Abstract
The adult hippocampus is one of the primary neural structures involved in memory formation. In addition to synapse-specific modifications thought to encode information at the sub-cellular level, changes in the intrahippocampal neuro-populational activity and dynamics at the circuit-level may contribute substantively to the functional capacity of this region. Within the hippocampus, the dentate gyrus has the potential to make a preferential contribution to neural circuit modification owing to the continuous addition of new granule cell population. The integration of newborn neurons into pre-existing circuitry is hypothesized to deliver a unique processing capacity, as opposed to merely replacing dying granule cells. Recent studies have begun to assess the impact of hippocampal neurogenesis by examining the extent to which adult-born neurons participate in hippocampal networks, including when newborn neurons become engaged in ongoing network activity and how they modulate circuit dynamics via their unique intrinsic physiological properties. Understanding the contributions of adult neurogenesis to hippocampal function will provide new insight into the fundamental aspects of brain plasticity, which can be used to guide therapeutic interventions to replace neural populations damaged by disease or injury.
Introduction
The seminal discovery of postnatal neurogenesis by Altman and Das in the 1960s overturned a long-held dogma that the adult mammalian brain is mainly a post-mitotic structure lacking the capacity to regenerate neurons . Two discrete “neurogenic” regions have since been identified in the adult rodent and primate brains using tritiated thymidine labeling of proliferating cells – namely, the subventricular zone (SVZ) of the lateral brain ventricles, and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus (Lledo et al., 2006; Zhao et al., 2008). New neurons in these regions originate from a residential population of adult neural stem cells (NSCs) (Gage, 2000; Alvarez-Buylla and Lim, 2004; Ma et al., 2009). Although NSCs may arise in other regions of the adult brain under pathological conditions and following injuries (Ming and Song, 2005), it remains controversial whether, and to what extent, active neurogenesis occurs outside of the SVZ and SGZ under physiological conditions. In the present review, we will focus on adult SGZ neurogenesis within the local hippocampal network.
Adult hippocampal neurogenesis is a complex, multi-step process that is highly regulated by existing neuronal network activity (Kempermann et al., 2004; Ma et al., 2009; Ming and Song, 2011). At the cellular level, the origin and development of NSCs in the adult mouse brain have been examined using a combination of immunohistological, electrophysiological, imaging and genetic approaches. Given the central role of the hippocampus in many forms of learning and memory, the potential contribution of adult neurogenesis to these processes at the system level has been a central question in the field. Specifically, how does the dynamic composition of the dentate granule cell population alter the information processing capacity of the hippocampus as a whole? Recently, much progress have been made in understanding how adult neurogenesis is regulated by mature circuitry in an activity-dependent manner, and in turn how newborn neurons affect the existing circuitry at the circuit and behavioral levels. Understanding the basic mechanism regulating adult neurogenesis and their contribution to brain functions is important for both basic biology and for clinical applications if we are to harness cell replacement potential to help repair the injured, diseased and aged central nervous system (Goh et al., 2003).
Adult mammalian hippocampal circuitry
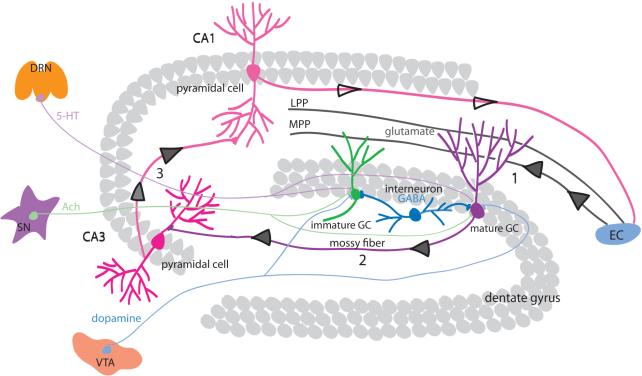
The hippocampal neural network is highly dynamic, with the capacity to modify its connectivity by changing the number and strength of synaptic contacts in an activity-dependent manner. Hippocampal principal neurons are located in three primary subregions: granule cells in the dentate gyrus (DG), and pyramidal neurons in CA1 and CA3. The principal neurons are synaptically connected to form the “tri-synaptic circuit” (Figure 1). Within this tri-synaptic circuit, information flows from entorhinal cortex (EC), the afferent input to the DG through medial and lateral perforant pathways, then to CA3 pyramidal cells via mossy fibers (axons of DG granule cells), then to CA1 pyramidal cells via Schaffer collateral projections (axons of CA3 neurons), then to the subiculum and back to the EC (Claiborne et al., 1986; Kohler, 1986). This primary hippocampal projection pattern forms a closed loop wherein sensory information from specific cortical areas converges onto the EC through excitatory pathways, processed through the hippocampal circuitry, and returns to the cortical region of origin EC (Li et al., 2009). Besides principal excitatory neurons that form the tri-synaptic circuit, another major component in the hippocampus is the inhibitory interneurons that release GABA, which is important for the generation of field potential oscillations. Furthermore, adult hippocampal circuitry is under the influence of multiple modulatory neurotransmitters, such as acetylcholine from septal nucleus, serotonin from dorsal rophe nucleus, and dopamine from ventral tegmental area. In addition to activity-induced changes at the synapse, a unique feature to this circuitry is the large-scale structural modification via the addition of new granule cells arising from ongoing neurogenesis. The entire milieu must preserve the functional integrity of the existing circuitry, and at the same time, it needs to provide a niche to support the development of adult-born granule cells and allowing these cells to modify the local neuronal network.
Figure 1. Trisynaptic circuits of the hippocampal network. A diagram of the transversal section of the hippocampus shows the different subregions: DG, CA3, and CA1.
The principle cells located in densely packed cell layers are synaptically connected via “tri-synaptic circuit”. Mature and newborn granule cells receive their major synaptic input from the medial and lateral perforant path (MPP and LPP) that originates from the entorhinal cortex (EC) (1). Granule cells send their axons (mossy fibers) that terminate on the proximal dendrites of CA3 pyramidal cells (2). CA3 pyramidal cells send axons (Schafer collateral axons) to CA1 pyramidal cells (3), whose axons in turn projects back to the EC. Inhibitory interneurons (such as basket cells) provide a dense network of GABAergic synaptic boutons within the granule cell layer and SGZ, innervating both mature and newborn granule cells. Hippocampal circuitry is subject to the regulation by various synaptic inputs: glutamate from EC, GABA from local interneurons, serotonin (5-HT) from dorsal raphe nucleus (DRN), Ach (acetylcholine) from septal nucleus (SN), and dopamine from ventral tegmental area (VTA). NSC: neural stem cell; NPC: neural progenitor cell.
Development of adult neural stem cells
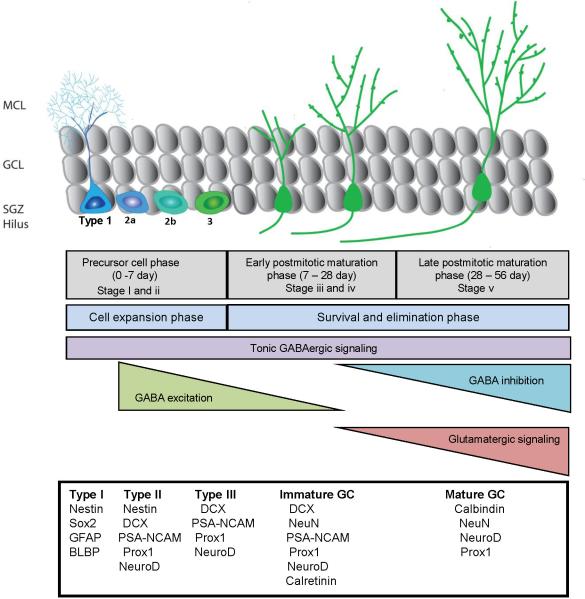
Among hippocampal principal cells, only granule cells are continuously generated throughout adulthood in the dentate gyrus. Self-renewing and multipotent NSCs residing in the SGZ, a narrow band of tissue lying between the granule cell layer and the hilus, give rise to both neurons and astrocytes, but not oligodendrocytes (Bonaguidi et al., 2011). The development of newborn neurons in the adult hippocampus can be divided into five distinct phases: i) Stem cell maintenance, activation and fate specification; ii) Expansion of intermediate neural progenitors; iii) migration and initial pruning of newborn granule cells; iv) Maturation and functional integration of newborn neurons; v) Late-phase maturation and maintenance of adult-born neurons (Figure 2). It is estimated that the entire neurodevelopmental process takes approximately 7-8 weeks in the young adult mouse brain (Zhao et al., 2006).
Figure 2. Adult neurogenesis in the dentate gyrus of the hippocampus.
Shown is a schematic summary of the developmental stages of dentate neurogenesis as characterized by estimated timeline for each developmental stage. Also shown are physiological properties, and expression of specific molecular markers at each stage. MCL, molecular cell layer; GCL, granule cell layer; SGZ, subgranular zone. GFAP, glial fibrillary acidic protein; BLBP, brain lipid-binding protein; DCX, doublecortin; NeuN, neuronal nuclei; PSA-NCAM: the polysialylated form of the neural cell adhesion molecule NCAM; GABA, γ-aminobutyric acid.
i) Stem cell maintenance, activation and fate specification
Adult hippocampal neurogenesis originates from a population of NSCs in the SGZ. It appears that multiple types of NSCs, such as radial and non-radial precursors, co-exist in the adult SGZ (Lugert et al., 2010). Radial glia-like cells have been traditionally classified as Type I cells, which are infrequently labeled by retroviruses or BrdU, indicative of their quiescence state. Morphologically, radial glia-like NSCs possess a long process that extends and branches into the inner molecular layer. Biochemically, these cells express glial fibrillary acidic protein (GFAP), the intermediate filament protein Nestin, a radial glia marker BLBP, and the Sry-related HMG-box transcription factor Sox2. Despite expressing several astrocytic markers, the radial glia-like NSCs are morphologically and functionally distinct from mature astrocytes. Recent fate mapping studies using inducible Cre recombinase driven by various promoters or enhancers, including Gli, GFAP, Nestin, and GLAST (glutamate aspartate transporter), have provided evidence in support of radial glia-like cells as the primary NSCs in the adult brain (Dhaliwal and Lagace, 2011). In vivo clonal analysis using a novel genetic labeling approach has demonstrated that radial glia-like cells are capable of both self-renewal and multipotent differentiation into neurons and astrocytes (Bonaguidi et al., 2011). Critically, the maintenance and activation of these quiescent population of cells appears to be dynamically regulated by experience and aging (Dranovsky et al., 2011; Encinas et al., 2011). The clonal properties of non-radial NSCs and how the behavior of these cells is regulated in vivo remain to be further characterized.
ii) Expansion of intermediate neural progenitors
In the adult SGZ, NSCs give rise to Type II intermediate progenitors, which in turn generate neuroblasts (Type III). Distinct subpopulations of actively proliferating intermediate progenitors can be identified according to their specific morphologies, electrophysiological properties, and molecular marker expression (Seri et al., 2001; Seri et al., 2004). Morphologically, horizontal cellular processes are prominent in these progenitor cells (Fukuda et al., 2003; Steiner et al., 2006). Interestingly, the proliferation of Type II cells is subject to activity-dependent regulation through either physiological stimuli such as voluntary wheel running (Kronenberg et al., 2003) or pharmacological stimulation, such as antidepressant treatment (Encinas et al., 2006). Type III cells express markers of neuronal lineage (DCX, PSA-NCAM, NeuroD, and Prox1), and transiently express the calcium-binding protein calretinin (Brandt et al., 2003). Morphologically, Type III cells possess processes of various lengths and complexities, and the orientation of their processes incrementally changes from horizontal to vertical. These cells are believed to have limited proliferative activity and thus considered to be a transitional cell type from proliferating neuroblasts to postmitotic immature neurons. Under pathological conditions, such as seizures, Type III cells display an aberrant state characterized by dramatically increased proliferation (Jessberger et al., 2005).
iii) Migration and initial pruning of newborn neurons
In the dentate gyrus, newborn neurons migrate to the inner granule cell layer and elaborate axon and dendritic processes to CA3 and molecular layer, respectively. Recent studies suggest that migration of newborn granule cells is tightly regulated. For example, knockdown of Disrupted-in-Schizophrenia 1 (DISC1) in newborn granule cells leads to overextended migration to the molecular layer (Duan et al., 2007). On the other hand, loss of Reelin expression from local interneurons in an animal model of seizures results in the ectopic hilar localization of newborn granule cells (Gong et al., 2007). During this early stage of development, a significant percentage of newborn progeny is eliminated through apoptosis and microglia-mediated phagocytosis (Sierra et al., 2010). The survival of newborn neurons can also be influenced by the experience of animals, such as spatial learning and exposure to an enriched environment (Tashiro et al., 2006; Tashiro et al., 2007).
iv) Maturation and synaptic integration of newborn neurons
Functional integration of newborn granule cells in vivo depends on the formation of synaptic input and output with other neurons in the hippocampal circuitry. Dendritic and axonal (mossy fiber) development initiates immediately upon exit of immature neurons from the cell cycle. It was recently demonstrated that synaptic integration with afferent projections from the entorhinal cortex depends on the emergence and assembly of primary cilia that occurs between 14 and 21 after the birth of adult born granule cells (Kumamoto et al., 2012). Mossy fibers can be detected around 10 days after cell birth and gradually grow and reach CA3 region before spines are formed (Hastings and Gould, 1999; Zhao et al., 2006). Newborn neurons exhibit very similar axonal and dendritic projection patterns to the neighboring mature neurons upon maturation, as revealed by retrovirus-mediated labeling (Zhao et al., 2006). For example, at 6-8 weeks of age, newborn neurons display overall morphological and functional characteristics very similar to that of fully mature dentate granule cells (van Praag et al., 2002; Ambrogini et al., 2004; Schmidt-Hieber et al., 2004). Additionally, high-resolution morphological studies using electron tomography and serial section electron microscopy confirmed that new granule cells at 30 days of cell age receive a variety of synaptic inputs from axosomatic, axodendritic, and axospinous connections comparable to mature neurons. The functional integration of newborn granule cells into the existing hippocampal circuitry have been studied in much detail by electrophysiological recording of these cells, either labeled by engineered onco-retrovirus (Song et al., 2005; Ge et al., 2006) or marked in transgenic reporter mice (Overstreet et al., 2004; Overstreet-Wadiche et al., 2006) (Markwardt and Overstreet-Wadiche, 2008; Markwardt et al., 2009). These studies have revealed that synaptic integration of newborn granule cells follows a stereotypic sequence, similar to that observed in embryonic and early postnatal development (Figure 2): 1) Initial activation of newborn neurons is non-synaptic, which is mediated by ambient GABA present in the local milieu; 2) Newborn neurons become activated by synaptic transmission from local interneurons through input-specific GABAergic signaling; 3) GABAergic inputs are converted from excitatory to inhibitory, and concomitantly, excitatory glutamatergic dendritic inputs begin to activate the developing neurons; 4) Finally, inhibitory GABA synaptic inputs begin to appear at perisomatic synapses to complete the mature granule cell innervation pattern.
v) Late-stage maturation and maintenance of adult-born neurons
This phase represents the fine-tuning stage of the hippocampal circuitry in response to ongoing activity. To date, little is known about the adaptive changes that occur in this late neurodevelopmental phase of adult neurogenesis. Although new granule cells undergo a switch in the expression of calcium-binding proteins from calretinin to calbindin, it takes several more weeks to become electrophysiologically indistinguishable from their mature counterparts (van Praag et al., 2002). One unique trait of new neurons at this stage is that they exhibit enhanced synaptic plasticity (Schmidt-Hieber et al., 2004; Ge et al., 2007), which may facilitate integration in order to achieve long-term changes in the network (Ramirez-Amaya et al., 2006; Wiskott et al., 2006). Such enhanced plasticity is suggested to give adult-born neurons an advantage in competing with mature neurons for selective formation and stabilization of afferent and efferent synaptic connections (Tashiro et al., 2006; Toni et al., 2008). Functionally, this unique physiological property of newborn granule cells after integration may contribute to the plasticity of the hippocampal network (Ge et al., 2008). Once mature, adult-born neurons are maintained throughout life (Kempermann et al., 2003).
Activity-dependent regulation of adult hippocampal neurogenesis
Adult neurogenesis is highly sensitive to neuronal activity within the hippocampal circuitry and is regulated at multiple developmental stages, including alterations in the activation of radial glia-like NSCs and in the rate of precursor cell proliferation, changes in the survival of newborn neurons, and strategic synaptic integration of newborn neurons.
Activity-dependent regulation of hippocampal neurogenesis has been examined under several physiological conditions. For instance, enriched physical environment affects neurogenesis by enhancing the survival of newborn neurons (Leuner et al., 2006; Drapeau et al., 2007; Sisti et al., 2007). On the other hand, the neurogenic effects of physical exercise, such as voluntary wheel running or forced treadmill, appear to be due to an increase in the proliferation of intermediate precursor cells (van Praag et al., 1999). Interestingly, some forms of learning and memory behavioral paradigm, such as trace eye-blink conditioning and Morris water maze, two hippocampal dependent tasks, increase the number of newly generated neurons (Leuner et al., 2006). In contrast, hippocampal-independent learning tasks, such as delay eye-blink conditioning and active shock avoidance, have little to no effect on neurogenesis (Gould et al., 1999; Leuner et al., 2006). An interesting example comes from the study of interaction of spatial learning and hippocampal neurogenesis (Dupret et al., 2007). The acquisition of spatial memory appears to have a differential effect on the survival of newborn neurons at different developmental stages. When 7-d-old newborn neurons were monitored, the water maze paradigm elicits an increase in neurogenesis. However, when 3d-old newborn neurons were monitored, there is actually a decrease in survival due to increased apoptosis. This study demonstrates that spatial learning can selectively stabilize a population of new neurons, and this tightly regulated sculpture of hippocampal network is in turn important for memory formation. Similarly, LTP induction in the hippocampus, which is thought to be associated with the mechanism for memory formation and retention, has been shown to increase dentate progenitor proliferation and promote survival of new neurons of 1-2 weeks of cell age (Bruel-Jungerman et al., 2006).
Hippocampal neurogenesis is also affected by aging, seizures, and antidepressant treatments. For example, seizure activity induces aberrant proliferation of both intermediate neural progenitors and neuroblasts, and results in abnormal morphological development and ectopic migration of newborn granule cells (Overstreet-Wadiche et al., 2006; Parent, 2007). Furthermore, this seizure-induced production of newborn neurons can integrate into the hippocampal circuitry and contribute to seizure-associated cognitive deficits. A drastic reduction of adult hippocampal neurogenesis is known to associate with aging, resulting from the depletion of NSCs, alterations in precursor properties, and changes in the hippocampal niche (Fabel and Kempermann, 2008). Antidepressants used in clinics have also been shown to regulate adult hippocampal neurogenesis by increasing neural progenitor proliferation, accelerating dendritic development, and enhancing survival of newborn neurons, presumably though indirect modulation of the niche (Warner-Schmidt and Duman, 2006; Sahay and Hen, 2007).
Molecular mechanisms underlying activity-dependent regulation of adult hippocampal neurogenesis
A critical question is how hippocampal network activity is translated into regulation of adult neurogenesis and which signals are responsible for driving the changes at different stages. SGZ NSCs and progenitor cells reside within a complex microenvironment and their behavior can be influenced by a plethora of modulatory factors from many brain areas through different neurotransmitters (Figure 1) - in particular, GABA and glutamate, the major inhibitory and excitatory neurotransmitters in the adult brain, respectively. The release of neurotransmitters is a direct outcome of network activity and can affect hippocampal neurogenesis via two forms: either through synaptic transmission, sometimes called “phasic activation” or through “tonic activation” (Ge et al., 2007). Tonic activation describes a phenomenon that the activation of receptors is triggered by an ambient level of neurotransmitter due to their diffusion from the synapses within the local microenvironment. Tonic signaling has distinct attributes that make it an important regulator of neurodevelopment. First, tonic signaling transcends individual pre-synaptic/post-synaptic cellular pairs, thus it provides a means for a spatial regulation that can influence cells located at some distance. Second, the ambient neurotransmitter level may represent an integrated signal to translate the overall local network activity. Indeed, several studies show that GABA is released from local interneurons and tonically activate progenitors and immature neurons (Ge et al., 2006; Platel et al., 2008). Interestingly, a recent study showed that both tonic and phasic GABA activation of neural progenitors and immature neurons are modulated by the chemokine stromal cell-derived factor 1 (SDF-1), which is co-released with GABA from local interneurons (Bhattacharyya et al., 2008; Kolodziej et al., 2008).
Glutamate signaling has long been implicated in regulating adult hippocampus neurogenesis. Injection of NMDA rapidly decreases cell proliferation in the adult rat DG, whereas injection of an NMDAR antagonist exhibits the opposite effect (Cameron et al., 1995; Nacher et al., 2001). On the other hand, induction of LTP at the glutamatergic medial perforant path-granule cell synapses promotes the proliferation of adult neural progenitors and survival of newborn neurons in a NMDAR-dependent fashion (Bruel-Jungerman et al., 2006; Chun et al., 2006). These findings highlight the complexity of glutamate signaling in regulating adult neurogenesis, which is likely to involve both cell autonomous effects in immature neurons and non-cell autonomous effects through existing neural circuits. Genetic deletion of NR1 in proliferating adult neural progenitors reduces the survival of their neuronal progeny 2 to 3 weeks after they are born (Tashiro et al., 2006). Interestingly, injection of an NMDAR antagonist diminishes differences in NMDA receptor signaling in all new neurons and promotes the survival of these NR1 knockdown neurons, suggesting a critical period for NMDAR-dependent competitive survival of newborn neurons in the adult brain. Of note, this critical period coincide with a transition from excitatory to inhibitory GABA signaling. Whether GABA cooperates with glutamate signaling in regulating the survival of new neurons during this critical period remains to be determined. Analysis of the plasticity of glutamatergic synaptic inputs onto newborn granule cells during their maturation process has identified another critical period during which recently born neurons exhibit enhanced LTP. New neurons within 4-6 weeks of birth exhibit both a reduced induction threshold and increased LTP amplitude in response to a physiological pattern of stimulation (Ge et al., 2007). Interestingly, this critical period is associated with developmentally regulated NR2B-containing NMDARs in adult-born neurons, since pharmacological inhibition of these receptors completely abolished LTP in these young neurons, but not in mature neurons.
Other neurotransmitters such as serotonin and dopamine also exert regulatory effects on adult SGZ neurogenesis (Figure 1). Serotonergic neurons originating from the dorsal raphe nuclei (DGN) send projections to the dentate gyrus and serotonergic signaling has been implicated in mood regulation (Jacobs et al., 2000; Suh et al., 2009). Increased serotonin transmission is positively linked to hippocampal neurogenesis, as evidenced by enhanced neurogenesis following antidepressant treatments that act on serotonin transporters (Malberg et al., 2000; Santarelli et al., 2003). Similar to serotonergic effects, denervation of dopaminergic neurons decreases proliferation of NSCs in the SGZ (Hoglinger et al., 2004). A recent study showed that dopamine is particularly effective in modulating the activities of hyper-excitable young neurons (but not mature neurons) by decreasing their capacity to express LTP, suggesting that dopamine may play a role in gating afferent information to the hippocampus (Mu et. al., 2011).
Diffusible molecules produced by local cells can also influence hippocampal neurogenesis. Growth factors, including neurotrophins and developmental cues, play critical roles in regulating NSCs and their progeny (Ma et al., 2005; Schmidt and Duman, 2007). Given the possibility that systemic diffusible factors might be involved in the activity-dependent control of adult neurogenesis, early efforts have been made to identify circulating factors that mediate enhanced neurogenesis by exercise. Two factors identified from these studies are insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF), both of which were shown to be necessary in mediating the exercise-induced increase of neurogenesis (Trejo et al., 2001; Fabel et al., 2003). Blocking either factor prevented the exercise-induced increase in SGZ neurogenesis. In addition, growth factors such as epidermal growth factor (EGF) and fibroblast growth factor 2 (FGF2) are critical for the maintenance of adult NSCs in vitro. Although infusion of FGF2 does not affect SGZ proliferation in young mice, deletion of fgfr1 (FGF receptor 1) in the CNS decreases SGZ neurogenesis, indicative of a permissive role of FGF2 signaling in SGZ proliferation (Zhao et al., 2007). Furthermore, conditional ablation of TrkB, the high-affinity receptor for BDNF, in both embryonic and adult-born NSCs led to an impairment of hippocampal neurogenesis and prevented behavioral improvements induced by chronic antidepressant administration or wheel-running (Rossi et al., 2006; Babu et al., 2009). In contrast, deleting TrkB in differentiated neurons of the same brain regions does not affect neurogenesis or the behavioral responses to antidepressants, supporting the notion that dentate NSCs are a required component in the amelioration of depression (Li et al., 2008). Other extrinsic factors, such as Wnts and their antagonists, VEGF, and the neuropeptide VGF, are likely to be regulated by various neuronal stimuli in the dentate to modulate activity-dependent neurogenesis (Schmidt and Duman, 2007).
Regulation of the hippocampal circuitry by newborn neurons
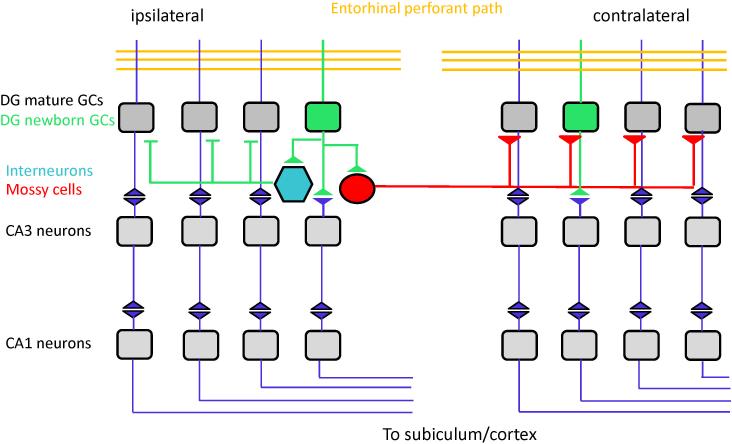
One fundamental question in adult neurogenesis is how addition of a small number of newborn neurons can affect global network activity and specific brain functions. Accumulating evidence supports the notion that newborn neurons do not merely replace lost neurons in the adult hippocampus; but are rather part of a continuously ongoing process that sculpt the existing neuronal circuitry of the adult brain as it responds to experiences encountered throughout life (Ge et al., 2008). Adult-born neurons exhibit a number of unique transient properties that are distinct from their neighboring mature neurons (Schmidt-Hieber et al., 2004; Ge et al., 2007). For instance, they display high-input resistance and activation of low threshold T-type Ca2+-channels which leads to enhanced excitability and generation of action potentials in response to weak excitatory inputs. A recent study using calcium imaging and electrophysiology demonstrated that immature neurons respond at higher rates to a given stimulus at both the single cell and population levels (Marin-Burgin et al., 2012). This increased intrinsic excitability of immature granule cells is coupled with a resistance to GABA-mediated inhibition to result in a lower activation threshold and the potential of this population to serve as an integrator of weak afferent signals. Furthermore, associative LTP can be induced more easily in young neurons than in mature neurons under identical conditions. The enhanced synaptic plasticity with increased LTP amplitude and decreased LTP induction threshold is present only within a fairly narrow time window, between 4-6 weeks of cell age. These unique properties may allow newborn neurons to function differently from their mature neighbors, thus bringing special properties to the local microcircuits, with or without changing the characteristics of their mature afferent and efferent neuronal targets. In addition to serve as an independent encoding unit, adult-born neurons may play an important role in modulate activity at the circuitry level. For example, adult-born dentate granule cells innervate tens of basket interneuron; each basket cell in turn inhibits hundreds of mature granule cells (Figure 3) (Freund and Buzsaki, 1996). Dentate granule neurons are also known to innervate hilar mossy cells, which in turn activate many mature granule cells contralaterally (Figure 3). In vivo recording from the adult mouse dentate gyrus showed that elimination of newborn neurons leads to a marked increase in the spontaneous gamma-frequency burst amplitude in the dentate gyrus and hilus, supporting a role of adult-born neurons in inhibiting recurrent network activity in the dentate gyrus, likely through regulation of interneurons (Lacefield et al., 2012).
Figure 3. Basic circuit architecture of adult hippocampus and a model on how new neurons impact the local circuitry.
Entorhinal cortical inputs innervate dentate gyrus granule cells, and dentate granule cells innervate CA3 neurons, which in turn innervate CA1 neurons. New granule cells innervate hilar interneurons, each of which inhibit hundreds of mature dentate granule cells. New granule cells also innervate hilar mossy cells, which activate many granule cells on the contralateral dentate gyrus. Through innervations of local interneurons and mossy cells, a single adult-born granule cell has the capacity to modulate the network activity at the circuitry level.
In supporting a functional role of adult-born neurons in hippocampal circuitry, numerous studies have shown a correlation between changes in hippocampal neurogenesis and effects on learning and memory. Early studies utilized two major approaches to reduce the number of adult-generated neurons: anti-mitotic drugs, such as methylazoxy - methonol (MAM), and X-irradiation (Shors et al., 2001; Snyder et al., 2005). Mice treated with MAM displayed obvious deficits in some but not all hippocampus-dependent tasks, such as trace eye-blink conditioning and trace fear conditioning, but not contextual fear conditioning (Shors et al., 2001). MAM treatment also prevents the long-term memory improvement normally induced by environmental enrichment (Bruel-Jungerman et al., 2005). Similarly, mice treated with cranial ionic irradiation reveal significant impairments in the acquisition of several hippocampus-dependent tasks, such as T-maze place recognition, spatial learning in Barnes maze, contextual fear conditioning, and nonmatching-to-sample (NMTS) tasks, whereas water maze spatial learning, and hippocampus-independent tasks were largely unaffected (Raber et al., 2004; Snyder et al., 2005; Saxe et al., 2006; Winocur et al., 2006). Though several learning deficits were identified in these studies, these acute manipulations are not anatomically restricted to the hippocampus and therefore nonspecific effects may account for some of the observed impairments. To eliminate the side effects associated with anti-mitotic drugs and irradiation, genetic approaches have been employed to manipulate adult neurogenesis. By exploiting transient expression of markers in developing adult-born neurons, it is possible to selectively ablate adult-born populations of cells in an inducible manner. Introducing herpes virus thymidine kinase (TK) under either the GFAP or nestin promoters, followed by treatment with the antiviral pro-drug, ganciclovir, results in a 50-75% reduction in neurogenesis and impairments in long-term spatial memory and contextual fear memory acquisition and extinction (Saxe et al., 2006; Deng et al., 2009). Using a similar approach to induce expression of the pro-apoptotic gene, Bax, under the nestin promoter resulted in a deficit in the acquisition of spatial relational memory, which was associated with a ~60% reduction in proliferating cells (Dupret et al., 2008). Interestingly, manipulating this system in the other direction, through suppression of endogenous Bax under the nestin promoter resulted in an expansion of the adult-born neuronal population and an enhancement in pattern separation in a contextual discrimination task (Sahay et al., 2011). Cumulative evidence thus suggests that adult hippocampal neurogenesis contributes significantly to long-term spatial memory retention, spatial pattern separation, trace conditioning and contextual fear conditioning, clearance of hippocampal memory traces, reorganization of memory to extrahippocampal substrates, and certain antidepressant-induced behavioral responses in specific mouse strains (Sahay and Hen, 2007; Deng et al., 2010; Aimone et al., 2011; Sahay et al., 2011). Collectively, these studies provide substantial evidence to support a functional role of adult hippocampal neurogenesis in learning and memory. In parallel with experimental evidence, a number of computational models of adult neurogenesis have provided further clues on how addition of new neurons may alter the global neural network properties and have suggested distinct roles of adult-born neurons in mediating information processing in the hippocampus (Aimone et al., 2011), which can guide the future experimental design for validation.
Adult neurogenesis has also been suggested to play a critical role in mood regulation, another hippocampal-associated higher brain function (Santarelli et al., 2003; Sahay and Hen, 2007). The potential impact of adult neurogenesis in pharmacological treatment of mood disorders was first demonstrated in a study showing that ablated SGZ neurogenesis by xirradiation prevents the ameliorative behavioral effects of antidepressants fluoxetine and imipramine (Santarelli et al., 2003). However, the dependence of behavioral effects of antidepressants on neurogenesis is complicated by variability in factors such as species, genetic background, nature of antidepressants, and behavioral protocols (Duman et al., 2001; Sahay and Hen, 2007; Surget et al., 2011). Therefore, it remains to be tested whether impaired hippocampal neurogenesis is an etiological factor for depression. Further studies are needed to test whether and to what extent the reduction in neurogenesis contributes to hippocampal pathology associated with depressed patients.
Concluding remarks
Rapid progress in the field over the past decade has led to a better understanding of the distinct developmental milestones of adult neurogenesis. Mounting evidence clearly demonstrate that adult-born hippocampal neurons possess unique electrophysiological features, making synaptic contacts with pre- and postsynaptic partners, responding to tonic and synaptic stimulation, and most importantly, integrating into the existing hippocampal network in an activity-dependent fashion. However, the physiological significance of these unique properties at the circuit level is much less clear. The exact manner by which adult NSCs and their progeny interact with and exert impact on the existing network in the adult brain remains poorly understood. To address these issues, future research targeting the specific contribution of newborn neurons at different developmental stages to the circuit and behavior will be of great importance. To dissect the functional contributions of newborn neurons to different brain functions, such as memory formation, consolidation, and retrieval, more refined behavioral paradigms with higher sensitivity and consistency are required. Future studies, using in vitro and in vivo electrophysiology and light-based network stimulation, will help interpreting the network properties associated with adult-born neurons. Finally, a better understanding of endogenous neurogenesis under physiological and pathological conditions may lead to the therapeutic interventions for various neurological diseases associated with neurodegeneration, aging, and depression.
Acknowledgements
This work was supported by grants from NIH (NS047344) to H.S., NIH (NS048271, HD069184) to G.L., postdoctoral fellowships from Life Sciences Research Foundation to J.S., and from Maryland Stem Cell Research Foundation to J.S. and K.C.
References
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Lattanzi D, Ciuffoli S, Agostini D, Bertini L, Stocchi V, Santi S, Cuppini R. Morpho-functional characterization of neuronal cells at different stages of maturation in granule cell layer of adult rat dentate gyrus. Brain Res. 2004;1017:21–31. doi: 10.1016/j.brainres.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Babu H, Ramirez-Rodriguez G, Fabel K, Bischofberger J, Kempermann G. Synaptic Network Activity Induces Neuronal Differentiation of Adult Hippocampal Precursor Cells through BDNF Signaling. Front Neurosci. 2009;3:49. doi: 10.3389/neuro.22.001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya BJ, Banisadr G, Jung H, Ren D, Cronshaw DG, Zou Y, Miller RJ. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. J Neurosci. 2008;28:6720–6730. doi: 10.1523/JNEUROSCI.1677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, Bick-Sander A, von der Behrens W, Kempermann G. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci. 2003;24:603–613. doi: 10.1016/s1044-7431(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26:5888–5893. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun SK, Sun W, Park JJ, Jung MW. Enhanced proliferation of progenitor cells following long-term potentiation induction in the rat dentate gyrus. Neurobiol Learn Mem. 2006;86:322–329. doi: 10.1016/j.nlm.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal J, Lagace DC. Visualization and genetic manipulation of adult neurogenesis using transgenic mice. Eur J Neurosci. 2011;33:1025–1036. doi: 10.1111/j.1460-9568.2011.07600.x. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Picchini AM, Moadel T, Sisti AC, Yamada A, Kimura S, Leonardo ED, Hen R. Experience dictates stem cell fate in the adult hippocampus. Neuron. 2011;70:908–923. doi: 10.1016/j.neuron.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau E, Montaron MF, Aguerre S, Abrous DN. Learning-induced survival of new neurons depends on the cognitive status of aged rats. J Neurosci. 2007;27:6037–6044. doi: 10.1523/JNEUROSCI.1031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Chang JH, Ge S, Faulkner RL, Kim JY, Kitabatake Y, Liu XB, Yang CH, Jordan JD, Ma DK, Liu CY, Ganesan S, Cheng HJ, Ming GL, Lu B, Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Malberg J, Nakagawa S. Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther. 2001;299:401–407. [PubMed] [Google Scholar]
- Dupret D, Fabre A, Dobrossy MD, Panatier A, Rodriguez JJ, Lamarque S, Lemaire V, Oliet SH, Piazza PV, Abrous DN. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5:e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Fabel K, Kempermann G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular Med. 2008;10:59–66. doi: 10.1007/s12017-008-8031-4. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci. 2003;23:9357–9366. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Ge S, Sailor KA, Ming GL, Song H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J Physiol. 2008;586:3759–3765. doi: 10.1113/jphysiol.2008.155655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh EL, Ma D, Ming GL, Song H. Adult neural stem cells and repair of the adult central nervous system. J Hematother Stem Cell Res. 2003;12:671–679. doi: 10.1089/15258160360732696. [DOI] [PubMed] [Google Scholar]
- Gong C, Wang TW, Huang HS, Parent JM. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. J Neurosci. 2007;27:1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Hastings N, Gould E. Erratum: rapid extension of axons into the CA3 region by adult-generated granule cells. J comp neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991206)415:1<144::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]; J Comp Neurol. 415:144. doi: 10.1002/(sici)1096-9861(19991206)415:1<144::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Romer B, Babu H, Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol. 2005;196:342–351. doi: 10.1016/j.expneurol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kohler C. Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J Comp Neurol. 1986;246:149–169. doi: 10.1002/cne.902460202. [DOI] [PubMed] [Google Scholar]
- Kolodziej A, Schulz S, Guyon A, Wu DF, Pfeiffer M, Odemis V, Hollt V, Stumm R. Tonic activation of CXC chemokine receptor 4 in immature granule cells supports neurogenesis in the adult dentate gyrus. J Neurosci. 2008;28:4488–4500. doi: 10.1523/JNEUROSCI.4721-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Reuter K, Steiner B, Brandt MD, Jessberger S, Yamaguchi M, Kempermann G. Subpopulations of proliferating cells of the adult hippocampus respond differently to physiologic neurogenic stimuli. J Comp Neurol. 2003;467:455–463. doi: 10.1002/cne.10945. [DOI] [PubMed] [Google Scholar]
- Kumamoto N, Gu Y, Wang J, Janoschka S, Takemaru KI, Levine J, Ge S. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat Neurosci (Epub ahead of print) 2012 doi: 10.1038/nn.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2012;22:106–116. doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mu Y, Gage FH. Development of neural circuits in the adult hippocampus. Curr Top Dev Biol. 2009;87:149–174. doi: 10.1016/S0070-2153(09)01205-8. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- Lugert S, Basak O, Knuckles P, Haussler U, Fabel K, Gotz M, Haas CA, Kempermann G, Taylor V, Giachino C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19:672–682. doi: 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Kim WR, Ming GL, Song H. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann N Y Acad Sci. 2009;1170:664–673. doi: 10.1111/j.1749-6632.2009.04373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DK, Ming GL, Song H. Glial influences on neural stem cell development: cellular niches for adult neurogenesis. Curr Opin Neurobiol. 2005;15:514–520. doi: 10.1016/j.conb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Burgin A, Mongiat LA, Pardi MB, Schinder AF. Unique Processing During a Period of High Excitation/Inhibition Balance in Adult-Born Neurons. Science (Epub ahead of print) 2012 doi: 10.1126/science.1214956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt S, Overstreet-Wadiche L. GABAergic signalling to adult-generated neurons. J Physiol. 2008;586:3745–3749. doi: 10.1113/jphysiol.2008.155713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt SJ, Wadiche JI, Overstreet-Wadiche LS. Input-specific GABAergic signaling to newborn neurons in adult dentate gyrus. J Neurosci. 2009;29:15063–15072. doi: 10.1523/JNEUROSCI.2727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J, Rosell DR, Alonso-Llosa G, McEwen BS. NMDA receptor antagonist treatment induces a long-lasting increase in the number of proliferating cells, PSA-NCAM-immunoreactive granule neurons and radial glia in the adult rat dentate gyrus. Eur J Neurosci. 2001;13:512–520. doi: 10.1046/j.0953-816x.2000.01424.x. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bromberg DA, Bensen AL, Westbrook GL. Seizures accelerate functional integration of adult-generated granule cells. J Neurosci. 2006;26:4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet LS, Hentges ST, Bumaschny VF, de Souza FS, Smart JL, Santangelo AM, Low MJ, Westbrook GL, Rubinstein M. A transgenic marker for newly born granule cells in dentate gyrus. J Neurosci. 2004;24:3251–3259. doi: 10.1523/JNEUROSCI.5173-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM. Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res. 2007;163:529–540. doi: 10.1016/S0079-6123(07)63028-3. [DOI] [PubMed] [Google Scholar]
- Platel JC, Dave KA, Bordey A. Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. J Physiol. 2008;586:3739–3743. doi: 10.1113/jphysiol.2008.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011;70:582–588. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisti HM, Glass AL, Shors TJ. Neurogenesis and the spacing effect: learning over time enhances memory and the survival of new neurons. Learn Mem. 2007;14:368–375. doi: 10.1101/lm.488707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Song H, Kempermann G, Overstreet Wadiche L, Zhao C, Schinder AF, Bischofberger J. New neurons in the adult mammalian brain: synaptogenesis and functional integration. J Neurosci. 2005;25:10366–10368. doi: 10.1523/JNEUROSCI.3452-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B, Klempin F, Wang L, Kott M, Kettenmann H, Kempermann G. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54:805–814. doi: 10.1002/glia.20407. [DOI] [PubMed] [Google Scholar]
- Suh H, Deng W, Gage FH. Signaling in Adult Neurogenesis. Annu Rev Cell Dev Biol. 2009 doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: a critical period during an immature stage. J Neurosci. 2007;27:3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Sandler VM, Toni N, Zhao C, Gage FH. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442:929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 2006;16:239–249. doi: 10.1002/hipo.20156. [DOI] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wiskott L, Rasch MJ, Kempermann G. A functional hypothesis for adult hippocampal neurogenesis: avoidance of catastrophic interference in the dentate gyrus. Hippocampus. 2006;16:329–343. doi: 10.1002/hipo.20167. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr., Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Li D, Shimazu K, Zhou YX, Lu B, Deng CX. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry. 2007;62:381–390. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]