Abstract
Severe childhood infections can occasionally be accompanied by bone marrow suppression. It is unusual for infection induced marrow aplasia to evolve into acute leukemia. We share our experience in managing four children with severe sepsis and pancytopenia which in due course evolved into acute leukemia. This report emphasizes that sepsis related pancytopenia can be a harbinger of evolving hematopoietic disorders.
Keywords: Infection associated pancytopenia, Children, Acute lymphoblastic leukemia, Spontaneous remission
Introduction
In about 2 % of cases of childhood acute lymphoblastic leukemia (ALL) clinical presentation of leukemia may be preceded by a transient, remitting phase of nonclassical aplastic anemia [1]. This latent period can be as long as 9 months, and occurs usually in association with an infection. Infections, predominantly bacterial are either the cause or effect of aplastic anemia. Bone marrows of patients with this prodromal aplastic phase of ALL may have features distinguishing them from true aplastic anemia which includes fibrosis, lymphocytic infiltration, and hypercellularity [2]. In this article we describe four patients who presented with sepsis and an infection associated aplastic prodrome prior to evolving into ALL.
Patients
Case 1
A 2.5 year male child presented with recurrent episodes (submandibular abscess and pneumonia) of infection over a period of 6 months. At initial presentation, hemoglobin: 6 g/dL, total leukocyte count: 1.1 × 109/L (too low for a differential count) and platelet count: 12 × 109/L. Each incident of infection had similar severe pancytopenia. Counts recovered to near normal levels during convalescence. Bone marrow (BM) examination done during pancytopenia was normal except for increased reticulin fibrosis (Table 1). After 6 months, child developed arthritis along with concomitant thrombocytopenia. A repeat BM examination at this time was consistent with a diagnosis of ALL.
Table 1.
Clinico hematological profile of patients
| Clinical presentation | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Age at presentation | 2.5 years | 3 years | 9.5 years | 5 years |
| Latent period | 6 months | 3 months | 1.5 months | 3 months |
| Focus of infection | Submandibular abscess and 2 episodes of pneumonia | Parapharyngeal abscess | Submandibular abscess | Maxillary and ethmoidal sinusitis |
| Organism cultured | Nil | Blood C/S E. Coli | Pus C/S-Pseudomonas aeruginosa | Aspergillus flavus |
| At presentation | ||||
| Lymphadenopathy/hepato splenomegaly | Hepatomegaly | Cervical lymphadenitis and tonsillitis | Splenomegaly | Axillary lymphadenopathy hepatosplenomegaly |
| BM findings during pancytopenia | ||||
| Cellularity | Hypocellular | Hypercellular | Hypo to normocellular | Hypocellular |
| Specific findings | Diffuse reticulin fibrosis | 1. Lymphoplasmacytoid cells—54 % 2. Promyelocytes—45 % | Lymphoid follicles diffuse reticulin fibrosis | Diffuse reticulin fibrosis |
C/S Culture and sensitivity, E. coliEscherichia coli
Case 2
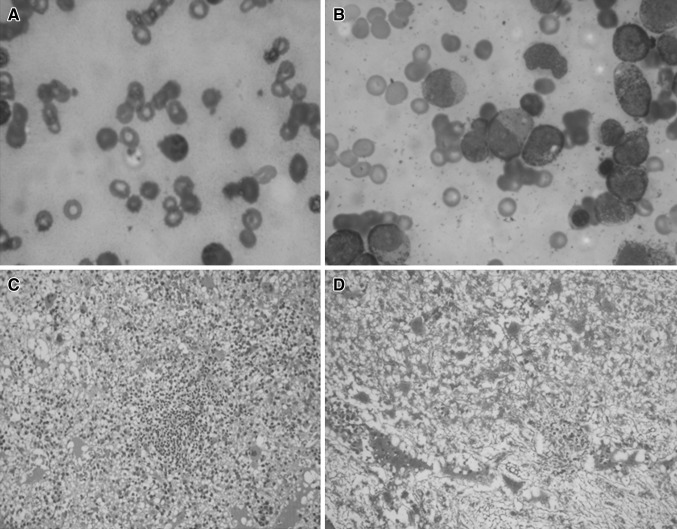
A 3 year male child presented with a parapharyngeal abscess. His hemogram showed hemoglobin: 8.6 gm/dL, total leukocyte count: 2.1 × 109/L, absolute neutrophil count (ANC): 378/mm3 and a platelet count: 13 × 109/L. The blood culture grew Escherichia coli. BM examination done initially revealed excess of lymphoplasmacytoid cells (54 %). A repeat marrow done after a month had a high promyelocytic count (45 %) (Fig. 1). Molecular tests for PML–RARA were negative. Three months later, child presented with fever and pancytopenia. Repeat BM examination revealed ALL–B cell lineage.
Fig. 1.
Bone marrow findings during the aplastic phase. A Case 2: lymphoplasmacytoid cell in bone marrow aspirate, B Case 2: high promyelocytic count in bone marrow aspirate, C Case 3: lymphoid follicle in bone marrow biopsy, D Case 3: marrow fibrosis in bone marrow biopsy (Reticulin stain)
Case 3
A 9.5 year male child presented with pallor, splenomegaly and had submandibular sialadenitis. The hemogram revealed hemoglobin: 6.9 gm/dL, total leukocyte count: 2.5 × 109/L, ANC-900/mm3 and a platelet count: 41 × 109/L. Pus grew Pseudomonas aeruginosa. BM aspiration revealed aplasia with increased marrow fibrosis and many lymphoid nodules. Counts recovered and splenomegaly regressed at discharge. A month later, child had recurrence of pancytopenia with fever. Repeat BM examination showed ALL–B cell lineage.
Case 4
A 5 year old boy presented with fever, epistaxis, hepatosplenomegaly and massive facial edema. He had a hemoglobin of 6.7 gm/dL, total leukocyte count: 1.5 × 109/L, ANC: 150/mm3 and a platelet count: 11 × 109/L. Due to airway compromise and suspected angioneurotic edema, child had received two doses of steroids. Imaging studies showed evidence of maxillary and ethmoidal sinusitis. Tissue from endoscopic debridement cultured Aspergillus flavus. BM examination revealed hypocellular marrow with myelofibrosis. Splenomegaly disappeared and counts recovered at the time of discharge. Six weeks later, hepatosplenomegaly reappeared along with thrombocytopenia, with blasts in peripheral smear. He was diagnosed to have ALL, B lineage.
Discussion
The above described cases presented with severe infections, predominantly bacterial [E. coli and P. aeruginosa], one child having fungal sinusitis [A. flavus]. During the period of acute infection, there was pancytopenia followed by recovery to near normal counts in convalescence. Bone marrow (BM) examination showed diffuse marrow fibrosis in three cases and a hypercellular marrow in one child. The marrow in case 2 had lymphoplasmacytoid cells and a high promyelocytic count during the period of pancytopenia. Case 3 had marrow fibrosis and many lymphoid nodules in the bone marrow biopsy (Fig. 1). All these children had received blood products (packed red cells, platelets) which were not leuko-reduced or irradiated. No granulocyte colony stimulating factors (G-CSF) were used.
Spontaneous remission of a malignancy was initially described in 1878. This has been attributed mainly to infections, hormonal factors, tumour necrosis, angiogenesis, apoptosis and surgical trauma. It has been described in hypernephroma, malignant melanoma, neuroblastomas, leukemias and non Hodgkin lymphomas [3]. It is estimated to occur in 1 in 60,000–100,000 cases of malignancies. Severe systemic infection induced suppression of the leukemic clone is described in acute leukemias [4, 6]. Infections resulting in an aplastic anemia like picture are usually bacterial [4]. Viral (parvovirus) and fungal (aspergillosis, pneumocystis jirovecii) etiology has also been described [5–7]. Preleukemic phase is usually short, lasting 2–9 months, although a prolonged phase of up to 36 months has been observed. At initial presentation, it might be difficult to distinguish this transient hypoplasia preceding leukemic presentation from true aplastic anemia. Presence of organomegaly and demonstration of an abnormal clone by molecular studies might help in differentiation. However, in most cases regular follow up may be the only answer to reveal the natural course of the disease.
Spontaneous remissions in acute leukemia are consistently associated with infections with or without use of G-CSF and transfusions [4, 8]. Cytokines released in the infection cascade which includes the tumor necrosis factor (TNF α &γ), interleukin-2 (IL-2), combined with increased activity of the NK cells, cytotoxic T cells and macrophages have a negative effect on leukemic cell proliferation and have been implicated in inducing spontaneous remission [9]. Similarly, natural killer cells and cytotoxic T cells transfused in blood products are thought to induce a graft versus leukemia effect in the recipient [4]. Growth factors stimulate the normal marrow clones, masking the leukemic clone [8].
The other postulated mechanism is an autoimmune response against the altered malignant stem cells which triggers an acute onset bone marrow failure like picture [10]. This anti neoplastic effect of autoimmunity leaves an empty marrow which if sampled earlier would have shown the primary disease. Tumor regression parallels the course of aplastic anemia. Induction and maintenance of this anti-tumor autoimmunity is probably T cell mediated and further research in this area might help us develop new biological agents augmenting endogenous anti-cancer potential.
Conclusions
This case series reiterates the dictum of keeping patients with pancytopenia on close monitoring, as hematopoietic malignancies can emerge with time.
Acknowledgments
Conflict of interest
There is no conflict of interest
References
- 1.Breatnach F, Chessells JM, Greaves MF. The aplastic presentation of childhood leukaemia: a feature of common-ALL. Br J Haematol. 1981;49:387–393. doi: 10.1111/j.1365-2141.1981.tb07241.x. [DOI] [PubMed] [Google Scholar]
- 2.Reid MM, Summerfield GP. Distinction between a leukaemic prodrome of childhood acute lymphoblastic leukaemia and aplastic anaemia. J Clin Pathol. 1992;45:697–700. doi: 10.1136/jcp.45.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papac RJ. Spontaneous regression of cancer. Cancer Treat Rev. 1996;22:395–423. doi: 10.1016/S0305-7372(96)90023-7. [DOI] [PubMed] [Google Scholar]
- 4.Muller CI, Trepel M, Kunzmann R, et al. Hematologic and molecular spontaneous remission following sepsis in acute monoblastic leukemia with translocation (9;11): a case report and review of the literature. Eur J Haematol. 2004;73:62–66. doi: 10.1111/j.1600-0609.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 5.Heegaard ED, Madsen HO, Schmiegelow K. Transient pancytopenia preceding acute lymphoblastic leukaemia (pre-ALL) precipitated by parvovirus B19. Br J Haematol. 2001;114:810–813. doi: 10.1046/j.1365-2141.2001.03021.x. [DOI] [PubMed] [Google Scholar]
- 6.Tzankov A, Ludescher C, Duba HC, et al. Spontaneous remission in a secondary acute myelogenous leukaemia following invasive pulmonary aspergillosis. Ann Hematol. 2001;80:423–425. doi: 10.1007/s002770100300. [DOI] [PubMed] [Google Scholar]
- 7.Fassas A, Sakellari I, Anagnostopoulos A, Saloum R. Spontaneous remission of acute myeloid leukemia in a patient with concurrent Pneumocystis carinii pneumonia. Nouv Rev Fr Hematol. 1991;33:363–364. [PubMed] [Google Scholar]
- 8.Takahashi M, Koike T, Aizawa Y, et al. Complete remission in three patients with acute myeloblastic leukemia by administration of G-CSF without antileukemic agents. Am J Hematol. 1997;56:42–44. doi: 10.1002/(SICI)1096-8652(199709)56:1<42::AID-AJH9>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Musto P, D’Arena G, Melillo L, et al. Spontaneous remission in acute myeloid leukaemia: a role for endogenous production of tumour necrosis factor and interleukin-2? Br J Haematol. 1994;87:879–880. doi: 10.1111/j.1365-2141.1994.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 10.Nissen C, Stern M. Acquired immune mediated aplastic anemia: is it antineoplastic? Autoimmun Rev. 2009;9:11–16. doi: 10.1016/j.autrev.2009.02.032. [DOI] [PubMed] [Google Scholar]