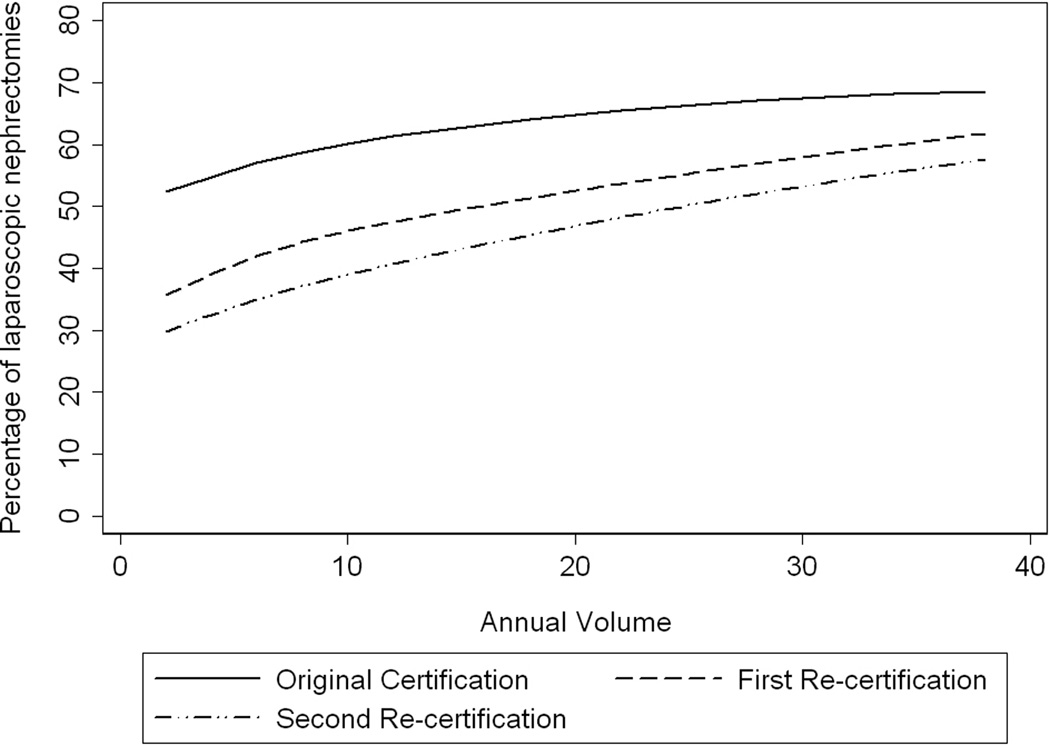
Percentage of nephrectomy procedures performed as laparoscopic partial or radical nephrectomies by urologist’s annual volume of nephrectomies, stratified by certification type. (Initial certification = solid line, first recertification = dashed line, second recertification = dotted dash line.)
Lastly, we assessed the factors associated with LPN. Use of LPN was associated with higher annual volume of nephrectomies (OR 1.09, p<0.0001), initial certification (OR 1.14, p = 0.016), endourology subspecialty (OR 1.13, p = 0.031) and practicing in a population area >500,000. Urologists subspecializing in urolithiasis (OR 0.62, p=0.001) and being in private practice (OR 0.74, p<0.0001) were associated with lower odder odds of performing LPN.
DISCUSSION
Our present analysis of case log data from certifying and recertifying urologists provides insight into the contemporary practice patterns of American urologists. The optimal proportion of PN and laparoscopic operations that should have been performed in this cohort is unknown. The surgical approach is clearly influenced by many variables such as comorbid conditions, tumor location/size, and patient preferences, but this work highlights the importance of surgeon characteristics. Several important observations can be derived from the present study.
Our data are consistent with prior findings that partial and laparoscopic nephrectomy are associated with a urologist’s volume of nephrectomies.8, 15, 16 Higher-volume surgeons perform a greater absolute number and higher proportion of partial and laparoscopic nephrectomies. In addition, the usage of partial and laparoscopic nephrectomy were intimately associated with a urologist’s certification status. Even at lower annual nephrectomy volume, PN was more likely to be performed by initial certifying urologists than more experienced, recertifying surgeons. Thus, their surgical practice is reflective of current techniques practiced at academic centers and reinforces the concept of high quality surgical training.
The trend for the relevance of certification status extended to the adoption of laparoscopy (Figure 3). Overall laparoscopic usage increased during the study period, but the further the urologist was from training, the less likely he or she was to perform a laparoscopic nephrectomy (LRN or LPN). Filson et al. had similar findings in an analysis of patients undergoing nephrectomy for early-stage kidney cancer, using linked NCI SEER-Medicare data (1995–2005).17 In this data set, LRN increased from 1.4% in 1995 to 44.9% in 2005; the authors identified recent medical school graduation (after 1991) as a predictor of laparoscopy usage. They reasoned that younger surgeons would have had formal laparoscopic training during residency; however, even among the subset of recent graduates, laparoscopy usage varied significantly depending on whether the surgeon had an affiliation with an academic hospital and/or NCI-designated center or practiced in an urban setting.17
Another noteworthy observation in our analysis is that urologists who self-identified their specialty as “endourology” or “urolithiasis” were more likely to perform a laparoscopic nephrectomy (OR 2.12 and 1.37, respectively), but “urolithiasis”-defined practitioners were less likely to do PN (OR 0.71). Conversely, urologists with an “oncology”-defined practice were less likely to perform laparoscopy (OR 0.52) but more likely to perform PN (OR 1.34). This divergence may be partly accounted by different patient populations but likely also reflects differences in practice patterns and in adoption of new technology among subspecialty groups. These types of “surgical signatures”18, 19 in kidney cancer surgery have also been described in an analysis of linked SEER-Medicare data (1997–2002) by Miller et al.19 While the dataset reflects an older transitional time period, these authors robustly revealed that for many patients the likelihood of undergoing PN or laparoscopic surgery was more dependent on the surgeon’s practice style than any patient factor.19
Given laparoscopy’s introduction over two decades ago, its slow integration into routine care of kidney cancer is intriguing when contrasted to the explosive adoption of robotic surgery for prostate cancer.20 Despite the steep learning curve,21 the significant capital investment required, and the questionable clinical benefit,22 Lowrance et al. reviewed ABU case log data and found that 67% of RPs done by certifying or recertifying urologists in 2010 were done robotically as compared to only 8% in 2004.20 It is unclear if robotic renal surgery will ultimately be adopted in the same fashion as robotic RP, but the impact will likely not be inconsequential.
The increase in laparoscopic nephrectomy we observed included robotic-assisted procedures as we could not distinguish robotic procedures from traditional laparoscopic ones due to lack of a specific CPT code. The growing familiarity with robotic RP has likely eased the transition into minimally invasive renal surgery for many urologists. Indeed, we noted a dramatic increase in LPN usage over the study period; in 2010, there was a greater usage of LPN than OPN. We hypothesize that this largely reflects an increase in robotic-assisted LPN. The robotic platform facilitates laparoscopic suturing and renorrhaphy when compared to the traditional laparoscopy. Minimally invasive-oriented urologists who once favored the LRN for the shorter convalescence period may be opting for a robotic-assisted LPN in select patients.
The strength of this study is that the data represent the contemporary experience of urologists from all geographic locations and practice types in the United States. The dataset is not limited to operations done at specific hospital sites or patients within a specific insurance coverage. However, several limitations need to be mentioned. Case logs are self-reported and certifying urologists may report a high volume six-month period that is not representative of their average practice to insure certification. Subspecialty designations were self-reported and do not reflect specific fellowship training. Urologists certified prior to 1985 are not required to submit case logs for recertification, thus the data are skewed towards younger urologists’ practices and the use of OPN and laparoscopy may be overestimated. Furthermore, as we lacked any clinical or pathologic data, the indication for nephrectomy was unknown. Approximately 20–30% of tumors are not amenable to kidney-sparing approaches,23 but this percentage should have remained stable during the study period. The management of small renal masses also includes ablative techniques and active surveillance. Ablative techniques were not analyzed due to small numbers and the inability to capture patients being referred to interventional radiologists. We were unable to capture active surveillance usage due to limitations of case log data.
CONCLUSIONS
While the use of laparoscopy and PN has increased between 2002 and 2010, surgeon characteristics and practice patterns play a clear role in the type of nephrectomy a patient receives. Higher surgical volume, practicing in areas over 1,000,000 and northeast region were associated with higher usage of laparoscopy and partial nephrectomy. Initial certifying urologists were more likely to utilize laparoscopy and PN than recertifying urologists, underscoring the role of surgical training during residency and fellowship. Further research is required on how to reduce barriers for surgeons, particularly those already in practice, to adopt NSS and MIS approaches. Educational programs and resources for urologists and patients alike are needed to ensure the medical and oncologic value of PN is widely understood.
Acknowledgments
Funding:
This work was supported by the Sidney Kimmel Center for Prostate and Urologic Cancers. SAP and JLS are supported by the NCI T32 CA082088-11 training grant. The authors were solely responsible for the following: study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the article for publication.
ABBREVIATIONS AND ACRONYMS
- ABU
American Board of Urology
- AUA
American Urological Association
- CKD
chronic kidney disease
- CPT
Common Procedural Terminology
- LPN
laparoscopic PN
- LRN
laparoscopic RN
- MIS
minimally invasive surgery
- NCI
National Cancer Institute
- NSS
nephron-sparing surgery
- OPN
open PN
- ORN
open RN
- PN
partial nephrectomy
- RN
radical nephrectomy
- RP
radical prostatectomy
- SEER
Surveillance, Epidemiology, and End Results
Appendix. Current Procedural Terminology codes queried for renal surgery
50220 – Nephrectomy, including partial ureterectomy, any open approach including rib resection
50225 – Nephrectomy, including partial ureterectomy, any open approach including rib resection; complicated because of previous surgery on same kidney
50230 – Nephrectomy, including partial ureterectomy, any open approach including rib resection; radical, with regional lymphadenectomy and/or vena caval thrombectomy
50240 – Nephrectomy, partial
50543 – Laparoscopy, surgical; partial nephrectomy
50545 – Laparoscopy, surgical; radical nephrectomy (includes removal of Gerota's fascia and surrounding fatty tissue, removal of regional lymph nodes, and adrenalectomy)
50546 – Laparoscopy, surgical; nephrectomy, including partial ureterectomy
Footnotes
Data Source/IRB:
The data was extracted from the log review database that is maintained by the staff and resources of the American Board of Urology (ABU), a member organization of the American Board of Medical Specialties. All data were de-identified, and therefore, IRB approval was not required. The authors are responsible for the content of this paper, and the presented views do not reflect endorsement by the ABU.
Authors’ contributions:
The study was conceived by SAP and JLS. All authors were responsible for the overall study design. LYC conducted statistical analyses. All authors contributed to the manuscript and approved the final version.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2012. [Accessed September 1, 2012]. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf. [Google Scholar]
- 2.Russo P. Renal cell carcinoma: presentation, staging, and surgical treatment. Semin Oncol. 2000;27:160. [PubMed] [Google Scholar]
- 3.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Urological Association. Guideline for management of the clinical stage 1 renal mass. Linthicum, MD: American Urological Association Education and Research, Inc.; 2009. [Accessed August 1, 2012]. http://www.auanet.org/content/media/renalmass09.pdf. [Google Scholar]
- 5.Thompson RH, Siddiqui S, Lohse CM, et al. Partial versus radical nephrectomy for 4 to 7 cm renal cortical tumors. J Urol. 2009;182:2601. doi: 10.1016/j.juro.2009.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clayman RV, Kavoussi LR, Soper NJ, et al. Laparoscopic nephrectomy: initial case report. J Urol. 1991;146:278. doi: 10.1016/s0022-5347(17)37770-4. [DOI] [PubMed] [Google Scholar]
- 7.Dunn MD, Portis AJ, Shalhav AL, et al. Laparoscopic versus open radical nephrectomy: a 9-year experience. J Urol. 2000;164:1153. [PubMed] [Google Scholar]
- 8.Hollenbeck BK, Taub DA, Miller DC, et al. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006;67:254. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 9.Miller DC, Hollingsworth JM, Hafez KS, et al. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol. 2006;175:853. doi: 10.1016/S0022-5347(05)00422-2. [DOI] [PubMed] [Google Scholar]
- 10.Miller DC, Taub DA, Dunn RL, et al. Laparoscopy for renal cell carcinoma: diffusion versus regionalization? J Urol. 2006;176:1102. doi: 10.1016/j.juro.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 11.Abouassaly R, Alibhai SM, Tomlinson G, et al. Unintended consequences of laparoscopic surgery on partial nephrectomy for kidney cancer. J Urol. 2010;183:467. doi: 10.1016/j.juro.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Howards SS. Information for applicants and candidates. 58 ed. Charlottesville: American Board of Urology, Inc.; 2011. [Google Scholar]
- 13.Howards SS. Information for applicants for recertification. 20 ed. Charlottesville: American Board of Urology, Inc.; 2011. [Google Scholar]
- 14.Tan HJ, Norton EC, Ye Z, et al. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307:1629. doi: 10.1001/jama.2012.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller DC, Wei JT, Dunn RL, et al. Trends in the diffusion of laparoscopic nephrectomy. JAMA. 2006;295:2480. doi: 10.1001/jama.295.21.2480. [DOI] [PubMed] [Google Scholar]
- 16.Patel SG, Penson DF, Pabla B, et al. National trends in the use of partial nephrectomy: a rising tide that has not lifted all boats. J Urol. 2012;187:816. doi: 10.1016/j.juro.2011.10.173. [DOI] [PubMed] [Google Scholar]
- 17.Filson CP, Banerjee M, Wolf JS, Jr, et al. Surgeon characteristics and long-term trends in the adoption of laparoscopic radical nephrectomy. J Urol. 2011;185:2072. doi: 10.1016/j.juro.2011.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baicker K, Chandra A, Skinner JS, et al. Who you are and where you live: how race and geography affect the treatment of medicare beneficiaries. Health affairs. 2004 doi: 10.1377/hlthaff.var.33. Suppl Variation: VAR33. [DOI] [PubMed] [Google Scholar]
- 19.Miller DC, Saigal CS, Banerjee M, et al. Diffusion of surgical innovation among patients with kidney cancer. Cancer. 2008;112:1708. doi: 10.1002/cncr.23372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowrance WT, Eastham JA, Savage C, et al. Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. J Urol. 2012;187:2087. doi: 10.1016/j.juro.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrell SD, Smith JA., Jr Robotic-assisted laparoscopic prostatectomy: what is the learning curve? Urology. 2005;66:105. doi: 10.1016/j.urology.2005.06.084. [DOI] [PubMed] [Google Scholar]
- 22.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302:1557. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 23.Russo P. The role of surgery in the management of early-stage renal cancer. Hematology/oncology clinics of North America. 2011;25:737. doi: 10.1016/j.hoc.2011.04.009. [DOI] [PubMed] [Google Scholar]


