Abstract
There are few areas in cardiology where the impact of genetics and of genetic testing on clinical management has been as great as in cardiac channelopathies, arrhythmic disorders of genetic origin related to the ionic control of the cardiac action potential. Among the growing number of diseases identified as channelopathies, three are sufficiently prevalent to represent significant clinical and societal problems and to warrant adequate understanding by practicing cardiologists: long QT syndrome, catecholaminergic polymorphic ventricular tachycardia, and Brugada syndrome.
This review will focus selectively on the impact of genetic discoveries on clinical management of these three diseases. For each disorder, we will discuss to what extent genetic knowledge and clinical genetic test results modify the way cardiologists should approach and manage affected patients. We will also address the optimal use of genetic testing including its potential limitations and the potential medico-legal implications when such testing is not performed. We will highlight how important can be to understand the ways by which genotype can impact clinical manifestations, risk stratification, and responses to the therapy. We will also illustrate the close bridge between molecular biology and clinical medicine, and will emphasize that consideration of the genetic basis for these hereditable arrhythmia syndromes, as well as the proper use and interpretation of clinical genetic testing, should remain the standard-of-care.
Keywords: Channelopathies, Gene-specific management, Genetic testing, Heart rhythm disorder, Sudden death
The discovery of the first three long QT syndrome (LQTS)-susceptibility genes in 1995-96 (1-3) had a transformative effect on the diagnosis and treatment of arrhythmias. It opened the way to the realization that molecular biology could no longer be regarded as “something weird, with an unfriendly jargon and of no interest for a clinician”; it allowed the understanding of how even simple aminoacid substitutions (missense mutations) due to a single nucleotide substitution could produce significant functional alterations in cellular electrophysiology. In addition, by showing that most of the disease genes for a number of arrhythmic disorders of genetic origin were involved in the ionic control of the cardiac action potential, it led to describe these disorders as “cardiac channelopathies”. Besides some very rare disorders, there are three truly important genetic heart rhythm diseases whose ignorance by practicing cardiologists could cost the life of the unfortunate patients seeking their medical advice. They are LQTS, catecholaminergic polymorphic ventricular tachycardia (CPVT), and Brugada syndrome (BrS).
Here, we will only touch briefly on the main features of these potentially life-threatening yet highly treatable diseases (at least LQTS and CPVT), as many thorough clinical reviews are available (4-8). Instead, our focus will be centered selectively on discussing the impact exerted on clinical management by the progressive unraveling of the genetic mechanisms underlying these diseases. Specifically, we will examine for each of them whether, how, and to what extent genetic test results should modify the way a cardiologist should approach and manage the mutation-positive (i.e. affected) patients.
The space given to the 3 diseases will be different. LQTS is undoubtedly the one that demonstrates best how close is the bridge between molecular biology and clinical medicine because it is the one in which the genotype-phenotype relationship has been best understood in terms of clinical manifestations, risk stratification, and response to the therapy. We trust that the readers will realize that, for patients with channelopathies, molecular genetics and clinical management should go hand in hand.
Long QT Syndrome
LQTS represents a leading cause of autopsy-negative sudden death in the young (9). It is characterized typically by a prolongation of the QT interval on the ECG and by the occurrence of syncope or cardiac arrest, mainly precipitated by emotional or physical stress; however, some deaths occur when patients are at rest or asleep. LQTS includes the relatively common Romano-Ward (RW) variant, which has a prevalence of 1:2000 live births (10), and the rare and extremely severe Jervell and Lange-Nielsen (JLN) syndrome accompanied by congenital deafness (11). The inheritance mode for RW is autosomal dominant or sporadic, whereas JLN shows autosomal recessive inheritance or sporadic cases of compound heterozygosity (11). LQTS contributes to Sudden Infant Death Syndrome (12) and even to stillbirths (13).
The ventricular tachyarrhythmia that underlies the cardiac events of LQTS is torsades-de-pointes (TdP). This highly specific type of ventricular tachycardia is often self-limiting, thus producing transient syncope, but TdP can also degenerate into ventricular fibrillation and cause cardiac arrest or sudden death. We still don't know why in certain patients TdP stops after few seconds whereas in others it continues with devastating consequences.
The morphology of the T wave is often useful for the diagnosis, and the lateral precordial leads are especially informative when they reveal biphasic or notched T waves (14). T-wave alternans in polarity or amplitude is a marker of major electrical instability and, when observed, is diagnostic (15). In the presence of syncopal episodes occurring under stressful conditions in an individual with marked prolongation of the QT interval, the diagnosis of LQTS is rather simple. For the more questionable situations, the so-called Schwartz score, originally proposed in 1993 (16) and updated more recently (6) (Table 1) can help. The standard and very effective therapy for LQTS is based on β-blockers [propranolol and nadolol, whereas metoprolol has been linked to frequent recurrences (17)] which should be administered also to still asymptomatic patients with QT prolongation, given the potential for sudden death as first disease manifestation. For patients on full-dose β-blocker therapy in the case of a first recurrence of syncope left cardiac sympathetic denervation (18,19) is the treatment of choice whereas in the case of cardiac arrest, recurrent syncope or of signs of very high risk an implantable cardioverter defibrillator (ICD) is appropriate (8,20).
Table 1. 1993-2012 LQTS Diagnostic Criteria.
| Points | |||
|---|---|---|---|
| ELECTROCARDIOGRAPHIC FINDINGS# | |||
| ≥480 ms | 3 | ||
| A | QTcˆ | 460 – 479 ms | 2 |
| 450 – 459 (male) ms | 1 | ||
| B | QTcˆ 4th minute of recovery from exercise stress test ≥ 480 ms | 1 | |
| C | TORSADE DE POINTES * | 2 | |
| D | T WAVE ALTERNANS | 1 | |
| E | NOTCHED T WAVE IN 3 LEADS | 1 | |
| F | LOW HEART RATE FOR AGE @ | 0.5 | |
| CLINICAL HISTORY | |||
| A | SYNCOPE * | WITH STRESS | 2 |
| WITHOUT STRESS | 1 | ||
| B | CONGENITAL DEAFNESS | 0.5 | |
| FAMILY HISTORY | |||
| A | FAMILY MEMBERS WITH DEFINITE LQTS $ | 1 | |
| B | UNEXPLAINED SUDDEN CARDIAC DEATH BELOW | 0.5 | |
| AGE 30 AMONG IMMEDIATE FAMILY MEMBERS $ | |||
In the absence of medications or disorders known to affect these electrocardiographic features
QTc calculated by Bazett's formula where QTc = QT/√RR
Mutually exclusive
Resting heart rate below the 2nd percentile for age
The same family member cannot be counted in A and B
Score: ≤ 1 point: low probability of LQTS
1.5 to 3 points: intermediate probability of LQTS
≥ 3.5 points high probability
(From ref. 6)
Updated Genetics
Sixteen genes have been identified so far as either responsible for or associated to LQTS (Table 2). The three main genes, KCNQ1 (LQT1), KCNH2 (LQT2), and SCN5A (LQT3), account for approximately 75% clinically definite LQTS whereas the minor genes contribute an additional 5% collectively. An estimated 20% of LQTS remains genetically elusive.
Table 2. Molecular Basis of Cardiac Channelopathies.
| Gene | Locus | Protein |
|---|---|---|
| LONG QT SYNDROME | ||
| Major LQTS Genes | ||
| KCNQ1 (LQT1) | 11p15.5 | IKs potassium channel alpha subunit (KVLQT1, KV7.1) |
| KCNH2 (LQT2) | 7q35-36 | IKr potassium channel alpha subunit (HERG, KV11.1) |
| SCN5A (LQT3) | 3p21-p24 | Cardiac sodium channel alpha subunit (NaV1.5) |
| Minor LQTS Genes (listed alphabetically) | ||
| AKAP9 | 7q21-q22 | Yotiao |
| CACNA1C | 12p13.3 | Voltage gated L-type calcium channel (CaV1.2) |
| CALM1 | 14q32.11 | Calmodulin 1 |
| CALM2 | 2p21.3-p21.1 | Calmodulin 2 |
| CAV3 | 3p25 | Caveolin-3 |
| KCNE1 | 21q22.1 | Potassium channel beta subunit (MinK) |
| KCNE2 | 21q22.1 | Potassium channel beta subunit (MiRP1) |
| KCNJ5 | 11q24.3 | Kir3.4 subunit of IKACH channel |
| SCN4B | 11q23.3 | Sodium channel beta 4 subunit |
| SNTA1 | 20q11.2 | Syntrophin-alpha 1 |
| ANDERSEN-TAWIL SYNDROME | ||
| KCNJ2 (ATS1) | 17q23 | IK1 potassium channel (Kir2.1) |
| ANKYRIN-B SYNDROME | ||
| ANKB | 4q25-q27 | Ankyrin B |
| TIMOTHY SYNDROME | ||
| CACNA1C (TS) | 12p13.3 | Voltage gated L-type calcium channel (CaV1.2) |
| CATECHOLAMINERGIC POLYMORPHIC VENTRICULAR TACHYCARDIA | ||
| RYR2 (CPVT1) | 1q42.1-q43 | Ryanodine Receptor 2 |
| CASQ2 (CPVT2) | 1p13.3 | Calsequestrin 2 |
| KCNJ2 (CPVT3) | 17q23 | IK1 potassium channel (Kir2.1) |
| CALM1 | 14q32.11 | Calmodulin 1 |
| TRDN | 6q22.31 | Triadin |
| BRUGADA SYNDROME | ||
| SCN5A (BrS1) | 3p21-p24 | Cardiac sodium channel alpha subunit (NaV1.5) |
| Minor BrS Genes (listed alphabetically) | ||
| CACNA1C | 2p13.3 | Voltage gated L-type calcium channel (CaV1.2) |
| CACNA2D1 | 7q21-q22 | Voltage gated L-type calcium channel 2 delta 1 subunit |
| CACNB2 | 10p12 | Voltage gated L-type calcium channel beta 2 subunit |
| DLG1 | 3q29 | Synapse-associated protein 97 |
| GPD1L | 3p22.3 | Glycerol-3-phosphate dehydrogenase 1-like |
| HCN4 | 15q24.1 | Hyperpolarization-activated cyclic nucleotide-gated channel 4 |
| KCND3 | 1p13.2 | Voltage-gated potassium channel (Ito) subunit Kv4.3 |
| KCNE3 | 11q13.4 | Potassium channel beta subunit 3 (MiRP2) |
| KCNE5 | Xq22.3 | Potassium channel beta subunit 5 |
| KCNJ8 | 12p12.1 | Inward rectifier K(+) channel Kir6.1 |
| MOG1 | 17p13.1 | RAN guanine nucleotide release factor 1 |
| SCN1B | 19q13 | Sodium channel beta 1 |
| SCN3B | 11q24.1 | Sodium channel beta 3 |
| S LMAP | 3p14.3 | Sarcolemma associated protein |
KCNQ1 encodes the α-subunit of the K+ channel Kv7.1, generating IKs, which is physiologically increased by sympathetic activation and is essential for QT adaptation during heart rate increases. When IKs is diminished or dysfunctional, the QT interval fails to shorten appropriately during tachycardia, thus leading to a potentially arrhythmogenic condition. Heterozygous KCNQ1 mutations cause the dominant RW LQT1 syndrome and is the most common LQTS genotype accounting for 30-35% of LQTS. Homozygous mutations in KCNQ1, or compound heterozygous mutations, can cause the autosomal recessive JLN variant. Different effects may be produced by mutations in this multimeric K+ channel. If the mutation prevents the co-assembly such that only the wild-type subunit can tetramerize, then a mechanism of haploinsufficiency whereby IKs is reduced by 50% emerges. On the other hand, if the mutant-containing allele can tetramerize and “poison” the tetramer, then a dominant negative mechanism emerges resulting in a minimum residual of 6% current density. The dominant-negative effect of certain KCNQ1 mutations may manifest as a failure to modulate Iks by (β-adrenergic signaling (21,22).
The second most common gene harboring LQTS mutations is KCNH2, encoding HERG, the α -subunit of the K+ channel conducting the Ikr current. Ikr and Iks are two components of the delayed rectifier Ik current, the major determinant of phase 3 repolarization in ventricular cardiomyocytes. LQT2-causative mutations in KCNH2 provoke a reduction in Ikr current, most commonly by mechanisms involving impaired trafficking of the protein to the plasma membrane (23).
The third major LQTS gene is SCN5A, which encodes the α-subunit of the cardiac sodium channel (NaV 1.5) that conducts the depolarizing inward sodium current. Few months after its identification as a LQTS gene in 1995 (1), it was shown that the SCN5A-ΔKPQ mutation produces the LQTS phenotype by increasing the persistent (or late) Na+ inward current and, therefore, prolonging action potential duration (24). This study provided the first evidence linking a mutation to a functional alteration in the ionic control of ventricular repolarization and paved the way to all subsequent functional studies that have become the gold standard to establish that a novel mutation in a LQTS patient is likely a disease-causing one.
After the identification of the first 3 major LQTS genes (1-3), several others were identified. KCNE1 and KCNE2 encode K+ channel auxiliary subunits that are associated with the α -subunits encoded by KCNQ1 and KCNH2. Mutations in KCNE1 may cause either the dominant RW (LQT5) or, if present in homozygosity or compound heterozygosity, the recessive JLN syndrome (11). There are few cases of KCNE2 mutations associated with LQTS and most of them represent acquired LQTS associated with specific drugs, almost all Ikr blockers.
Among the sodium channel interacting protein-coding genes, CAV3 (25), SCN4B (26), and SNTA1 (27) are regarded as additional LQTS genes (LQT9, LQT10, and LQT12) that essentially mimic LQT3. The AKAP9-encoded yotiao is involved in the phosphorylation of Kv7.1 and its mutation has been described in LQT11 which functionally mimics LQT1. Two missense mutations in CACNA1C, encoding a voltage-gated calcium channel, are linked to Timothy syndrome (TS; LQT8), a rare and extremely malignant LQTS variant. In a large Chinese family, a heterozygous mutation was identified in the inwardly rectifying K+ channel subunit Kir3.4, encoded by KCNJ5. The variant was present in all the 9 affected family members and was absent in >500 ethnically matched controls, suggesting a role in the pathogenesis of LQT13. On the other hand, the ANKB, KCNJ2, and CACNA1C genes, often referred to as LQT4, LQT7, and LQT8, are associated with complex clinical disorders: ankyrin B syndrome, Andersen-Tawil syndrome, and Timothy syndrome respectively. In the first two prolongation of the QT interval is modest. Until LQTS-causing mutations are found in these genes in patients with clinically definite LQTS, these 3 genes should not be strictly considered as part of LQTS.
Quite recently, a most malignant form of LQTS that causes recurrent cardiac arrest due to ventricular fibrillation manifesting in infancy has been found associated with mutations in CALM1 and CALM2, two of the three human genes encoding calmodulin (28). Calmodulin is a ubiquitous multifunctional Ca2+ binding protein and overexpression of calmodulin mutants with defective Ca2+ binding produces major prolongation of ventricular action potentials (29,30). In two unrelated infants with QT prolongation and very early occurrence of cardiac arrest due to ventricular fibrillation, whole exome sequencing revealed de novo mutations in either CALM1 or CALM2. A subsequent candidate gene screening in a cohort of 82 LQTS genotype-negative subjects identified two more CALM1 mutation carriers (28). All 4 patients share strikingly similar clinical manifestations: major QT prolongation (all > 600 ms), T wave alternans, cardiac arrest in infancy, multiple episodes of ICD-terminated ventricular fibrillation mostly triggered by sympathetic activation, poor response to pharmacological and non-pharmacological interventions.
Genetics and Arrhythmia Triggers
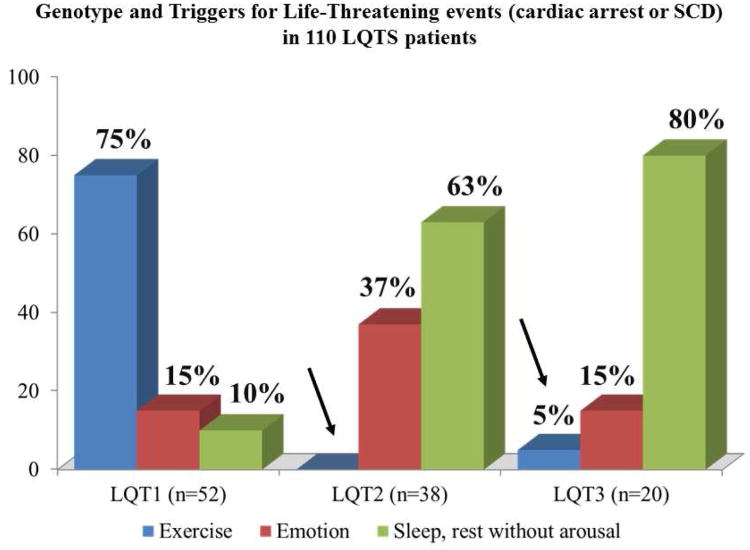
A study performed on almost 700 patients of known genotype and all with arrhythmic events demonstrated that the triggers for arrhythmias in LQTS are gene-specific (31). LQT1 patients are at risk especially during sympathetic activation, as with physical exercise or emotional stress (Fig. 1), and this stems from the fact that they have a lower-than-normal IKs and therefore their ability to shorten the QT interval when heart rate increases is impaired. LQT2 and LQT3 patients, who have a normal level of IKs, are at no special risk during physical exercise and sport activity. LQT2 patients are exquisitely sensitive to sudden noises, such as alarm clocks or telephone ringing whereas LQT3 patients tend to have their events while at rest or while asleep. The fact that in that large study 99% of the events occurring while swimming were in LQT1 patients and that 80% of the events triggered by sudden noises were in LQT2 patients allow the shrewd clinician to suspect the correct genotype based on simple clinical history and well before obtaining the genetic results.
Figure 1. Triggers for lethal cardiac events in LQT1, LQT2, and LQT3 patients.
The arrows point out the rare occurrence of these events during sympathetic activation in patients without mutations affecting the IKs current. (Modified from ref. 31)
Genetics and Risk Stratification
Since the early days of molecular genetics for LQTS attempts were made to correlate genotypes with outcomes. The first large study reported on 647 LQTS patients and suggested interactions between genotype, QTc and gender (32). The risk of cardiac events, higher for LQT2 females and LQT3 males, increased in the presence of marked QT prolongation (QTc >500 ms). LQT1 patients were less likely to experience events probably because of the very high percentage (36%) of patients with the disease-causing mutation but with a QTc<440 ms. The existence of these genotype-positive/phenotype-negative patients is related to the low penetrance existing in LQTS, which was postulated in 1980 (33) and demonstrated in 1999 (34).
A significant step forward came with the realization that besides genotype-based risk stratification, intragenic risk stratification was possible for LQT1 and LQT2 based on molecular/structural location and cellular function. In 2002 (35) and 2007 (36), Moss et al indicated first that LQT2 patients with pore-localizing mutations were at higher risk and then that in LQT1 patients both the transmembrane location of the mutations and their dominant-negative effect are independent risk factors for cardiac events. These studies indicated that not all mutations on the same gene produce a similar clinical phenotype and initiated a series of intriguing revelations on the complexity of the genotype-phenotype correlation. An evolution of these studies led to the realization that there are areas in the genes, such as the Kv7.1 cytoplasmic loops, not only associated to higher arrhythmic risk but also to a particularly good response to β-blocker therapy (21).
However, neither the localization of a mutation nor its cellular electrophysiological effect is sufficient to consistently predict the impact on clinical manifestations. The most striking example of mutation-specific behavior is probably that of KCNQ1-A341V, a relative hot-spot mutation characterized by unusual clinical severity demonstrated by 80% of the patients being symptomatic, with >30% experiencing cardiac arrest or sudden death (37,38). What is puzzling is that A341V is only a mildly dominant negative mutation producing a relatively modest IKs loss.
Genetics, Response to Therapy, and Clinical Management
Since the identification of the first LQTS genes high hopes were generated that understanding the molecular underpinnings of the disease would inspire novel therapeutic approaches. So far, this has been only partially true. Still, progress in the management of these patients based on genotype-phenotype studies is impressive and undeniable and so far, the diagnostic, prognostic, and therapeutic impact of genetic testing has been realized most fully for LQTS compared to all other genetically-mediated channelopathies and cardiomyopathies (7).
The response to β-blocker therapy is in part gene-specific, but not as much as previously thought. Clearly, β-blockers are extremely effective for LQT1 patients (31,39,40) and are also effective for LQT2 patients but LQT2 females are less fully protected. Contrary to some previous opinion, largely dependent on the inclusion in the analysis of a subgroup largely unresponsive to therapy and represented by patients with events in the first year of life (41), β-blockers are effective also for LQT3 patients as indicated by a study in > 400 patients (42).
Within months after the cellular demonstration, in August 1995, that the electrophysiological consequence of a SCN5A mutation is an increase in persistent Na+ current (24), clinical (43) and experimental (44) evidence was provided that a sodium channel blocker, mexiletine, could markedly shorten the QT interval in LQT3, but not in LQT1 and LQT2, patients and block the persistent Na+ current. It was immediately warned that mexiletine should have never been used instead of β-blockers but as potentially useful addition. Indeed, despite mexiletine-mediated clear evidence of benefit in certain patients there have been failures in others. The response to Na+ channel blockers is clearly mutation-specific and this dictates the correct clinical approach: to always test the QT shortening effect of mexiletine using the acute oral drug testing approach (45) with half of the daily dose while monitoring for two hours the patient's ECG: if the QTc shortens by more than 40 ms without undue PR lengthening then it is reasonable to add mexiletine to the β-blocker therapy. There is current interest as potential LQT3-specific therapy in ranolazine, a drug more selective for the persistent Na+ current, but the available clinical data are scanty and short term (46). It should not be forgotten that Na+ channel blockers have the potential to impair cardiac conduction, and vigilant ECG monitoring is necessary when LQT3 patients are treated with mexiletine to avoid serious consequences In LQT3 patients with specific mutations, mexiletine and propranolol may have beneficial synergistic effects in correcting major electrophysiological abnormalities (47). Flecainide should be, and is, seldom considered for LQTS patients because of its IKr blocking effect.
The major impact of genetics has been in the general management of the patients and of their families. As to the patients, the identification of specific triggers for the arrhythmic events (31) has led to rational attempts to avoid or counter the at-risk situations. LQT1 are advised to avoid excessive stress, be it physical or mental, and specific activities such as swimming unless under proper protection. For LQT2 patients it is important to minimize sudden noises, especially when resting; this means to avoid telephones and alarm clocks in the bedroom and to wake up the affected children without yelling. Also, as sleep deprivation and disruption is particularly bad for LQT2 women in the post-partum period it is advisable that fathers find the proper way to feed the infants nighttime without waking the mothers. LQT2 and LQT3 patients, because of their “normal” IKs, are not expected to be at special risk during physical activity.
As to the families, the issue is that of “cascade screening” (48), i.e. once the disease-causing mutation is identified in the proband the entire family should undergo testing for that specific mutation, which is rapid and inexpensive, in order to identify the mutation carriers with normal QT. This concept is the direct consequence of the fact that low penetrance is common in LQTS (34), which confirms the original hypothesis (33) that some patients may be affected by LQTS and nonetheless have a normal QT interval. This implies that normal findings on an electrocardiogram cannot be used to exclude LQTS and mandates the necessity to perform molecular screening in all family members once the disease-causing mutation had been identified in the proband.
Cascade screening allows the identification, as mutation positive, of individuals who would have been otherwise considered unaffected and therefore would have remained at risk for potentially life-threatening arrhythmias either as spontaneous event or more likely as provoked by a variety of drugs with an IKr blocking effect. This leads to prophylactic treatment in over 70% of mutation positive individuals (49). Also important is the fact that those family members who are found to be negative for the family's disease-causing mutation will be relieved to learn that they are not at risk and that they should not fear for their offspring.
The magnitude of the impact that genetic screening has on clinical cardiology is exemplified by the fact that not performing cascade screening could lead to a number of otherwise avoidable deaths among those genotype positive/phenotype negative family members of affected patients. These are deaths that could be prevented partly by therapy, when appropriate, and partly by providing on a regular basis an updated list of drugs to carefully avoid. Cascade screening forcefully demonstrates that molecular biology and genetics can no longer be regarded as tools for researchers, but nowadays represent an essential component of good medical care.
Catecholaminergic Polymorphic Ventricular Tachycardia
Akin to LQT1, catecholaminergic polymorphic ventricular tachycardia is characterized phenotypically by exercise-induced syncope, seizures, or sudden death in the setting of a structurally normal heart (50-52). However, in contrast to LQTS, the electrocardiogram at rest is typically normal with only subtle, non-diagnostic bradycardia and U waves occasionally present. Instead, provocative stress testing by treadmill, cycle, or isoproterenol is the key diagnostic test to elicit CPVT's trademark signature of exercise-induced bidirectional ventricular tachycardia. Importantly, although fairly specific for CPVT, exercise-induced bidirectional VT is insensitive as the majority of patients with mutation proven CPVT do not manifest this arrhythmia (52). Moreover, CPVT1 patients with a negative exercise stress test are not at zero risk (52). Rather, in the context of a positive personal or family history, CPVT should be suspected when a stress test exhibits the onset of premature ventricular contractions (PVCs) when the heart rate reaches around 110–130 beats per minute. At this work load, this exercise-induced ectopy will commence with single, intermittent PVCs and will progress typically to PVCs in bigeminy and couplets. Only occasionally will more complex ectopy ensue. Often the exercise-induced ectopy will burn out (return to normal sinus rhythm) at highest work load/peak heart rate and normal sinus rhythm almost always persists throughout the recovery phase.
Because CPVT is more arrhythmic than LQTS with higher estimated lethality and higher breakthrough rates during conventional β-blocker therapy, it is critical to distinguish CPVT from LQTS (53). Often, CPVT patients have been misdiagnosed as having “atypical” LQTS (54). As a diagnostic pearl, in the setting of an exercise-triggered cardiac event, a resting QTc < 460 ms, and a structurally normal heart, CPVT rather than “concealed” or “normal QT interval” LQTS, is far more likely to be the root cause.
Pathogenetically, approximately 50-60% of CPVT stems from heritable or sporadic mutations in the RYR2-encoded cardiac ryanodine receptor/calcium release channel, a critical regulator of intracellular calcium (55). RYR2 is one of the largest genes in the human genome with its 105 translated exons that encodes for a protein containing 4967 amino acids. However, there are 3 particular domains/clusters encoded by 16 exons where two-thirds of the current CPVT1-associated mutations localize and all published mutations currently reside within less than half of RYR2's exons (56,57).
Besides CPVT1, rare autosomal recessive subtypes of CPVT stem from mutations in CASQ2-encoded calsequestrin 2 (CPVT2) (58) or TRDN encoding the junctional protein triadin (59) (CPVT4). Recently, mutations in CALM1 encoding calmodulin were discovered in one family with autosomal dominant CPVT-like phenotype, and in a de novo single case, all genotype-negative for RYR2 or CASQ2 mutations (60).(CPVT5). Also, mutations in the KCNJ2-encoded Kir2.1 can express a clinical phenotype that mimics autosomal dominant CPVT (61) (CPVT3). Generally, loss-of-function KCNJ2 mutations cause type 1 Andersen-Tawil syndrome (ATS1), a heritable channelopathy readily distinguishable from CPVT with characteristic U waves, facial/skeletal stigmata, and with a much more benign prognosis. However, several KCNJ2 mutations have been identified in patients clinically diagnosed with CPVT because of complex ventricular ectopy including bi-directional VT with no clinical features to suggest ATS1.
CPVT genetic testing is recommended for any patient in whom a cardiologist has established a clinical index of suspicion for CPVT based on examination of the patient's clinical history, family history, and expressed electrocardiographic phenotype during provocative stress testing with cycle, treadmill, or catecholamine infusion and mutation-specific genetic testing is recommended for family members and appropriate relatives following the identification of the CPVT-causative mutation in an index case (62). Presently, the primary purpose of CPVT genetic testing is diagnostic, to genetically confirm a clinically suspected case of CPVT and establish the particular genotype and to identify the potentially at-risk relatives (63).
However, in stark contrast to the genotype-specific, region-specific, and even mutation-specific risk stratifying information that has emerged in LQTS, no definitive domain-specific or mutation-specific prognostication can be made for RYR2-mediated CPVT (i.e. CPVT1). Preliminary data suggest that relatives carrying a RYR2 mutation in the C-terminal channel-forming domain may be at greater arrhythmic risk than those with N-terminal domain (64).
Thus, all patients with clinically manifest CPVT1 are treated based upon their phenotype without regard to any details about the particular mutation. Here, the genotype does not guide therapy. Phenotype-guided therapy generally consists of β-blocker therapy and/or left cardiac sympathetic denervation therapy and if necessary, combination therapy with the addition of flecainide (53,65-68). Device therapy with an implantable defibrillator should be the last intervention (rather than the observed all too often first one) for only the highest risk CPVT subjects because of the uncommon but concerning issue of a CPVT-related ICD storm whereby the ICD ultimately fails to rescue the patient (69,70).
Although the chief purpose of CPVT genetic testing is diagnostic rather than either prognostic or therapeutic, the genotype influences management/treatment of a patient with genetically confirmed CPVT in two important ways. First, it is important to distinguish KCNJ2-mediated CPVT (CPVT3) from the more common CPVT1 as the treatment strategy is different for the two genotypes. In contrast to the phenotype-guided treatment strategy for either CPVT1 or genotype negative/phenotype positive CPVT, patients with KCNJ2-mediated CPVT may be more responsive to primary therapy with flecainide or mexiletine rather than β-blocker therapy (71). In addition, the protective, anti-fibrillatory effect of LCSD has been demonstrated much more clearly for patients with CPVT1 than CPVT3 patients (65).
Second, mutation-specific confirmatory testing in relatives enables prophylactic β-blocker therapy to be initiated at a young age if deemed necessary (64). Here, without the genotype, potentially at-risk family members would only be revealed after they are old enough to do a provocative stress test or if they manifest a concerning symptom. Considering that the first concerning symptom can be sudden death and that up to 15% of autopsy negative sudden unexplained death victims are CPVT1 positive (72), identifying a potentially vulnerable CPVT1-positive relative as early as possible is an indirect but potentially lifesaving therapeutic contribution of CPVT genetic testing.
Brugada Syndrome
The Brugada syndrome (BrS) is a hereditary disease characterized by its “signature sign”, a coved-type ST-segment elevation in the anterior precordial leads (V1 to V3), referred to as a type 1 Brugada ECG pattern, and by the presence of right ventricular conduction abnormalities and life threatening ventricular arrhythmias (73,74). The typical case is a 40-year-old resuscitated male without clear evidence for structural heart disease and with a family history for (nocturnal) sudden cardiac death (SCD). Indeed, up to 75% of those clinically affected are of male gender, and the mean age of onset of events is around 40 years but with a wide range. A family history of SCD is reported in 20-50% of cases. There is an autosomal dominant pattern of transmission with highly variable and often low penetrance. Several aspects are still unclear, especially the pathophysiology of the right precordial ST-segment elevation (75).
BrS is a genetically heterogeneous disease, with the involvement of at least 13 different genes (76,77). Most mutations occur in genes with impact on the function of cardiac Na+ channels. SCN5A, the gene encoding for the α-subunit of the cardiac sodium channel, is involved in 20% to 25% of patients. More than 200 SCN5A BrS-related mutations have been described to date (78). All mutations induce a reduction in the sodium current amplitude and do so through several mechanisms, including altered channel kinetics (e.g., faster inactivation or slower recovery from inactivation), trafficking defects and generation of truncated proteins. Recently, a pure, self-sufficient causative role of loss-of-function SCN5A mutations has been challenged as in several large SCN5A-related BrS families affected individuals did not carry the presumed familial disease causing mutation thus suggesting that SCN5A may actually represent a strong modifier (79).
Other genes with impact on sodium channel function are the sodium channel β-subunit genes (SCN1B, SCN3B) affecting channel kinetics, glycereol-3 phosphate dehydrogenase 1-like enzyme (GPD1L), MOG1 and SLMAP that affects trafficking of sodium channels. Potentially causative variants are also found in the calcium channel genes (CACNA1C, CACNB2B, CACNA2D1), in genes that affect the transient outward current (Ito) (KCNE3, KCND3, KCNE5) and in the gene that forms the pore forming unit of the ATP sensitive potassium current (IKATP KCNJ8) (76). Involvement of most of these genes has been described in single patients and families with BrS, although mutations in CACNA1C and CACNB2B are reported to contribute to up to 11% of BrS. In basic electrophysiological studies, the calcium channel genes lead to loss of function of basal L-type calcium current (ICa,L), a mutation in KCNE3, KCND3, or KCNE5 lead to a gain of function of Ito and mutations in KCNJ8 increase IKATP.
Genotype-phenotype correlation studies in Brugada syndrome are sparse. Initial studies indicate that SCN5A-associated BrS typically presents with longer conduction intervals in all cardiac compartments (80). Meta-analyses consistently show that the presence or absence of a SCN5A mutation does not impact clinical outcome (81). However, within the SCN5A cohort, the type of SCN5A mutation may be useful for risk stratification, with nonsense mutations giving rise to truncated protein leading to more severe conduction disorders and more symptoms (82). Calcium channel-related BrS seems to associate with shorter than normal QTc intervals, but it is not clear whether this impacts on prognosis (83).
As the diagnosis of BrS is made on clinical grounds, genetic testing is not required for this goal. Yet, the finding of a loss-of-function SCN5A mutation might help in a clinically uncertain diagnosis. As indicated above, knowledge of a mutation does not impact on prognosis, with the possible exception of specific findings in SCN5A. BrS genetic testing can be useful for any patient in whom there is reasonable suspicion for BrS based on examination of the patient's and of his/her family clinical history, and a clear electrocardiographic phenotype based on either resting 12-lead ECGs and/or provocative drug challenge testing. Because the presence of a disease-causing mutation does impact life style [e.g., fever and specific drugs should be avoided (84)], cascade genetic screening is recommended for family members following the identification of the BrS-causative mutation in an index case. In the setting of an isolated type 2 or type 3 Brugada ECG pattern genetic testing has no place (61).
Issues with Genetic Testing
Advances in deciphering the molecular basis for heritable cardiac arrhythmia susceptibility have re-shaped the diagnostic paradigm and clinical management of these three familial arrhythmia syndromes (cardiac channelopathies) to include genetic testing, gene specific considerations in therapy and increased awareness of the need to assess disease risk in family members. The accurate ascertainment of family history, the proper use and interpretation of genetic test results, and the identification and management of at-risk family members have become the standard-of-care in this field.
Optimal Use of Genetic Testing
Ascertaining a comprehensive family history of cardiac arrhythmias and unexpected death is of utmost importance when considering the diagnosis of a heritable arrhythmia syndrome. In performing a detailed family history, special attention should be paid to the occurrence of syncope or sudden unexpected death especially among young adults and children in the family, noting special circumstances surrounding unexpected death (e.g., drowning, seizures), and identifying any relatives with implanted cardiac devices along with the indication. A pedigree drawing is essential for the recognition of a mode of inheritance compatible with a monogenic disorder (e.g. autosomal dominant, autosomal recessive, X-linked). Information about the patient and close relatives is ultimately helpful in making final decisions about the use of genetic testing to confirm clinical suspicions. However, incomplete penetrance or subclinical disease expression may obscure the pattern of disease segregation in a family. This is unfortunately a common problem in families with BrS due to low penetrance (79) and may also obfuscate the recognition of inheritance patterns in LQTS (31). Especially severe and early-onset forms of LQTS and other syndromes may be caused by de novo mutations in which case no family history is expected (47,85,86).
Genetic testing is a specialized diagnostic procedure available for LQTS, BrS, and CPVT through commercial and research laboratories (87). In the United States, clinical genetic testing laboratories must meet stringent criteria for quality standards that conform to the federal Clinical Laboratory Improvement Amendments (CLIA) passed in 1988 (88). Further, unlike more commonly used laboratory tests, genetic testing should be performed after informing the patient regarding the potential risks, benefits and limitations. Involvement of a genetic counselor is ideal in circumstances where either physician time or knowledge is limited. Despite these requirements, genetic testing can have tremendous value in identifying mutations that help confirm clinical suspicions, select genotype-specific therapy and direct specific testing of at-risk relatives.
Pitfalls and Limitations of Genetic Testing
The current yield of genetic testing for each of these syndromes ranges from 25% (BrS) to 80% (LQTS). Further, the methods to identify mutations are not 100% sensitive and therefore a negative genetic test cannot exclude the disorder by itself. Also, certain detectable DNA sequence variants may not have a clear causal role in a patient's condition either because they are rare polymorphisms (89) or are located in regions of the channel protein that have unknown functional importance. Based upon several years of genetic testing experience in the academic and commercial sectors, we now know that most mutations are ‘private’ (i.e., occurring in a single family) missense mutations with uncertain functional or pathophysiological consequences (77,90). Therefore, interpreting genetic test results is often confounded by discovery of ‘variants of unknown significance’ for which there is insufficient data or predictive tools to assess accurately the likelihood that a particular variant predisposes to an arrhythmia or whether the change is merely a benign rare variant (91). Only a small fraction of all identified genetic variants in the myriad genes associated with LQTS, BrS, CPVT have been investigated functionally to elucidate a biologically plausible contribution to pathogenesis. Even fewer mutations have been studied in a genetically engineered animal model or native cardiac cell. Computational strategies have been developed to predict the functional consequences of mutations but none of these methods have been tested rigorously as valid clinical predictors. The lack of functional or biological validation of mutation effects remains the most severe limitation of genetic test interpretation in the cardiac channelopathies (88).
Interpretation of a negative genetic test in a symptomatic person is a challenge. Despite more than 18 years of genetic discovery in the cardiac channelopathies, there remain a substantial number of cases having classic symptoms and signs for one of the heritable arrhythmia syndromes who test negative for the many known genes. This may be explained by either a false negative or a true negative test result. One potential cause for a false negative genetic test is the location of a mutation outside the region of the gene normally interrogated by the test (92). Alternatively, certain types of mutation may be missed by standard testing strategies. For example, DNA sequencing can miss multi-exon deletion or duplication mutations (93-95). False negative results may sometimes be overcome by repeat testing in situations where the clinical diagnosis has a high level of certainty (96). A true negative test result may be a clue to the existence of an as yet undefined gene involved with arrhythmia susceptibility. These situations are excellent opportunities to pursue genetic discovery in a research setting as was recently done to identify novel calmodulin gene mutations in severe infantile presentations of LQTS (28). The potential negative impact of learning the results of a genetic test must also to be considered. Patients should be properly educated and carefully counseled about their long-term risks of cardiac arrhythmia without inciting excessive apprehension by implying that genotype is an absolute predictor of sudden death risk. Physicians should also be sensitive to the potential socioeconomic fallout (e.g., insurability) from a genetic diagnosis and vigorously guard confidentiality. Fortunately, in the United States, the recently enacted Genetic Information Non-discrimination Act (GINA) prohibits workplace and insurance discrimination based on genetic predisposition.
Genetic Modifiers
Another important conceptual barrier to extrapolating genetic test results to patient management is the variable disease expression and penetrance common among these disorders. For example, in congenital LQTS, not all individuals carrying disease associated mutations have equal risk for expressing the clinical manifestations of the disease (34,97). Clinical heterogeneity is a common feature in LQTS and BrS. Members of the same family that share the same mutation may have varying phenotypes, ranging from no symptoms to sudden death. In rare cases, multiple mutations or combinations of a mutation with a common variant have accounted for unusual severity of one member of a larger family. Compound mutations help explain exaggerated disease severity in 4-8% of LQTS probands (98,99). On the other hand, predicting the likelihood of life-threatening arrhythmias in an asymptomatic mutation carrier continues to be most challenging.
These and related observations have inspired the hypothesis that genetic factors other than the primary disease-associated mutation can modify the risk for disease-related morbidity and mortality. Conceptually, hypotheses proposed to explain variable penetrance in the genetic arrhythmias may be separated into two categories: 1) factors that modify the underlying arrhythmogenic myocardial substrate, and 2) factors that affect the probability and magnitude of arrhythmia-triggering events. Genetic factors that could impact on the myocardial substrate include genes that encode proteins that contribute to the balance of inward and outward currents operating during the cardiac action potential. Genetic factors responsible for inter-individual differences in sympathetic and parasympathetic tone may alter one's susceptibility to triggered arrhythmias. Similarly, the magnitude of catecholamine responses to stress and exercise vary among individuals and some of this variability may have a genetic basis (100). Therefore, genes that participate in autonomic responses are candidate genetic modifiers.
Sorting out the relative effects of genetic modifiers is very challenging. Demonstrating association of particular genetic variants with phenotype requires a large population, and there are sample size restrictions with any rare disease such as inherited arrhythmias. Exploiting unique cohorts such as founder populations may have particular value in identifying modifier genes (97,101). For example, common variants in NOS1AP originally tagged in association with variable QT interval duration in healthy adults have been demonstrated to be modifiers of QT interval and the probability of symptoms in LQTS (102,103). Also, common variants in the 3′ untranslated region of KCNQ1 modify disease severity in an allele specific manner (104).
Ideally, risk stratification schemes based on presence of a primary mutation and one or more modifier alleles will emerge to improve prediction of cardiac events. However, there are challenges to extrapolating from population-based results to predicting an individual's risk. Clearly, more work is needed before we can take full advantage of this information for the ultimate goal of assessing risk even in asymptomatic mutation carriers.
Genetics and Medico-Legal Implications
The effectiveness of cascade screening for the early identification of affected family members also carries medico-legal implications. Cascade screening requires positive genotyping of the proband because identification of the disease-causing mutation is the necessary first step. It follows that the physician who does not attempt to genotype the proband clinically affected by one channelopathy has willfully decided to ignore whether some of his or her family members are carriers of the disease and thereby exposed to the risk of life-threatening arrhythmias. Similarly, the physician who, after having obtained positive genotyping, does not propose to initiate cascade screening within the family of the proband has similarly willfully decided to leave the affected family members - approximately one-half of the first-degree relatives - uninformed about their status and unprotected.
Future Impact of Genetics
Advances in DNA sequencing technology have inspired a vision of widespread use of genome sequencing in clinical medicine. Exactly how this vision will be realized is uncertain at the present time, but there is enormous potential for impacting risk prediction for common and less common diseases and for predicting responses to drug therapy, including drug-induced TdP which can be favored by specific genetic variants (105,106). Whole genome sequencing will eventually achieve an accuracy level and price point that will supplant targeted genetic testing for rare diseases such as inherited arrhythmia syndromes. Although this may make diagnosing genetic disorders technically more feasible, the art of making a clinical diagnosis will remain important particularly when genetic information reveals unexpected findings. There is already information from large scale exome sequencing efforts to anticipate that many incidentally discovered genetic variants in disease-associated genes, including those associated with LQTS, will be discovered in many individuals (107). Many of these variants could be merely false positive results and strategies to properly interpret and handle these incidental findings will be critical to avoid evoking needless concern or implementing unnecessary therapies in an asymptomatic person.
Conclusion
The progress in understanding channelopathies, and the underlying molecular biology, proceeds at mind-boggling speed. It should be clear to everyone in cardiology and medicine that genetics and clinical management of these diseases are tied together and that nowadays it is seldom possible to efficiently treat the affected patients without keeping into account what has been learnt from genetic testing.
Acknowledgments
The Authors are grateful to Pinuccia De Tomasi, BS, for expert editorial support.
Funding Support and Disclosuress: NIH grants HL068880 and HL083374; Dr. George has a Research funding from Gilead Sciences, Inc. Foster City, CA; Dr. Wilde is a member of an advisory board of Sorin and of Transgenomic; Dr. Ackerman is a consultant for Boston Scientific, Medtronic, St. Jude Medical, and Transgenomic. Intellectual property derived from Dr. Ackerman's research program resulted in license agreements in 2004 between Mayo Clinic Health Solutions (formerly Mayo Medical Ventures) and PGxHealth (formerly Genaissance Pharmaceuticals and now Transgenomic); Dr. Schwartz's research activity is supported by Telethon-Italy grant GGP09247.
List of Abbreviations
- BrS
Brugada Syndrome
- CPVT
Catecholaminergic Polymorphic Ventricular Tachycardia
- ICD
Implantable Cardioverter Defibrillator
- JLN
Jervell and Lange-Nielsen syndrome
- LQTS
Long QT Syndrome
- PVCs
Premature Ventricular Contractions
- RW
Romano Ward syndrome
- SCD
Sudden Cardiac Death
- TdP
Torsades-de-Pointes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang Q, Shen J, Splawski I, et al. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell. 1995;80:805–11. doi: 10.1016/0092-8674(95)90359-3. [DOI] [PubMed] [Google Scholar]
- 2.Curran ME, Splawski I, Timothy KW, et al. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Curran ME, Splawski I, et al. Positional cloning of a novel potassium channel gene: KvLQT1 mutations cause cardiac arrhythmias. Nature Genetics. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz PJ, Periti M, Malliani A. The long Q-T syndrome. Am Heart J. 1975;89:378–90. doi: 10.1016/0002-8703(75)90089-7. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz PJ. Idiopathic long QT syndrome: Progress and questions. Am Heart J. 1985;109:399–411. doi: 10.1016/0002-8703(85)90626-x. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz PJ, Crotti L, Insolia R. Long QT syndrome: from genetics to management. Circ Arrhythm Electrophysiol. 2012;5:868–77. doi: 10.1161/CIRCEP.111.962019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tester DJ, Ackerman MJ. Genetic testing for potentially lethal, highly treatable inherited cardiomyopathies/channelopathies in clinical practice. Circulation. 2011;123:1021–37. doi: 10.1161/CIRCULATIONAHA.109.914838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwartz PJ, Ackerman MJ. The long QT syndrome A transatlantic clinical approach to diagnosis and therapy. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht089. [DOI] [PubMed] [Google Scholar]
- 9.Tester DJ, Ackerman MJ. Postmortem long QT syndrome genetic testing for sudden unexplained death in the young. J Am Coll Cardiol. 2007;49:240–6. doi: 10.1016/j.jacc.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz PJ, Stramba-Badiale M, Crotti L, et al. Prevalence of the congenital long-QT syndrome. Circulation. 2009;120:1761–7. doi: 10.1161/CIRCULATIONAHA.109.863209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz PJ, Spazzolini C, Crotti L, et al. The Jervell and Lange-Nielsen Syndrome. Natural history, molecular basis, and clinical outcome. Circulation. 2006;113:783–90. doi: 10.1161/CIRCULATIONAHA.105.592899. [DOI] [PubMed] [Google Scholar]
- 12.Arnestad M, Crotti L, Rognum TO, et al. Prevalence of long QT syndrome gene variants in sudden infant death syndrome. Circulation. 2007;115:361–367. doi: 10.1161/CIRCULATIONAHA.106.658021. [DOI] [PubMed] [Google Scholar]
- 13.Crotti L, Tester DJ, White WM, et al. Long QT syndrome associated mutations in intrauterine fetal death. JAMA. 2013;309:1473–1482. doi: 10.1001/jama.2013.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malfatto G, Beria G, Sala S, Bonazzi O, Schwartz PJ. Quantitative analysis of T wave abnormalities and their prognostic implications in the idiopathic long QT syndrome. J Am Coll Cardiol. 1994;23:296–301. doi: 10.1016/0735-1097(94)90410-3. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz PJ, Malliani A. Electrical alternation of the T wave. Clinical and experimental evidence of its relationship with the sympathetic nervous system and with the long QT syndrome. Am Heart J. 1975;89:45–50. doi: 10.1016/0002-8703(75)90008-3. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz PJ, Moss AJ, Vincent GM, Crampton RS. Diagnostic criteria for the long QT syndrome: an update. Circulation. 1993;88:782–4. doi: 10.1161/01.cir.88.2.782. [DOI] [PubMed] [Google Scholar]
- 17.Chockalingam P, Girardengo G, Crotti L, et al. Not all beta-blockers are equal in the management of long QT syndrome types 1 and 2. J Am Coll Cardiol. 2012;60:2092–9. doi: 10.1016/j.jacc.2012.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz PJ. Cutting nerves and saving lives. Heart Rhythm. 2009;6:760–3. doi: 10.1016/j.hrthm.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 19.Collura CA, Johnson JN, Moir C, Ackerman MJ. Left cardiac sympathetic denervation for the treatment of long QT syndrome and catecholaminergic polymorphic ventricular tachycardia using video-assisted thoracic surgery. Heart Rhythm. 2009;6:752–9. doi: 10.1016/j.hrthm.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz PJ, Spazzolini C, Priori SG, et al. Who are the long-QT syndrome patients who receive an implantable cardioverter defibrillator and what happens to them? Data from the European long-QT syndrome implantable cardioverter-defibrillator (LQTS ICD) Registry. Circulation. 2010;122:1272–1282. doi: 10.1161/CIRCULATIONAHA.110.950147. [DOI] [PubMed] [Google Scholar]
- 21.Barsheshet A, Goldenberg I, O-Uchi J, et al. Mutations in cytoplasmic loops of the KCNQ1 channel and the risk of life-threatening events: implications for mutation-specific response to β-blocker therapy in type 1 long-QT syndrome. Circulation. 2012;125:1988–96. doi: 10.1161/CIRCULATIONAHA.111.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heijman J, Spätjens RL, Heijman J, et al. Dominant-negative control of cAMP-dependent IKs upregulation in human long-QT syndrome type 1. Circ Res. 2012;110:211–9. doi: 10.1161/CIRCRESAHA.111.249482. [DOI] [PubMed] [Google Scholar]
- 23.Anderson CL, Delisle BP, Anson BD, et al. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113:365–73. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- 24.Bennett PB, Yazawa K, Makita N, George AL., Jr Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–5. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 25.Vatta M, Ackerman MJ, Ye B, et al. Mutant caveolin-3 induces persistent late sodium current and is associated with long QT syndrome. Circulation. 2006;114:2104–12. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- 26.Medeiros-Domingo A, Kaku T, Tester DJ, et al. Sodium channel β4 subunit (SCN4β) mutation causes congenital long QT syndrome. Circulation. 2007;116:134–42. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueda K, Valdivia C, Medeiros-Domingo A, et al. Syntrophin mutation associated with long QT syndrome through activation of the nNOS-SCN5A macromolecular complex. Proc Natl Acad Sci U S A. 2008;105:9355–60. doi: 10.1073/pnas.0801294105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crotti L, Johnson CN, Graf E, et al. Calmodulin mutations associated with recurrent cardiac arrest in infants. Circulation. 2013;127:1009–1017. doi: 10.1161/CIRCULATIONAHA.112.001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier LS, Bers DM, Brown JH. Calmodulin and Ca2+/calmodulin kinases in the heart -physiology and pathophysiology. Cardiovasc Res. 2007;73:629–30. doi: 10.1016/j.cardiores.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT. Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. Proc Natl Acad Sci U S A. 2002;99:17185–90. doi: 10.1073/pnas.262372999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz PJ, Priori SG, Spazzolini C, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 32.Priori SG, Schwartz PJ, Napolitano C, et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–74. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz PJ. The long QT syndrome. In: Kulbertus HE, Wellens HJJ, editors. SUDDEN DEATH. M Nijhoff, The Hague; 1980. pp. 358–378. [Google Scholar]
- 34.Priori SG, Napolitano C, Schwartz PJ. Low penetrance in the long-QT syndrome: clinical impact. Circulation. 1999;99:529–33. doi: 10.1161/01.cir.99.4.529. [DOI] [PubMed] [Google Scholar]
- 35.Moss AJ, Zareba W, Kaufman ES, Gartman E, et al. Increased risk of arrhythmic events in long-QT syndrome with mutations in the pore region of the human ether-ago-go-related gene potassium channel. Circulation. 2002;105:794–99. doi: 10.1161/hc0702.105124. [DOI] [PubMed] [Google Scholar]
- 36.Moss AJ, Shimizu W, Wilde AA, et al. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation. 2007;115:2481–89. doi: 10.1161/CIRCULATIONAHA.106.665406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brink PA, Crotti L, Corfield V, et al. Phenotypic variability and unusual clinical severity of congenital Long QT Syndrome in a founder population. Circulation. 2005;112:2602–10. doi: 10.1161/CIRCULATIONAHA.105.572453. [DOI] [PubMed] [Google Scholar]
- 38.Crotti L, Spazzolini C, Schwartz PJ, et al. The common Long QT syndrome mutation KCNQ1/A341V causes unusually severe clinical manifestations in patients with different ethnic backgrounds: toward a mutation-specific risk stratification. Circulation. 2007;116:2366–75. doi: 10.1161/CIRCULATIONAHA.107.726950. [DOI] [PubMed] [Google Scholar]
- 39.Priori SG, Napolitano C, Schwartz PJ, et al. Association of long QT syndrome loci and cardiac events among patients treated with β-blockers. JAMA. 2004;292:1341–44. doi: 10.1001/jama.292.11.1341. [DOI] [PubMed] [Google Scholar]
- 40.Vincent GM, Schwartz PJ, Denjoy I, et al. High efficacy of beta-blockers in long-QT syndrome type 1: contribution of noncompliance and QT-prolonging drugs to the occurrence of beta-blocker treatment “failures”. Circulation. 2009;119:215–21. doi: 10.1161/CIRCULATIONAHA.108.772533. [DOI] [PubMed] [Google Scholar]
- 41.Schwartz PJ, Spazzolini C, Crotti L. All LQT3 patients need an ICD. True or false? Heart Rhythm. 2009;6:113–20. doi: 10.1016/j.hrthm.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 42.Wilde AA, Kaufman ES, Shimizu W, et al. Sodium channel mutations, risk of cardiac events, and efficacy of beta-blocker therapy in type 3 long QT syndrome. (abstr) Heart Rhythm. 2012;9(May Suppl):S321. [Google Scholar]
- 43.Schwartz PJ, Priori SG, Locati EH, et al. Long QT syndrome patients with mutations of the SCN5A and HERG genes have differential responses to Na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation. 1995;92:3381–6. doi: 10.1161/01.cir.92.12.3381. [DOI] [PubMed] [Google Scholar]
- 44.Dumaine R, Wang Q, Keating MT, et al. Multiple mechanisms of Na+ channel-linked long-QT syndrome. Circ Res. 1996;78:916–24. doi: 10.1161/01.res.78.5.916. [DOI] [PubMed] [Google Scholar]
- 45.Lown B. Management of patients at high risk of sudden death. Am Heart J. 1982;103(4 Pt 2):689–97. doi: 10.1016/0002-8703(82)90475-6. [DOI] [PubMed] [Google Scholar]
- 46.Moss AJ, Zareba W, Schwarz KQ, Rosero S, McNitt S, Robinson JL. Ranolazine shortens repolarization in patients with sustained inward sodium current due to type-3 long-QT syndrome. J Cardiovasc Electrophysiol. 2008;19:1289–93. doi: 10.1111/j.1540-8167.2008.01246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang DW, Crotti L, Shimizu W, et al. Malignant perinatal variant of long QT syndrome caused by a profoundly dysfunctional cardiac sodium channel. Circ Arrhythm Electrophysiol. 2008;1:370–8. doi: 10.1161/CIRCEP.108.788349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz PJ. Cascades or waterfalls, the cataracts of genetic screening are being opened on clinical cardiology. J Am Coll Cardiol. 2010;55:2577–9. doi: 10.1016/j.jacc.2009.12.064. [DOI] [PubMed] [Google Scholar]
- 49.Hofman N, Tan HL, Alders M, van Langen IM, Wilde AA. Active cascade screening in primary inherited arrhythmia syndromes: does it lead to prophylactic treatment? J Am Coll Cardiol. 2010;55:2570–6. doi: 10.1016/j.jacc.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 50.Coumel P, Fidelle J, Lucet V, et al. Catecholaminergic-induced severe ventricular arrhythmias with Adams-Stokes syndrome in children: report of four cases. Br Heart J. 1978;40:28–37. [Google Scholar]
- 51.Leenhardt A, Lucet V, Denjoy I, et al. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–9. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 52.Hayashi M, Denjoy I, Extramiana F, et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–34. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 53.Horner JM, Ackerman MJ. Ventricular ectopy during treadmill exercise stress testing in the evaluation of long QT syndrome. Heart Rhythm. 2008;5:1690–4. doi: 10.1016/j.hrthm.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 55.Tester DJ, Kopplin LJ, Will ML, Ackerman MJ. Spectrum and prevalence of cardiac ryanodine receptor (RyR2) mutations in a cohort of unrelated patients referred explicitly for long QT syndrome genetic testing. Heart Rhythm. 2005;2:1099–105. doi: 10.1016/j.hrthm.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 56.Priori SG, Napolitano C, Tiso N, et al. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 57.Medeiros-Domingo A, Bhuiyan ZA, Tester DJ, et al. The RYR2-encoded ryanodine receptor/calcium release channel in patients diagnosed previously with either catecholaminergic polymorphic ventricular tachycardia or genotype negative, exercise-induced long QT syndrome: a comprehensive open reading frame mutational analysis. J Am Coll Cardiol. 2009;54:2065–74. doi: 10.1016/j.jacc.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eldar M, Pras E, Lahat H. A missense mutation in the CASQ2 gene is associated with autosomal-recessive catecholamine-induced polymorphic ventricular tachycardia. Trends Cardiovasc Med. 2003;13:148–51. doi: 10.1016/s1050-1738(03)00025-2. [DOI] [PubMed] [Google Scholar]
- 59.Roux-Buisson N, Cacheux M, Fourest-Lieuvin A, et al. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum Mol Genet. 2012;21:2759–67. doi: 10.1093/hmg/dds104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nyegaard M, Overgaard MT, Søndergaard MT, et al. Mutations in calmodulin cause ventricular tachycardia and sudden cardiac death. Am J Hum Genet. 2012;91:703–12. doi: 10.1016/j.ajhg.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ackerman MJ, Priori SG, Willems S, et al. Heart Rhythm Society; European Heart Rhythm Association. HRS/EHRS expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association. Heart Rhythm. 2011;8:1308–39. doi: 10.1016/j.hrthm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 62.Tester DJ, Arya P, Will M, et al. Genotypic heterogeneity and phenotypic mimicry among unrelated patients referred for catecholaminergic polymorphic ventricular tachycardia genetic testing. Heart Rhythm. 2006;3:800–5. doi: 10.1016/j.hrthm.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 63.Tester DJ, Ackerman MJ. Genetic testing for potentially lethal, highly treatable inherited cardiomyopathies/channelopathies in clinical practice. Circulation. 2011;123:1021–37. doi: 10.1161/CIRCULATIONAHA.109.914838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Werf C, Nederend I, Hofman N, et al. Familial evaluation in catecholaminergic polymorphic ventricular tachycardia: disease penetrance and expression in cardiac ryanodine receptor mutation-carrying relatives. Circ Arrhythm Electrophysiol. 2012;5:748–56. doi: 10.1161/CIRCEP.112.970517. [DOI] [PubMed] [Google Scholar]
- 65.Wilde AA, Bhuiyan ZA, Crotti L, et al. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med. 2008;358:2024–29. doi: 10.1056/NEJMoa0708006. [DOI] [PubMed] [Google Scholar]
- 66.Coleman MA, Bos JM, Johnson JN, et al. Videoscopic left cardiac sympathetic denervation for patients with recurrent ventricular fibrillation/malignant ventricular arrhythmia syndromes besides congenital long QT syndrome. Circ Arrhyth Electrophysiol. 2012;5:782–8. doi: 10.1161/CIRCEP.112.971754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watanabe H, Chopra N, Laver D, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nature Med. 2009;15:380–3. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van der Werf C, Kannankeril PJ, Sacher F, et al. Flecainide therapy reduces exercise-induced ventricular arrhythmias in patients with catecholaminergic polymorphic ventricular tachycardia. J Am Coll Cardiol. 2011;57:2244–54. doi: 10.1016/j.jacc.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohamed U, Gollob MH, Gow RM, Krahn AD. Sudden cardiac death despite an implantable cardioverter-defibrillator in a young female with catecholaminergic ventricular tachycardia. Heart Rhythm. 2006;3:1486–9. doi: 10.1016/j.hrthm.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 70.Pizzale S, Gollob MH, Gow R, Birnie DH. Sudden death in a young man with catecholaminergic polymorphic ventricular tachycardia and paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:1319–21. doi: 10.1111/j.1540-8167.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- 71.Bokenkamp R, Wilde AA, Schalij MJ, Blom NA. Flecainide for recurrent malignant ventricular arrhythmias in two siblings with Andersen-Tawil syndrome. Heart Rhythm. 2007;4:508–11. doi: 10.1016/j.hrthm.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 72.Tester DJ, Medeiros-Domingo A, Will ML, Haglund CM, Ackerman MJ. Cardiac channel molecular autopsy: insights from 173 consecutive cases of autopsy-negative sudden unexplained death referred for postmortem genetic testing. Mayo Clinic Proc. 2012;87:524–39. doi: 10.1016/j.mayocp.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20:1391–6. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 74.Wilde AA, Antzelevitch C, Borggrefe M, et al. Proposed diagnostic criteria for the Brugada syndrome: consensus report. Circulation. 2002;106:2514–9. doi: 10.1161/01.cir.0000034169.45752.4a. [DOI] [PubMed] [Google Scholar]
- 75.Wilde AAM, Postema PG, Diego JM, et al. The pathophysiological mechanism underlying Brugada Syndrome. Depolarization versus repolarization. point/counterpoint. J Mol Cell Cardiol. 2010;49:543–53. doi: 10.1016/j.yjmcc.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mizusawa Y, Wilde AAM. Arrhythmogenic disorders of genetic origin: Brugada Syndrome. Circulation Arrhtyh Electrophysiol. 2012;5:606–16. doi: 10.1161/CIRCEP.111.964577. [DOI] [PubMed] [Google Scholar]
- 77.Crotti L, Marcou CA, Tester DJ, et al. Spectrum and prevalence of mutations involving BrS1- through BrS12-susceptibility genes in a cohort of unrelated patients referred for Brugada syndrome genetic testing: implications for genetic testing. J Am Coll Cardiol. 2012;60:1410–8. doi: 10.1016/j.jacc.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kapplinger JD, Tester DJ, Alders M, et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Probst V, Wilde AA, Barc J, et al. SCN5A mutations and the role of genetic background in the pathophysiology of Brugada syndrome. Circ Cardiovasc Genet. 2009;2:552–7. doi: 10.1161/CIRCGENETICS.109.853374. [DOI] [PubMed] [Google Scholar]
- 80.Smits JPP, Eckardt L, Probst V, et al. Genotype-phenotype relationship in Brugada syndrome; Electrocardiographic features differentiate SCN5A-related patients from non SCN5A related patients. J Am Coll Cardiol. 2002;40:350–6. doi: 10.1016/s0735-1097(02)01962-9. [DOI] [PubMed] [Google Scholar]
- 81.Gehi AK, Duong TD, Metz LD, Gomes JA, Mehta D. Risk stratification of individuals with the Brugada electrocardiogram: a meta-analysis. J Cardiovasc Electrophysiol. 2006;17:577–83. doi: 10.1111/j.1540-8167.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 82.Meregalli PG, Tan HL, Probst V, et al. Type of SCN5A mutation determines clinical severity and degree of conduction slowing in loss-of-function sodium channelopathies. Heart Rhythm. 2009;6:341–8. doi: 10.1016/j.hrthm.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 83.Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–9. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Postema PG, Wolpert C, Amin AS, et al. Drugs and Brugada syndrome patients: review of the literature, recommendations, and an up-to-date website. Heart Rhythm. 2009;6:1335–41. doi: 10.1016/j.hrthm.2009.07.002. www.brugadadrugs.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang CC, Acharfi S, Wu MH, Chiang FT, Wang JK, Sung TC, Chahine M. A novel SCN5A mutation manifests as a malignant form of long QT syndrome with perinatal onset of tachycardia/bradycardia. Cardiovasc Res. 2004;64:268–278. doi: 10.1016/j.cardiores.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 86.Bankston JR, Yue M, Chung W, et al. A novel and lethal de novo LQT-3 mutation in a newborn with distinct molecular pharmacology and therapeutic response. PLoS ONE. 2007;2:e1258. doi: 10.1371/journal.pone.0001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tester DJ, Will ML, Haglund CM, Ackerman MJ. Compendium of cardiac channel mutations in 541 consecutive unrelated patients referred for long QT syndrome genetic testing. Heart Rhythm. 2005;2:507–17. doi: 10.1016/j.hrthm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 88.Schwartz MK. Genetic testing and the clinical laboratory improvement amendments of 1988: present and future. Clin Chem. 1999;45:739–45. [PubMed] [Google Scholar]
- 89.Ackerman MJ, Splawski I, Makielski JC, et al. Spectrum and prevalence of cardiac sodium channel variants among black, white, Asian, and Hispanic individuals: Implications for arrhythmogenic susceptibility and Brugada/long QT syndrome genetic testing. Heart Rhythm. 2004;1:600–7. doi: 10.1016/j.hrthm.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 90.Kapplinger JD, Tester DJ, Salisbury BA, et al. Spectrum and prevalence of mutations from the first 2,500 consecutive unrelated patients referred for the FAMILION long QT syndrome genetic test. Heart Rhythm. 2009;6:1297–303. doi: 10.1016/j.hrthm.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kapa S, Tester DJ, Salisbury BA, et al. Genetic testing for Long QT Syndrome - Distinguishing pathogenic mutations from benign variants. Circulation. 2009;120:1752–60. doi: 10.1161/CIRCULATIONAHA.109.863076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crotti L, Lewandowska MA, Schwartz PJ, et al. A KCNH2 branch point mutation causing aberrant splicing contributes to an explanation of genotype-negative long QT syndrome. Heart Rhythm. 2009;6:212–8. doi: 10.1016/j.hrthm.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 93.Tester DJ, Benton AJ, Train L, Deal B, Baudhuin LM, Ackerman MJ. Prevalence and spectrum of large deletions or duplications in the major long QT syndrome-susceptibility genes and implications for long QT syndrome genetic testing. Am J Cardiol. 2010;106:1124–8. doi: 10.1016/j.amjcard.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Eddy CA, Maccormick JM, Chung SK, et al. Identification of large gene deletions and duplications in KCNQ1 and KCNH2 in patients with long QT syndrome. Heart Rhythm. 2008;5:1275–81. doi: 10.1016/j.hrthm.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 95.Koopmann TT, Alders M, Jongbloed RJ, et al. Long QT syndrome caused by a large duplication in the KCNH2 (HERG) gene undetectable by current polymerase chain reaction-based exon-scanning methodologies. Heart Rhythm. 2006;3:52–5. doi: 10.1016/j.hrthm.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 96.Medlock MM, Tester DJ, Will ML, Bos JM, Ackerman MJ. Repeat long QT syndrome genetic testing of phenotype-positive cases: prevalence and etiology of detection misses. Heart Rhythm. 2012;9:1977–82. doi: 10.1016/j.hrthm.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 97.Brink PA, Crotti L, Corfield V, et al. Phenotypic variability and unusual clinical severity of congenital long-QT syndrome in a founder population. Circulation. 2005;112:2602–10. doi: 10.1161/CIRCULATIONAHA.105.572453. [DOI] [PubMed] [Google Scholar]
- 98.Schwartz PJ, Priori SG, Napolitano C. How really rare are rare diseases?: the intriguing case of independent compound mutations in the long QT syndrome. J Cardiovasc Electrophysiol. 2003;14:1120–1. doi: 10.1046/j.1540-8167.2003.03339.x. [DOI] [PubMed] [Google Scholar]
- 99.Westenskow P, Splawski I, Timothy KW, Keating MT, Sanguinetti MC. Compound mutations: a common cause of severe long-QT syndrome. Circulation. 2004;109:1834–41. doi: 10.1161/01.CIR.0000125524.34234.13. [DOI] [PubMed] [Google Scholar]
- 100.Schwartz PJ, Vanoli E, Crotti L, et al. Neural control of heart rate is an arrhythmia risk modifier in long QT syndrome. J Am Coll Cardiol. 2008;51:920–929. doi: 10.1016/j.jacc.2007.09.069. [DOI] [PubMed] [Google Scholar]
- 101.Fodstad H, Swan H, Laitinen P, et al. Four potassium channel mutations account for 73% of the genetic spectrum underlying long-QT syndrome (LQTS) and provide evidence for a strong founder effect in Finland. Ann Med. 2004;36(Suppl 1):53–63. doi: 10.1080/17431380410032689. [DOI] [PubMed] [Google Scholar]
- 102.Crotti L, Monti MC, Insolia R, et al. NOS1AP is a genetic modifier of the long-QT syndrome. Circulation. 2009;120:1657–63. doi: 10.1161/CIRCULATIONAHA.109.879643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tomas M, Napolitano C, De Giuli L, et al. Polymorphisms in the NOS1AP gene modulate QT interval duration and risk of arrhythmias in the long QT syndrome. J Am Coll Cardiol. 2010;55:2745–52. doi: 10.1016/j.jacc.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 104.Amin AS, Giudicessi JR, Tijsen AJ, et al. Variants in the 3′ untranslated region of the KCNQ1-encoded Kv7.1 potassium channel modify disease severity in patients with type 1 long QT syndrome in an allele-specific manner. Eur Heart J. 2012;33:714–23. doi: 10.1093/eurheartj/ehr473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nishio Y, Makiyama T, Itoh H, et al. D85N, a KCNE1 polymorphism, is a disease-causing gene variant in long QT syndrome. J Am Coll Cardiol. 2009;54:812–9. doi: 10.1016/j.jacc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 106.Kääb S, Crawford DC, Sinner MF, et al. A large candidate gene survey identifies the KCNE1 D85N polymorphism as a possible modulator of drug-induced torsades de pointes. Circ Cardiovasc Genet. 2012;5:91–9. doi: 10.1161/CIRCGENETICS.111.960930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Refsgaard L, Holst AG, Sadjadieh G, Haunso S, Nielsen JB, Olesen MS. High prevalence of genetic variants previously associated with LQT syndrome in new exome data. Eur J Hum Genet. 2012;20:905–8. doi: 10.1038/ejhg.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]