Abstract
Background
A growing body of evidence suggests that deficits in long-chain omega-3 (LCn-3) fatty acids may contribute to major depressive disorder (MDD) and principal causes of excess mortality including suicide and cardiovascular disease. In the present study we compared concentrations of docosahexaenoic acid (DHA, 22:6n-3), the principal LCn-3 fatty acid in brain, in the postmortem prefrontal cortex (BA10) of adult depressed suicide victims and controls with and/or without cardiovascular disease.
Methods
DHA concentrations (μmol/g) in the prefrontal cortex (PFC, BA10) of adult male and female suicide victims (n=20) and controls with (n=8) or without (n=12) cardiovascular disease were determined by gas chromatography.
Results
There was a non-significant trend for lower DHA concentrations in suicide victims compared with all controls (−10%, p=0.06, d = 0.5). Significantly lower DHA concentrations were observed in suicide victims compared with controls without cardiovascular disease (−14%, p=0.03, d = 0.7) but not controls with cardiovascular disease (−4%, p=0.71, d = 0.1). There was a non-significant trend for lower DHA concentrations in controls with cardiovascular disease compared with controls without cardiovascular disease (−11%, p=0.1, d = 0.6).
Conclusions
Adult depressed suicide victims exhibit lower postmortem PFC DHA concentrations compared with controls without cardiovascular disease. These data add to a growing body of evidence implicating DHA deficits in the pathophysiology of MDD, suicide, and cardiovascular disease.
Keywords: Suicide, adolescent, prefrontal cortex, postmortem brain, omega-3 fatty acid, docosahexaenoic acid (DHA)
1. Introduction
Major depressive disorder (MDD) is associated with excess premature mortality primarily due to suicide and cardiovascular-related diseases (Angst et al., 2002; Osby et al., 2001). A growing body of evidence has emerged over the past two decades that suggest that a deficiency in dietary essential long-chain omega-3 (LCn-3) fatty acids, eicosapenaenoic acid (EPA) and docosahexaenoic acid (DHA), may represent a modifiable risk factor for depression and comorbid cardiovascular disease (McNamara, 2009a). The principal LCn-3 fatty acid found in mammalian gray matter is DHA (Chen et al., 2011; Carver et al., 2001), which preferentially accumulates in gray matter synaptasomal and mitochondrial membranes (Suzuki et al., 1997). DHA is mobilized from membrane phospholipids by the calcium-independent phospholipase A2 (iPLA2) isoform (Farooqui & Horrocks, 2004) and free DHA is a substrate for anti-inflammatory docosanoids (Groeger et al., 2010; Hong et al., 2003). We originally reported that MDD patients exhibit significant and selective DHA deficits in postmortem prefrontal cortex (PFC)(Brodmann area, BA 10)(McNamara et al., 2007). However, other postmortem studies did not observe lower DHA levels in the PFC of adult or adolescent suicide victims with or without comorbid MDD (Lalovic et al., 2007; McNamara et al., 2009b; Tatebayashi et al., 2012), and a number of different pre and postmortem variables may contribute to this discrepancy (McNamara & Jandacek, 2011).
A large percentage of control subjects typically used in postmortem brain studies died from cardiovascular-related diseases which may represent a potential confound, particularly when investigating DHA levels. Cardiovascular disease is associated with low erythrocyte DHA levels similar to those observed in MDD patients (Block et al., 2008; McNamara, 2009a), and erythrocyte DHA levels are positively correlated with frontal cortex DHA levels (Carver et al., 2001; Connor et al., 1990). Furthermore, low DHA levels are associated with clinical depression in patients with cardiovascular disease (Frasure-Smith et al., 2004; Parker et al., 2006). The primary goal of the present study was to determine DHA concentrations in the postmortem PFC (BA 10) of adult MDD patients that died from suicide and controls with and/or without cardiovascular disease. Based on this evidence, our a priori prediction was that depressed suicide victims would exhibit DHA deficits compared with controls dying from non-cardiovascular related diseases, but not compared with controls dying from cardiovascular disease.
2. Methods and materials
2.1. Postmortem brain tissues
Frozen, unfixed, postmortem prefrontal cortex (BA 10) tissue from adult suicide victims (n=20) and adult controls (n=20) were obtained from the Maryland Brain Collection at the Maryland Psychiatric Research Center, Baltimore, MD. Brain samples were free of neuropathologic abnormalities and human immunodeficiency virus antibodies. All subjects in this study were diagnosed using the Schedule for Clinical Interviews for the DSM-IV (SCID) (First et al., 1997). The SCID was administered by a trained interviewer using a family member as an informant and included a review of all obtainable medical and psychiatric records. The SCID diagnoses were validated by two trained psychiatrists (Kappa >0.9). This has been found to be a very accurate way to make diagnoses (Ramirez Basco et al., 2000). All procedures were approved by the University of Maryland Institutional Review Board.
2.2. Gas chromatography
DHA concentrations were determined using the saponification and methylation methods described previously (McNamara et al., 2009b). A known mass (0.5 mg) of heptadecanoic acid (17:0, 99%, Matreya LLC Inc., Pleasant Gap PA) was added to each sample prior to adding the saponification solution, and tissue fatty acid concentrations (μmol/g tissue weight) calculated from the relative mass of the heptadecanoic acid peak. Samples were analyzed with a Shimadzu GC-2010 equipped with an auto-injector (Shimadzu Scientific Instruments Inc., Columbia MD). The column was a DB-23 (123–2332): 30 m (length), I.D. 0.32 mm wide bore, film thickness of 0.25 μM (J&W Scientific, Folsom CA). Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap PA). Analysis of fatty acid methyl esters is based on areas calculated with EZstart 7.4 software. Both DHA composition (mg fatty acid/100 mg fatty acids) and concentration (μmol/g) data were obtained. All samples were processed by a technician that was blind to subject diagnosis.
2.3. Statistical analysis
For the a priori analysis, case-control differences in DHA concentrations were evaluated with a one-tailed t-test (α=0.05). Analysis of gender effects was performed with a two-way ANOVA using gender (male, female) and cause-of-death (suicide, control) as the main factors. Parametric linear regression analyses were performed to determine the relationship between DHA concentrations, postmortem tissue variables, and selected demographic variables (α=0.05). Effect size was calculated using Cohen’s d, with small, medium, and large effect sizes being equivalent to d-values of 0.30, 0.50, and 0.80, respectively (Cohen, 1997). All statistical tests were performed with GB-STAT software (Dynamic Microsystems, Inc., Silver Springs MD).
3. Results
3.1. Demographic variables
A comparison of subject and tissue variables is presented in Table 1, and a list of individual subject demographic, clinical, and toxicological variables are presented in Supplemental Table 1. The majority (n=19/20) of suicide victims had a history of MDD, and n=1 had a history of bipolar disorder. Controls were verified as free from psychiatric illness and substance abuse. A subset of controls died from atherosclerotic cardiovascular disease (n=7) or cardiac arrhythmia (n=1), and remaining controls (n=12) died from non-cardiac causes. There were no significant differences between depressed suicides and controls dying from non-cardiovascular-related diseases in age (p=0.83) or gender (p=0.72), or between depressed suicides and controls dying from cardiovascular-related diseases in age (p=0.46) or gender (p=0.19). DHA concentrations were not correlated with age in suicides (r = +0.30, p=0.2), controls (r = −0.16, p=0.5), or when both groups were combined (r = +0.08, p=0.6).
Table 1.
Demographic and Tissue Characteristics
| Controls (n=20) | Suicides (n=20) | P-value1 | |
|---|---|---|---|
| Subject Characteristics: | |||
| Age at death, mean ± S.D. | 41.5 ± 16.1 | 39.9 ± 15.7 | 0.75 |
| Male | 39.1 ± 15.1 | 44.9 ± 13.7 | 0.83 |
| Female | 51.8 ± 14.7 | 37.8 ± 16.5 | 0.39 |
| Gender (n ) | 5F,15M | 9F,11M | 0.32 |
| Race (n ) | 14C,6AA | 18C,2AA | 0.23 |
| Cause of death (n ) | |||
| Suicide | 0 | 20 | – |
| Cardiovacular disease | 8 | 0 | |
| Other | 12 | 0 | |
| Diagnosed with MDD | 0 | 19 | – |
| Diagnosed with Bipolar Disorder | 0 | 1 | – |
| Tissue Characteristics: | |||
| Brain hemisphere | 18R/2L | 14R/4L/2UN | 0.23 |
| Postmoretm Interval (mean hrs ± S.D.) | 18.3 ± 7.4 | 19.1 ± 6.7 | 0.72 |
| Tissue pH (mean ± S.D.) | 6.2 ± 0.4 | 6.2 ± 0.6 | 0.55 |
Two-tailed t-test or Chi-squared test
Race: C = Caucasian, AA = African American
UN = Unknown
3.2. Postmortem variables
Within the control group (n=20), there were no significant correlations between DHA concentrations and brain pH (r = −0.11, p=0.64) or postmortem interval (r = −0.03, p=0.89). Within the suicide group (n=20), there were no significant correlations between DHA concentration and brain pH (r = +0.30, p=0.2) or postmortem interval (r = +0.29, p=0.21). After combining control and suicide groups (N=40), there were no significant correlations between DHA concentration and brain pH (r = +0.12, p=0.49) or postmortem interval (r = +0.11, p=0.48). There were no significant differences between depressed suicides and controls dying from non-cardiovascular-related diseases in pH (p=0.97) or postmortem interval (p=0.76), or between depressed suicides and controls dying from cardiovascular-related diseases in pH (p=0.27) and postmortem interval (p=0.77).
3.3. Group differences
A list of individual fatty acid concentrations and compositions are presented in Supplemental Tables 2 and 3. There was a non-significant trend for lower DHA concentrations in depressed suicide victims compared with all control subjects (−10%, p=0.06, d = 0.5). Significantly lower DHA concentrations were observed in suicide victims compared with controls without cardiovascular disease (−14%, one-tailed, p=0.03, d = 0.7) but not when compared with controls with cardiovascular disease (−4%, p=0.71, d = 0.1)(Fig. 1). There was a non-significant trend for lower DHA concentrations in controls with cardiovascular disease compared with controls without cardiovascular disease (−11%, p=0.2). The main effect of gender and the group x gender interaction were not significant (p>0.05). Among all subjects (N=40) DHA concentrations (μmol/g) were positively correlated with DHA composition (mg fatty acid/100 mg fatty acids)(r = +0.86, p≤0.0001). There was a non-significant trend for lower DHA composition in depressed suicide victims compared with controls without cardiovascular disease (−14%, p=0.1, d = 0.4) but not compared to controls with cardiovascular disease (+7%, p=0.71, d = 0.1).
Figure 1.
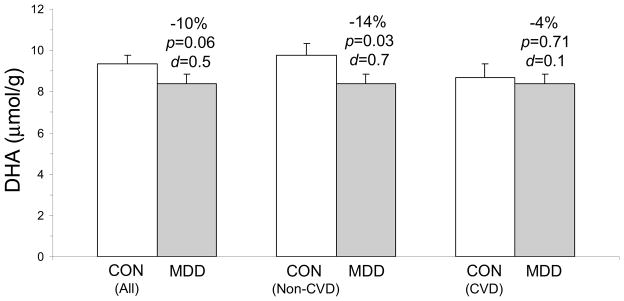
Comparison of postmortem PFC (BA10) DHA concentrations (μmol/g) in depressed suicide victims (MDD, n=20) and all controls (n=20), controls dying from non-cardiovascular-related diseases (No CVD, n=12), and controls dying from cardiovascular-related diseases (CVD, n=8). Percent differences, p-values (one-tail t-test), and Cohen d-values are presented. Values are group means ± S.E.M.
4. Discussion
The main finding of the present study is that adult depressed suicide victims exhibit significantly lower DHA concentrations when compared with controls without cardiovascular disease but not when compared with controls with cardiovascular disease. Moreover, there was a non-significant trend for lower DHA concentrations in controls with cardiovascular disease compared with controls without cardiovascular disease. This observation emphasizes the importance of selecting controls without cardiovascular disease when evaluating case-control differences in postmortem DHA levels in psychiatric patients (McNamara & Jandacek, 2011). Moreover, this finding is congruent with results from antemortem studies finding lower blood DHA levels in suicide attempters/completers (Huan et al., 2004; Lewis et al., 2011; Garland et al., 2007) and MDD patients (McNamara et al., 2010; Lin et al., 2010), which are positively correlated with frontal cortex DHA levels (Carver et al., 2001; Connor et al., 1990). Lastly, because cardiovascular disease is associated with low erythrocyte DHA levels similar to those observed in MDD patients (Block et al., 2008; McNamara, 2009a, 2010), the present findings may have implications understanding central effects of LCn-3 fatty acid insufficiency in the pathophysiology of depression in cardiovascular disease.
Previous postmortem brain studies investigating fatty acid levels in patients with MDD used composition (i.e., weight percent of total fatty acids) rather than absolute concentration data. In the present study, although DHA concentration and composition data were positively correlated, the deficit in DHA composition observed in depressed suicide victims compared with controls without cardiovascular disease (−14%) did not reach statistical significance (p=0.1). Because fatty acid ‘composition’ data are expressed as a percentage of total fatty acids, and are therefore influenced by changes in other fatty acids, they may be associated with greater variability. Indeed, in the present study the standard deviation for DHA composition data (4.1) was twice as large as the standard deviation for DHA concentration data (2.0). It is notable that a previous postmortem brain fatty acid study also observed discrepancies between fatty acid concentration and composition data (Taha et al., 2013), and a prior postmortem study found that PFC DHA composition (vs. concentration) did not differ between depressed suicides and controls regardless of cause of death (Lalovic et al., 2007). Together these findings suggest that absolute concentration data may represent a more rigorous and sensitive measure of fatty acid levels within a lipid pool.
The finding that adult depressed suicide victims exhibit PFC DHA deficits is in general agreement with our prior postmortem study (McNamara et al., 2007). It is notable, however, that our previous study observed more robust PFC DHA deficits (−22%) in MDD patients despite a high percentage of controls (77%) that died from cardiovascular-related diseases. It may be relevant, therefore, that 50 percent of MDD patients in our prior study also died from cardiovascular-related diseases, and these patients had significantly lower DHA levels compared with controls that died from cardiovascular-related diseases. In view of the present findings, these data suggest that MDD patients with comorbid cardiovascular disease may exhibit more robust PFC DHA deficits.
While the size of the PFC DHA deficit observed in the present study was small (−14%), the effect size was medium-large and preclinical studies suggest that a DHA deficit of this size is sufficient to have functional consequences. For example, we previously reported that a similar dietary-induced deficit in adult mouse PFC DHA (−13%) was associated with augmented amphetamine-induced behavioral sensitization (McNamara et al., 2008). Another study found that a similar decrease in mouse brain DHA (−12%) was associated with reduced prepulse inhibition (Fedorova et al., 2009). Furthermore, 70 d dietary n-3 fatty acid deficiency initiated in young adulthood is associated with a 10% reduction in rat PFC DHA levels (McNamara et al., 2009c). It is also relevant that peri-adolescent dietary n-3 fatty acid insufficiency is associated with reductions in DHA turnover (DeMar et al., 2004) and iPLA2 expression/activity (Rao et al., 2007) in the rat PFC. Therefore, even moderate deficits in PFC DHA levels may lead to compensatory reductions in DHA mobilization. This is significant because free DHA is a substrate for anti-inflammatory docosanoids (Groeger et al., 2010; Hong et al., 2003), and a previous human postmortem brain study found elevated markers of pro-inflammatory signaling in the PFC of suicide victims (Pandey et al., 2012).
The observation of a trend for lower DHA concentrations in controls with cardiovascular disease compared with controls without cardiovascular disease may have implications for understanding the role of LCn-3 fatty acid deficits observed in cardiovascular disease patients with depression. Indeed, cardiovascular disease is associated with low erythrocyte DHA levels similar to those observed in MDD patients (Block et al., 2008; McNamara, 2009a, 2010), and erythrocyte DHA levels are correlated with both cardiac biopsies (Harris et al., 2004) and frontal cortex (Carver et al., 2001; Connor et al., 1990) DHA levels. Furthermore, low DHA levels are associated with clinical depression in patients with cardiovascular disease (Frasure-Smith et al., 2004; Parker et al., 2006). However, depression symptom severity scores were not available for controls with cardiovascular disease in the present study, and future studies are warranted to further elucidate the relationship between cardiovascular disease, depression, and PFC DHA concentrations.
In summary, the present case-control study observed lower DHA concentrations in the postmortem PFC of depressed suicide victims compared with controls with but not without cardiovascular disease. These data highlight the importance of selecting controls that died of acute trauma versus cardiovascular-related diseases in future case-control postmortem brain studies investigating DHA concentrations. Collectively, these data add to a growing body of evidence implicating LCn-3 fatty acid insufficiency in the pathophysiology of MDD, suicide, and cardiovascular disease.
Supplementary Material
Acknowledgments
This work was supported in part by National Institute of Mental Health grants MH024773 to R.K.M, and MH 98554 and MH48153 grants to G.N.P. The authors thank Miljana Petkovic for her technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angst F, Stassen HH, Clayton PJ, Angst J. Mortality of patients with mood disorders: follow-up over 34–38 years. Journal of Affective Disorders. 2002;68:167–181. doi: 10.1016/s0165-0327(01)00377-9. [DOI] [PubMed] [Google Scholar]
- Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA. EPA and DHA in blood cell membranes from acute coronary syndrome patients and controls. Atherosclerosis. 2008;197:821–828. doi: 10.1016/j.atherosclerosis.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Carver JD, Benford VJ, Han B, Cantor AB. The relationship between age and the fatty acid composition of cerebral cortex and erythrocytes in human subjects. Brain Research Bulletin. 2001;56:79–85. doi: 10.1016/s0361-9230(01)00551-2. [DOI] [PubMed] [Google Scholar]
- Chen CT, Liu Z, Bazinet RP. Rapid de-esterification and loss of eicosapentaenoic acid from rat brain phospholipids: an intracerebroventricular study. Journal of Neurochemistry. 2011;116:363–373. doi: 10.1111/j.1471-4159.2010.07116.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York: Academic Press; 1997. [Google Scholar]
- Connor WE, Neuringer M, Lin DS. Dietary effects on brain fatty acid composition: the reversibility of n-3 fatty acid deficiency and turnover of docosahexaenoic acid in the brain, erythrocytes, and plasma of rhesus monkeys. Journal of Lipid Research. 1990;31:237–247. [PubMed] [Google Scholar]
- DeMar JC, Jr, Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. Journal of Neurochemistry. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA. Brain phospholipases A2: a perspective on the history. Prostaglandins, Leukotrienes & Essential Fatty Acids. 2004;71:161–169. doi: 10.1016/j.plefa.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Fedorova I, Alvheim AR, Hussein N, Salem N., Jr Deficit in prepulse inhibition in mice caused by dietary n-3 fatty acid deficiency. Behavioral Neuroscience. 2009;123:1218–1225. doi: 10.1037/a0017446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Arlington, Virginia, USA: American Psychiatric Publishing, Inc; 1997. [Google Scholar]
- Frasure-Smith N, Lespérance F, Julien P. Major depression is associated with lower omega-3 fatty acid levels in patients with recent acute coronary syndromes. Biological Psychiatry. 2004;55:891–896. doi: 10.1016/j.biopsych.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Garland MR, Hallahan B, McNamara M, Carney PA, Grimes H, Hibbeln JR, Harkin A, Conroy RM. Lipids and essential fatty acids in patients presenting with self-harm. British Journal of Psychiatry. 2007;190:112–117. doi: 10.1192/bjp.bp.105.019562. [DOI] [PubMed] [Google Scholar]
- Groeger AL, Cipollina C, Cole MP, et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nature Chemical Biology. 2010;6:433–441. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–1649. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. Journal of Biological Chemistry. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Huan M, Hamazaki K, Sun Y, Itomura M, Liu H, Kang W, Watanabe S, Terasawa K, Hamazaki T. Suicide attempt and n-3 fatty acid levels in red blood cells: a case control study in China. Biological Psychiatry. 2004;56:490–496. doi: 10.1016/j.biopsych.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Lalovic A, Levy E, Canetti L, Sequeira A, Montoudis A, Turecki G. Fatty acid composition in postmortem brains of people who completed suicide. Journal of Psychiatry and Neuroscience. 2007;32:363–370. [PMC free article] [PubMed] [Google Scholar]
- Lewis MD, Hibbeln JR, Johnson JE, Lin YH, Hyun DY, Loewke JD. Suicide deaths of active-duty US military and omega-3 fatty-acid status: a case-control comparison. Journal of Clinical Psychiatry. 2011;72:1585–1590. doi: 10.4088/JCP.11m06879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. Journal of Clinical Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Hahn C-G, Jandacek R, Rider T, Tso P, Stanford K, Richtand NM. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biological Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Sullivan J, Richtand NM. Omega-3 fatty acid deficiency augments amphetamine-induced behavioral sensitization in adult mice: Prevention by chronic lithium treatment. Journal of Psychiatric Research. 2008;42:458–468. doi: 10.1016/j.jpsychires.2007.05.009. [DOI] [PubMed] [Google Scholar]
- McNamara RK. Omega-3 fatty acid deficiency as a preventable risk factor for comorbid coronary heart disease in major depressive disorder. Cardiovascular Psychiatry and Neurology. 2009a;9:1–13. doi: 10.1155/2009/362795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Roberts RC, Conley RR, Pandey GN. Fatty acid composition of the postmortem prefrontal cortex of male and female adolescent suicide victims. Prostaglandins, Leukotrienes & Essential Fatty Acids. 2009b;80:19–26. doi: 10.1016/j.plefa.2008.10.002. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Able JA, Liu Y, Jandacek R, Rider T, Tso P, Lipton JW. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: Dissociation from estrogenic effects. Journal of Psychiatric Research. 2009c;43:656–663. doi: 10.1016/j.jpsychires.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. Journal of Affective Disorders. 2010;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Jandacek R. Investigation of postmortem brain polyunsaturated fatty acid composition in psychiatric disorders: Limitations, challenges, and future directions. Journal of Psychiatric Research. 2011;45:44–46. doi: 10.1016/j.jpsychires.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osby U, Brandt L, Correia N, Ekbom A, Sparén P. Excess mortality in bipolar and unipolar disorder in Sweden. Archives of General Psychiatry. 2001;58:844–850. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Rizavi HS, Ren X, Conley RR. Decreased catalytic activity and expression of protein kinase C isozymes in teenage suicide victims: a postmortem brain study. Archives of General Psychiatry. 2004;61:685–693. doi: 10.1001/archpsyc.61.7.685. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Conley RR, Dwivedi Y. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. Journal of Psychiatric Research. 2012;46:57–63. doi: 10.1016/j.jpsychires.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker GB, Heruc GA, Hilton TM, Olley A, Brotchie H, Hadzi-Pavlovic D, Friend C, Walsh WF, Stocker R. Low levels of docosahexaenoic acid identified in acute coronary syndrome patients with depression. Psychiatry Research. 2006;141:279–286. doi: 10.1016/j.psychres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Ramirez Basco M, Bostic JQ, Davies D, Rush AJ, Witte B, Hendrickse W, Barnett V. Methods to improve diagnostic accuracy in a community mental health setting. American Journal of Psychiatry. 2000;157:1599–1605. doi: 10.1176/appi.ajp.157.10.1599. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, DeMar JC, Jr, Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Molecular Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Manabe S, Wada O, Crawford MA. Rapid incorporation of docosahexaenoic acid from dietary sources into brain microsomal, synaptosomal and mitochondrial membranes in adult mice. International Journal for Vitamin and Nutrition Research. 1997;67:272–278. [PubMed] [Google Scholar]
- Taha AY, Cheon Y, Ma K, Rapoport SI, Rao JS. Altered fatty acid concentrations in prefrontal cortex of schizophrenic patients. Journal of Psychiatric Research. 2013;47:636–643. doi: 10.1016/j.jpsychires.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebayashi Y, Nihonmatsu-Kikuchi N, Hayashi Y, Yu X, Soma M, Ikeda K. Abnormal fatty acid composition in the frontopolar cortex of patients with affective disorders. Translational Psychiatry. 2012;2:e204. doi: 10.1038/tp.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


