Abstract
Aims
To evaluate the incremental prognostic value of reserve-pulse pressure (reserve-PP: exercise-PP minus rest-PP) to standard risk factors among patients with suspected coronary artery disease (CAD) but normal exercise myocardial perfusion imaging (MPI).
Methods and results
We studied 4269 consecutive symptomatic patients without known CAD who were referred for exercise MPI but had normal MPI results (mean age 58 ± 12 years, 56% females, 84% referred for evaluation of chest pain or dyspnoea, 95% with intermediate pretest likelihood of CAD). There were 202 deaths over 5.1 ± 1.4 years of follow-up. Reserve-PP was abnormal (<44 mmHg increase in PP from rest) in 1894 patients (44%). Patients with an abnormal reserve-PP had a higher risk of death compared with patients with normal reserve-PP [hazard ratio (HR): 2.47, 95% CI, 1.8–3.3]. In multivariable models adjusting for age, sex, ejection fraction, medications, heart rate recovery, Duke treadmill score (DTS), and rest-PP, each 10 mmHg lower reserve-PP was associated with a 20.6% increase in risk-adjusted mortality (adjusted HR 0.83, 95% CI 0.76–0.91). Models incorporating reserve-PP significantly reclassified risk compared with models without these parameters (net reclassification index 14.3%, P = 0.0007; integrated discrimination index 0.69, P = 0.01).
Conclusion
In patients without a history of CAD and a normal MPI, an abnormal reserve-PP identified and reclassified those at higher risk of death independent of known risk factors and DTS.
Keywords: Myocardial perfusion imaging, Pulse pressure, SPECT, Prognosis, Normal
See page 2028 for the editorial comment on this article (doi:10.1093/eurheartj/eht138)
Introduction
Exercise myocardial perfusion imaging (MPI) with single photon emission computed tomography (SPECT) is an excellent tool to stratify risk of future cardiovascular events.1–3 Patients with a normal exercise MPI have an excellent prognosis with <1% of the patients with a normal scan experiencing adverse cardiac events (cardiac death or myocardial infarction) each year. However, in most laboratories the majority of the patients (∼60–70%) have normal or low-risk scans or treadmill test results. Hence, when we count the absolute number of adverse cardiac events, more events are observed in the normal MPI compared with the abnormal MPI group.4,5 Indeed, among patients who suffered an adverse cardiovascular event, 50% of them had a low risk MPI (normal or mildly abnormal MPI) and about 85% of patients had a low or intermediate risk Duke Treadmill Score (DTS).4,5 These findings highlight the fact that conventional tests of ischaemia such as exercise treadmill testing and MPI may underestimate a significant proportion of future clinical events, likely from failure to detect non-obstructive coronary artery disease (CAD). Several reports have suggested incremental risk stratification with measures of atherosclerosis such as calcium score in patients with normal MPI.6,7 The current study attempts to improve the risk stratification of symptomatic patients without known CAD and with a normal exercise MPI, using exercise-induced changes in pulse pressure (PP) as a novel risk marker.
Increased rest-PP is a marker of arterial stiffness, signifies underlying vascular disease, and predicts subsequent cardiovascular events.8 Under physiological conditions, there is a several fold increase in PP from rest to peak exercise (reserve-PP). This is likely mediated by the exercise-induced increase in stroke volume and decrease in peripheral vascular resistance that leads to an increase in arterial flow and increased shear stress on the endothelium with release of nitric oxide and vasodilation.9 Indeed, a suboptimal increase in PP with exercise has been described in older individuals, individuals with dyslipidaemia,10 and those with endothelial dysfunction (impaired brachial artery flow mediated dilation).11 These findings suggest that with dynamic exercise, suboptimal increase in PP may relate to vascular dysfunction from atherosclerosis.
To date, prior studies have not evaluated the incremental prognostic utility of changes in PP with exercise to standard risk factors for classifying risk of death after exercise MPI. We hypothesized that an abnormal reserve-PP (exercise-PP minus rest-PP) could add to existing parameters in identifying patients at high risk of death after a normal MPI. The primary objective of this study was to evaluate the incremental prognostic value of reserve-PP compared with conventional risk markers, including the DTS and heart rate recovery (HRR), among patients with suspected CAD but normal exercise MPI.
Methods
Study population
We evaluated 5417 consecutive patients with suspected CAD and a normal clinically indicated symptom-limited exercise Technetium-99m SPECT (2002–2006). Patients with known CAD (prior coronary revascularization or Q-wave myocardial infarction, n = 937, 17.3%) or a >10 mmHg decrease in systolic blood pressure during exercise (n = 12) were excluded (due to the concern of balanced ischaemia). Patients with left bundle branch block, paced rhythm or uninterpretable baseline electrocardiogram (n = 180, 3.3%), severe valvular disease (n = 84, 1.6%), patients on haemodialysis (n = 22, 0.4%), and those with <365 days follow-up (n = 51) were excluded. Some patients had overlapping exclusion criteria. The remaining 4269 patients comprised the study cohort for this analysis. The Partners human research committee (Boston, MA, USA) approved this study and waived the requirement of informed consent.
Exercise protocol
A structured interview and a chart review were performed for every patient prior to the exercise test, documenting medical history including symptoms, coronary risk factors, prior cardiac events and medications as well as height, and weight, into a database. The pre-test likelihood of CAD was calculated using the logistic-based formula developed and reported by Pryor et al.12
All patients underwent maximal symptom-limited treadmill exercise according to the Bruce protocol. Heart rate, blood pressure, and a 12-lead electrocardiogram were obtained before exercise, at the beginning of each stage, during the first minute of recovery, and then every minute for 5 min or until the electrocardiogram returned to baseline (in the presence of ST-segment changes or arrhythmia). Exercise endpoints included physical exhaustion, limiting symptoms of claudication, angina pectoris or dyspnoea, horizontal or down-sloping ST-segment depression ≥3 mm (80 ms after the J-point), ST-segment elevation >1 mm, sustained ventricular tachycardia, and exertional hypotension (≥10 mmHg decrease in systolic blood pressure). Workload in metabolic equivalents (METs) was estimated based on treadmill time.
Duke treadmill score was determined as previously described,13 duration of exercise in minutes − (5 × maximum ST-segment deviation in millimetres) − (4 × treadmill angina index).14 A DTS of <−5 (intermediate or high risk) was considered abnormal. Heart rate recovery was computed as peak exercise heart rate minus heart rate 1 min after exercise (with 1 min cool down at 1.7 mph) in 4218 patients. A HRR of ≤12 bpm was considered abnormal based on the prior literature.15
Pulse pressure
Systolic and diastolic blood pressure was measured manually before the test and during each exercise stage using an appropriate sized cuff and mobile aneroid sphygmomanometers (Welch Allen CE0297). Pulse pressure (arithmetic difference between the systolic and diastolic blood pressure) was computed at rest and at peak exercise. Reserve-PP was defined as PP at peak exercise (exercise-PP) minus PP at rest (rest-PP). A reserve-PP of <44 mmHg was considered abnormal based on receiver operator characteristic analysis for the prediction of mortality.
Myocardial perfusion imaging analysis
Patients underwent rest-stress Tc-99m sestamibi SPECT MPI per standard protocol16 Two experienced observers (S.D., M.D.C.) assessed all studies using the standard 17-segment model and a 5-point scoring system. Global summed scores were computed for the stress and rest images. The scan was considered to be normal if the summed stress score was ≤3. Left ventricular ejection fraction was analysed on the post stress images with commercially available software.
Study endpoint
The primary endpoint of this study was all-cause mortality, ascertained using the Partners Health Care Research Patient Data Registry (linked to the Social Security Death Index, updated 31 December 2009). The mean follow-up was 5.2 ± 1.4 years after the index MPI study.
Statistical analysis
Continuous variables are reported as means and standard deviations and compared using Student's t-test. Categorical variables are reported as proportions and compared using a Chi-square test. A two-sided P-value < 0.05 was considered significant. After verifying the proportionality assumption, univariable and multivariable Cox regression analyses were used to analyse the relation between exercise and reserve-PP with mortality. Because of the strong correlation between systolic blood pressure and PP, we stratified the baseline hazard rate by history of hypertension. We developed separate models with clinical variables (model 1), clinical variables + rest-PP (model 2), model 2 variables + exercise-PP (model 3), and model 2 variables + reserve-PP (model 4). The clinical multivariable Cox model included cardiovascular risk factors (age, sex, history of diabetes, smoking), medication use (diuretic or beta-blocker use), haemodynamics (rest systolic blood pressure), exercise stress parameters (peak heart rate, DTS, HRR), and left ventricular ejection fraction.
Model performance was assessed with the Akaike's Information Criterion (AIC, the model with lowest AIC value being the best fitting model), Harrell's c-statistic, net reclassification improvement (NRI),17 and integrated discrimination improvement (IDI).18 For the NRI and IDI, we right-censored the data and studied mortality risk within 3 years (minimum follow-up 3.12 years in all alive subjects) using a published SAS macro17 and categories of <1%, 1–3%, >3% over a 3 year follow-up (for NRI). The survival analyses were performed with the SAS software (version 9.12, SAS Institute, Cary, NC, USA). The R software was used to compute log hazard and age-adjusted Kaplan–Meier curves and Harrell's c-statistic.
Results
The mean age of the cohort was 58 years, with 56% women (Table 1). The majority of the patients (95%) had an intermediate pretest likelihood of CAD, referred for evaluation of suspected CAD. A total of 72% of patient had low DTS and another 28% intermediate DTS.
Table 1.
Baseline characteristics of patients with normal and abnormal reserve-pulse pressure
| Characteristics | Normal reserve-PP ≥ 44 mmHg | Abnormal reserve-PP < 44 mmHg | P-value |
|---|---|---|---|
| n = 2375 | n = 1894 | ||
| Age (years) mean ± SD | 56.4 ± 11 | 60.8 ± 12 | <0.001 |
| Female (%) | 50.4 | 63.6 | <0.001 |
| Body mass index (kg/m2) mean ± SD | 28.9 ± 5.8 | 27.8 ± 5.8 | <0.001 |
| Coronary risk factors | |||
| Hypertension (%) | 52.5 | 47.5 | <0.001 |
| Diabetes (%) | 14.2 | 17.2 | 0.004 |
| Dyslipidaemia (%) | 49.3 | 48.9 | 0.98 |
| Smoking (%) | 13.3 | 14.8 | 0.4 |
| Family history of premature CAD (%) | 41.9 | 38.8 | 0.01 |
| Post-menopausal (%) | 27.7 | 44.4 | <0.001 |
| Medications | |||
| Beta-blockers (%) | 28.8 | 40.9 | <0.001 |
| Calcium channel blockers (%) | 10.1 | 12.6 | 0.06 |
| ACEI inhibitors (%) | 24.0 | 25.6 | 0.36 |
| Aspirin (%) | 44.2 | 45.9 | 0.12 |
| Diuretics (%) | 17.2 | 21.8 | 0.008 |
| Insulin (%) | 4.2 | 5.0 | 0.03 |
| Oral hypoglycaemic agents (%) | 6.7 | 7.7 | 0.15 |
| Lipid lowering agents (%) | 39 | 40 | 0.43 |
| Reason for test | |||
| Chest pain (%) | 57.2 | 58.5 | 0.39 |
| Dyspnoea (%) | 23.6 | 28.5 | 0.005 |
| Preoperative evaluation (%) | 5.3 | 7.3 | 0.02 |
| LVEF | 67 ± 10.6 | 68.6 ± 11.2 | <0.001 |
| Haemodynamics | |||
| Rest heart rate (bpm) | 68.7 ± 12 | 70.4 ± 13.3 | <0.001 |
| Rest systolic BP (mmHg) | 124.5 ± 15.7 | 131.0 ± 18.8 | <0.001 |
| Rest diastolic BP (mmHg) | 77.2 ± 9 | 76 ± 9.9 | <0.001 |
| Rest-PP (mmHg) | 47.5 ± 12.7 | 55.1 ± 16 | <0.001 |
| Peak exercise heart rate (bpm) | 147.88 ± 18.5 | 139.3 ± 19.5 | <0.001 |
| Peak systolic BP (mm Hg) | 185.1 ± 19.2 | 163.0 ± 19.7 | <0.001 |
| Peak diastolic BP (mm Hg) | 77.2 ± 10.1 | 77.8 ± 9.9 | 0.02 |
| Exercise-PP (mmHg) | 109.2 ± 16.4 | 86 ± 16.8 | <0.001 |
| Heart rate recovery (bpm) | 19.2 ± 10.7 | 16.5 ± 11.4 | <0.001 |
| Duke treadmill score | 7.4 ± 4.1 | 5.6 ± 4.1 | <0.001 |
| Metabolic equivalents (METs) | 10.1 ± 3.5 | 8.3 ± 3.1 | <0.001 |
| Reserve-PP (mm Hg) | 60.4 ± 12.2 | 29.9 ± 10.9 | <0.0001 |
CAD, coronary artery disease; ACE, angiotensin converting enzyme; BMI, body mass index; LVEF, left ventricular ejection fraction; BP, blood pressure; PP, pulse pressure.
Compared with patients with a normal reserve-PP, those with an abnormal reserve-PP (<44 mm Hg) were older, more likely to be women, and had lower BMI, but they had a higher percentage of hypertension, diabetes, and use of beta-blockers and diuretics (Table 1). Patients with an abnormal reserve-PP were more likely to have dyspnoea as the test indication and a higher baseline left ventricular ejection fraction.
Haemodynamics and exercise test characteristics
Compared with patients who had a normal reserve-PP, those with an abnormal reserve-PP had higher risk exercise test characteristics, such as a higher rest heart rate and lower values of HRR, DTS, maximal workload, and maximal heart rate attained (Table 1). Patients with an abnormal reserve-PP had a higher rest-PP as well as a lower exercise-PP compared with those with a normal reserve-PP.
Reserve- pulse pressure and mortality
Overall, there were 202 deaths (4.7%) over a mean follow-up of 5.2 ± 1.4 years (annualized mortality of 0.92%). In patients with HRR data (n = 4218) there were 190 deaths, and this cohort was used for the multivariable models. About two-thirds of the patients who died (132/202, 65.3%) had an abnormal reserve-PP. Patients with an abnormal reserve-PP had a significantly higher unadjusted mortality risk compared with patients with abnormal reserve-PP [hazard ratio (HR): 2.47, 95% CI, 1.8–3.3].
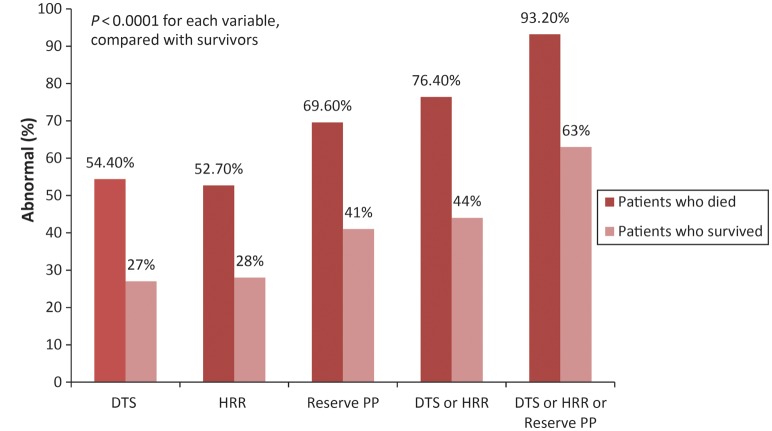
The proportion of subjects with abnormal DTS, HRR, reserve PP, or any one or more abnormal parameters was significantly higher in patients who subsequently died compared with survivors (P < 0.0001 for each comparison, Figure 1). Conventional exercise parameters of DTS and HRR demonstrated a large detection gap in identifying patients who died (Figure 1). Of the patients who subsequently died, only 54.4% of them had an abnormal DTS (83/158), 52.7% (78/148) had an abnormal HRR, but 69.6% (110/158) had an abnormal reserve PP during their index stress test. When abnormal DTS or abnormal HRR were considered a greater proportion of patients with events were identified (76.4%, 113/148). When one of the three parameters of DTS, HRR, and reserve-PP was abnormal, only a few deaths (6.8%, n = 10) were missed by using the combination of any one of the three abnormal parameters.
Figure 1.
The proportion of patients with abnormal reserve pulse pressure (PP), Duke treadmill score (DTS), and heart rate recovery (HRR) alone or when considered together (either DTS or HRR abnormal or either DTS, HRR, or reserve-PP abnormal) was significantly greater among the 158 patients who died compared with the 4111 survivors.
Univariable correlates of mortality
In this cohort, older age, male gender, smoking, lower left ventricular ejection fraction, diuretic use, lower DTS, lower HRR, higher rest systolic blood pressure, higher rest-PP, lower peak PP, and a lower reserve-PP were all significant univariable predictors of mortality (Table 2). Kaplan–Meier survival curves adjusted for age show significantly better survival in patients with a normal reserve-PP compared with those with abnormal reserve-PP (Figure 2).
Table 2.
Univariable predictors of mortality
| Patient characteristics (n) | Hazard ratio (95% CI) | P-value |
|---|---|---|
| Demographics | ||
| Agea | 2.05 (1.77–2.36) | <0.001 |
| Female | 0.53 (0.38–0.72) | <0.001 |
| Diabetes | 1.06 (0.69–1.63) | 0.78 |
| Hypertension | 1.18 (0.86–1.61) | 0.31 |
| Smoking | 1.53 (1.03–2.26) | 0.04 |
| LVEFa | 0.77 (0.66–0.89) | <0.001 |
| Medications | ||
| Beta-blocker use | 1.22 (0.88–1.68) | 0.23 |
| Diuretic use | 1.48 (1.04–2.12) | 0.03 |
| Exercise parameters | ||
| DTS, per unit change | 0.90 (0.88–0.93) | <0.001 |
| HRR, per unit change | 0.95 (0.93–0.96) | <0.001 |
| Blood pressure parameters | ||
| Rest SBPa | 1.15 (1.06–1.25) | 0.001 |
| Rest-PPa | 1.12 (1.11–1.13) | 0.04 |
| Peak-PPa | 0.84 (0.78–0.91) | <0.001 |
| Reserve-PPa | 0.75 (0.70–0.81) | <0.001 |
LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; PP, pulse pressure; DTS, Duke treadmill score; HRR, heart rate recovery.
aPer 10 unit change in variable.
Figure 2.
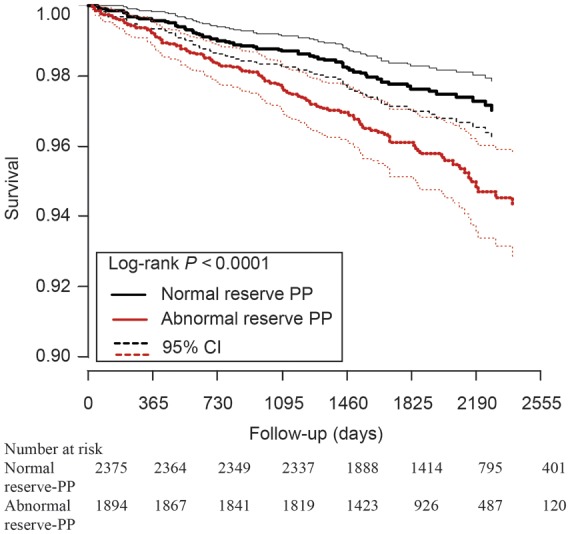
Patients with normal reserve-pulse pressure (PP) have better survival compared with those with abnormal reserve-PP. PP, pulse pressure. The three lines represent mean survival and 95% CI (confidence intervals).
Multivariable Cox regression analysis to predict risk-adjusted mortality
We developed four separate models to evaluate the independent and incremental value of rest-PP, exercise-PP, and reserve-PP over known clinical and exercise risk markers in predicting mortality (Table 3). Model 1 incorporating clinical risk factors, medications, left ventricular ejection fraction, DTS, and HRR was the referent model with several independent predictors of mortality (Table 3). After adjustment for the model 1 covariates, rest-PP (HR 0.84; 95% confidence interval, 0.70–1.01; P = 0.07) was only a borderline significant predictor of mortality (model 2). Exercise-PP and reserve-PP were independent predictors of mortality (HR 0.83; 95% CI, 0.76–0.91; P < 0.0001) (models 3 and 4). Each 10 mmHg lower exercise-PP and reserve-PP was associated with a 20.6% (95% CI) higher risk-adjusted mortality. Models 3 and 4 were identical, except that rest-PP was not an independent predictor of mortality in model 3. We found no significant interactions between sex and LVEF, or between age and rest-PP, exercise-PP or reserve-PP.
Table 3.
Multivariable predictors of mortality
| Parameter | Model 1 |
Model 2 |
Model 3 |
Model 4 |
||||
|---|---|---|---|---|---|---|---|---|
| NRI, P-value | 3.0%, P = 0.25 | 14.3%, P = 0.0007 | 14.3%, P = 0.0007 | |||||
| IDI, P-value | 0.13, P = 0.13 | 0.69, P = 0.01 | 0.69, P = 0.01 | |||||
| C-statistic | 0.77 | 0.76 | 0.78 | 0.78 | ||||
| AIC |
2403 |
2402 |
2386 |
2386 |
||||
| |
HR (95% CI) |
P-value |
HR (95% CI) |
P-value |
HR (95% CI) |
P-value |
HR (95% CI) |
P-value |
| Age | 1.67 (1.41–1.97) | <0.0001 | 1.72 (1.45–2.04) | <0.0001 | 1.69 (1.43–1.99) | <0.0001 | 1.69 (1.43–1.99) | <0.0001 |
| Female | 0.66 (0.47–0.93) | 0.02 | 0.66 (0.47–0.94) | 0.02 | 0.61 (0.43–0.86) | 0.01 | 0.61 (0.43–0.86) | 0.01 |
| Diabetes | 0.89 (0.59–1.34) | 0.58 | 0.93 (0.62–1.41) | 0.74 | 0.96 (0.64–1.46) | 0.86 | 0.96 (0.64–1.46) | 0.86 |
| LVEF | 0.76 (0.66–0.87) | <0.0001 | 0.76 (0.66–0.87) | <0.0001 | 0.78 (0.68–0.89) | 0.0003 | 0.78 (0.68–0.89) | 0.0003 |
| Diuretic | 1.32 (0.92–1.90) | 0.13 | 1.31 (0.92–1.89) | 0.14 | 1.27 (0.88–1.83) | 0.20 | 1.27 (0.88–1.83) | 0.20 |
| Beta-blocker | 0.78 (0.55–1.09) | 0.14 | 0.78 (0.55–1.09) | 0.15 | 0.71 (0.51–1.01) | 0.06 | 0.71 (0.51–1.01) | 0.06 |
| Rest SBP | 0.97 (0.88–1.06) | 0.46 | 1.09 (0.93–1.28) | 0.28 | 1.14 (0.97–1.33) | 0.12 | 1.14 (0.97–1.33) | 0.12 |
| Duke treadmill score | 0.94 (0.90–0.97) | 0.0007 | 0.94 (0.90–0.97) | 0.0007 | 0.95 (0.91–0.99) | 0.01 | 0.95 (0.91–0.99) | 0.01 |
| Peak heart rate | 0.86 (0.78–0.94) | 0.002 | 0.85 (0.77–0.94) | 0.001 | 0.87 (0.79–0.96) | 0.004 | 0.87 (0.79–0.96) | 0.004 |
| Heart rate recovery | 0.96 (0.95–0.98) | <0.0001 | 0.96 (0.95–0.98) | <0.0001 | 0.97 (0.95–0.98) | <0.0001 | 0.97 (0.95–0.98) | <0.0001 |
| Smoking | 1.51 (1.03–2.22) | 0.04 | 1.55 (1.05–2.28) | 0.03 | 1.58 (1.08–2.33) | 0.02 | 1.58 (1.08–2.33) | 0.02 |
| Rest-PP | 0.84 (0.70–1.01) | 0.07 | 0.92 (0.76–1.12) | 0.41 | 0.76 (0.63–0.92) | 0.01 | ||
| Exercise-PP | 0.83 (0.76–0.91) | <0.0001 | ||||||
| Reserve-PP | 0.83 (0.76–0.91) | <0.0001 | ||||||
The variables included in model 1 were: cardiovascular risk factors [age (10 year increments), gender, history of diabetes, smoking], medication use (diuretic and beta blocker use), haemodynamics (rest systolic blood pressure, 10 unit increments), exercise stress parameters (peak heart rate, DTS, HRR), and left ventricular ejection fraction (10 unit increments). Models 2, 3, and 4 included all the variables in model 1 and rest-PP, exercise-PP, and reserve-PP (10 unit increments), respectively. NRI and IDI were computed for the same models including events in 3.12 years of follow-up.
NRI, net reclassification improvement; IDI, integrated discrimination improvement; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure.
Addition of exercise-PP or reserve-PP to the base model (model 1) appropriately reclassified patients into lower or higher risk categories (Table 3, NRI, 14.3% P = 0.0007). Models incorporating METs instead of DTS were very similar with an NRI of 10.7%, P = 0.01 (Appendix Table A1); METs remained a significant independent predictor or mortality in addition to reserve-PP. Using IDI, a measure of model performance that does not depend on risk categories, the difference in average predicted probabilities between case (patients who died) and control patients (patients that were alive) increased by 0.69%, P = 0.01, when exercise or reserve PP was considered.
Finally, as shown in Figure 3, adjusted mortality risk was inversely and exponentially related to reserve-PP. In patients at the normal end of reserve-PP, risk was similar for patients with normal or abnormal DTS/ HRR. However, in patients at the abnormal end of reserve-PP, the curves diverge, suggesting that the risk is higher in patients with abnormal DTS and or HRR compared with those with normal DTS and HRR.
Figure 3.
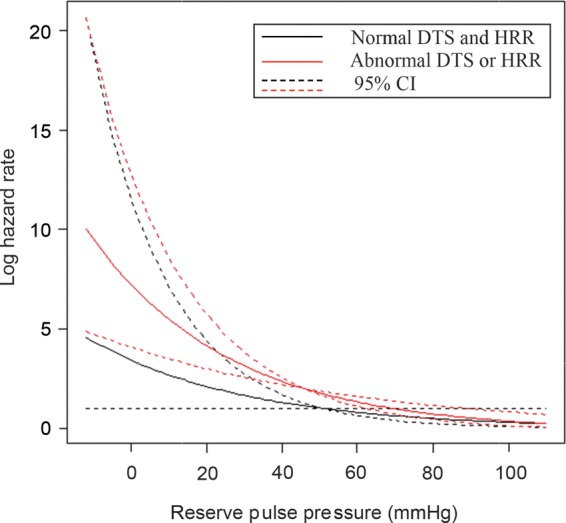
Mortality hazard increased with lower reserve-pulse pressure (PP). Patients with normal reserve PP showed low mortality hazard irrespective of Duke treadmill score (DTS) and heart rate recovery (HRR). However, among patients with abnormal reserve PP, those with abnormal HRR or DTS had a higher hazard compared with those with normal HRR and DTS.
Discussion
Reducing mortality from cardiovascular disease is an important health care goal, but risk stratification with existing strategies of ischaemia evaluation is suboptimal.4 Despite excellent prognosis with a normal MPI, a small though not inconsequential proportion of events occur in patients with normal/mildly abnormal MPI.4 In the current study, we evaluated a simple risk marker, reserve-PP, to further risk stratification of patients with normal MPI. In this cohort, ∼24% of patients who died were not identified as high risk by DTS and HRR. However, by adding reserve-PP to DTS and HRR, identification of patients at risk of mortality improved significantly, with only ∼7% of deaths not identified by the combination of normal DTS, HRR, and reserve-PP. On multivariable analysis, reserve-PP and exercise-PP were significant independent predictors of mortality and improved risk reclassification in 14.3% of patients when compared with models without reserve-PP or exercise-PP information. Each 10 mm lower reserve-PP conferred a 20.6% higher risk-adjusted mortality.
Comparison to prior exercise studies
The relationship between rest-PP,19–21 MPI,5 DTS,5 HRR,15,22,23 and cardiovascular risk, respectively, have been independently examined previously. A 10 mmHg increment in rest-PP was associated with an increase in the risk of coronary heart disease (12%), congestive heart failure (14%), and mortality (6%) (15). Likewise, abnormal DTS14 and HRR15 are established predictors of mortality. Few studies have evaluated changes in PP during exercise. In one study, adolescent offspring of hypertensive patients demonstrated diminished PP responses to psychological stress.24 In another study of 20 athletes, exercise-PP was directly related to physiological left ventricular hypertrophy and VO2 max, suggesting that a higher exercise-PP may be physiological and protective.25 In the Framingham Heart Study, dynamic blood pressure response and exercise diastolic blood pressure were incremental to rest BP information for predicting the risk of incident cardiovascular disease, but that study did not specifically evaluate changes in PP with exercise.26
To the best of our knowledge, this is the first study evaluating the value of reserve-PP to further risk stratify patients with normal MPI. A decrease in systolic blood pressure ≥10 mmHg, a flat blood pressure response, and a peak systolic blood pressure of <120 mmHg during dynamic exercise are known high risk markers.27 Our results extend these prior observations by showing that a failure to increase PP with exercise portends worse prognosis in certain patients.
Comparison to prior imaging studies
Also, patients with prior CAD, a high DTS, undergoing pharmacologic stress testing, elderly and female diabetics have higher event rates despite a normal MPI.5,28 Our study findings extend those results by demonstrating that an abnormal reserve-PP identifies a group at high risk of mortality despite no apparent CAD, a low/intermediate risk DTS, normal HRR, and independent of functional capacity. Markers of atherosclerosis such as coronary calcium score6,7,29 or imaging markers such as transient cavity dilation, increased lung uptake30,31 improve risk stratification of patients with normal MPI. Likewise, it is unknown if abnormal reserve-PP provides similar prognostic information as an abnormal coronary artery calcium score in patients with normal MPI needs to be further explored. Finally, the relatively high mortality in patients with an abnormal reserve-PP despite normal MPI could be mediated via atherosclerotic risk factors and non-obstructive CAD, balanced ischaemia (extensive CAD with normal MPI), coronary microvascular dysfunction, or complications arising from systemic vascular dysfunction. Although myocardial blood flow quantitation using PET MPI can better identify these factors, it can be challenging with exercise stress and PET MPI is not as widely available, making reserve-PP an attractive option. Also, vasodilator coronary flow reserve on MPI reflects a combination of endothelium independent and endothelium dependent flow abnormalities. In healthy adults, PP measures correlate inversely with global endothelial function and measures of central artery stiffness and augmentation index.32 Whether abnormal reserve-PP provides similar prognostic information as an abnormal endothelial function in patients with normal MPI is not known. The greatest value of reserve-PP appears to be in identifying the lowest risk patients, particularly when used in conjunction with conventional risk markers such as DTS, HRR, and workload (METs).
Potential mechanistic links between reserve-pulse pressure and mortality
The precise mechanistic links between lower reserve-PP and mortality cannot be determined from this study. Abnormal central arterial compliance is related to cardiovascular risk factors and predicts adverse cardiovascular outcomes.33 Rest-PP reflects the state of vascular compliance.34,35 Exercise-PP is related to higher cardiac output (by higher heart rate and stroke volume) and relative arterial compliance during exercise. Therefore, diminished reserve-PP may reflect abnormalities in exercise-induced left ventricular stroke volume (ischaemic myocardial dysfunction, heart failure) or arterial compliance and may indicate a mismatch between exercise-induced stroke volume and arterial compliance (impediment to stroke output, chamber, or vascular stiffness). Also, vascular compliance decreases with age and with hypertension and as such increasing age and heart failure from uncontrolled hypertension may be another link between abnormal PP response and mortality. Impaired baroreceptor sensitivity in the elderly and those with hypertension is associated with increased arterial stiffness33 and can result in inadequate PP response with exercise.
Thus, reserve-PP likely represents a functional measure of overall cardiac and vascular health obtained during routine treadmill testing.
Study strengths and limitations
This was observational, single-centre study, although large in size and prospective in design. Since abnormal PP signifies vascular disease, we felt that all-cause mortality was a more appropriate endpoint than cardiac specific mortality, and it was also less subject to bias. We examined symptomatic patients referred for evaluation of suspected CAD, and therefore these results and the cutoff values for normal reserve-PP from this study are most applicable to similar individuals. Assessment of peak exercise diastolic blood pressure may be difficult. But, we routinely report peak exercise diastolic blood pressure in clinical practice, and it has also been used in research studies.36,37 The majority of the patients in our cohort were treated with anti-hypertensive medications that may affect aortic stiffness and endothelial function and alter the true relationship between these physiologic markers. The widespread use of anti-hypertensive medications in the general population makes this limitation difficult to avoid. Finally, because of PP amplification38 measures of brachial artery, PP cannot be extrapolated to indicate central arterial stiffness; however, the requirement of aortic catheterization limits the widespread clinical use of measures of central arterial stiffness. Despite known limitations, peripheral PP measures are easily available and appear to be excellent tools for risk stratification.
Conclusions
In patients with suspected CAD and normal MPI, suboptimal reserve-PP is a simple and useful marker of risk that provides incremental prognostic value by appropriately reclassifying a significant proportion of subjects with normal MPI into higher or lower risk of mortality. The ease of measurement without adding cost makes reserve-PP a particularly attractive risk marker in this era where containing costs and accurate risk assessment is a major priority.
Acknowledgements
The authors are indebted to the patients and the dedicated exercise physiologists at the Brigham and Women's hospital for making this study possible. The authors thank Shawn Murphy and Henry Chueh and the Partners Health Care Research Patient Data Registry group for facilitating the use of their database.
Appendix
Appendix: Table I.
Value of Reserve Pulse Pressure in Improving the Risk Stratification of Patients With Normal Myocardial Perfusion Imaging
| Appendix Table 1: Models Including Exercise and Reserve Pulse Pressure and METS instead of Duke Score | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Model 1 | Model 2 | Model 3 | Model 4 | ||||
| NRI, P-value | 1.3%, P = 0.54 | 10.7%, P = 0.01 | 10.7%, P = 0.01 | |||||
| IDI, P-value | 0.10, P = 0.15 | 0.52, P = 0.07 | 0.52, P = 0.07 | |||||
| C-statistic | 0.78 | 0.78 | 0.80 | 0.79 | ||||
| AIC | 2340 | 2340 | 2331 | 2331 | ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 1.46 (1.24–1.72) | <.0001 | 1.50 (1.27–1.77) | <.0001 | 1.48 (1.25–1.75) | <.0001 | 1.48 (1.25–1.75) | <.0001 |
| Female | 0.47 (0.33–0.67) | <.0001 | 0.47 (0.33–0.68) | <.0001 | 0.45 (0.32–0.65) | <.0001 | 0.45 (0.32–0.65) | <.0001 |
| Diabetes | 0.81 (0.54–1.23) | 0.33 | 0.85 (0.56–1.28) | 0.43 | 0.88 (0.58–1.33) | 0.53 | 0.88 (0.58–1.33) | 0.53 |
| LVEF | 0.79 (0.69–0.91) | 0.0008 | 0.79 (0.69–0.91) | 0.0009 | 0.81 (0.70–0.93) | 0.002 | 0.81 (0.70–0.93) | 0.002 |
| Diuretic | 1.23 (0.85–1.77) | 0.27 | 1.23 ( 0.85–1.77) | 0.28 | 1.21 (0.83–1.74) | 0.32 | 1.21 (0.83–1.74) | 0.32 |
| Betablocker | 0.87 (0.62–1.23) | 0.43 | 0.87 (0.62–1.23) | 0.43 | 0.81 (0.57–1.14) | 0.22 | 0.81 (0.57–1.14) | 0.22 |
| Rest SBP | 0.97 (0.89–1.06) | 0.44 | 1.07 (0.91–1.26) | 0.39 | 1.11 (0.94–1.30) | 0.21 | 1.11 (0.94–1.30) | 0.21 |
| METS | 0.79 (0.74–0.85) | <.0001 | 0.79 (0.74–0.85) | <.0001 | 0.81 (0.76–0.87) | <.0001 | 0.81 (0.76–0.87) | <.0001 |
| Peak heart rate | 0.92 (0.84–1.01) | 0.09 | 0.91 (0.83–1.01) | 0.07 | 0.92 (0.83–1.01) | 0.08 | 0.92 (0.83–1.01) | 0.08 |
| Heart rate recovery | 0.97 (0.96–0.99) | 0.0007 | 0.97 (0.96–0.99) | 0.0006 | 0.97 (0.96–0.99) | 0.0009 | 0.97 (0.96–0.99) | 0.0009 |
| Smoking | 1.37 (0.93–2.02) | 0.11 | 1.40 (0.95–2.06) | 0.09 | 1.43 (0.97–2.10) | 0.08 | 1.43 (0.97–2.10) | 0.08 |
| ST Depression | 1.12 (0.56–2.21) | 0.75 | 1.14 (0.58–2.26) | 0.71 | 1.14 (0.58–2.26) | 0.71 | 1.14 (0.58–2.26) | 0.71 |
| Rest PP | 0.87 (0.72–1.04) | 0.13 | 0.93 (0.77–1.12) | 0.45 | 0.86 (0.79–0.94) | 0.001 | ||
| Exercise PP | 0.86 (0.79–0.94) | 0.001 | ||||||
| Reserve PP | 0.86 (0.79–0.94) | 0.001 | ||||||
The variables included in the model 1 were: cardiovascular risk factors [age (10 year increments), gender, history of diabetes, smoking], medication use (diuretic & beta blocker use), hemodynamics (rest systolic blood pressure, 10 unit increments), exercise stress parameters (peak heart rate, DTS, HRR) and left ventricular ejection fraction (10 unit increments). Models 2, 3 and 4 included all the variables in model 1 and rest-PP, exercise-PP and reserve-PP (10 unit increments), respectively. NRI and IDI were computed for the same models including events in 3.12 years of follow-up.
NRI, net reclassification improvement; IDI, integrated discrimination improvement; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure.
Funding
This work was supported by the National Institutes of Health grant K23HL092299. S.D. was supported by NIH, Grant number: 5K23HL92299-3.
Conflict of interest: S.D has board membership in Astellas, consultant for Astellas, Bracco Diagnostics, received grants from Astellas.
References
- 1.Fox K, Garcia MA, Ardissino D, Buszman P, Camici PG, Crea F, Daly C, De Backer G, Hjemdahl P, Lopez-Sendon J, Marco J, Morais J, Pepper J, Sechtem U, Simoons M, Thygesen K, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Osterspey A, Tamargo J, Zamorano JL. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27:1341–1381. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 2.Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 3.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR, Jr, Smith SC, Jr, Spertus JA, Williams SV. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Pasternak RC, Abrams J, Greenland P, Smaha LA, Wilson PW, Houston-Miller N. 34th Bethesda Conference: Task force #1—identification of coronary heart disease risk: is there a detection gap? J Am Coll Cardiol. 2003;41:1863–1874. doi: 10.1016/s0735-1097(03)00358-9. [DOI] [PubMed] [Google Scholar]
- 5.Hachamovitch R, Berman DS, Kiat H, Cohen I, Cabico JA, Friedman J, Diamond GA. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93:905–914. doi: 10.1161/01.cir.93.5.905. [DOI] [PubMed] [Google Scholar]
- 6.Chang SM, Nabi F, Xu J, Peterson LE, Achari A, Pratt CM, Mahmarian JJ. The coronary artery calcium score and stress myocardial perfusion imaging provide independent and complementary prediction of cardiac risk. J Am Coll Cardiol. 2009;54:1872–1882. doi: 10.1016/j.jacc.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 7.Schenker MP, Dorbala S, Hong EC, Rybicki FJ, Hachamovitch R, Kwong RY, Di Carli MF. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: a combined positron emission tomography/computed tomography study. Circulation. 2008;117:1693–1700. doi: 10.1161/CIRCULATIONAHA.107.717512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dart AM, Kingwell BA. Pulse pressure—a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37:975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 9.Rowell LB, Brengelmann GL, Blackmon JR, Bruce RA, Murray JA. Disparities between aortic and peripheral pulse pressures induced by upright exercise and vasomotor changes in man. Circulation. 1968;37:954–964. doi: 10.1161/01.cir.37.6.954. [DOI] [PubMed] [Google Scholar]
- 10.Sharman JE, McEniery CM, Dhakam ZR, Coombes JS, Wilkinson IB, Cockcroft JR. Pulse pressure amplification during exercise is significantly reduced with age and hypercholesterolemia. J Hypertens. 2007;25:1249–1254. doi: 10.1097/HJH.0b013e3280be5911. [DOI] [PubMed] [Google Scholar]
- 11.Stewart KJ, Sung J, Silber HA, Fleg JL, Kelemen MD, Turner KL, Bacher AC, Dobrosielski DA, DeRegis JR, Shapiro EP, Ouyang P. Exaggerated exercise blood pressure is related to impaired endothelial vasodilator function. Am J Hypertens. 2004;17:314–320. doi: 10.1016/S0895-7061(03)01003-3. [DOI] [PubMed] [Google Scholar]
- 12.Pryor DB, Shaw L, McCants CB, Lee KL, Mark DB, Harrell FE, Jr, Muhlbaier LH, Califf RM. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993;118:81–90. doi: 10.7326/0003-4819-118-2-199301150-00001. [DOI] [PubMed] [Google Scholar]
- 13.Mark DB, Shaw L, Harrell FE, Jr, Hlatky MA, Lee KL, Bengtson JR, McCants CB, Califf RM, Pryor DB. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325:849–853. doi: 10.1056/NEJM199109193251204. [DOI] [PubMed] [Google Scholar]
- 14.Mark DB, Hlatky MA, Harrell FE, Jr, Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Ann Intern Med. 1987;106:793–800. doi: 10.7326/0003-4819-106-6-793. [DOI] [PubMed] [Google Scholar]
- 15.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. N Engl J Med. 1999;341:1351–1357. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 16.Dorbala S, Giugliano RP, Logsetty G, Vangala D, Mishra R, Crugnale S, Yang D, Di Carli MF. Prognostic value of SPECT myocardial perfusion imaging in patients with elevated cardiac troponin I levels and atypical clinical presentation. J Nucl Cardiol. 2007;14:53–58. doi: 10.1016/j.nuclcard.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 19.Domanski M, Mitchell G, Pfeffer M, Neaton JD, Norman J, Svendsen K, Grimm R, Cohen J, Stamler J. Pulse pressure and cardiovascular disease-related mortality: follow-up study of the Multiple Risk Factor Intervention Trial (MRFIT) JAMA. 2002;287:2677–2683. doi: 10.1001/jama.287.20.2677. [DOI] [PubMed] [Google Scholar]
- 20.Klassen PS, Lowrie EG, Reddan DN, DeLong ER, Coladonato JA, Szczech LA, Lazarus JM, Owen WF., Jr Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA. 2002;287:1548–1555. doi: 10.1001/jama.287.12.1548. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell GF, Moye LA, Braunwald E, Rouleau JL, Bernstein V, Geltman EM, Flaker GC, Pfeffer MA. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation. 1997;96:4254–4260. doi: 10.1161/01.cir.96.12.4254. [DOI] [PubMed] [Google Scholar]
- 22.Diaz LA, Brunken RC, Blackstone EH, Snader CE, Lauer MS. Independent contribution of myocardial perfusion defects to exercise capacity and heart rate recovery for prediction of all-cause mortality in patients with known or suspected coronary heart disease. J Am Coll Cardiol. 2001;37:1558–1564. doi: 10.1016/s0735-1097(01)01205-0. [DOI] [PubMed] [Google Scholar]
- 23.Nishime EO, Cole CR, Blackstone EH, Pashkow FJ, Lauer MS. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA. 2000;284:1392–1398. doi: 10.1001/jama.284.11.1392. [DOI] [PubMed] [Google Scholar]
- 24.Ewart CK, Kolodner KB. Diminished pulse pressure response to psychological stress: early precursor of essential hypertension? Psychosom Med. 1992;54:436–446. doi: 10.1097/00006842-199207000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Kasikcioglu E, Oflaz H, Akhan H, Kayserilioglu A, Umman S. Peak pulse pressure during exercise and left ventricular hypertrophy in athletes. Anadolu Kardiyol Derg. 2005;5:64–65. [PubMed] [Google Scholar]
- 26.Lewis GD, Gona P, Larson MG, Plehn JF, Benjamin EJ, O'Donnell CJ, Levy D, Vasan RS, Wang TJ. Exercise blood pressure and the risk of incident cardiovascular disease (from the Framingham Heart Study) Am J Cardiol. 2008;101:1614–1620. doi: 10.1016/j.amjcard.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Libby P, Bonow RO, Zipes DP. Braunwald's Heart Disease. 8th ed . Philadelphia: Saunders; 2008. [Google Scholar]
- 28.Hachamovitch R, Hayes S, Friedman JD, Cohen I, Shaw LJ, Germano G, Berman DS. Determinants of risk and its temporal variation in patients with normal stress myocardial perfusion scans: what is the warranty period of a normal scan? J Am Coll Cardiol. 2003;41:1329–1340. doi: 10.1016/s0735-1097(03)00125-6. [DOI] [PubMed] [Google Scholar]
- 29.Rozanski A, Gransar H, Wong ND, Shaw LJ, Miranda-Peats R, Polk D, Hayes SW, Friedman JD, Berman DS. Clinical outcomes after both coronary calcium scanning and exercise myocardial perfusion scintigraphy. J Am Coll Cardiol. 2007;49:1352–1361. doi: 10.1016/j.jacc.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 30.Abidov A, Bax JJ, Hayes SW, Hachamovitch R, Cohen I, Gerlach J, Kang X, Friedman JD, Germano G, Berman DS. Transient ischemic dilation ratio of the left ventricle is a significant predictor of future cardiac events in patients with otherwise normal myocardial perfusion SPECT. J Am Coll Cardiol. 2003;42:1818–1825. doi: 10.1016/j.jacc.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 31.Diamond JA, Makaryus AN, Sandler DA, Machac J, Henzlova MJ. Normal or near normal myocardial perfusion stress imaging in patients with severe coronary artery disease. J Cardiovasc Med (Hagerstown) 2008;9:820–825. doi: 10.2459/JCM.0b013e3282f88bc5. [DOI] [PubMed] [Google Scholar]
- 32.McEniery CM, Wallace S, Mackenzie IS, McDonnell B, Yasmin S, Newby DE, Cockcroft JR, Wilkinson IB. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48:602–608. doi: 10.1161/01.HYP.0000239206.64270.5f. [DOI] [PubMed] [Google Scholar]
- 33.Michas F, Manios E, Stamatelopoulos K, Koroboki E, Toumanidis S, Panerai RB, Zakopoulos N. Baroreceptor reflex sensitivity is associated with arterial stiffness in a population of normotensive and hypertensive patients. Blood Press Monit. 2012;17:155–159. doi: 10.1097/MBP.0b013e32835681fb. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility-Reykjavik Study. Hypertension. 2008;51:1123–1128. doi: 10.1161/HYPERTENSIONAHA.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dart AM, Kingwell BA, Gatzka CD, Willson K, Liang YL, Berry KL, Wing LM, Reid CM, Ryan P, Beilin LJ, Jennings GL, Johnston CI, McNeil JJ, MacDonald GJ, Morgan TO, West MJ, Cameron JD. Smaller aortic dimensions do not fully account for the greater pulse pressure in elderly female hypertensives. Hypertension. 2008;51:1129–1134. doi: 10.1161/HYPERTENSIONAHA.107.106310. [DOI] [PubMed] [Google Scholar]
- 36.Akhras F, Jackson G. Raised exercise diastolic blood pressure as indicator of ischaemic left ventricular dysfunction. Lancet. 1991;337:899–900. doi: 10.1016/0140-6736(91)90216-c. [DOI] [PubMed] [Google Scholar]
- 37.Singh JP, Larson MG, Manolio TA, O'Donnell CJ, Lauer M, Evans JC, Levy D. Blood pressure response during treadmill testing as a risk factor for new-onset hypertension. The Framingham heart study. Circulation. 1999;99:1831–1836. doi: 10.1161/01.cir.99.14.1831. [DOI] [PubMed] [Google Scholar]
- 38.Casey DP, Nichols WW, Braith RW. Impact of aging on central pressure wave reflection characteristics during exercise. Am J Hypertens. 2008;21:419–924. doi: 10.1038/ajh.2007.74. [DOI] [PubMed] [Google Scholar]