Abstract
Inactivation of the tumor suppressor p53 is a pathogenetic event in the development of head and neck squamous cell carcinoma (HNSCC). In the absence of functional wildtype p53, HNSCC cells have increased resistance to standard chemotherapeutics and radiation. Numerous approaches to restore p53 function in cancer cells have been developed over the past several decades. This review article focuses on viral approaches to deliver wildtype p53 to HNSCC cells, a designer virus that selectively eliminates mutant p53 HNSCC cells, and chemical approaches to reactivate p53 function in HNSCC cells. These promising studies provide evidence that p53 therapeutics may prove useful alone or in combination with conventional chemotherapy and/or radiation for the management of HNSCC patients.
Keywords: Tumor suppressor gene, p53, Anti-cancer therapeutics, Head and neck cancer, Oral cancer, Gene therapy, and Human papillomavirus
Introduction
Head and neck squamous cell carcinoma (HNSCC) is a complex and multi-factorial disease, with an estimated 50,000 new cases occurring in the United States in 2012.1 The most important risk factors for the development of HNSCC remain tobacco and alcohol use, but recent evidence indicates that the human papillomavirus (HPV) is associated with a particular HNSCC: oropharyngeal SCC.2,3 At this time, the exact sequence and importance of the genetic alterations necessary to transform normal epithelial cells into invasive HNSCC cells are not fully elucidated. However, it is clear that in a majority of HNSCC cases, mutations or inactivation of the tumor suppressor p53 are essential to initiate the tumorigenesis cascade.4
Since its discovery several decades ago,5 p53 has been reported to be mutated in numerous types of solid malignancies,6 including HNSCC.4 The gene encoding p53 has been reported to be mutated in one-third to two-thirds of HNSCC, with mutations most commonly occurring in exons 5 – 8.7,8,9 It has been shown that introduction of mutant p53 into HNSCC cells promotes resistance to cisplatin and radiation treatment.10 This is consistent with the finding that HNSCC patients with mutated p53 have worse overall survival than patients with p53 wildtype HNSCC.11 Moreover, sub-set analysis has revealed that HNSCC patients with mutations that leave p53 non-functional, known as disruptive mutations, have poorer prognosis than HNSCC patients with non-disruptive p53 mutations.11
Due to the survival disadvantage associated with non-functional p53, several strategies have been developed to restore p53 function in HNSCC. This review discusses various types of p53-based therapy for HNSCC: viral gene therapy to deliver wildtype p53; viruses designed to kill carcinoma cells without functional p53; small molecules to restore wildtype function to mutated p53; and small molecules to prevent endogenous or exogenous inactivation of wildtype p53.
Adenoviral p53 Gene Therapy
The tumor suppressor p53 is known to induce apoptosis in damaged cells, but its function is often lost in HNSCC, leading to increased resistance to conventional therapies, including cisplatin-based chemotherapy and radiation.10 Thus, one potential strategy to enhance treatment response in HNSCC cells is to deliver the wildtype p53 gene. Because of its affinity for the cells of the upper aerodigestive tract, adenovirus has been the most widely-used vector for p53 gene therapy in HNSCC. A modified adenovirus developed to deliver wildtype p53, Ad-p53 (AdCMV5-p53; INGN 201), was first demonstrated to induce apoptosis in HNSCC cell in vitro and in vivo nearly 20 years ago.12 Moreover, work on a different p53 adenovirus, Av1-p53, demonstrated that p53 gene therapy sensitized HNSCC cells to conventional radiotherapy in vitro and in vivo.13
Based on the exciting pre-clinical results with p53 gene therapy, a Phase I trial was initiated to determine the safety and efficacy of Ad-p53 in HNSCC patients. Ad-p53 is a modified adenovirus-5 with replacement of the E1 protein region with wildtype human p53 cDNA. The p53 gene is preceded by a cytomegalovirus promoter and followed by an SV40 polyadenylation signal as part of a mini-gene cassette.14 A series of 33 recurrent HNSCC patients were enrolled; 16 patients had tumors that were re-resected and 17 patients had non-resectable tumors.15 Resectable patients received six direct intratumoral injections pre-operatively, then an intraoperative administration, followed by administration through a catheter left in the surgical area 72 hours post-operatively. Non-resectable patients received direct intratumoral injections every other day until disease progression. No serious adverse events were reported. Of the 17 non-resectable patients, 9 had progressive disease, 6 had stable disease, and 2 had greater than 50% tumor regression. At 24 months of follow-up, 15/17 non-resectable patients (median survival, 127 days) and 6/16 resectable patients had died (median survival, 408 days). Interestingly, one resectable patient had no histologic evidence of disease in the resected specimen after receiving 6 preoperative intratumoral injections. This pivotal study showed that Ad-p53 was safe and a promising therapeutic strategy for managing HNSCC patients.
A phase II trial evaluating Ad-p53 in previously untreated HNSCC patients was reported in 2009.16 All patients (n=13) had resectable disease, and received direct injections of Ad-p53 into the surgical margins of the tumor bed intraoperatively. Injections were also given in the soft tissues of the ipsilateral neck for all patients, and three patients received bilateral neck injections. In addition, all patients received Ad-p53 by retrograde catheter instillation post-operatively. Adverse reactions were rare and included one orocutaneous fistula, one incisional separation, and one episode of leukopenia, neutropenia and vomiting leading to hypokalemia and hyponatremia. At one-year post-treatment, 12 (92%) patients were disease-free. This small study provides intriguing evidence that adenoviral p53 gene therapy is highly active in treatment-naïve HNSCC. However, additional studies with a larger cohort of patients are needed to confirm the therapeutic benefit of Ad-p53 for this population.
Since Ad-p53 was shown to be active in HNSCC in several clinical trials, a Phase III clinical trial was initiated to compare the efficacy of Ad-p53 to methotrexate for recurrent HNSCC patients.14 Patients were randomized to receive 6 intratumoral injections of Ad-p53 or 3 intravenous administrations of methotrexate over 3 weeks. This study included a primary end point of overall survival and a secondary endpoint of tumor response. The median overall survival was better in the methotrexate arm (6.1 months) than the Ad-p53 arm (4.4 months); however, this difference did not reach statistical significance (p=0.236). Interestingly, Ad-p53-treated HNSCC patients could be separated by “favorable” and “unfavorable” biomarkers based on their response to Ad-p53 therapy. “Favorable” biomarkers included wildtype p53 with either high or low p53 expression and mutant p53 with low p53 expression. The “unfavorable” biomarker was mutant p53 with high p53 expression. In the Ad-p53 treatment arm, median overall survival was 7.2 months for “favorable” biomarker patients and just 2.7 months for “unfavorable” biomarker patients (p<0.0001). These investigators concluded that the utilization of pre-treatment genetic and immunohistochemical analysis of the recurrent HNSCC tumor may allow for the selection of patients most likely to benefit from Ad-p53 therapy.
In addition to its use in HNSCC, p53 adenovirus gene therapy has been attempted in oral leukoplakia (OLK), a known precursor to squamous cell carcinoma of the oral cavity. Two independent studies evaluated the direct intralesional injection of rAd-p53 into sites of OLK. Zhang et al. evaluated the efficacy and safety of rAd-p53 in 18 OLK patients. Five total doses of rAd-p53 were delivered over two weeks.17 Six patients experienced transient fever, 3 had transient leukocytosis, and 2 had flu-like symptoms. At 6 months post-treatment, 4 (22%) patients had complete resolution of disease, 9 (50%) had decreased size of lesions, 3 (17%) had no change, and 2 (11%) had lesions that progressed to invasive squamous cell carcinoma. Immunohistochemical analysis of the tissues showed increased p53 in treated tissues compared to pre-treatment specimens. In the second study, Li et al. evaluated 22 patients who received intralesional rAd-p53 (5 doses over 2 weeks) for OLK.18 Mild adverse events were observed, including transient fever in 7 (32%) patients and flu-like symptoms in 2 (9%) patients. Patients were followed for 24 months, and over this time, 5 (23%) had complete regression, 11 (50%) had decreased size of lesions, 4 (18%) had no improvement, and 2 (9%) progressed to invasive squamous cell carcinoma. These two studies provide initial evidence that intraepithelial rAd-p53 is a safe and well-tolerated treatment option for patients with OLK. Additional studies to confirm the clinical benefit of adenoviral p53 gene therapy in the OLK setting are warranted.
Virus that Targets p53-deficient Cells
Adenoviruses used for p53 gene therapy are replication-incompetent and designed only to deliver p53 cDNA to cells to induce apoptosis. However, another way to eliminate cells with mutant p53 is to deliver a virus that preferentially target cells that lack functional p53. Adenoviruses contain an E1B gene producing a 55 kD protein that inactivates host p53 to promote host cell survival. An adenovirus lacking the E1B gene, Onyx-015, was developed to selectively eliminate cells without functional p53.19 Extensive in vitro and in vivo studies showed that Onyx-015 is capable of replicating in and lysing carcinoma cells from various solid malignancies, including cervical, colon, pancreatic, and glioblastoma.20 Moreover, Onyx-015 potentiated the anti-tumor efficacy of standard chemotherapeutic agents.21 Based on these pre-clinical results, Onyx-015 has since been used as monotherapy and in combination with conventional chemotherapeutics in several clinical trials for HNSCC.
In a Phase I trial, Ganly et al. treated 22 patients with recurrent HNSCC with direct intratumoral injection of Onyx-015.22 Treatment was performed initially then every four weeks up to five total injections. Overall, Onyx-015 was well-tolerated with 7 patients experiencing transient fever, 5 with pain at the injection site, 4 with transient leukocytosis, and 2 with flu-like symptoms. At the end of the study, 3 patients (14%) had greater than 50% reduction in tumor size, 2 patients (9%) had greater than 25% but less than 50% reduction, 8 patients (36%) had stable disease, and 9 patients (41%) had disease progression. Unexpectedly, in the patients with response to Onyx-015, no association was found between p53 status of the tumor and response to Onyx-015. In another Phase I trial, Nemunaitis et al. reported 10 patients with advanced cancer, including 2 with HSNCC, in whom Onyx-015 was delivered intravenously, once weekly for three weeks.23 One HNSCC patient had stable disease and the other had a mixed response. The only adverse effects associated with Onyx-015 for the entire cohort were fever and rigors. These two studies demonstrate that intravenous administration of Onyx-015 is safe and feasible for HNSCC patients.
Data from several Phase II trials indicate that Onyx-015 has modest activity in HNSCC as a single-agent or in combination with standard chemotherapeutics. Nemunaitis et al. studied 40 patients with recurrent HNSCC who received Onyx-015 by direct intratumoral injection.19 Thirty patients received a “standard schedule” of one daily injection for 5 days. Ten patients received 2 daily injections for 5 days in a “hyper-fractionated schedule.” Onyx-015 was well-tolerated; however, there were three deaths, though not directly attributed to the investigational drug. The most common adverse effect was transient fever in 28 patients. Of the evaluable patients who received the standard schedule, 4 (14%) had complete or partial tumor reduction, 12 (41%) had stable disease, and 13 (45%) had progressive disease. Of the patients who received the hyper-fractionated schedule, 1 (14%) had complete regression, 4 (57%) had stable disease, and 2 (29%) had progressive disease. In another trial, 9 recurrent HNSCC patients were enrolled to receive Onyx-015 in addition to cisplatin and 5-fluorouracil (5-FU).24 Onyx-015 was administered by direct intratumoral injection once daily for 5 days. Most patients experienced nausea and vomiting, which were attributed to concurrent chemotherapy. Three patients (33%) had complete regression, another 3 (33%) had partial regressions, 1 (11%) had minor regression, and 2 (22%) had stable disease. Overall survival was 7–8 months; however, this outcome was not much better than the 5–6 month survival that would be expected for HNSCC patients with the same clinical parameters. Khuri et al. enrolled 30 HNSCC patients with multi-foci tumors to received intratumoral Onyx-015 in just one tumor.25 All patients received systemic chemotherapy with cisplatin and 5-FU. Roughly half of all patients experienced pain at the injection site, and 20% had a mucous membrane disorder, either inflammation or infection. Eight (27%) patients had complete response, 11 (37%) had at least 50% size reduction, and 6 (20%) more had less than 50% response. One patient had a dramatic response which made tumor resection possible. Time to tumor progression was significantly longer (p=0.006) in tumors that had been injected.
Molecules that Reactive Mutant p53
Screening a library of small-molecular-weight compounds identified a molecule capable of altering the conformation of mutant p53 to wildtype, thus allowing binding to sequence-specific DNA resulting in an increase in p53-regulated genes.26,27 This molecule was named PRIMA (for “p53 reactivation and induction of massive apoptosis”) for its ability to restore p53-dependent transcription in osteosarcoma cells.26 PRIMA-1 is known to induce apoptosis through the p53-dependent c-Jun-NH2-kinase pathway but not through other p53-dependent apoptotic pathways.27 While PRIMA-1’s anti-cancer effects have been studied in other cancer cell lines, only one study to date has explored the ability of PRIMA-1 to induce apoptosis in HNSCC cell lines. Roh et al. treated a panel of HNSCC cell lines, JHU-028, JHU-029, UMSCC-22A, and Fadu, with PRIMA-1 as a single-agent and in combination with standard chemotherapeutics.28 PRIMA-1 inhibited the proliferation of all four HNSCC cell lines but was less active in a wildtype p53 HNSCC cell line. G2-arrest and increased nuclear p53 was observed in the mutant p53 HNSCC cell lines following PRIMA-1 treatment. Additionally, PRIMA-1 increased the expression of a panel of p53-regulated genes, including p21, Bax, Puma, and Noxa, and potentiated the efficacy of cisplatin in HNSCC cells.
Another small molecule that was identified from a small molecule library screen to enhance p53 activity was CP-31398. CP-31398 stabilizes the DNA-binding domain of p53 for both wildtype and mutant (V173A and R249S) p53.29 The same study that investigated PRIMA-1 in HNSCC also evaluated CP-31398.28 Similar to PRIMA-1, CP-31398 inhibited the proliferation of all four HNSCC cell lines but was least effective in wildtype p53 HNSCC cells. In mutant p53 HNSCC cell lines, CP-31398 promoted cell-cycle arrest and enhanced apoptosis.28
Molecules that Disrupt Endogenous p53 Inhibitors
Mutations in p53 are frequent events, however, p53 mutations are not always a prerequisite for HNSCC development. Wildtype p53 can be effectively compromised through up-regulation of endogenous p53 inhibitors, such as MDM2. MDM2 is an E3 ubiquitin ligase that binds to and inactivates p53 through ubiquitin-dependent proteasomal degradation.30 Development of small molecules that target the MDM2-p53 interaction has resulted in a novel class of p53 reactivation therapeutics.31
The nutlins are the prototypical class of small molecules that block the MDM2-p53 interaction. Nutlins were first discovered by screening a library of small molecules for their ability to antagonize MDM2.32 Nutlin-3 has been shown to interact with MDM2 at its p53-binding interface.32 Roh et al. reported that Nutlin-3 inhibited the proliferation of wildtype and mutant HNSCC cell lines, however, Nutlin-3 was most effective in HNSCC cells with wildtype p53.28 In wildtype p53 HNSCC cells, Nutlin-3 increased nuclear p53 levels, induced apoptosis, and potentiated the anti-tumor efficacy of cisplatin.28 Another small molecule that has been reported to block the interaction between MDM2 and p53 is RITA (for “reactivation of p53 and induction of tumor cell apoptosis”).33 Two separate reports have determined the effect of RITA on wildtype and mutant p53 HNSCC cells.28, 34 In both studies, RITA was found to be more effective against wildtype p53 HNSCC cells than mutant p53 HNSCC cells. RITA increased nuclear p53, p53-dependent transcription, and p53-induced apoptosis in wildtype p53 HNSCC cells. Similar to Nutin-3, the combination of RITA with cisplatin was more efficacious than either single-agent alone to block the proliferation of wildtype p53 HNSCC cells.
Molecules that Disrupt Exogenous p53 Inhibitors
The causative role of human papillomavirus-16 (HPV16) in HNSCC is largely attributed to two HPV16 oncogenes, E6 and E7. p53 is predominantly wildtype but inactivated in HPV16-positive HNSCC by HPV16 E6 through two distinct mechanisms. HPV16 E6 associates with E6AP to degrade p53 through the proteasome pathway and associates with p300 to block p300-mediated p53 acetylation.35–40 Since inactivation of p53 by HPV16 E6 is critical for HPV-mediated tumorigenesis, reactivation of p53 may be an efficient strategy to eliminate HPV16-positive HNSCC cells. Recent work from our group identified CH1iB as a small molecule that disrupts the interaction between HPV16 E6 and p300 in HPV16-positive UMSCC47 and UPCI-SCC090 HNSCC cells.41 CH1iB increased total and acetylated p53 levels, enhanced p53 transcriptional activity, and increased the expression of p53-regulated genes, p21, miR-34a, and miR-200c. CH1iB had a global anti-cancer effect, in part through a reduction in the cancer stem cell population, and potentiated the anti-tumor activity of cisplatin in HPV16-positive HNSCC cells. Our work demonstrates that CH1iB is a novel p53 reactivation therapeutic for the HPV-positive HNSCC population.
Conclusion
The prognosis of HNSCC has remained largely unchanged over several decades despite advances in multi-disciplinary treatment modalities. Thus, there is a critical need for the development of active and innovative therapeutics for this recalcitrant disease. The tumor suppressor p53 is frequently mutated or inactivated by endogenous or exogenous mechanisms suggesting that approaches to reactivate p53 function may be an attractive strategy to eliminate HNSCC cells.
Adenoviral approaches, Ad-p53 and Onyx-015, have shown great promise in pre-clinical studies and subsequently advanced to mid to late stage clinical trials in HNSCC patients. The clinical development of Ad-p53 in HNSCC has been halted in the United States soon after the FDA rejected approval due in part to the disappointing results in the pivotal randomized Phase III clinical trial. Similar to Ad-p53, Onyx-015 was abandoned as a result of limited therapeutic efficacy in recurrent HNSCC patients. Although Ad-p53 showed modest efficacy in recurrent HNSCC patients, the promising data using adenoviral p53 gene therapy in two small studies in OLK patients suggests that Ad-p53 may be an ideal therapeutic to prevent the progression of pre-malignant lesions to HNSCC. Additional clinical trials to validate the benefit of Ad-p53 in OLK should be prioritized in the near future.
Besides adenoviral p53 gene therapy, several chemical approaches to reactivate p53 in HNSCC have shown great promise in vitro and in pre-clinical animal models. PRIMA-1 and CP-31398 restore the ability of mutant p53 to transactivate p53-regulated genes, whereas Nutlin-3 and RITA inhibit an endogenous p53 inhibitor, MDM2, to restore wildtype p53 function in HNSCC. To date, there is very limited evidence to confirm the efficacy of these novel small molecules in cancer patients. Nutlin-3 and other nutlin family members have been studied in early stage clinical trials in hematologic and solid malignancies; however, the clinical activity of nutlins remains to be reported. PRIMA-1, CP-31398, and RITA have not progressed into clinical development suggesting that additional structure-activity refinement may be necessary to enhance potency prior to advancement into clinical trials.
High-risk HPV, including HPV16, is now recognized as an etiological agent for HNSCC, in particular oropharyngeal SCC. The current standard of care treatment for HPV-positive HNSCC is cis-platinum-based chemoradiation. However, this therapeutic regimen is associated with severe adverse events and thus, alternate treatment approaches for HPV-positive HNSCC patients are needed. Past attempts to develop therapeutics to target HPV-positive cancers have been limited to cervical carcinomas and moreover, these strategies have not progressed past pre-clinical testing. Recent work from our group demonstrates that CH1iB, a small molecule that blocks the interaction between HPV16 E6 and p300, reactivates p53 function and has activity as a single-agent and in combination with cisplatin in HPV-positive HNSCC. To our knowledge, CHiB is the first therapeutic to be rationally designed to target HPV-positive HNSCC. Although CH1iB is early in pre-clinical development, the potential of this molecule is high and further development of CH1iB as an anti-cancer therapeutic in HPV-positive HNSCC and other HPV-associated carcinomas is warranted.
Various approaches to modulate p53 function have been assessed for HNSCC. Adenoviral strategies to deliver wildtype 53 to HNSCC cells or selectively eliminate p53-inactive HNSCC cells have progressed to mid to late stage clinical trials but failed to demonstrate a robust clinical benefit. Small molecules to reactivate p53 in p53-inactivated HNSCC, due to mutations, MDM2, or HPV, have demonstrated promising pre-clinical efficacy. Further investigation of these molecules should be prioritized to expedite the progression to the clinical development phase. It is clear that the full potential of p53 reactivation therapeutics has yet to materialize in HNSCC. As further insight in p53 biology in HNSCC is gained, additional therapeutic strategies may be developed to optimally reactivate p53 to better manage this patient population.
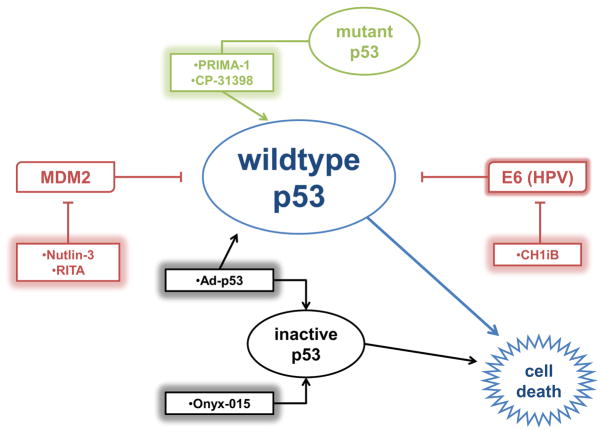
Figure 1. Current p53-based therapeutics for HNSCC.
Adenoviral p53 gene therapy, Ad-p53, increases wildtype p53 levels in HNSCC cells. Onyx-015 selectively eliminates inactive p53 HNSCC cells. PRIMA-1 restores the ability of mutant p53 to bind to the p53 transcriptional response elements of target genes and CP-31398 stabilizes the DNA binding domain of mutant and wildtype p53. Nutlin-3 and RITA restore p53 function by blocking the p53-MDM2 interaction. CH1iB disrupts the HPV16 E6-p300 interaction to reactivate p53 in HPV16-positive HNSCC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Gillison ML, Lowy DR. A causal role for human papillomavirus in head and neck cancer. Lancet. 2004;363(9420):1488–9. doi: 10.1016/S0140-6736(04)16194-1. [DOI] [PubMed] [Google Scholar]
- 3.Sinha P, Logan HL, Mendenhall WM. Human papillomavirus, smoking, and head and neck cancer. Am J Otolaryngology. 2012;33(1):130–6. doi: 10.1016/j.amjoto.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somers KD, Merrick MA, Lopez ME, Incognito LS, Schechter GL, Casey G. Frequent p53 mutations in head and neck cancer. Cancer Res. 1992;52(21):5997–6000. [PubMed] [Google Scholar]
- 5.Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979;278(5701):261–3. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- 6.Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, Bigner SH, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989;342(6250):705–8. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- 7.Velculescu VE, El-Deiry WS. Biological and clinical importance of the p53 tumor suppressor gene. Clin Chem. 1996;42(6 Pt 1):858–68. [PubMed] [Google Scholar]
- 8.Gasco M, Crook T. The p53 network in head and neck cancer. Oral Oncol. 2003;39(3):222–31. doi: 10.1016/s1368-8375(02)00163-x. [DOI] [PubMed] [Google Scholar]
- 9.Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum Mutat. 2002;19(6):607–14. doi: 10.1002/humu.10081. [DOI] [PubMed] [Google Scholar]
- 10.Ohnishi K, Ota I, Takahashi A, Yane K, Matsumoto H, Ohnishi T. Transfection of mutant p53 gene depresses X-ray- or CDDP-induced apoptosis in a human squamous cell carcinoma of the head and neck. Apoptosis. 2002;7(4):367–72. doi: 10.1023/a:1016131614856. [DOI] [PubMed] [Google Scholar]
- 11.Poeta ML, Manola J, Goldwasser MA, Forastiere A, Benoit N, Califano JA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med. 2007;357(25):2552–61. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu TJ, el-Naggar AK, McDonnell TJ, Steck KD, Wang M, Taylor DL, et al. Apoptosis induction mediated by wild-type p53 adenoviral gene transfer in squamous cell carcinoma of the head and neck. Cancer Res. 1995;55(14):3117–22. [PubMed] [Google Scholar]
- 13.Pirollo KF, Hao Z, Rait A, Jang YJ, Fee WE, Jr, Ryan P, et al. p53 mediated sensitization of squamous cell carcinoma of the head and neck to radiotherapy. Oncogene. 1997;14(14):1735–46. doi: 10.1038/sj.onc.1201116. [DOI] [PubMed] [Google Scholar]
- 14.Nemunaitis J, Nemunaitis J. Head and neck cancer: response to p53-based therapeutics. Head Neck. 2011;33(1):131–4. doi: 10.1002/hed.21364. [DOI] [PubMed] [Google Scholar]
- 15.Clayman GL, el-Naggar AK, Lippman SM, Henderson YC, Frederick M, Merritt JA, et al. Adenovirus-mediated p53 gene transfer in patients with advanced recurrent head and neck squamous cell carcinoma. J Clin Oncol. 1998;16(6):2221–32. doi: 10.1200/JCO.1998.16.6.2221. [DOI] [PubMed] [Google Scholar]
- 16.Yoo GH, Moon J, Leblanc M, Lonardo F, Urba S, Kim H, et al. A phase 2 trial of surgery with perioperative INGN 201 (Ad5CMV-p53) gene therapy followed by chemoradiotherapy for advanced, resectable squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, and larynx: report of the Southwest Oncology Group. Arch Otolaryngol Head Neck Surg. 2009;135(9):869–74. doi: 10.1001/archoto.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Li Y, Li L, Zhang Y, Gao N, Zhang Z, et al. Phase I study of repeated intraepithelial delivery of adenoviral p53 in patients with dysplastic oral leukoplakia. J Oral Maxillofac Surg. 2009;67(5):1074–82. doi: 10.1016/j.joms.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Li LJ, Zhang ST, Wang LJ, Zhang Z, Gao N, et al. In vitro and clinical studies of gene therapy with recombinant human adenovirus-p53 injection for oral leukoplakia. Clin Cancer Res. 2009;15(21):6724–31. doi: 10.1158/1078-0432.CCR-09-1296. [DOI] [PubMed] [Google Scholar]
- 19.Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19(2):289–98. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 20.Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An Adenovirus Mutant that Replicates Selectively in p53-Deficient Human Tumor Cells. Science. 1996;264:373–6. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 21.Heise H, Sampson-Johannes A, Williams A, McCormack F, Von Hoff DD, Kirn DH. Onyx-015, an E1B gene-attenuated adenovirus, causes tumour specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997;3:639–45. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 22.Ganly I, Kirn D, Eckhardt G, Rodriguez GI, Soutar DS, Otto R, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6(3):798–806. [PubMed] [Google Scholar]
- 23.Nemunaitis J, Cunningham C, Buchanan A, Blackburn A, Edelman G, Maples P, et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 2001;8(10):746–59. doi: 10.1038/sj.gt.3301424. [DOI] [PubMed] [Google Scholar]
- 24.Lamont JP, Nemunaitis J, Kuhn JA, Landers SA, McCarty TM. A prospective phase II trial of ONYX-015 adenovirus and chemotherapy in recurrent squamous cell carcinoma of the head and neck (the Baylor experience) Ann Surg Oncol. 2000;7(8):588–92. doi: 10.1007/BF02725338. [DOI] [PubMed] [Google Scholar]
- 25.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6(8):879–85. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 26.Bykov VJ, Issaeva N, Shilov A, Hultcrantz M, Pugacheva E, Chumakov P, et al. Restoration of the tumor suppressor function to mutant p53 by a low-molecular-weight compound. Nat Med. 2002;8(3):282–8. doi: 10.1038/nm0302-282. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Mao Y, Brandt-Rauf PW, Williams AC, Fine RL. Selective induction of apoptosis in mutant p53 premalignant and malignant cancer cells by PRIMA-1 through the c-Jun-NH2-kinase pathway. Mol Cancer Ther. 2005;4(6):901–9. doi: 10.1158/1535-7163.MCT-04-0206. [DOI] [PubMed] [Google Scholar]
- 28.Roh JL, Kang SK, Minn I, Califano JA, Sidransky D, Koch WM. p53-Reactivating small molecules induce apoptosis and enhance chemotherapeutic cytotoxicity in head and neck squamous cell carcinoma. Oral Oncol. 2011;47(1):8–15. doi: 10.1016/j.oraloncology.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foster BA, Coffey HA, Morin MJ, Rastinejad F. Pharmacological rescue of mutant p53 conformation and function. Science. 1999;286(5449):2507–10. doi: 10.1126/science.286.5449.2507. [DOI] [PubMed] [Google Scholar]
- 30.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420(1):25–7. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 31.Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, et al. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci U S A. 2006;103(6):1888–93. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 33.Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M, et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10(12):1321–8. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 34.Roh JL, Ko JH, Moon SJ, Ryu CH, Choi JY, Koch WM. The p53-reactivating small-molecule RITA enhances cisplatin-induced cytotoxicity and apoptosis in head and neck cancer. Cancer Lett. 2012;325(1):35–41. doi: 10.1016/j.canlet.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 35.Huibregtse JM, Scheffner M, Howley PM. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 37.Talis AL, Huibregtse JM, Howley PM. The role of E6AP in the regulation of p53 protein levels in human papillomavirus (HPV)-positive and HPV-negative cells. J Biol Chem. 1998;273:6439–6445. doi: 10.1074/jbc.273.11.6439. [DOI] [PubMed] [Google Scholar]
- 38.Zimmermann H, Degenkolbe R, Bernard HU, O’Connor MJ. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator BP/p300. J Virol. 1999;73:6209–6219. doi: 10.1128/jvi.73.8.6209-6219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel D, Huang SM, Baglia LA, McCance DJ. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 1999;18:5061–5072. doi: 10.1093/emboj/18.18.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas MC, Chiang CM. E6 oncoprotein represses p53-dependent gene activation via inhibition of protein acetylation independently of inducing p53 degradation. Mol Cell. 2005;17:251–264. doi: 10.1016/j.molcel.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Xie X, Piao L, Bullock BN, Smith A, Su T, Zhang M, et al. Targeting HPV16 E6-p300 interaction reactivates p53 and inhibits the tumorigenicity of HPV-positive head and neck squamous cell carcinoma. Oncogene. 2013 doi: 10.1038/onc.2013.25. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]