Abstract
Mucosal and parenteral immunizations elicit qualitatively distinct immune responses, and there is evidence that mucosal immunization can skew the balance of T helper 1 and T helper 2 responses. However, a clear picture of the effect of the route of infection on the balance of the T helper responses has not yet emerged. Our laboratory previously demonstrated that oral reovirus infection elicits specific serum immunoglobulin G2a (IgG2a), while parenteral reovirus infection elicits the mixed production of specific serum IgG2a and IgG1 in mice of the H-2d haplotype. Knowing that IgG2a production is indicative of a T helper 1 response and IgG1 production is indicative of a T helper 2 response, we hypothesized that the route of infection influences the development of T helper 1 and T helper 2 responses. Using quantitative reverse transcription-PCR, we found that mRNA for the T helper 1 cytokines gamma interferon and interleukin-12 (IL-12) were expressed in draining lymphoid tissues following both oral and parenteral infections. However, we observed that mRNA for the T helper 2 cytokine IL-10 was suppressed in the Peyer's patches and mesenteric lymph nodes and IL-4 mRNA was suppressed in the mesenteric lymph nodes compared to noninfected controls, following oral infection. Using recombinant cytokines and cytokine knockout mice, we confirmed that IL-4 plays a major role in mediating the route-of-infection-dependent differences in serum IgG subclass responses. Therefore, the route of infection needs to be taken into consideration when developing vaccines and adjuvant therapies.
Mucosal and parenteral immunizations elicit qualitatively distinct immune responses. Two well-characterized features unique to the mucosal immune response are production of immunoglobulin A (IgA) in secretions and serum (26, 35, 41) and, in some cases, induction of systemic immunologic hyporesponsiveness, a phenomenon commonly referred to as oral tolerance (32, 58). Both of these distinct mucosal responses are in part mediated by cytokines produced locally during the immune response (4, 57, 58). Cytokines play an important role in generating the appropriate immune response following both oral and parenteral infections. In mice, T helper 1 (Th1)-type proinflammatory cytokines, such as gamma interferon (IFN-γ), interleukin-12 (IL-12), and IL-2, are involved in inducing cell-mediated immunity and antibody class switching to IgG2a (11, 39). In contrast, Th2-type anti-inflammatory cytokines, such as IL-4, IL-5, IL-6, and IL-10, are involved in inducing humoral immunity and play a role in inducing IgG1 antibodies (11, 39). There is evidence that mucosal immunization skews the balance of Th1 and Th2 responses, but a clear picture of the effect that the route of infection has on the balance of Th responses has not yet emerged.
Several studies have shown that the nature of the antigen significantly contributes to the type of mucosal immune response that is induced in the gastrointestinal tract. This idea has been supported in large part by studies examining a predominant Th2 mucosal immune response in rodents, particularly following immunization with protein antigens with mucosal adjuvant properties such as cholera toxin (CT) (31, 59, 61), or inert particulate antigens, such as sheep red blood cells (60), and in studies of oral tolerance where immunosuppressive cytokines such as transforming growth factor-β are produced in response to oral feeding of protein antigens (58). In addition to protein and inert particulate antigens, protective Th2 responses can be induced following infection with intestinal nematode parasites (8, 9). However, predominant Th1 responses have been observed following oral infections with reovirus (10), Salmonella (17), Citrobacter rodentium (18), and Leishmania major (43, 50). In addition, oral rotavirus (EHPw) infection in mice induces a mixed Th1 and Th2 response (16).
While the specific pathogen affects the Th type of immune response that is induced, the effect of the route of infection on antiviral Th responses to infection is less clear. Morrison et al. (38) demonstrated route-dependent differences in the IgG subclass responses following subcutaneous (s.c.) and intranasal (i.n.) infections with a replication-defective mutant of herpes simplex virus type 2. They observed a Th1-skewed response as measured by robust IgG2a and weaker IgG1 production following i.n. infection compared to that following s.c. infection. Pacheco et al. (42) also observed an increased Th1 response following i.n. immunization with human immunodeficiency virus reverse transcriptase plus CT compared to that following intraperitoneal (i.p.) immunization, as measured by IgG subclass responses. Therefore, mucosal viral infections and viral antigens seem to be capable of inducing more-robust systemic Th1 responses than parenteral exposure. In contrast, Ramakrishna et al. (47) recently observed a more-robust IgG1 antibody response against Japanese encephalitis virus following oral infections compared to i.p. or s.c. infections in C57BL/6J and Swiss albino mice. In addition to viruses, other intracellular pathogens such as L. major can induce route-of-infection-dependent differences in the balance of Th1 and Th2 responses (7).
Reovirus is a nonenveloped double-stranded RNA-containing virus that has been used to study mucosal immune responses following both oral and respiratory infections (10, 27, 29, 56, 63). A difference in the reovirus-specific serum IgG subclass responses following oral or intradermal reovirus infections, which was genetically linked to the H-2d haplotype, was previously reported (27). Oral reovirus infections induce prominent reovirus-specific serum IgG2a production, while intradermal infections induce the mixed production of IgG2a and IgG1 in BALB/c and B10.D2 mice but not the C57BL/6 and C3H strains of mice (27), suggesting that oral reovirus infections induce predominant Th1 responses and parenteral infections induce mixed Th1 and Th2 responses. A possible mechanism to explain the difference was route-of-infection-dependent variations in the cytokine responses. Substantial IFN-γ levels in the peripheral lymph nodes and Peyer's patches (PP) following footpad (FP) and oral infections, respectively, were previously reported (10, 27). It was expected that intradermal reovirus infections would elicit a mixture of Th1 and Th2 cytokines. However, IL-4 and IL-6 were not detected, and IL-5 was only marginally detected in culture supernatants of peripheral lymph nodes (27). In the previous study robust Th2 cytokine responses were not detected 7 days postinfection, but earlier time points were not assessed. An additional study performed in C3H mice, which do not display the route-dependent dichotomy in the immune response, also demonstrated robust Th1 responses and very weak or absent Th2 responses following oral infection (10). Together, our previous work indicated that in all strains of mice tested thus far, oral infection with reovirus induced robust Th1 responses in intestinal tissues. In mouse strains that are predisposed to mounting Th2 responses, systemic infection resulted in antibody responses that suggested a mixed Th1 and Th2 response, but the cellular and molecular basis for this difference remained undefined.
We hypothesize that the route of infection influences the local Th1 and Th2 cytokine responses and is responsible for the serum IgG subclass differences observed following oral or parenteral infections. We report here that in contrast to parenteral infection, oral infection results in suppression of IL-4 and IL-10 mRNA in draining lymphatic tissues early in infection. Our hypothesis was further supported by experiments using recombinant cytokines and cytokine knockout mice. These results suggest that oral infections induce transient skewing of the immune response toward a Th1 response that suppresses serum IgG1 production.
MATERIALS AND METHODS
Animals and infections.
Male 6-week-old BALB/c mice and BALB/c-IL-4tm2Nnt (IL-4−/−) mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Mice were housed under specific-pathogen-free conditions in microisolator cages and used between the ages of 7 and 12 weeks. Mice were treated in accordance with West Virginia University laboratory animal guidelines. Reovirus serotype 1 strain Lang (T1/L) was purified according to previous protocols using a CsCl purification method (54). Mice were infected orally with doses of reovirus T1/L ranging from 3 × 106 to 1 × 109 PFU diluted in 50 μl of borate-buffered gelatin (gel saline), using a 20-gauge feeding needle. Parenteral infections were given as 10-μl volumes to both hind FP for a final dose ranging from 1 × 104 to 3 × 107 PFU. Nasal infections were given in 10-μl volumes at a final dose of 3 × 107 PFU diluted in gel saline.
Cytokine RT-PCR.
PP, popliteal lymph nodes (PLN), and mesenteric lymph nodes (MLN) were transferred to tubes containing 0.5 ml of RNALater (Ambion, Inc., Austin, Tex.). Approximately 35-μg pieces of tissues were homogenized sequentially by mechanical disruption in RLT buffer (Qiagen, Valencia, Calif.) followed by homogenization using Qiashredders (Qiagen). Total RNA isolation was performed using the RNeasy mini RNase-free DNase set (Qiagen) following the manufacturer's protocols. RNA was quantified by spectrophotometry at 260/280 nm and reverse transcribed to cDNA with Taqman reverse transcription (RT) reagents (Applied Biosystems, Foster City, Calif.) following the manufacturer's protocol, using random hexamers with 400 ng of RNA in 30-μl reaction mixtures. The RT reaction was carried out in a Genius thermocycler (Techne, Inc., Princeton, N.J.) according to the manufacturer's protocol for 10 min at 25°C, 30 min at 48°C, and 5 min at 95°C. Real-time PCR was performed using a LightCycler (Roche Molecular Biochemicals, Indianapolis, Ind.). Taqman IL-10, IL-4, IL-12, or IFN-γ predeveloped assay reagents (Applied Biosystems) and 1 μl of cDNA were used to perform the real-time PCRs according to the manufacturer's protocols with the following changes: reaction volumes were reduced to 20 μl, 2.5 μg of bovine serum albumin was added to each sample, and primer and probe concentrations were reduced to one-fourth the recommended concentrations (actual concentrations are proprietary). Protocol reaction times were also modified to 50°C for 2 min followed by 95°C for 10 min for enzyme activation, 94°C for 30 s followed by 60°C for 30 s for amplification cycles, and finally 40°C for 1 min. The cytokine mRNA was quantified using an external homologous standard that was serially diluted in 10-fold increments to generate a standard curve for absolute quantification. Standards were spiked with 1 μg of salmon sperm genomic DNA. The quantification was then normalized based on total RNA (2).
Reovirus-specific ELISA.
Ninety-six-well EIA/RIA (Costar) flat-bottom plates were coated with a 5 × 1010 particles/ml density of purified reovirus T1/L per well in 50 μl of 0.1 M NaHCO3 and incubated overnight at 4°C. Plates were washed twice in phosphate-buffered saline (PBS) supplemented with 0.05% Tween 20 (PBS-Tween) and then blocked with 3% bovine serum albumin (wt/vol) in PBS at room temperature for 2 h. Plates were washed an additional two times with PBS-Tween, and 100 μl of serially diluted serum samples, diluted in PBS supplemented with 10% (vol/vol) fetal bovine serum or bovine calf serum (10% serum), were added to the plates. Twofold serial dilutions of reference serum samples of known titer were also assayed on each plate. In some instances where absolute antibody concentrations were determined, additional lanes were coated with 1 μg of goat anti-mouse Ig (H+L) (Southern Biotechnology, Birmingham, Ala.), and mouse IgG1, IgG2a, or IgA standards (Southern Biotechnology) were assayed in place of the reference serum. After samples and standards were added, the plates were incubated overnight at 4°C. The plates were washed four times with PBS-Tween, and 100 μl of a 1-μg/ml biotinylated goat anti-mouse antibody specific for IgG1, IgG2a, or IgA (Southern Biotechnology) in 10% serum was added to the plates and incubated at room temperature for 45 min. Plates were washed six times with PBS-Tween, and 100 μl of avidin-conjugated peroxidase (Sigma) at 2.5 μg/ml in 10% serum was added to each plate and incubated at room temperature for 30 min. The plates were washed a final eight times with PBS-Tween, and 100 μl of substrate, 2,2′-azinobis-3-ethylbenzthiazoline-6-sulfonic acid (ABTS) (Sigma), plus 20 μl of a 30% H2O2 solution per 10 ml of ABTS was added to each well. Color development was assessed at 405 nm on an enzyme-linked immunosorbent assay (ELISA) plate reader.
In vivo treatments with cytokines. (i) rIL-10 treatments.
Mice were treated i.p. with 100 ng of mouse recombinant IL-10 (rIL-10; PeproTech, Rocky Hill, N.J.) (or PBS as vehicle control) every 12 h for the first 5 days of infection.
(ii) Anti-IL-10R treatment.
Mice received anti-IL-10 receptor (anti-IL-10R) antibody (clone 1B1.3a) or control rat IgG1 antibody (clone GL113) isotype control (DNAX, Palo Alto, Calif.). Treatments given i.p. were started on day −1 of infection with 1 mg of antibody/mouse and then 0.5 mg/mouse every other day for 10 days.
(iii) rIL-4 treatment.
Mice received 3 μg i.p. of rIL-4 with or without 30 μg of anti-IL-4 carrier (clone 11B.11 rat monoclonal antibody [MAb]; Biological Resources Branch, National Cancer Institute, Frederick, Md.) to increase cytokine stability (14). The molar ratio of cytokine to antibody was approximately 1:1. Cytokines were administered on days −1 and +2 of infection.
Lamina propria fragment cultures for IgA.
Small intestines were removed from mice, PP were excised, and intestines were cut longitudinally and then further cut into approximately 5-cm segments. Intestinal contents were removed by washing in Hanks balanced salt solution (HBSS) supplemented with 10 mM HEPES and 0.35 g of NaHCO3/liter. Epithelium was removed by washing the tissue fragments in HBSS medium plus 1 mM EDTA (HBSS-EDTA) for 30 min at 37°C. Medium was replaced with fresh HBSS-EDTA for an additional 30 min at 37°C. Tissue fragments were rinsed in HBSS and cultured in 5 ml of RPMI 1640 supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and penicillin (500 IU/ml)-streptomycin (0.5 mg/ml) in individual wells of a six-well plate. Lamina propria tissue cultures were incubated at 37°C and with 5% CO2 for 5 days. Day 5 supernatants were collected and clarified by centrifugation at 300 × g. Reovirus-specific IgA antibody concentrations were determined by ELISA (28).
Statistical analysis.
Data were analyzed by using one-way analysis of variance (ANOVA) followed by Dunnett's, Dunn's, or Student-Newman-Keuls' post-hoc tests. A P value of <0.05 was considered significant.
RESULTS
Antibody production following mucosal and parenteral infections.
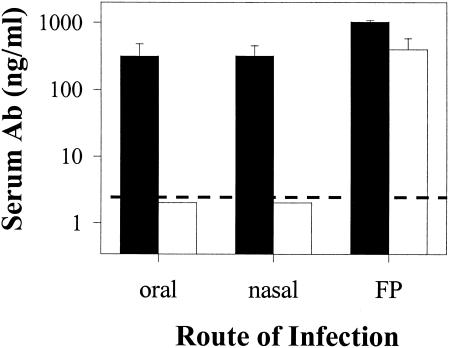
Our laboratory previously demonstrated that reovirus FP infections induced virus-specific serum IgG2a and IgG1, while oral infections induced predominately IgG2a with little or no IgG1 (27). To determine if mucosal infections in general induce a distinct serum antibody response, we infected groups of mice orally, nasally, or in the FP, and IgG subclass responses were assessed 10 days following infection. Both oral and nasal infections induced prominent IgG2a responses, with little or no IgG1. The FP infections induced both IgG2a and IgG1 (Fig. 1). This time point was used for these studies because reovirus-infected mice uniformly seroconvert by day 10. Sera from one set of orally infected mice were examined 1 month postinfection, and little or no IgG1 was detected (data not shown).
FIG. 1.
Reovirus-specific IgG2a (closed bars) and IgG1 (open bars) serum antibody concentrations following mucosal or parenteral infections. BALB/c mice were infected with reovirus orally, nasally, or in the FP with 3 × 106 PFU/mouse, and serum antibody responses were determined 10 days after infection. Bars indicate the standard errors among groups of three mice. The dashed line indicates the limit of detection. Data shown are from one of three similar experiments.
Th1 and Th2 cytokine mRNA expression profiles following oral or FP infections.
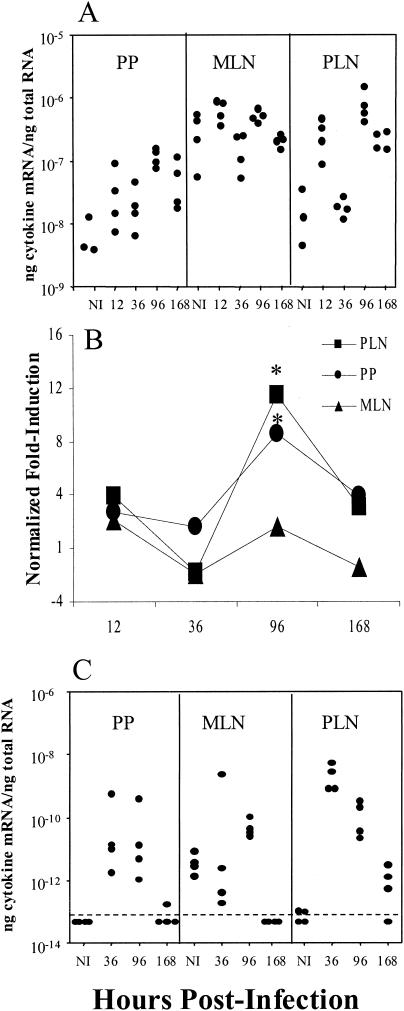
To determine mechanisms involved in inducing the distinct IgG subclass responses following oral and FP infections, mRNA levels for Th1 and Th2 cytokines were assessed by quantitative RT-PCR. Cytokine mRNA levels were analyzed at time points ranging from 12 to 168 h postinfection in PP and MLN from orally infected mice and PLN from FP-infected mice. The mRNA levels for the Th1-like cytokines IL-12 (Fig. 2A and B) and IFN-γ (Fig. 2C) were elevated in the PP and PLN with similar kinetics. At 96 h following infection, there was a statistically significant 8.5-fold increase in IL-12 mRNA expression in the PP from orally infected mice, but no significant increase in the MLN, and a statistically significant 11.5-fold increase in the PLN following FP infections. The IFN-γ mRNA levels increased by 36 h in the PP and PLN and by 96 h in the MLN (Fig. 2C). Because the control IFN-γ levels were below the limit of detection, fold change levels were not calculated for IFN-γ.
FIG. 2.
IL-12 (A and B) and IFN-γ (C) mRNA expression following oral or FP reovirus infections. Each dot in panels A and C represents an individual mouse at the represented time postinfection, with three to four mice per group. Values in panel B are the fold induction of mRNA expression relative to noninfected (NI) controls. Onefold induction in panel B indicates endogenous levels. The dashed line in panel C indicates the limit of detection. Because the control IFN-γ levels were below the limit of detection, fold change levels were not calculated for IFN-γ. Asterisks indicate a significant difference from NI controls as determined by one-way ANOVA and Dunnett's test (P < 0.05). The data are representative of two similar experiments.
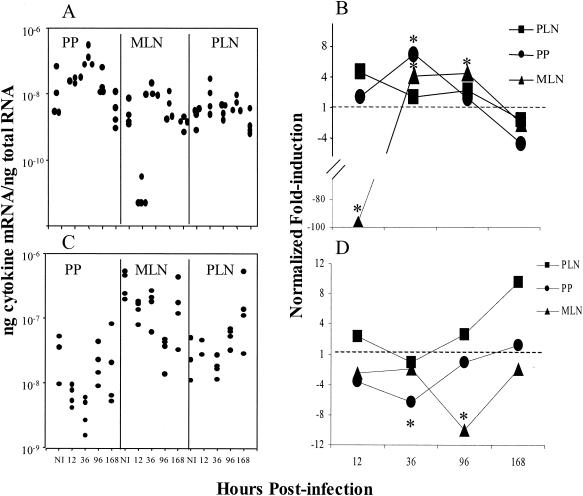
The mRNA levels for the Th2 cytokines IL-4 (Fig. 3A and B) and IL-10 (Fig. 3C and D) were also examined. There was a statistically significant 96-fold decrease in IL-4 mRNA in the MLN following oral infections and almost a 5-fold increase in the PLN following FP infections 12 h postinfection (Fig. 3A and B). IL-10 mRNA levels were reduced in the PP and MLN at 36 and 96 h, respectively. In contrast, IL-10 mRNA levels in the PLN were increased almost 10-fold following FP infections. (Fig. 3C and D). An earlier experiment using a less quantitative assay yielded similar results (unpublished data).
FIG. 3.
IL-4 (A and B) and IL-10 (C and D) mRNA expression following oral or FP reovirus infection. Each dot in panels A and C represents an individual mouse at the represented time postinfection, with three to four mice per group. In panels B and D values are expressed as the fold induction of mRNA expression relative to noninfected (NI) controls. The dashed lines in panels B and D at 1-fold induction indicate endogenous levels. Asterisks indicate a significant difference from NI controls by one-way ANOVA and post-hoc tests (P < 0.05). The data are representative of two similar experiments.
IL-4 involvement in the induction of the Th1-Th2 dichotomy following oral or FP infections.
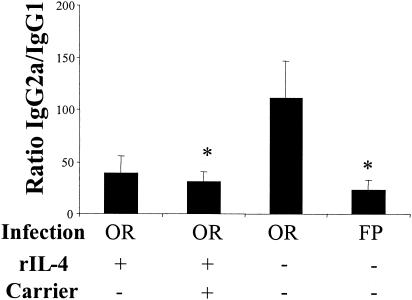
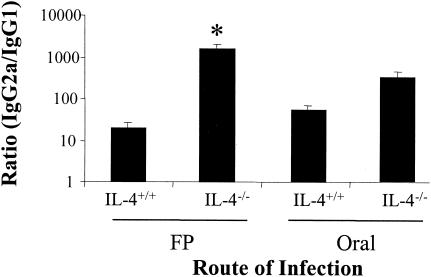
Cytokine analysis suggested that virus infection suppressed mucosal IL-4 and IL-10, which resulted in decreased serum IgG1 production. Therefore, groups of orally infected mice were treated with rIL-4 with or without an anti-IL-4 MAb carrier to enhance the efficacy of the IL-4 treatment (14). For clarity, the data are expressed as a ratio of the titers of reovirus-specific IgG2a and IgG1. There was a statistically significant (P < 0.05) decrease in the IgG2a/IgG1 ratio in orally infected mice that were treated with rIL-4 plus anti-IL-4 MAb carrier (Fig. 4). rIL-4 alone with no carrier antibody appeared to have an effect; however, the decrease was not statistically significant. To further support the hypothesis that IL-4 was the critical cytokine in inducing the route-of-infection-dependent difference in the IgG2a and IgG1 responses, we examined the IgG subclass responses in IL-4−/− mice and in anti-IL-4-treated mice. There was a statistically significant increase in the IgG2a/IgG1 ratio in FP-infected IL-4−/− mice (Fig. 5). The IgG subclass ratio was not affected in orally infected IL-4−/− mice (Fig. 5). Treatment of IL-4−/− mice with IL-4 skewed the response back toward a Th2 response in two of four mice, and a combination of IL-4 and IL-10 dramatically skewed the response toward Th2 (http://www.hsc.wvu.edu/micro/cuff/subclasspaper/supplementary.pdf).There was not a significant change in the IgG2a/IgG1 ratio in FP-infected mice treated with neutralizing anti-IL-4 (data not shown), which has been shown to fail to block IgG1 production in vivo (12, 13, 15).
FIG. 4.
Reduction of IgG2a/IgG1 ratios by rIL-4 treatment in orally infected mice. BALB/c mice were infected either orally or in the FP, and some orally infected mice received rIL-4 at 3 μg/dose (with or without 30 μg of anti-IL-4 MAb carrier/dose as described in the text) on day −1 and on day 2 of infection. The serum IgG subclass responses were determined by ELISA 10 days following infection. Data are represented as the ratio of the geometric means of IgG2a and IgG1 titers. Bars indicate the standard errors among groups of 5 to 11 mice from two independent experiments. Asterisks indicate significant differences from orally infected mice as determined by a one-way ANOVA and Dunn's post-hoc test (P < 0.05).
FIG. 5.
Increase in the IgG2a/IgG1 ratio in FP-infected IL-4−/− mice. IL-4−/− mice or BALB/c controls (IL-4+/+) were infected either orally or in the FP with 107 PFU of reovirus T1/L per mouse. Serum IgG subclass responses were determined by ELISA 10 days following infection. Data are represented as the ratio of the geometric mean titers of IgG2a and IgG1. Bars indicate the standard errors among groups of 4 to 10 mice from two independent experiments. The asterisk indicates a significant difference from FP-infected BALB/c control mice as determined by a one-way ANOVA and Dunn's post-hoc test (P < 0.05).
Local mucosal effects of in vivo IL-4.
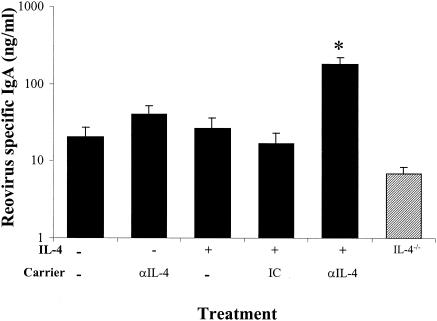
We assessed the effect of exogenous IL-4 on the intestinal IgA response following oral infections by quantitating reovirus-specific IgA in intestinal fragment cultures established from virus-infected IL-4-treated and IL-4−/− mice. Groups of control or IL-4-treated mice were infected orally with reovirus, and lamina propria fragment cultures were established for these mice 10 days following infection. Lamina propria cultures from IL-4-treated mice appeared to produce more reovirus-specific IgA than nontreated controls, but this difference was not statistically significant (Fig. 6). In addition, culture supernatants from orally infected IL-4−/− mice produced slightly less reovirus-specific IgA than controls (Fig. 6). IL-4−/− mice treated with IL-4 or a combination of IL-4 and IL-10 had a slightly enhanced IgA response in PP fragment cultures (http://www.hsc.wvu.edu/micro/cuff/subclasspaper/supplementary.pdf).
FIG. 6.
Enhancement of reovirus-specific IgA production by in vivo rIL-4 treatment. BALB/c mice (solid bars) or IL-4−/− mice (hatched bars) were infected orally with 107 PFU of reovirus T1/L per mouse. Groups of mice received rIL-4 at 3 μg/dose (with or without 30 μg of a carrier/dose as noted) on day −1 and on day 2 of infection. The fragment culture supernatants were collected, and IgA concentrations were determined by reovirus-specific ELISA. Bars indicate the standard errors among groups of 4 to 10 mice from two independent experiments. The asterisk indicates a significant difference from nontreated orally infected mice as determined by one-way ANOVA and Dunn's post-hoc test (P < 0.05).
IL-10 involvement in the induction of the Th1-Th2 dichotomy following oral or FP infections.
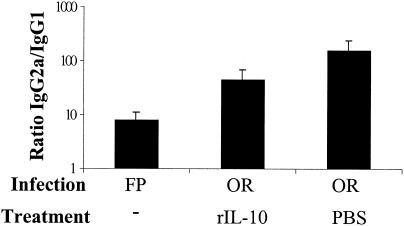
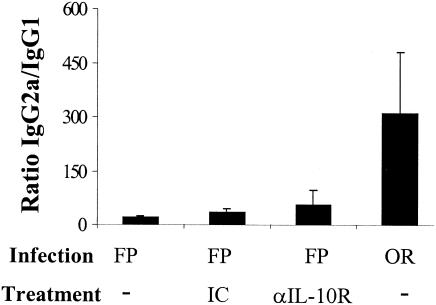
Because the route of infection also influenced the IL-10 mRNA response significantly, we hypothesized that IL-10 might also have a role in inducing the observed difference in the IgG subclass responses. In an effort to counteract the suppressed IL-10 response observed in orally infected mice, groups of orally infected mice were treated with rIL-10 or PBS as a control. In vivo treatment of BALB/c mice with rIL-10 partially but not significantly decreased the IgG2a/IgG1 ratio in orally infected mice (Fig. 7). In addition, anti-IL-10R MAb treatment (40, 44) did not significantly increase the IgG2a/IgG1 ratio in groups of FP-infected mice (Fig. 8). Consistent with another report (44), we observed elevated serum IL-12 levels in some of the anti-IL-10R MAb-treated mice (data not shown), suggesting that in vivo anti-IL-10R MAb treatment had a biologic effect.
FIG. 7.
Partial effect of IL-10 treatment on the IgG2a/IgG1 ratio in orally infected mice. BALB/c mice were infected either orally or in the FP with 3 × 107 PFU of reovirus/mouse. Orally infected mice received rIL-10 at 100 ng/dose (or PBS as vehicle where noted) every 12 h for the first 5 days postinfection, and serum antibody responses were determined 10 days following infection. Bars indicate the standard errors among groups of four mice. Representative data are from one of three independent experiments.
FIG. 8.
Failure of anti-IL-10R treatment to alter the IgG2a/IgG1 ratios in FP-infected mice. BALB/c mice were infected either orally or in the FP. FP-infected mice received anti-IL-10R MAb at 1.0 mg/mouse (or rat IgG1 as isotype control [IC] where noted) the day before infection and 0.5 mg/mouse every other day for 10 days. Data are represented as the ratio of the geometric means of IgG2a and IgG1 titers. Bars indicate the standard errors among groups of six to eight mice from one of two independent experiments.
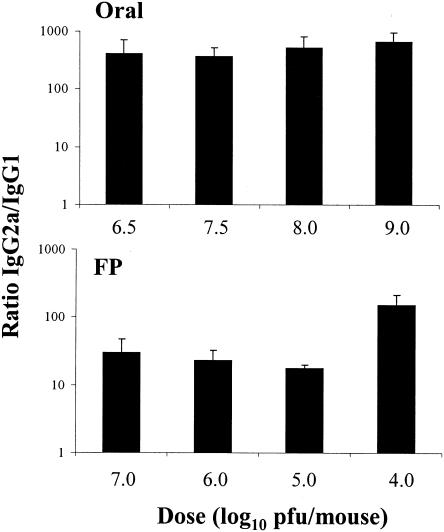
The effect of viral dose on the Th1 and Th2 response.
Antigen dose has been shown to affect Th1-Th2 differentiation (5, 6, 22, 34, 45, 48). Therefore, we examined the viral dose as a potential mechanism inducing the route-dependent difference in the IL-4 cytokine induction. Mice were infected with various doses of virus, and IgG subclass ratios of specific antibody were examined. We observed no consistent dose-response effect on the IgG2a/IgG1 ratios following infections with high or low doses of virus by either the oral or FP routes of infection (Fig. 9) The choice of doses used in these experiments was guided by previous unpublished work wherein we found that the minimum dose of virus given by the oral route that resulted in uniform seroconversion was approximately 106 PFU/mouse, whereas seroconversion was uniform in mice infected by the systemic route with doses as low as 105 PFU/mouse. In addition, Rubin et al. (49) reported that 5 of 10 mice died by day 6 following intestinal infection with 1010 PFU/mouse.
FIG. 9.
Reovirus serum IgG subclass ratio was not affected following mucosal or parenteral infections. BALB/c mice were infected with reovirus orally or in the FP with the indicated reovirus dose ranging from 104 to 109 PFU per mouse, and serum antibody responses were determined 10 days after infection. Bars indicate the standard errors among groups of four mice. Representative data are from two similar experiments.
DISCUSSION
The goal of this study was to determine the mechanisms responsible for route-of-infection-dependent differences in the serum IgG antibody responses following infection with reovirus. We first compared responses following oral, nasal, and FP routes of infection to determine if the decreased IgG1 response was a general characteristic of mucosal infection. We found reovirus-specific serum IgG2a with little or no IgG1 production following both oral and nasal infections, indicating that mucosal infections induced a response that differed from FP infections. It is likely that the mice swallowed some virus during nasal infection. Nevertheless, nasal infections did not elicit substantial virus-specific IgG1 production, which is a characteristic of FP infections. These results are consistent with a previous analysis of responses following oral and nasal reovirus infections (63). Because systemic immune responses that developed from mucosal infections were similar but distinct from responses that developed from FP infections, we determined the mechanisms responsible for inducing the route-dependent differences in serum responses by comparing oral and FP infections.
We hypothesized that oral infections induced either an overabundance of Th1 cytokines or a reduced Th2 cytokine responsiveness compared to FP infections. Both oral and FP infections induced similar levels of IFN-γ and IL-12 mRNA in the PP and PLN, and these cytokines likely promote reovirus-specific serum IgG2a following infections. Therefore, it does not appear that the route of infection skews the Th1 response.
The IL-12 mRNA expression in the PP and PLN peaked later than was expected for a cytokine involved in both innate and adaptive immune responses. However, Monteiro et al. (37) reported that IL-12 mRNA peaked at 4 days following i.n. influenza virus infection in the lung tissues of BALB/c mice and that replicating virus was required to induce IL-12. A requirement of viral replication may also explain why there was not a substantial increase in IL-12 mRNA in the MLN or spleen following oral reovirus infections (Fig. 2 and data not shown). We and others have previously reported that most replicating reovirus is found in the PP and small intestines and little if any is found in the MLN or spleen following oral reovirus infections (10, 21). Therefore, the initial Th1 immune response appears to be elicited in the PP following oral reovirus infections and in the PLN following FP infections.
Virus infection induces a weak Th2-type cytokine response, but this response can contribute to the production of neutralizing virus-specific IgG1 antibodies (10, 36, 55). We observed a significant reduction in IL-4 mRNA in the MLN following oral reovirus infections compared to noninfected controls. Furthermore, oral infection resulted in dramatically reduced IL-10 mRNA in both the PP and MLN compared to noninfected controls. Thus, enteric virus infection appears to suppress the normal Th2-skewed mucosal cytokine environment in mice of the H-2d haplotype, which likely influences the development of IgG subclasses.
These results led us to further refine our hypothesis to postulate that the reduced IgG1 production following oral infection is due to decreased expression of IL-4, IL-10, or a combination of these (or other) cytokines. We first examined the role of IL-4 in inducing reovirus-specific IgG1. Following rIL-4 treatment of orally infected mice we observed a decrease in the IgG2a/IgG1 ratio, which was due to an increase in reovirus-specific IgG1. In addition, FP-infected IL-4−/− mice produced lower levels of virus-specific IgG1, resulting in a higher IgG2a/IgG1 ratio compared to controls. Taken together these experiments suggest that IL-4 plays a major role in inducing the route-of-infection-dependent differences in the Th1 and Th2 immune responses following reovirus infection. In contrast, no change in the IgG2a/IgG1 ratio was observed in anti-IL-4-treated FP-infected mice. This could be due to the inability of the antibody to completely block the interaction of IL-4 with IL-4 receptors during cognate T-cell-B-cell interactions (12, 13, 15) or, perhaps more likely, the compensatory actions of additional cytokines, such as IL-13 (33).
Following rIL-10 treatment in orally infected mice, we observed a slight but not statistically significant decrease in the IgG2a/IgG1 ratio, but this decrease was not sufficient to account for the route-of-infection-dependent responses. Treatment of FP-infected mice with anti-IL-10R also did not affect the IgG2a/IgG1 ratio. It is possible that the rIL-10 and anti-IL-10R MAb doses were not high enough to affect the immune response. However, we used doses reported to induce immunologic changes (44, 52, 53, 62) and observed increased IL-12 in sera of some of the treated mice, indicating that the treatment had a biologic effect. In orally infected IL-4−/− mice, IL-10 alone or in combination with IL-4 skewed the response toward Th2, but this effect was due mainly to reduction of serum IgG2a levels. Therefore, we conclude that IL-10 contributes to the route-of-infection-dependent difference in the balance of Th1 and Th2 responses, particularly in combination with IL-4, but is not the essential regulating factor in the difference in reovirus-specific IgG1 responses.
The role of IL-4 in inducing IgG1 during in vivo infection has been studied using IL-4−/− and IL-10−/− mice (24, 25). Khan et al. (24) demonstrated that IL-4−/− mice that were infected with Streptococcus pneumoniae (strain R36A) had a reduced capacity to produce IgG1. In contrast, infected IL-10−/− mice produced more IgG1 than controls (24). Furthermore, Kendall et al. (23) demonstrated that BALB/c IL-4−/− mice have reduced IL-10 mRNA expression following infection with cilium-associated respiratory bacillus. Therefore, while there might be a modest contribution of IL-10 to the route-of-infection-dependent differences in IgG1 production, it is perhaps more likely that the decrease in IL-10 mRNA expression is a product of the reduced IL-4 response.
We observed a substantial increase in the reovirus-specific mucosal IgA production following treatment with IL-4, but only a slight and nonsignificant reduction in reovirus-specific IgA was observed in orally infected IL-4−/− mice. Coffman et al. (4) demonstrated that IL-4 could enhance the secretion of IgA in lipopolysaccharide-stimulated, T-cell-depleted spleen cells cultured with IL-5. This effect was confirmed in vivo by Chen et al. (3), who observed no decrease in serum specific IgA in IL-4−/− or control mice infected with Helicobacter pylori but did see a significant increase in specific serum IgA in infected IL-4 transgenic mice. These results are partially consistent with experiments examining the mucosal immune responses in IL-4−/− mice where adjuvant (CT or QS-21) plus protein antigen (keyhole limpet hemocyanin or tetanus toxoid) resulted in a reduced antigen-specific IgA response (1, 57). However, the CT-specific IgA response was not affected in IL-4−/− mice (57). Therefore, it appears that IL-4 can enhance IgA production following enteric virus infection, although significant IgA is produced during the intestinal response that is strongly skewed towards a Th1 response.
Why does the route of infection influence the IL-4 response? We hypothesized that the route-dependent difference in IL-4 induction might be a reflection of the amount of virus that accesses the inductive lymphoid tissue following parenteral or oral infection. Previous reports have demonstrated that the dose of antigen can have varying effects on the differentiation of the Th1 and Th2 immune responses (5, 6, 19, 22, 34, 45, 48). Studies examining the effect of antigen dose on Th1 and Th2 differentiation using T-cell receptor-transgenic mice demonstrated that low antigen doses induced IL-4, intermediate doses induced IFN-γ, and high doses induced a mixed response (5, 19, 48). In contrast, infections with the replicating intracellular pathogens L. major and Mycobacterium bovis BCG indicated that lower doses induced predominant Th1 responses while the highest doses induced predominant Th2 responses (34, 45). However, reovirus dose-response experiments with oral or FP infections did not induce a consistent change in the IgG2a/IgG1 ratio. We did observe a slight increase in the IgG2a/IgG1 ratio at the lowest dose following FP infections. Nevertheless, a similar effect was not observed following oral infections with high doses of reovirus. Similar IgG subclass responses have been observed following infections with serotype 3 reovirus, but UV light-inactivated virus did not induce serum IgG antibody responses (data not shown). Therefore, while dose has been shown to affect Th1-Th2 differentiation, the route-of-infection-dependent difference in IgG subclass responses following reovirus infection does not appear to be dose dependent.
Mechanisms such as differential activation of dendritic cell (DC) subpopulations may play a role in differentially inducing IL-4 following oral and FP reovirus infections. DC subpopulations are capable of inducing the differentiation of Th1 and Th2 cells. It appears that myeloid DC preferentially induce Th2 responses, while lymphoid DC preferentially induce Th1 responses (30, 46). In addition, DC from the PP have been observed to react differently to CD40L stimulation than those in the spleen. For instance, myeloid DC in the PP but not spleen secreted IL-10 upon CD40L stimulation (20). However, in preliminary studies Shreedhar et al. did not observe any DC movement in the PP 24 h after oral reovirus infection (51). Still, work is under way to examine the potential role of DC subpopulations on inducing the route-dependent differences in the reovirus immune responses.
In summary, our studies have established a potential mechanism for the route-dependent difference in the reovirus-specific IgG1 immune response. It appears that although certain mouse strains are predisposed to mounting Th2 responses to antigen, intestinal infection with an intracellular enteric virus induces a robust Th1 response and transiently suppresses the basal Th2 environment of the mucosa-associated lymphoid tissue. This transient alteration in the cytokine balance does not block mucosal IgA production but does regulate serum IgG1. Therefore, both the nature of the pathogen and the route of infection need to be considered when developing vaccines and adjuvant therapies for optimal cellular and humoral immune responses.
Acknowledgments
We thank Fred Finkelman (Children's Hospital Medical Center, Cincinnati, Ohio) and Fiona Powrie (University of Oxford, Oxford, United Kingdom) for their helpful advice. We thank DNAX for the release of their anti-IL-10R and control antibody hybridomas. We also thank the Biological Resources Branch, National Cancer Institute, for anti-IL-4 antibody.
This research was supported by grants AI34544 and RR16440 from the National Institutes of Health and by the National Cell Culture Center (Minneapolis, Minn.).
REFERENCES
- 1.Boyaka, P. N., M. Marinaro, R. J. Jackson, F. W. van Ginkel, E. Cormet-Boyaka, K. L. Kirk, C. R. Kensil, and J. R. McGhee. 2001. Oral QS-21 requires early IL-4 help for induction of mucosal and systemic immunity. J. Immunol. 166:2283-2290. [DOI] [PubMed] [Google Scholar]
- 2.Bustin, S. A. 2000. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 25:169-193. [DOI] [PubMed] [Google Scholar]
- 3.Chen, W., D. Shu, and V. S. Chadwick. 1999. Helicobacter pylori infection in interleukin-4-deficient and transgenic mice. Scand. J. Gastroenterol. 34:987-992. [DOI] [PubMed] [Google Scholar]
- 4.Coffman, R. L., B. Shrader, J. Carty, T. R. Mosmann, and M. W. Bond. 1987. A mouse T cell product that preferentially enhances IgA production. I. Biologic characterization. J. Immunol. 139:3685-3690. [PubMed] [Google Scholar]
- 5.Constant, S., C. Pfeiffer, A. Woodard, T. Pasqualini, and K. Bottomly. 1995. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. J. Exp. Med. 182:1591-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constant, S. L., and K. Bottomly. 1997. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 15:297-322. [DOI] [PubMed] [Google Scholar]
- 7.Constant, S. L., K. S. Lee, and K. Bottomly. 2000. Site of antigen delivery can influence T cell priming: pulmonary environment promotes preferential Th2-type differentiation. Eur. J. Immunol. 30:840-847. [DOI] [PubMed] [Google Scholar]
- 8.Else, K. J., and F. D. Finkelman. 1998. Intestinal nematode parasites, cytokines and effector mechanisms. Int. J. Parasitol. 28:1145-1158. [DOI] [PubMed] [Google Scholar]
- 9.Fallon, P. G., H. E. Jolin, P. Smith, C. L. Emson, M. J. Townsend, R. Fallon, P. Smith, and A. N. McKenzie. 2002. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 17:7-17. [DOI] [PubMed] [Google Scholar]
- 10.Fan, J. Y., C. S. Boyce, and C. F. Cuff. 1998. T-helper 1 and T-helper 2 cytokine responses in gut-associated lymphoid tissue following enteric reovirus infection. Cell. Immunol. 188:55-63. [DOI] [PubMed] [Google Scholar]
- 11.Finkelman, F. D., J. Holmes, I. M. Katona, J. F. Urban, Jr., M. P. Beckmann, L. S. Park, K. A. Schooley, R. L. Coffman, T. R. Mosmann, and W. E. Paul. 1990. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 8:303-333. [DOI] [PubMed] [Google Scholar]
- 12.Finkelman, F. D., J. Holmes, J. F. Urban, Jr., W. E. Paul, and I. M. Katona. 1989. T help requirements for the generation of an in vivo IgE response: a late acting form of T cell help other than IL-4 is required for IgE but not for IgG1 production. J. Immunol. 142:403-408. [PubMed] [Google Scholar]
- 13.Finkelman, F. D., I. M. Katona, J. F. Urban, Jr., C. M. Snapper, J. Ohara, and W. E. Paul. 1986. Suppression of in vivo polyclonal IgE responses by monoclonal antibody to the lymphokine B-cell stimulatory factor 1. Proc. Natl. Acad. Sci. USA 83:9675-9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finkelman, F. D., K. B. Madden, S. C. Morris, J. M. Holmes, N. Boiani, I. M. Katona, and C. R. Maliszewski. 1993. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J. Immunol. 151:1235-1244. [PubMed] [Google Scholar]
- 15.Finkelman, F. D., J. F. Urban, Jr., M. P. Beckmann, K. A. Schooley, J. M. Holmes, and I. M. Katona. 1991. Regulation of murine in vivo IgG and IgE responses by a monoclonal anti-IL-4 receptor antibody. Int. Immunol. 3:599-607. [DOI] [PubMed] [Google Scholar]
- 16.Fromantin, C., L. Piroth, I. Petitpas, P. Pothier, and E. Kohli. 1998. Oral delivery of homologous and heterologous strains of rotavirus to BALB/c mice induces the same profile of cytokine production by spleen cells. Virology 244:252-260. [DOI] [PubMed] [Google Scholar]
- 17.George, A. 1996. Generation of gamma interferon responses in murine Peyer's patches following oral immunization. Infect. Immun. 64:4606-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins, L. M., G. Frankel, G. Douce, G. Dougan, and T. T. MacDonald. 1999. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect. Immun. 67:3031-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosken, N. A., K. Shibuya, A. W. Heath, K. M. Murphy, and A. O'Garra. 1995. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J. Exp. Med. 182:1579-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwasaki, A., and B. L. Kelsall. 1999. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 190:229-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kauffman, R. S., J. L. Wolf, R. Finberg, J. S. Trier, and B. N. Fields. 1983. The sigma 1 protein determines the extent of spread of reovirus from the gastrointestinal tract of mice. Virology 124:403-410. [DOI] [PubMed] [Google Scholar]
- 22.Kehoe, K. E., M. A. Brown, and F. Imani. 2001. Double-stranded RNA regulates IL-4 expression. J. Immunol. 167:2496-2501. [DOI] [PubMed] [Google Scholar]
- 23.Kendall, L. V., L. K. Riley, R. R. Hook, Jr., C. L. Besch-Williford, and C. L. Franklin. 2001. Differential interleukin-10 and gamma interferon mRNA expression in lungs of cilium-associated respiratory bacillus-infected mice. Infect. Immun. 69:3697-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan, A. Q., Y. Shen, Z. Q. Wu, T. A. Wynn, and C. M. Snapper. 2002. Endogenous pro- and anti-inflammatory cytokines differentially regulate an in vivo humoral response to Streptococcus pneumoniae. Infect. Immun. 70:749-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhn, R., K. Rajewsky, and W. Muller. 1991. Generation and analysis of interleukin-4 deficient mice. Science 254:707-710. [DOI] [PubMed] [Google Scholar]
- 26.Lamm, M. E. 1988. The IgA mucosal immune system. Am. J. Kidney Dis. 12:384-387. [DOI] [PubMed] [Google Scholar]
- 27.Major, A. S., and C. F. Cuff. 1996. Effects of the route of infection on immunoglobulin G subclasses and specificity of the reovirus-specific humoral immune response. J. Virol. 70:5968-5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Major, A. S., and C. F. Cuff. 1997. Enhanced mucosal and systemic immune responses to intestinal reovirus infection in β2-microglobulin-deficient mice. J. Virol. 71:5782-5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Major, A. S., D. H. Rubin, and C. F. Cuff. 1998. Mucosal immunity to reovirus infection. Curr. Top. Microbiol. Immunol. 233:163-177. [DOI] [PubMed] [Google Scholar]
- 30.Maldonado-Lopez, R., T. De Smedt, P. Michel, J. Godfroid, B. Pajak, C. Heirman, K. Thielemans, O. Leo, J. Urbain, and M. Moser. 1999. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189:587-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marinaro, M., H. F. Staats, T. Hiroi, R. J. Jackson, M. Coste, P. N. Boyaka, N. Okahashi, M. Yamamoto, H. Kiyono, and H. Bluethmann. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 155:4621-4629. [PubMed] [Google Scholar]
- 32.Matthews, J. B., B. H. Fivaz, and H. F. Sewell. 1981. Serum and salivary antibody responses and the development of oral tolerance after oral and intragastric antigen administration. Int. Arch. Allergy Appl. Immunol. 65:107-113. [DOI] [PubMed] [Google Scholar]
- 33.McKenzie, G. J., P. G. Fallon, C. L. Emson, R. K. Grencis, and A. N. McKenzie. 1999. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J. Exp. Med. 189:1565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menon, J. N., and P. A. Bretscher. 1998. Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route and strain of host or parasite. Eur. J. Immunol. 28:4020-4028. [DOI] [PubMed] [Google Scholar]
- 35.Mestecky, J. 1988. Immunobiology of IgA. Am. J. Kidney Dis. 12:378-383. [DOI] [PubMed] [Google Scholar]
- 36.Mo, X. Y., S. R. Sarawar, and P. C. Doherty. 1995. Induction of cytokines in mice with parainfluenza pneumonia. J. Virol. 69:1288-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Monteiro, J. M., C. Harvey, and G. Trinchieri. 1998. Role of interleukin-12 in primary influenza virus infection. J. Virol. 72:4825-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrison, L. A., X. J. Da Costa, and D. M. Knipe. 1998. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 243:178-187. [DOI] [PubMed] [Google Scholar]
- 39.Mosmann, T. R., H. Cherwinski, M. W. Bond, M. A. Giedlin, and R. L. Coffman. 1986. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348-2357. [PubMed] [Google Scholar]
- 40.O'Farrell, A. M., Y. Liu, K. W. Moore, and A. L. Mui. 1998. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 17:1006-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Neal, C. M., G. R. Harriman, and M. E. Conner. 2000. Protection of the villus epithelial cells of the small intestine from rotavirus infection does not require immunoglobulin A. J. Virol. 74:4102-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pacheco, S. E., R. A. Gibbs, A. Ansari-Lari, and P. Rogers. 2000. Intranasal immunization with HIV reverse transcriptase: effect of dose in the induction of helper T cell type 1 and 2 immunity. AIDS Res. Hum. Retrovir. 16:2009-2017. [DOI] [PubMed] [Google Scholar]
- 43.Park, A. Y., B. D. Hondowicz, and P. Scott. 2000. IL-12 is required to maintain a Th1 response during Leishmania major infection. J. Immunol. 165:896-902. [DOI] [PubMed] [Google Scholar]
- 44.Phillips, J. M., N. M. Parish, M. Drage, and A. Cooke. 2001. Interactions through the IL-10 receptor regulate autoimmune diabetes. J. Immunol. 167:6087-6091. [DOI] [PubMed] [Google Scholar]
- 45.Power, C. A., G. Wei, and P. A. Bretscher. 1998. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect. Immun. 66:5743-5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pulendran, B., J. L. Smith, G. Caspary, K. Brasel, D. Pettit, E. Maraskovsky, and C. R. Maliszewski. 1999. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA 96:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramakrishna, C., V. Ravi, A. Desai, D. K. Subbakrishna, S. K. Shankar, and A. Chandramuki. 2003. T helper responses to Japanese encephalitis virus infection are dependent on the route of inoculation and the strain of mouse used. J. Gen. Virol. 84:1559-1567. [DOI] [PubMed] [Google Scholar]
- 48.Rogers, P. R., and M. Croft. 1999. Peptide dose, affinity, and time of differentiation can contribute to the Th1/Th2 cytokine balance. J. Immunol. 163:1205-1213. [PubMed] [Google Scholar]
- 49.Rubin, D. H., M. J. Kornstein, and A. O. Anderson. 1985. Reovirus serotype 1 intestinal infection: a novel replicative cycle with ileal disease. J. Virol. 53:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scott, P., P. Natovitz, R. L. Coffman, E. Pearce, and A. Sher. 1988. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J. Exp. Med. 168:1675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shreedhar, V. K., B. L. Kelsall, and M. R. Neutra. 2003. Cholera toxin induces migration of dendritic cells from the subepithelial dome region to T- and B-cell areas of Peyer's patches. Infect. Immun. 71:504-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva, R. A., and R. Appelberg. 2001. Blocking the receptor for interleukin 10 protects mice from lethal listeriosis. Antimicrob. Agents Chemother. 45:1312-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slavin, A. J., R. Maron, and H. L. Weiner. 2001. Mucosal administration of IL-10 enhances oral tolerance in autoimmune encephalomyelitis and diabetes. Int. Immunol. 13:825-833. [DOI] [PubMed] [Google Scholar]
- 54.Smith, R. E., H. J. Zweerink, and W. K. Joklik. 1969. Polypeptide components of virions, top component and cores of reovirus type 3. Virology 39:791-810. [DOI] [PubMed] [Google Scholar]
- 55.Su, H. C., L. P. Cousens, L. D. Fast, M. K. Slifka, R. D. Bungiro, R. Ahmed, and C. A. Biron. 1998. CD4+ and CD8+ T cell interactions in IFN-gamma and IL-4 responses to viral infections: requirements for IL-2. J. Immunol. 160:5007-5017. [PubMed] [Google Scholar]
- 56.Thompson, A. H., J. G. McRoberts, S. R. Crowe, L. London, and S. D. London. 1999. Optimal induction of upper respiratory tract immunity to reovirus 1/L by combined upper and lower respiratory tract inoculation. Vaccine 17:1404-1415. [DOI] [PubMed] [Google Scholar]
- 57.Vajdy, M., M. H. Kosco-Vilbois, M. Kopf, G. Kohler, and N. Lycke. 1995. Impaired mucosal immune responses in interleukin 4-targeted mice. J. Exp. Med. 181:41-53. [DOI] [PubMed] [Google Scholar]
- 58.Weiner, H. L. 2001. Oral tolerance: immune mechanisms and the generation of Th3-type TGF-beta-secreting regulatory cells. Microbes Infect. 3:947-954. [DOI] [PubMed] [Google Scholar]
- 59.Wilson, A. D., M. Bailey, N. A. Williams, and C. R. Stokes. 1991. The in vitro production of cytokines by mucosal lymphocytes immunized by oral administration of keyhole limpet hemocyanin using cholera toxin as an adjuvant. Eur. J. Immunol. 21:2333-2339. [DOI] [PubMed] [Google Scholar]
- 60.Xu-Amano, J., W. K. Aicher, T. Taguchi, H. Kiyono, and J. R. McGhee. 1992. Selective induction of Th2 cells in murine Peyer's patches by oral immunization. Int. Immunol. 4:433-445. [DOI] [PubMed] [Google Scholar]
- 61.Xu-Amano, J., H. Kiyono, R. J. Jackson, H. F. Staats, K. Fujihashi, P. D. Burrows, C. O. Elson, S. Pillai, and J. R. McGhee. 1993. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J. Exp. Med. 178:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin, Z., G. Bahtiyar, N. Zhang, L. Liu, P. Zhu, M. E. Robert, J. McNiff, M. P. Madaio, and J. Craft. 2002. IL-10 regulates murine lupus. J. Immunol. 169:2148-2155. [DOI] [PubMed] [Google Scholar]
- 63.Zuercher, A. W., S. E. Coffin, M. C. Thurnheer, P. Fundova, and J. J. Cebra. 2002. Nasal-associated lymphoid tissue is a mucosal inductive site for virus-specific humoral and cellular immune responses. J. Immunol. 168:1796-1803. [DOI] [PubMed] [Google Scholar]