Abstract
Herpes simplex virus (HSV) type 1 and bovine herpesviruses 1 and 5 (BHV-1 and BHV-5) can use the same cellular receptor for entry, but only HSV is known to cause disease in mice. We hypothesized that components of either the innate or the adaptive immune system, or a combination of both, were responsible for curbing replication of BHVs in mice. Therefore, wild-type mice as well as mice with various combined genetic deficiencies in the alpha/beta interferon receptor or gamma interferon receptor and in the ability to produce mature B and T lymphocytes (RAG-2 deletion) were infected with BHV-1 and BHV-5 and monitored clinically, serologically, histopathologically, and virologically. A functional immune system protected the mice from disease and death due to BHV infection, and the immune response was Th1 like. BHV-5 was transported to the central nervous system by the axonal pathway, whereas viremia was required for this outcome with BHV-1. The alpha/beta interferon system was able to obstruct quantitative spread of the viruses in the infected organism. The gamma interferon system had a protective effect against BHV-1, even in mice with the RAG-2 deletion. In contrast, the same mice succumbed to neurological disease and death upon infection with BHV-5. Productively infected neurons were detected only in BHV-5-infected mice with an intact gamma interferon system. We conclude that the alpha/beta interferon system had a protective effect, while an intact gamma interferon system was required for efficient replication of BHV-5 in mouse neurons and for the development of neurological disease.
Bovine herpesviruses 1 and 5 (BHV-1 and BHV-5) belong to the subfamily Alphaherpesvirinae and are closely related pathogens of cattle (17). BHV-1 is the causative agent of respiratory and genital tract infections such as infectious rhinotracheitis, pustular vulvovaginitis, and abortion (44). The virus has only rarely been associated with central nervous disorders (4, 5, 21, 37). In contrast, BHV-5 causes severe encephalitis in calves and, upon experimental infection, in rabbits (31-34, 39).
Alphaherpesviruses commonly have a broad host range (10, 19, 40, 43). Nevertheless, mice were hitherto assumed to be resistant to infection with either BHV-1 or BHV-5. BHV-1, at least, can make use of the same receptor for infection of cells as herpes simplex virus type 1 (HSV-1) (10, 15). Since HSV-1 can easily infect mice, we assumed that factors relating to permissiveness rather than factors relating to adsorption and entry were responsible for the restricted ability of BHVs to replicate in mice.
The interferon systems can influence viral replication directly by antiviral activity or indirectly by modulation of the immune response (6-8, 16, 29). Immediately after infection, they provide protection against viral replication and dissemination (6, 24, 35). Therefore, we hypothesized that one of these systems or a combination of both might be responsible for the protection of mice against infections with BHVs. To address these questions, we used mice with genetic deficiencies in the alpha/beta interferon receptor and/or the gamma interferon receptor, which therefore have a nonfunctional alpha/beta interferon and/or gamma interferon system (25). However, it was of interest to scrutinize the effects of the innate defense against the BHVs either in the presence or in the absence of a functional adaptive immune system. Therefore, mice which were unable to produce mature B and T lymphocytes, i.e., mice with both copies of the RAG-2 gene deleted, were also included in this study (24).
Indeed, mice with intact interferon systems were able to rapidly curb replication of the BHVs. Interestingly, the serological immune response was of a Th1 type, even in mice without the gamma interferon receptor. Dissemination of the viruses was greatly enhanced in infected mice lacking the alpha/beta interferon receptor. Mice lacking both interferon receptors in combination with RAG-2 gene deletions died within a few days following infection with either BHV-1 or BHV-5. Most interestingly, a functional gamma interferon system was exposed as an important determinant for effective replication of BHV-5 in neurons and for associated symptoms of central nervous disease.
MATERIALS AND METHODS
Experimental design.
A first series of experiments was designed to trace disease progression and viral distribution through the infected organism. For this purpose, groups of 21 mice (8 weeks of age) with various deletions in the interferon system or the specific immune system (see below) were infected intraperitoneally at day 0 with 107 50% tissue culture infective doses (TCID50) of either BHV-1 (12 mice per group) or BHV-5 (9 mice per group). Three animals of each group were euthanatized at days 3 and 6 postinfection (dpi 3 and 6). The third sampling was done as soon as infected animals presented disease symptoms (dpi 16 or 21, depending on the mouse strain). The last sampling was restricted to the remaining BHV-1-infected mice, which were euthanatized at dpi 46.
At necropsy the blood, brains, lungs, livers, spleens, and kidneys were collected and frozen at −70°C until further analysis. The brains were carefully removed and sagitally divided into two parts. One part was fixed with 4% paraformaldehyde solution in order to be used for hematoxylin and eosin (HE) staining, immunohistochemistry, and in situ hybridization. The remaining part was shock frozen in liquid nitrogen before being pulverized in a sterile mortar and conserved at −70°C until further analysis by PCR methods.
A second series of experiments was designed to analyze the antiviral antibody response of BHV-1- or BHV-5-infected mice. In this series, four animals per group were infected with 107 TCID50 of either of BHV-1 or BHV-5. Blood samples were taken at dpi 6, 16, and 43. The animals were euthanatized at dpi 43, when whole blood as well as organs (brains, livers, lungs, spleens, and kidneys) were collected. Clotted whole blood was fractioned into serum for serology and blood clot for PCR. All samples were kept frozen until further use.
In a third experiment, a group of 12 mice without the adaptive immune system and without the gamma interferon receptor (GR129 mice) were inoculated with BHV-5 as described above and sacrificed at dpi 16 (6 mice) and dpi 43 (6 mice). At necropsy, organs and blood samples were harvested for PCR.
Viruses and cells.
BHV-1 strain Jura (36) and BHV-5 strain N569 (9, 20) were used for the animal experiments. Both viruses were propagated and titrated in MDBK cells.
Mice.
All animals were cared for and used in accordance with the Swiss laws for animal experimentation. The mouse strains used throughout this study (wild type, AGR129, AR129, A129, AG129, and GR129) are derived from 129Sv/Ev (H-2b) mice and have been described previously (24). The relevant details of their genetic properties are discussed elsewhere in this paper and summarized in Table 1. RAG-2-deficient mice were on a C57BL/6 background.
TABLE 1.
Courses of BHV-1- and BHV-5-infections in mice
| Mouse straina | Outcome of infection withb:
|
|
|---|---|---|
| BHV-1 | BHV-5 | |
| Wild typec | No diseased | No diseased |
| A129e | No diseased | No diseased |
| AG129f | No diseased | No diseased |
| AR129g | No diseaseh | Disease and death (dpi 16)i |
| AGR129k | Disease and death (dpi 16)l | Disease and death (dpi 11)l |
| GR129 | Not done | No diseasem |
All mouse strains are derived from strain 129Sv/Ev.
Mice were infected intraperitoneally with 107 TCID50 of the indicated virus.
Wild-type mice with normal innate and adaptive defense systems.
No disease signs for at least 43 dpi; antiviral antibodies detectable.
Mice with genetically deleted alpha/beta interferon receptor.
Mice with genetically deleted alpha/beta and gamma interferon receptors.
Mice with genetically deleted alpha/beta interferon receptor combined with RAG-2 knockout.
No disease signs until the end of the experiment at dpi 21.
Symptoms of central nervous disease as described in the text. The time of onset of disease is indicated in parentheses.
Mice with genetically deleted alpha/beta and gamma interferon receptors combined with RAG-2 knockout.
Disease signs and death as described in the text.
No disease signs for at least 43 dpi.
DNA detection and quantification.
Primers and probes for quantitative real-time PCR (TaqMan) were designed with Primer Express software (version 1.0; Applied Biosystems [AB], Foster City, Calif.) to amplify sequences within the open reading frames of the glycoprotein B genes of BHV-1 and BHV-5 (Table 2). Oligonucleotide primers and 6-carboxyfluorescein-labeled probes were synthesized by AB, Weiterstadt, Germany. Amplifications were performed according to a standard protocol. Briefly, quantitative real-time PCR amplifications were carried out in a volume of 25 μl containing 12.5 μl of Mastermix (AB); 2.5 μl of a mixture of primer, probe, and diethyl pyrocarbonate-treated water; and 10 μl of DNA sample. The final concentrations of primers and probes were as follows: BHV-1 forward primer, BHV-1 reverse primer, and BHV-5 forward primer, 240 nM each; BHV-1 probe, 160 nM; BHV-5 reverse primer, 60 nM; and BHV-5 probe, 80 nM. The PCR conditions for the reactions were set as follows: 2 min at 50°C, 10 min at 95°C, and then 40 cycles consisting of a denaturation step at 95°C for 15 s and an annealing-elongation step at 60°C for 1 min. For the internal control, the predeveloped TaqMan assay reagent for the 18S rRNA kit was used, and the PCRs were carried out according to the protocol of the manufacturer (AB).
TABLE 2.
Primers and probes for fluorogenic PCR at the gB locus
| Oligo- nucleotide | Sequence (5′-3′) | Product length (bp) |
|---|---|---|
| BHV-1 forward | TGT GGA CCT AAA CCT CAC GGT | 97 |
| BHV-1 probea | AGG ACC GCG AGT TCT TGC CGC | |
| BHV-1 reverse | GTA GTC GAG CAG ACC CGT GTC | |
| BHV-5 forward | ACA TCA TCT ACA TGT CGC CCT TC | 103 |
| BHV-5 probea | ACC GCG AGC ACA CCA GCT ACT | |
| BHV-5 reverse | TTG TAG TAG CCC TCG ATT TGC T |
6-Carboxyfluorescein-labeled.
The data were analyzed on an ABI PRISM 7700 detector (AB) with the appropriate sequence detector software (version 1.6). Signals were regarded as positive if the fluorescence intensity exceeded 10 times the standard deviation of the baseline fluorescence (threshold cycle, ct). The sensitivity and specificity of the system were determined essentially as described before for ovine herpesvirus 2 and feline herpesvirus 1 (26, 42).
Plasmid standards.
A 97-nucleotide (nt) fragment of the BHV-1 gB gene (nt 2106 to 2202) and a 103-nt fragment of the BHV-5 gB gene (nt 932 to 1034) were amplified by conventional PCR, using specific primers with additional EcoRI linkers, and cloned into the EcoRI site of the pBSKS vector to obtain the BHV-1 and BHV-5 gB plasmid standards. Quantitation of viral DNA was done according to AB User Bulletin 2 with the standard curves obtained by using Excel.
Extraction of viral DNA from mouse tissue.
DNA from 25 to 30 mg of brain, kidney, liver, and lung; 10 mg of spleen; and blood clots of mice was extracted by using the QIAamp DNA minikit (Qiagen, Basel, Switzerland) according to the manufacturer's tissue protocol.
In situ hybridization.
In situ hybridization was performed with paraffin-embedded brain tissue sections fixed in 4% paraformaldehyde. The slides were deparaffinized in xylene and rehydrated by being dipped in a graded ethanol series (100, 96, and 70% and distilled water). The tissue was digested with 50 μl of proteinase K (100 μg/ml) for 15 min at 37°C. Thereafter, sections were fixed with 50 μl of 0.4% paraformaldehyde for 5 min at 4°C. Twenty microliters of the probe cocktail, containing a randomly digoxigenin-dUTP-labeled BHV-1 or BHV-5 glycoprotein C probe (31), was added to each section (DIG DNA labeling and detection kit; Roche Diagnostics GmbH, Mannheim, Germany). The samples were denatured by placing the slides on a 95°C heating plate for 6 min. Hybridization was done overnight at 42°C. Labeled hybrid molecules were detected by using alkaline phosphatase-conjugated antidigoxigenin antibody (150 mU/ml) and the nitroblue tetrazolium chloride-bromo-chloro-indolyl-phosphate color substrate solution that was included in the kit.
Histology and antigen detection.
Tissue sections were deparaffinized and rehydrated as described above. After a counterstaining in hemalaun for 2 min and washing in distilled water at ambient temperature, the sections were digested with 0.05% pronase for 5 min at 37°C. The procedure for the detection of viral antigen was according to the instructions of the manufacturer of the En Vision kit (Dako Corporation, Carpinteria, Calif.). Incubations were done at room temperature. Endogenous peroxidase activity was blocked by incubation in 3% H2O2-0.2% NaN3 for 15 min. Nonspecific binding of antibodies was eliminated by treatment with blocking solution (serum-free protein block; Dako) for 10 min. The sections were then incubated for 30 min with monoclonal antibody 141 (specific for gC of both BHV-1 and BHV-5 [31]) diluted 1:50 in phosphate-buffered saline (PBS) (pH 8) and for 30 min with the secondary antibody conjugate containing a dextran polymer labeled with horseradish peroxidase (Dako). The development of the color reaction by the aminoethyl carbazole substrate (Zymed Laboratories Inc., San Francisco, Calif.), added after washings, was under the microscope and stopped by rinsing with PBS monitored after 15 to 30 min. Cells showing gC appeared red.
Serology.
Titers of antiviral antibodies were determined by indirect enzyme-linked immunosorbent assay (ELISA) (38). BHV-1 and BHV-5 are sufficiently related so that the serological response can be analyzed with BHV-1 antigens (32, 34). Commercially available 96-well plates coated with extracts from BHV-1-infected cells were used (Chekit Trachitest; Bommeli AG, Liebefeld, Switzerland). Binding to BHV-1 antigen of either total immunoglobulin (Ig) or IgG isotypes IgG2a, IgG2b, and IgG1 was measured (24). Fivefold dilutions (in PBS containing 0.1% Tween 20; 100 μl/well) of serum were added and incubated for 1 h at 37°C. After washing with PBS-Tween, a 1/4,000 dilution of Ig (total) and IgG isotype-specific horseradish peroxidase-conjugated goat anti-mouse serum (Southern Biotechnology Associates Inc., Birmingham, Ala.) were added. After incubation for 1 h at 37°C, the plates were washed and ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate was added. The reaction was stopped after 15 min, and the optical density at 405 nm was measured in an ELISA reader. The optical density at 405 nm resulting from the incubation of the serum with negative control antigen was subtracted from the result obtained with viral antigen. The antibody titer was defined as the reciprocal of the highest serum dilution which gave twice the value of the background reaction.
RESULTS
Successful infection of mice with BHVs.
Mouse models for the study of the pathogenesis of BHV-1 or BHV-5 have not been developed. It was assumed that these viruses were unable to infect mice, and it was hypothesized that elements of either the innate or the adaptive immune system or both were responsible for this.
To address these questions, mice with specific deletions of their innate or adaptive defense mechanisms were infected intraperitoneally and observed for disease symptoms and death or survival. The results are summarized in Table 1.
Wild-type 129Sv/Ev mice, A129 mice with an intact specific immune system but with a genetically deleted alpha/beta interferon receptor, and AG129 mice with genetically deleted alpha/beta and gamma interferon receptors survived the infection with either BHV-1 or BHV-5 without showing any disease symptoms. In contrast, AGR129 mice without the adaptive immune system (RAG-2 knockout) and with both the alpha/beta and gamma interferon receptors deleted succumbed to a lethal disease. The disease symptoms included weakness, marked depression, and hunched back. Animals infected with BHV-5 were moribund at around dpi 11. AGR129 mice infected with BHV-1 showed the same range and severity of symptoms but commencing only around dpi 16. Thus, even in these severely immunocompromised mice, there was a marked difference in the survival time following infection with either BHV-5 or BHV-1. In AR129 mice, which are similar to AGR129 mice but have an intact gamma interferon system, these differences were even more pronounced. BHV-1-infected mice survived without disease symptoms. In contrast, typical signs of central nervous system disease, including reduced reflexes and circling, developed in AR129 mice infected with BHV-5. However, compared to that in AGR129 mice, the onset of the disease symptoms in BHV-5-infected AR129 mice was delayed by at least 5 days. Thus, it seemed as if the gamma interferon system, supported by activated NK cells (2, 18, 45), was able to protect mice from BHV-1-associated disease and death. In contrast, the same elements appeared to be unable to protect AR129 mice from BHV-5-associated disease.
BHV infection of mice with a functional adaptive immune system leads to a Th-1-like antibody response.
The infection studies described above raised questions as to how the specific immune system coped with the infections. To analyze the antibody response to the infection, sera collected at intervals from mice with an intact adaptive immune system (wild type, A129, and AG129) and infected with either BHV-1 or BHV-5 were analyzed by ELISA with BHV-1 antigens. BHV-1 and BHV-5 are sufficiently related for that purpose (32, 34). Mice with both copies of the RAG-2 gene deleted, which are unable to produce antibodies because they lack mature B and T lymphocytes, were used as controls. The results of the serological analysis are shown in Fig. 1. At dpi 6, most of the A129 and AG129 mice inoculated with BHV-1 had already seroconverted. BHV-1-inoculated wild-type mice had low titers of IgG2a, whereas, with one exception (Fig. 1B), no antibodies against BHV-1 were detected in wild-type mice inoculated with BHV-5.
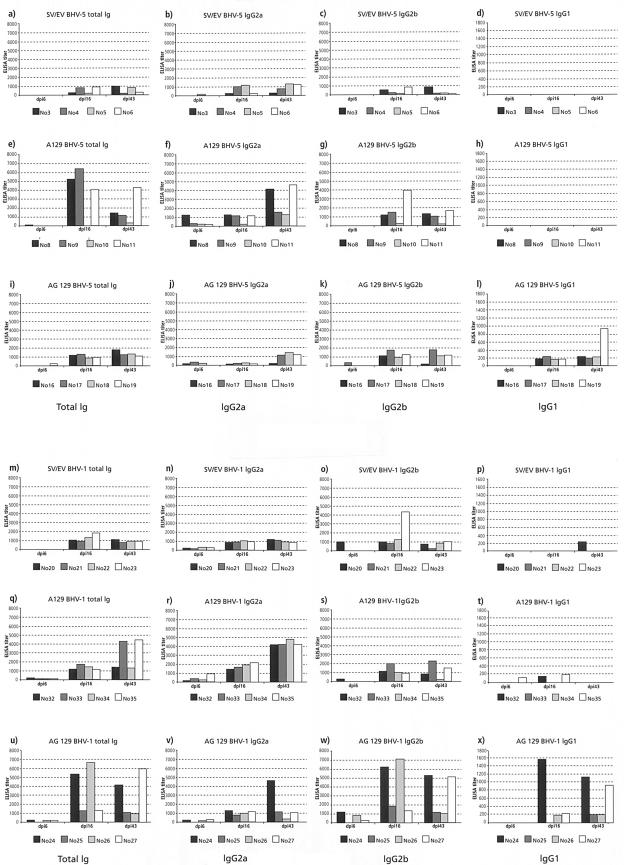
FIG.1.
Serological immune response of mice inoculated with either BHV-5 (a to l) or BHV-1 (m to x). Individual ELISA titers of antibodies against BHV-1 are shown (y axis), each at three different occasions (x axis), i.e., at dpi 6, 16, and 43. Each row represents an experiment in which a specific mouse strain was infected with either BHV-5 or BHV-1. (a to d and m to p) Wild-type mice; (e to h and q to t) A129 mice; (i to l and u to x) AG129 mice. Each column compares the outcome of the different experimental settings with regard to isotype(s) of immunoglobulin measured. First column, total Ig; second column, IgG2a; third column, IgG2b; fourth column, IgG1. Note that the scaling of the y axis is identical in the first three columns but differs in the fourth.
At dpi 16 all of the animals had elevated titers of antibodies against BHV-1. The lowest titers were present in wild-type mice inoculated with BHV-5, whereas the highest titers were found in A129 mice inoculated with BHV-5 and AG129 mice inoculated with BHV-1. Interestingly, IgG1 was of very low titer, whereas IgG2a and IgG2b were dominant. In BHV-1-inoculated A129 mice, the titers of IgG2a dominated over IgG2b, whereas just the opposite was observed with AG129 mice inoculated with either BHV-1 or BHV-5. Furthermore, significant titers of IgG1 against BHV-1 were detected in AG129 mice.
Similar results were obtained with sera obtained at dpi 43. The antibody titers in wild-type mice inoculated with either BHV-1 or BHV-5 did not further increase compared to those at dpi 16, suggesting that viral replication probably had been terminated. Yet, in A129 mice inoculated with either BHV-1 or BHV-5, the titers of anti-BHV-1 IgG2a antibodies had further increased and were dominant. IgG2b antibodies were still dominant in AG129 mice inoculated with BHV-1. Rearrangement of the IgG isotypes indicated that a Th-1-like immune response had taken place in the immunocompetent mice inoculated with the BHVs and that this type of response was independent of either one or both of the interferon systems. In RAG-2 mice, as expected, antibodies against the BHVs were not detected at all at any time point after infection (data not shown).
Mice are productively infected by BHVs.
To investigate replication and spread of the BHVs in the infected mice, tissues were collected at the end of the experiment at dpi 43 and analyzed. Virus isolation in cell culture gave qualitative insight into the fate of BHV in mice, but low virus titers were difficult to detect because undiluted organ tissue was often toxic for cell culture (data not shown). However, both qualitative and quantitative analyses of PCR for viral DNA revealed interesting results. At dpi 43, DNAs of BHVs were found in several organs of all of the inoculated mice, irrespective of the composition of their defense system. This indicates that the two BHVs had been able to infect all of these animals and to spread within the organism in a productive manner. The results are summarized in Table 3.
TABLE 3.
PCR analysis of BHV DNA in selected organs of different mouse strains (dpi 43)
| Mouse straina | Detection of BHVb in:
|
|||||
|---|---|---|---|---|---|---|
| Spleen | Liver | Kidney | Blood | Lung | Brain | |
| Wild type | 1, 5 | 1, 5 | 5 | (1) | Neg | Neg |
| AG129 | (1), 5 | 1, 5 | 5 | (1) | Neg | (5) |
| A129 | 1, 5 | 1, 5 | 5 | Neg | 1, 5 | 5 |
| RAG-2 | 1, 5 | 1, 5 | (1), 5 | (1) | Neg | Neg |
| GR129c | 5 | Neg | 5 | Neg | 5 | Neg |
For mouse strain definitions, see Table 1 and Materials and Methods.
Numbers indicate the BHV type for which DNA was consistently detected. Numbers in parentheses indicate that viral DNA was occasionally detected. Neg, viral DNA was not detected
Infected solely with BHV-5.
Viral DNA was consistently detected in both spleen and liver. In addition, the kidneys of BHV-5-infected animals, but not those of BHV-1-infected animals, were reliably positive. Blood samples at dpi 43 were all negative for viral DNA, with a single exception, where traces of BHV-1 DNA were detected in one out of four wild-type mice. Similarly, a few individual blood samples from BHV-1-infected mice, collected at earlier time points, reacted weakly positive.
Interestingly, DNAs of the BHVs were consistently detected in lung tissues of A129 mice. Finally, three out of four A129 mice as well as one out of four AG129 inoculated with BHV-5 had detectable amounts of BHV-5 DNA in their brain tissue. These results suggested that the two interferon systems may influence, in a virus type-specific fashion, the pathogenesis and therefore the tissue distribution of the two herpesviruses.
Both viral factors and defense mechanisms influence the course of BHV dissemination in mice.
To investigate the types and time courses of viral dissemination in relation to the different types of defense mechanisms, groups of mice were inoculated with either BHV-1 or BHV-5, sacrificed at intervals, and monitored for the presence or absence of viral DNA in various organs. The results are summarized in Table 4. Long-lasting viremia was observed with both BHV-1 and BHV-5 in all animals except A129 mice. However, since even in those animals DNAs of both viruses were qualitatively detected in kidney, lung, liver, and spleen, the occurrence of at least a transient period of viremia had to be assumed. In contrast to the results of the previous experiment, this time neither virus was detected in the brains of A129 mice. Differences between BHV-1 and BHV-5 were observed with regard to the time of onset of the viremia. While BHV-5 was detected in the bloodstream already at dpi 3 in both the AGR129 and AR129 groups of mice, the onset of viremia with BHV-1 was observed only 3 days later, i.e., at dpi 6. Moreover, BHV-5 was detected at dpi 3 in the brains of both AGR129 and AR129 mice. In contrast, BHV-1 was able to gain access to the brains of either AGR129 or AR129 mice only following a prolonged viremia. Surprisingly, viremia of BHV-5 in AR129 mice was no longer detectable at dpi 16.
TABLE 4.
Qualitative time courses of viral DNA in organs, blood, and brain of various mouse strains
| Mouse straina and virus | Times (dpi)c of BHV detection in:
|
||
|---|---|---|---|
| Organsb | Blood | Brain | |
| AGR129 | |||
| BHV-1 | 3, 6, 11, 16 | 6, 11, 16 | 11, 16 |
| BHV-5 | 3, 6, 11 | 3, 6, 11 | 3, 6, 11 |
| AR129 | |||
| BHV-1 | 3, 6, 16, 22 | 6, 16, 22 | 16, 22 |
| BHV-5 | 3, 6, 16 | 3, 6 | 3, 6, 16 |
| GR129, BHV-5 | 16, 43d | Not detectedd | Not detectedd |
| A129, BHV-5 | 3, 6, 43 | Not detectede | Not detected |
For mouse strain definitions, see Table 1 and Materials and Methods.
Kidney, lung, liver, and spleen.
Numbers indicate the dpi at which groups of three animals were sacrificed and viral DNA was detected by PCR in extracts of the organs indicated.
Not tested on dpi 3 and 6.
Negative results with materials from this series of experiments.
Under the experimental conditions used, the spread of either virus to the brain was apparently inefficient in the presence of the functional specific immune system, even in those mice with genetic deletion of the alpha/beta interferon receptor. In contrast, in the absence of the alpha/beta interferon receptor and without specific immune functions (AGR129 and AR129 mice), BHV-5 gained immediate (at dpi 3) access to the brain, while with BHV-1, viremia was required to this end. The gamma interferon system alone, without any help from the specific immune system or the alpha/beta interferon system, was apparently able to curb BHV-5 viremia, to retard the access of BHV-1 to the brain, and to prolong the survival time of mice infected with either BHV-1 or BHV-5.
Quantitative analysis of viral dissemination.
To study the dynamics of viral dissemination to organs, the load of viral DNA was determined quantitatively at different time points. Although there was some variation among the individual animals in each group, the results within the individuals were reproducible.
(i) BHV-5.
In A129 mice with an intact specific immune system, the largest amounts of BHV-5 DNA were found at dpi 3 in several organs, but BHV-5 DNA was still detectable in all animals at dpi 43 (data not shown). Among the organs affected, the largest amounts of BHV-5 DNA were consistently found in the kidneys. The lung, liver, and spleen were also positive, while no virus DNA was detected in the brain. With one exception BHV-5 was not detected in the blood of these mice.
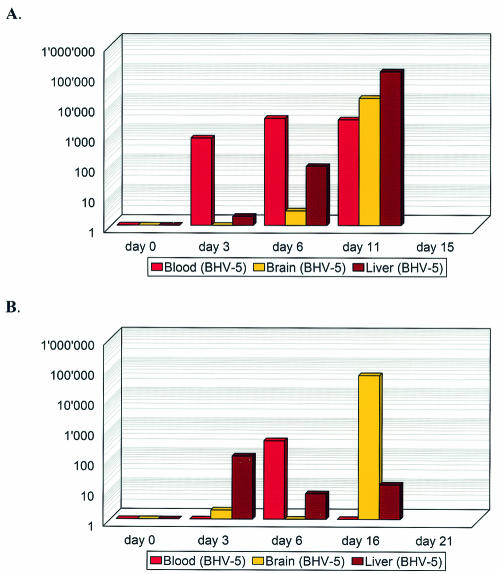
In RAG-2- and interferon-deficient mice (AGR129 and AR129), the BHV-5 DNA load continued to increase with each time point measured (Fig. 2A), probably due to the absence of a specific immune system. The highest BHV-5 DNA load was first observed in the blood (at dpi 3). Starting from dpi 6, the viral DNA load started to dramatically increase in both liver and brain. In AR129 mice the viral DNA load first peaked in the liver (dpi 3) and bloodstream (dpi 6). However, on dpi 16, coinciding with the onset of central nervous system symptoms, vast amounts of viral DNA were detected in the brains of those animals (Fig. 2B). While BHV-5 readily gained access to the bloodstream, the titers on dpi 3 in the kidneys and, less pronounced, in the lungs were below those found in the A129 mice. In contrast, the viral loads found in liver and spleen on dpi 3 were higher in the RAG-2- and interferon-deficient mice than in the A129 mice (data not shown).
FIG. 2.
Quantitative time course of viral DNA in blood, brain, and liver of AGR129 (A) and AR129 (B) mice inoculated with BHV-5. Viral DNA copy numbers per 1,000 cells (y axis) detected by quantitative real-time PCR in the various tissues collected at various time points after inoculation (x axis) are shown.
(ii) BHV-1.
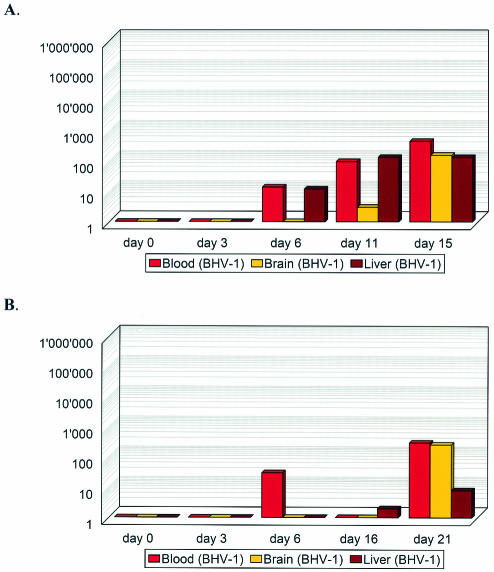
In A129 mice, the largest amounts of BHV-1 were found early after inoculation (dpi 3) in the spleens and kidneys. Lungs and livers were also positive. Yet, BHV-1 was not detected at any time in the bloodstream or, with one exception, in the brain. Over time, decreasing titers of BHV-1 DNA were found in these mice. In contrast, increasing viral loads were found in AGR129 and AR129 mice (Fig. 3). Starting from dpi 6, increasing amounts of BHV-1 DNA were detected in the bloodstream. Only subsequent to viremia was BHV-1 DNA found in brain tissue, where it continued to accumulate over time.
FIG. 3.
Quantitative time course of viral DNA in blood, brain, and liver of AGR129 (A) and AR129 (B) mice inoculated with BHV-1. Viral DNA copy numbers per 1,000 cells (y axis) detected by quantitative real-time PCR in the various tissues collected at various time points (x axis) after inoculation are shown.
Contribution of the gamma interferon system to replication of BHV-5 in neurons.
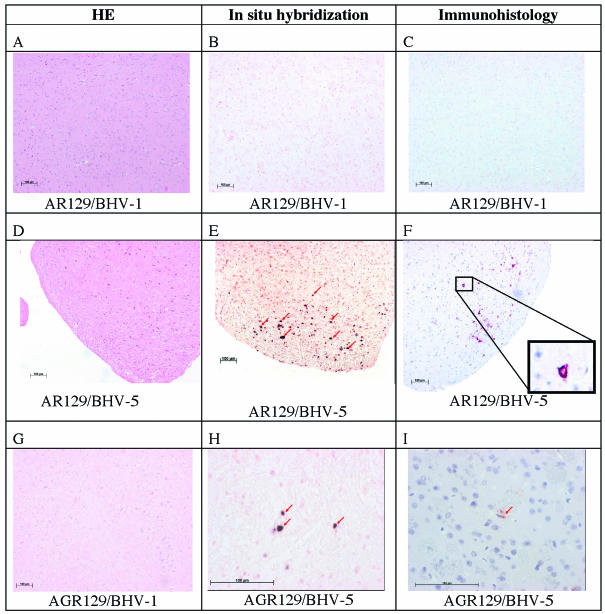
As described above (Table 4), viral DNA had been detected in the brains of four groups of mice (AGR and AR mice inoculated with either BHV-1 or BHV-5), although mice of only three groups had developed disease and only AR129 mice inoculated with BHV-5 showed clinical symptoms indicative of central nervous system disease (Table 1). Histopathological evidence of morphological changes in brain tissues was not detected. Importantly, no signs of inflammation were apparent (Fig. 4A, D, and G). It was therefore of interest to analyze mouse brains in situ for the presence and distribution of either viral DNA or viral proteins.
FIG. 4.
Analysis of mouse brains from BHV-1- and BHV-5-infected mice by histology, in situ hybridization, and immunohistology. AR129 (A to F) and AGR129 (G to I) mice were inoculated with BHV-1 or BHV-5 as indicated. At intervals, groups of mice were euthanatized to be analyzed at various time points after infection. (A, D, and G) Examples of histological examination following hematoxylin and eosin (HE) staining at dpi 22 (A) or 16 (D and G), where no differences between various time points were recognized. (B, E, and H) Examples of results after in situ hybridization at dpi 22 (B), 16 (E) (arrows point to positive neuronal nuclei), or 11 (H). Viral DNA was not present at detectable levels in brain tissue collected from BHV-1-inoculated mice or in BHV-5-infected mice, before the onset of clinical symptoms (an example is shown in panel B). BHV-5 DNA detected by in situ hybridization is shown in panels E and H (arrows point to positive neuronal nuclei). (C, F, and I) Examples from immunohistological analyses at dpi 22 (C), 16 (F), and 11(I). Sections from BHV-1-inoculated mice and from BHV-5-infected mice, before the onset of clinical symptoms, reacted negatively (an example is shown in panel C). Structural glycoprotein C was detected by immunohistology following monoclonal antibody staining of brains from AR129 mice at dpi 16 after inoculation with BHV-5 (F) (the inset shows a positive neuron at a higher magnification) and from AGR129 mice at dpi 11 after BHV-5-infection (I) (the arrow points to a weakly positive cell that cannot be clearly identified as a neuron). Bars, 100 μm.
In situ hybridization.
In a first series of experiments, digoxigenin-labeled DNA fragments corresponding to the gC genes of either BHV-1 or BHV-5 were used for in situ hybridization. Detectable amounts of viral DNA were present exclusively in neuronal nuclei in the brain stem regions of BHV-5-infected AGR129 (dpi 11) and AR129 (dpi 16) mice (Fig. 4H and E). In AR129 mice, positive cells were also detected in the cortex. The brains of either AGR129 or AR129 mice which had been sacrificed at earlier time points and had tested positive for BHV-5 DNA by PCR remained negative by in situ hybridization (not shown). Viral DNA was not detected by in situ hybridization in the brains of BHV-1 infected mice (Fig. 4B). Surprisingly, even BHV-1-infected AGR129 mice, which had shown clinical disease symptoms at dpi 15 remained negative by in situ hybridization. Furthermore, mouse 12 in the group of AR129 mice, which had been sacrificed at day 21 after infection with BHV-1 and which contained high amounts of viral DNA in the brain as determined by quantitative real-time PCR, tested negative in this test. Based on semiquantitative estimations, higher numbers of neurons containing BHV-5 DNA were present in the brains of AR129 mice compared to AGR129 mice. This result was confirmed by the observation that viral titers, determined by virus isolation and titration (data not shown), found in the brains of AR mice exceeded those in AGR mice by at least one order of magnitude.
Immunohistology.
In a second series of experiments, mouse brains were analyzed by immunohistology for the presence of BHV antigens. Either a monoclonal antibody against the late structural protein gC or immune sera from infected A129 mice were used for antigen detection. The highest number of positive cells was found in the brain stems of BHV-5-infected AR129 mice (Fig. 4F). At higher magnifications (4F, inset), neurons carrying intracytoplasmic viral protein could easily be identified. Positive neurons were detected in the area ranging from the diencephalon (thalamus and hypothalamus) to the brain stem. Furthermore, antigen-positive neurons colocalized with cells that had been previously found to be positive by in situ hybridization. Surprisingly, only a few antigen-positive cells were detected in the brains of BHV-5-infected AGR129 mice (Fig. 4I). However, the few antigen-positive cells were detected in the same locations that had previously shown a high number of strongly hybridization-positive cells.
GR129 mice, lacking both the gamma interferon system and the adaptive immune system, are protected from BHV-5-associated disease and death.
Conservatively, one might conclude from the above-described experiments that the adaptive immune system played the most important role in protection of mice against BHV-5. However, there was evidence (Table 3) that BHV-5 was able to reach the brains of mice with the alpha/beta interferon receptor knocked out (A129 and AG129 mice) but not those of mice with a functional alpha/beta interferon system (wild-type and RAG-2 mice). In order to test the protective capacity of the alpha/beta interferon system without help of the other branches of the defense, GR129 mice were inoculated with BHV-5 and monitored for disease signs and virus spread to various organs, including the brain. The results (Tables 1, 3, and 4) showed that those mice were entirely protected from disease and death. Although BHV-5 was able to spread to the spleen, kidney, and lung, there was no evidence of BHV-5 DNA in the brains of these mice on either dpi 16 or dpi 43. It was concluded from these results that the alpha/beta interferon system was as important and powerful for protection of mice against BHV-5 as the adaptive immune system.
DISCUSSION
In the present work, we provide evidence that mice can be infected by BHVs and that both BHV-1 and BHV-5 are able to disseminate within infected mice. Infections of normal mice as well as of mice with combined genetic deficiencies in their innate and/or adaptive immune systems revealed new knowledge concerning the pathogenesis of BHV-1 and BHV-5 in experimentally infected animals with regard to infection, disease, and death.
The alpha/beta interferon system is known to influence viral replication directly and immediately by antiviral activity on the cellular level (24, 35). Previous studies have also shown reduced natural killer (NK) cell responses in alpha/beta interferon receptor-deficient mice (35). In contrast, the effectors of an adaptive immune response are available only after a delay. In return, they are broadly active against intracellular as well as extracellular virus. Finally, the gamma interferon system is thought to primarily modulate the specific antiviral immune response and to some extent the innate immunity (8, 29). For example, gamma interferon receptor-deficient mice showed poor granule formation of NK cells (3).
To address the specific roles of the various branches of the defense system in the context of BHV-1 and BHV-5 infections, we used mice with genetic deficiencies in the alpha/beta and/or the gamma interferon receptor, which therefore have a nonfunctional alpha/beta and/or gamma interferon system, respectively. Disruption of both interferon receptors also leads to loss of NK cell activity. We also used mice which were, additionally, unable to produce mature specific B and T lymphocytes, i.e., mice with both copies of the RAG-2 gene deleted. Knowing that mice are not bovines, we should caution that results obtained with the mouse system may not automatically be valid for the bovine system.
The salient features of these new findings are as follows.
The specific immune system and the alpha/beta interferon system are equally and independently protective.
Mice with an intact specific immune system remained healthy upon infection with either BHV-1 or BHV-5, even when both types of interferon systems were deficient as a result of receptor knock out (AG129 mice). This is in good agreement with reports for BHV-5 infections in a rabbit model in which the immunosuppressive drug dexamethasone is used to enhance the frequency of encephalitis (11, 30). Yet, disease associated with these infections was seen only in the absence of a functional specific immune system combined with an additional defect in the alpha/beta interferon system (AR129 and AGR129 mice). Surprisingly, GR129 mice with the alpha/beta interferon system and NK cells as their only defense did not succumb to disease and death from BHV-5 infection. Therefore, the alpha/beta, but not the gamma, interferon system can mediate such a protective effect, which is based on reducing, if not precluding, viral replication at the level of infected cells. Thus, the specific immune system and the alpha/beta interferon system provide for equally good protection. However, both health-protective systems were unable to qualitatively prevent viral dissemination within the organism (Table 3). Yet, BHV-5 was only occasionally detected in the brains of infected mice provided that either the specific immune system or the alpha/beta interferon system was intact. This indicated that invasion of the central nervous system (neuroinvasion) was either ineffective or terminated by components of the specific immune system (Table 4, A129 mice). Since the alpha/beta interferon system cannot affect cell-free virus, these results also suggested that the alpha/beta interferon system, without help from the specific immune system, was able to successfully suppress replication of BHV-5 in the brain (Table 4, GR129 mice).
Influence of viral and host factors.
Thus far, the basis for virulence of a specific virus concerning the brain had been attributed mostly to individual genes of the infecting virus rather than to host factors (12, 13, 31). We provide evidence that both the virus type used for infection and the two interferon systems contributed individually to the outcome of the infections and to neurovirulence. Independent of the mouse strain used for infection, BHV-1 replicated more slowly and to lesser final viral loads than BHV-5. BHV-5 was qualitatively detected in the brains of AGR129 and AR129 mice at dpi 3, whereas it took until dpi 11 (AGR129 mice) or 16 (AR129 mice) before BHV-1 DNA was detectable in extracted brain tissue of these mice. However, successful dissemination, including neuroinvasion, of the two viruses studied did not always lead to disease and death. AR129 mice, in which only the defense mechanisms connected to an intact gamma interferon system and residual NK cell activity remained (18), stayed healthy upon infection with BHV-1, whereas they succumbed to disease and death upon infection with BHV-5. Quantitative real-time PCR indicated that the gamma interferon system with the help of NK cells of AR129 mice was able to curb the viral load in most organs, with the exception of the brain. Furthermore, in situ hybridization and immunohistology suggested that productive BHV-5 infection, leading to accumulation of viral proteins in neurons, was facilitated in the presence of an intact gamma interferon system (AR129 mice) but not in its absence (AGR129 and GR129 mice). Since GR129 mice, with the alpha/beta interferon system and residual NK cell activity as their only defense, were protected from disease and death due to BHV-5 infection, this provides subtractive evidence host factors associated with the gamma interferon system being required for abundant productive replication of BHV-5 in neurons. Indeed, others have described a protective role of gamma interferon against HSV-1-mediated neuronal death (22). Yet, the exact circumstances representing the basis for our observations remain to be elucidated. BHV-5 replication in neurons of BHV-5-infected AR129 mice was associated with clinically evident neurological disorder, although no histological signs of encephalitis were observed; i.e., immigration of granulocytes, monocytes, or lymphocytes was never seen. Therefore, these clinical symptoms may be attributed to impairment of neuronal function due to or at least in association with BHV-5 replication.
Serological immune response.
The serological immune response of immunocompetent mice showed the following (Fig. 1). At around dpi 6, all infected mice with an intact specific immune system had already seroconverted towards BHV-1. Although BHV-1 and BHV-5 are sufficiently related that the serological response can be analyzed with BHV-1 antigens (32, 34), it was surprising that some of the highest antibody titers were found among mice infected with BHV-5. A sustained antibody titer against BHV-1 at dpi 43 in wild-type mice infected with either BHV-1 or BHV-5 was indicative of a de novo antigen synthesis in those mice, which can be explained only by successful viral replication. Although some lymphocytes in the infected AG129 mice produced specific antibodies of the IgG1 isotype, all mice, even AG129, were able to mount antibodies of isotype IgG2 against BHV-1. Thus, similar to pseudorabies virus (24), the BHVs were able to force a Th1-like immune response on the infected mice, even in the absence of a functional gamma interferon system. Although Ig isotype switching may be a very complex process, it is assumed that Ig isotype switching from IgG1 to IgG2a is primarily dependent on gamma interferon, whereas switching from IgG1 to IgG2b depends on transforming growth factor β (23). It was therefore surprising that both IgG2a and IgG2b were detected at high titers in AG129 mice. However, a dominance of IgG2b over IgG2a antibodies was evident in those mice. In contrast, A129 mice, with an intact gamma interferon system, had higher titers of IgG2a than of IgG2b. The same mice, in contrast to the wild type, showed ever-increasing antiviral antibody titers until the end of the experiment at dpi 43. The most constant increase was associated with antibodies of the IgG2a isotype. These findings suggest that active replication of and protein synthesis by these herpesviruses are not terminated in mice without the alpha/beta interferon receptor. This may open new possibilities in the context of producing antisera to unconventional antigens in these mice and the establishment of models for autoimmune diseases (1, 41).
Viremia and axonal transport.
Earlier observations of experimentally infected rabbits had indicated that not only BHV-5 but also BHV-1 possesses, at least in principle, the ability to access and infect the brain and to replicate in neurons (31, 33). Viremia was observed either before or at the same time as detection of BHV-1 DNA as well as BHV-5 DNA in brains of infected AGR129 and AR129 mice. Therefore, the question of whether or not viremia was responsible for the transport of the viruses to the brain arose. Interestingly, invasion of the neuronal tissue by BHV-1 was prevented in mice with a functional adaptive immune system. Since BHV-1 viremia was observed at around dpi 6, a time point at which antiviral antibodies were detected in the sera of immunocompetent mice, these observations suggest that spread of BHV-1 to the brain may actually depend on viremia. In contrast, BHV-5 was able to invade the brain even in the face of an intact adaptive immune system, although lack of the alpha/beta interferon receptor was required (A129 and AG129 mice) to this end. Productively infected neurons, as detected in AR129 mice, were not found distributed evenly over the brain but were localized mainly to the brain stem. Since these mice had been infected intraperitoneally, axonal transport (nervus vagus) rather than viremia was responsible for neuroinvasion by BHV-5. This is in good agreement with the olfactory pathway reported for BHV-5 upon intranasal infection of rabbits (14, 30) and is also reminiscent of the progression of BHV-5 infection in cattle, the natural host of BHV-5 (34).
The present work exposes the alpha/beta interferon system as being important for the inability of BHVs to spread efficiently in normal mice and cause disease. On the basis of mice lacking the alpha/beta interferon receptor, a wealth of genetically modified experimental animals may now be available for future research on the pathogenesis of BHVs, especially BHV-5. On the other hand, these BHVs have properties that differ from those of other alphaherpesviruses. This will make them useful tools for studying neuropathogenesis in a manner that cannot be achieved with the well-established models that use HSV or pseudorabies virus. For example, the individual molecules within the alpha/beta interferon-associated cascade, which are responsible for the protective effect, can be identified and functionally studied. Similarly, efficient replication of BHV-5 in mouse neurons occurs only in the presence of an intact gamma interferon system. Therefore, BHV-5 may be used in the future to elucidate the factors responsible for efficient viral replication in neurons, which are contributed by the gamma interferon system. The possibility of dissecting contributions to neurovirulence by either viral or host factors may be of general importance, given the great regularity with which new viral infections of the nervous system emerge (27, 28).
Acknowledgments
We thank Eva Loepfe for excellent technical support, Rolf Kocherhans and Kurt Tobler for enlightening discussions, Martin Schwyzer for critically reading the manuscript, and Irene Schweizer for help with graphics.
This work was supported by the Swiss Federal Veterinary Office (1.01.08), the Robert and Dorothea Wyler foundation, and the Canton of Zurich.
REFERENCES
- 1.Abu-Shakra, M., and Y. Shoenfeld. 1991. Chronic infections and autoimmunity. Immunol. Ser. 55:285-313. [PubMed] [Google Scholar]
- 2.Aguilar-Delfin, I., P. J. Wettstein, and D. H. Persing. 2003. Resistance to acute babesiosis is associated with interleukin-12- and gamma interferon-mediated responses and requires macrophages and natural killer cells. Infect. Immun. 71:2002-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashkar, A. A., G. P. Black, Q. Wei, H. He, L. Liang, J. R. Head, and B. A. Croy. 2003. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J. Immunol. 171:2937-2944. [DOI] [PubMed] [Google Scholar]
- 4.Bagust, T. J., and L. Clark. 1972. Pathogenesis of meningo-encephalitis produced in calves by infectious bovine rhinotracheitis herpesvirus. J. Comp. Pathol. 82:375-383. [DOI] [PubMed] [Google Scholar]
- 5.Barenfus, M., C. A. Delliquadri, R. W. McIntyre, and R. J. Schroeder. 1963. Isolation of infectious bovine rhinotracheitis virus from calves with meningo-encephalitis. J. Am. Vet. Med. Assoc. 143:725-728. [PubMed] [Google Scholar]
- 6.Biron, C. A. 1999. Initial and innate responses to viral infections—pattern setting in immunity or disease. Curr. Opin. Microbiol. 2:374-381. [DOI] [PubMed] [Google Scholar]
- 7.Biron, C. A. 1998. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin. Immunol. 10:383-390. [DOI] [PubMed] [Google Scholar]
- 8.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 9.Bulach, D. M., and M. J. Studdert. 1990. Comparative genome mapping of bovine encephalitis herpesvirus, bovine herpesvirus 1, and buffalo herpesvirus. Arch. Virol. 113:17-34. [DOI] [PubMed] [Google Scholar]
- 10.Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10:305-319. [DOI] [PubMed] [Google Scholar]
- 11.Chowdhury, M., and S. I. Chowdhury. 1997. Neuropathology of bovine herpesvirus type 5 (BHV-5) meningo-encephalitis in a rabbit seizure model. Mol. Hum. Reprod. 3:109-114. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury, S. I., B. J. Lee, M. Onderci, M. L. Weiss, and D. Mosier. 2000. Neurovirulence of glycoprotein C (gC)-deleted bovine herpesvirus type-5 (BHV-5) and BHV-5 expressing BHV-1 gC in a rabbit seizure model. J. Neurovirol. 6:284-295. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury, S. I., B. J. Lee, A. Ozkul, and M. L. Weiss. 2000. Bovine herpesvirus 5 glycoprotein E is important for neuroinvasiveness and neurovirulence in the olfactory pathway of the rabbit. J. Virol. 74:2094-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chowdhury, S. I., M. Onderci, P. S. Bhattacharjee, A. Al-Mubarak, M. L. Weiss, and Y. Zhou. 2002. Bovine herpesvirus 5 (BHV-5) Us9 is essential for BHV-5 neuropathogenesis. J. Virol. 76:3839-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72:9992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Waal Malefyt, R. 1997. The role of type I interferons in the differentiation and function of Th1 and Th2 cells. Semin. Oncol. 24:S9-94-S9-98. [PubMed] [Google Scholar]
- 17.Engels, M., and M. Ackermann. 1996. Pathogenesis of ruminant herpesvirus infections. Vet. Microbiol. 53:3-15. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez, N. C., A. Lozier, C. Flament, P. Ricciardi-Castagnoli, D. Bellet, M. Suter, M. Perricaudet, T. Tursz, E. Maraskovsky, and L. Zitvogel. 1999. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 5:405-411. [DOI] [PubMed] [Google Scholar]
- 19.Fraefel, C., T. Heister, and M. Ackermann. 2003. Herpes simplex type 1/adeno-associated virus hybrid vectors. Gene Ther. Regulat. 2:7-28. [Google Scholar]
- 20.French, E. L. 1962. A specific virus encephalitis in calves: isolation and characterization of the causal agent. Aust. Vet. J. 38:216-221. [Google Scholar]
- 21.Friedli, K., and A. E. Metzler. 1987. Reactivity of monoclonal antibodies to proteins of a neurotropic bovine herpesvirus 1 strain and to proteins of representative BHV-1 strains. Arch. Virol. 94:109-122. [DOI] [PubMed] [Google Scholar]
- 22.Geiger, K. D., T. C. Nash, S. Sawyer, T. Krahl, G. Patstone, J. C. Reed, S. Krajewski, D. Dalton, M. J. Buchmeier, and N. Sarvetnick. 1997. Interferon-gamma protects against herpes simplex virus type 1-mediated neuronal death. Virology 238:189-197. [DOI] [PubMed] [Google Scholar]
- 23.Goldsby, R. A., T. J. Kindt, and B. A. Osborne (ed.). 2002. Kuby immunology, 4th ed. W. H. Freeman and Company, New York, N.Y.
- 24.Grob, P., V. E. Schijns, M. F. van den Broek, S. P. Cox, M. Ackermann, and M. Suter. 1999. Role of the individual interferon systems and specific immunity in mice in controlling systemic dissemination of attenuated pseudorabies virus infection. J. Virol. 73:4748-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R. M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science 259:1742-1745. [DOI] [PubMed] [Google Scholar]
- 26.Hussy, D., N. Stauber, C. M. Leutenegger, S. Rieder, and M. Ackermann. 2001. Quantitative fluorogenic PCR assay for measuring ovine herpesvirus 2 replication in sheep. Clin. Diagn. Lab. Immunol. 8:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson, R. T. 2003. Emerging viral infections of the nervous system. J. Neurovirol. 9:140-147. [DOI] [PubMed] [Google Scholar]
- 28.Johnson, R. T. 1995. Neurovirology: evolution of a new discipline. J. Neurovirol. 1:2-4. [DOI] [PubMed] [Google Scholar]
- 29.Landolfo, S., G. Gribaudo, A. Angeretti, and M. Gariglio. 1995. Mechanisms of viral inhibition by interferons. Pharmacol Ther. 65:415-442. [DOI] [PubMed] [Google Scholar]
- 30.Lee, B. J., M. L. Weiss, D. Mosier, and S. I. Chowdhury. 1999. Spread of bovine herpesvirus type 5 (BHV-5) in the rabbit brain after intranasal inoculation. J. Neurovirol. 5:474-484. [DOI] [PubMed] [Google Scholar]
- 31.Liman, A., M. Engels, G. Meyer, M. Ackermann. 2000. Glycoprotein C of bovine herpesvirus 5 (BHV-5) confers a distinct heparin-binding phenotype to BHV-1. Arch. Virol. 145:2047-2059. [DOI] [PubMed] [Google Scholar]
- 32.Metzler, A. E., A. A. Schudel, and M. Engels. 1986. Bovine herpesvirus 1: molecular and antigenic characteristics of variant viruses isolated from calves with neurological disease. Arch. Virol. 87:205-217. [DOI] [PubMed] [Google Scholar]
- 33.Meyer, G., M. Lemaire, J. Lyaku, P. P. Pastoret, and E. Thiry. 1996. Establishment of a rabbit model for bovine herpesvirus type 5 neurological acute infection. Vet. Microbiol. 51:27-40. [DOI] [PubMed] [Google Scholar]
- 34.Meyer, G., M. Lemaire, C. Ros, K. Belak, A. Gabriel, D. Cassart, F. Coignoul, S. Belak, and E. Thiry. 2001. Comparative pathogenesis of acute and latent infections of calves with bovine herpesvirus types 1 and 5. Arch. Virol. 146:633-652. [DOI] [PubMed] [Google Scholar]
- 35.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 36.Probst, U., R. Wyler, U. Kihm, M. Ackermann, L. Bruckner, H. K. Muller, and F. Ehrensperger. 1985. Zur IBR-Virus-Ausscheidung experimentell infizierter Kühe insbesondere in der Milch. Schweizer Arch. Tierheilkunde. 127:723-733. [PubMed] [Google Scholar]
- 37.Roels, S., G. Charlier, C. Letellier, G. Meyer, F. Schynts, P. Kerkhofs, E. Thiry, and E. Vanopdenbosch. 2000. Natural case of bovine herpesvirus 1 meningoencephalitis in an adult cow. Vet. Rec. 146:586-588. [DOI] [PubMed] [Google Scholar]
- 38.Rosskopf, M., E. Staub, and M. Ackermann. 1994. Vergleich zweier ELISA Systeme zum Nachweis von Antikorpern gegen IBR/IPV sowie gegen EBL. Schweizer Arch. Tierheilkunde. 136:58-67. [PubMed] [Google Scholar]
- 39.Schudel, A. A., B. J. Carrillo, R. Wyler, and A. E. Metzler. 1986. Infections of calves with antigenic variants of bovine herpesvirus 1 (BHV-1) and neurological disease. Zbl. Vet. Reihe B 33:303-310. [DOI] [PubMed] [Google Scholar]
- 40.Six, A., M. Banks, M. Engels, C. R. Bascunana, and M. Ackermann. 2001. Latency and reactivation of bovine herpesvirus 1 (BHV-1) in goats and of caprine herpesvirus 1 (CapHV-1) in calves. Arch. Virol. 146:1325-1335. [DOI] [PubMed] [Google Scholar]
- 41.Vanderlugt, C. L., K. L. Neville, K. M. Nikcevich, T. N. Eagar, J. A. Bluestone, and S. D. Miller. 2000. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J. Immunol. 164:670-678. [DOI] [PubMed] [Google Scholar]
- 42.Vogtlin, A., C. Fraefel, S. Albini, C. M. Leutenegger, E. Schraner, B. Spiess, H. Lutz, and M. Ackermann. 2002. Quantification of feline herpesvirus 1 DNA in ocular fluid samples of clinically diseased cats by real-time TaqMan PCR. J. Clin. Microbiol. 40:519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogtlin, A., C. Fraefel, R. Kocherhans, C. M. Leutenegger, K. Frei, A. Fontana, and M. Ackermann. 2002. HSV-1-based amplicon particles are able to transduce cells of feline origin with genes encoding biologically functional feline IL-10 or IL-6. Vet. Microbiol. 86:103-113. [DOI] [PubMed] [Google Scholar]
- 44.Wyler, R., M. Engels, and M. Schwyzer. 1989. Infectious bovine rhinotracheitis/vulvovaginitis (BHV1). Kluwer Academic Publishers, Boston, Mass.
- 45.Zhang, G., R. D. Nichols, M. Taniguchi, T. Nakayama, and M. J. Parmely. 2003. Gamma interferon production by hepatic NK T cells during Escherichia coli infection is resistant to the inhibitory effects of oxidative stress. Infect. Immun. 71:2468-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]