Abstract
The circadian system of mammals is composed of a hierarchy of oscillators that function at the cellular, tissue and systems levels. A common molecular mechanism underlies the cell autonomous circadian oscillator throughout the body, yet this clock system is adapted to different functional contexts. In the central suprachiasmatic nucleus (SCN) of the hypothalamus, a coupled population of neuronal circadian oscillators acts as a master pacemaker for the organism to drive rhythms in activity and rest, feeding, body temperature and hormones. Coupling within the SCN network confers robustness to the SCN pacemaker which in turn provides stability to the overall temporal architecture of the organism. Throughout the majority of the cells in the body, cell autonomous circadian clocks are intimately enmeshed within metabolic pathways. Thus, an emerging view for the adaptive significance of circadian clocks is their fundamental role in orchestrating metabolism.
Keywords: Clock Genes, Suprachiasmatic Nucleus, Oscillator Coupling, Metabolism, Temperature
INTRODUCTION
Living systems possess an exquisitely accurate internal biological clock that times daily events ranging from sleep and wakefulness in humans to photosynthesis in plants (Takahashi et al 2008). These ‘circadian rhythms’ represent an evolutionarily conserved adaptation to the environment that can be traced back to the earliest life forms. In animals circadian behavior can be analyzed as an integrated system - beginning with genes and leading ultimately to behavioral outputs. In the last fifteen years, the molecular mechanism of circadian clocks has been uncovered by the use of phenotype-driven (forward) genetic analysis in a number of model systems (Lowrey & Takahashi 2011). Circadian oscillations are generated by a set of genes forming a transcriptional autoregulatory feedback loop. In mammals, these include: Clock, Bmal1, Per1, Per2, Cry1, and Cry2. Another dozen candidate genes have been identified and play additional roles in the circadian gene network such as the feedback loop involving Rev-erbα.
Early work on mammalian rhythms used rhythmic behavior as a readout of the clock, and the hypothalamic suprachiasmatic nucleus (SCN) was identified as the dominant circadian pacemaker driving behavioral rhythms (Welsh et al 2010). However, the discovery of ‘clock genes’ led to the realization that the capacity for circadian gene expression is widespread throughout the body (Dibner et al 2010). Using circadian gene reporter methods, one can demonstrate that most peripheral organs and tissues can express circadian oscillations in isolation, yet still receive and may require input from the SCN in vivo. The cell autonomous clock has been found to be ubiquitous, and almost every cell in the body contains a circadian clock (Balsalobre et al 1998; Nagoshi et al 2004; Welsh et al 2004; Yoo et al 2004). It is now evident that the circadian oscillators within individual cells respond differently to entraining signals, control different physiological outputs and interact with each other and with the system as a whole. These discoveries have raised a number of questions concerning synchronization and coherence of rhythms at the cellular level as well as the architecture of circadian clocks at the systems level (Hogenesch & Ueda 2011). Here we discuss recent work that addresses these organizational issues and examines a number of levels of complexity within the circadian system. We will review mechanisms by which circadian clocks govern biological processes, as well as mechanisms by which these processes feed back into the circadian system. Perhaps the most important example of this is the intimate and reciprocal interaction between the circadian clock system and fundamental metabolic pathways (Bass & Takahashi 2010; Green et al 2008; Rutter et al 2002). In addition, there exist additional oscillatory processes in the circadian time domain that are observable in the presence of scheduled meals or methamphetamine treatment. These oscillators can generate behavioral rhythms in vivo in the absence of the SCN (Honma & Honma 2009).
MOLECULAR MECHANISM OF THE CIRCADIAN CLOCK IN MAMMALS
In mammals, the mechanism of the circadian clock is cell autonomous and arises from an autoregulatory negative feedback transcriptional network (Lowrey & Takahashi 2004; Takahashi et al 2008) (Fig. 1). At the core of this clock network are the transcriptional activators, CLOCK (and its paralog, NPAS2) and BMAL1, which positively regulate the expression of the Period (Per1, Per2) and Cryptochrome (Cry1, Cry2) genes at the beginning of the cycle. Per and Cry gene products accumulate, dimerize, and form a complex which translocates into the nucleus to interact with CLOCK and BMAL1, repressing their own transcription. This feedback cycle takes ~24 hours, and the turnover of the PER and CRY proteins is tightly regulated by E3 ubiquitin ligase complexes. There are additional feedback loops interlocked with the core CLOCK-BMAL1/PER-CRY loop. Prominent among these is a loop involving Rev-erbα (Nr1d1) and Rora, which are also direct targets of CLOCK-BMAL1. The feedback effects of this loop impinge upon the transcription of Bmal1 (and to a lesser extent on Clock) to cause an antiphase oscillation of BMAL1. Other feedback loops involve the PAR-bZip family members, DBP, HLF and TEF; the bZip protein, E4BP4 (Nfil3); and the bHLH proteins, DEC1 and DEC2 (Bhlhb2, Bhlhb3), all of which are transcriptional targets of CLOCK-BMAL1 (Gachon 2007; Lowrey & Takahashi 2004; Takahashi et al 2008).
Figure 1.
The molecular mechanism of the circadian clock in mammals. An autoregulatory transcriptional feedback loop involving the activators, CLOCK and BMAL1, and their target genes, Per1, Per2, Cry1 and Cry2, whose gene products form a negative feedback repressor complex, constitute the core circadian clock mechanism. In addition to this core transcriptional feedback loop, there are other feedback loops driven by CLOCK:BMAL1. One feedback loop involving Rev-erbα and Rorα that represses Bmal1 transcription leads to an antiphase oscillation in Bmal1 gene expression. CLOCK:BMAL1 also regulates many downstream target genes known as clock-controlled genes (Ccg). At a post-transcriptional level, the stability of the PER and CRY proteins is regulated by SCF (Skp1-Cullin-F-box protein) E3 ubiquitin ligase complexes involving β-TrCP and FBXL3, respectively. The kinases, casein kinase 1ε/δ (CK1ε/δ) and AMP kinase (AMPK) phosphorylate the PER and CRY proteins, respectively, to promote polyubiquitination by their respective E3 ubiquitin ligase complexes, which in turn tag the PER and CRY proteins for degradation by the 26S proteasome complex.
The discovery of a ubiquitous, cell autonomous clock in mammals has led to a re-evaluation of central and peripheral oscillators: are they fundamentally similar in mechanism, how do they function in different cellular contexts, and what role does coupling in the central SCN clock play in its functional properties?
CENTRAL CIRCADIAN OSCILLATORS
The hypothalamic suprachiasmatic nucleus acts as a master pacemaker for the generation of circadian behavioral rhythms in mammals [for review see (Welsh et al 2010)]. Classic work not reviewed here has shown that the SCN are both necessary and sufficient for the generation of circadian activity rhythms in rodents. The SCN receives direct photic input from the retina from a recently discovered photoreceptor cell type known as ‘intrinsically photoreceptive retinal ganglion cells’ (ipRGCs) [reviewed in (Do & Yau 2010)]. These ipRGCs express a novel photopigment, melanopsin, which renders them intrinsically photosensitive to short wavelength irradiation. Interestingly, ipRGCs are depolarizing photoreceptors that employ a phototransduction mechanism that is analogous to that seen in invertebrate photoreceptors. The photoresponse in ipRGCs has slow kinetics and a relatively high threshold to light, making them ideally suited to function as circadian photoreceptors, which must integrate light information over relatively long durations and must be insensitive to transient light signals that are not associated with the solar light cycle. Although ipRGCs appear to be optimal circadian photoreceptors, they do not act alone, and rod and cone photoreceptors also have photic inputs to the SCN. Interestingly, these non-visual inputs from rods and cones to the SCN are mediated by the ipRGCs (Chen et al 2011; Guler et al 2008). An emerging theme is that melanopsin-positive ipRGCs are involved in a surprisingly broad array of non-visual photic responses in mammals. The complexity of the ipRGCs and their contribution to circadian rhythms and other behaviors is beyond the scope of this discussion, but recent reviews have covered this topic in depth (Do & Yau 2010; Schmidt et al 2011).
Suprachiasmatic nucleus
The SCN itself is composed of ~20,000 neurons, each of which is thought to contain a cell autonomous circadian oscillator. The SCN functions as a network in which the population of SCN cells are coupled together and oscillate in a coherent manner (Herzog 2007). The dynamics of the spatial and temporal coordination of rhythms in the SCN have been studied recently with the advent of single-cell circadian reporter technology, which has revealed unexpected complexity in the temporal architecture of the nucleus (Evans et al 2011; Foley et al 2011; Yamaguchi et al 2003). At the single-cell level, SCN neurons exhibit a wide range in cell autonomous circadian periods that vary from 22 to 30 hours (Ko et al 2010; Liu et al 1997; Welsh et al 1995). Intercellular coupling among SCN neurons acts to mutually couple the entire population to a much narrower range that corresponds to the circadian period of the locomotor activity rhythm which is extremely precise (a standard deviation in period that is ~0.2 hrs or 12 min in mice) (Herzog et al 2004). The heterogeneity in intrinsic period of the SCN cells confers at least two important functions: phase lability and phase plasticity. The phases of the rhythms of individual SCN neurons are highly stereotyped anatomically and appear as a wave that spreads across the nucleus over time (Evans et al 2011; Foley et al 2011; Yamaguchi et al 2003). Intrinsically shorter period cells have earlier phases and intrinsically longer period cells have later phases within the SCN – this is a reflection of phase lability (Yamaguchi et al 2003). Under different photoperiods (e.g., long vs. short photoperiod light cycles), the waveform of the SCN population rhythm is modulated such that in short photoperiods, the SCN waveform is narrow and high amplitude; whereas, in long photoperiods, the SCN waveform broad and low amplitude – this is a reflection of phase plasticity (Inagaki et al 2007; VanderLeest et al 2007). In addition to the heterogeneity of SCN oscillator period and phase, it has been proposed that the cell autonomous SCN oscillators are not uniformly robust intrinsically (Webb et al 2009). Rather, the intercellular coupling of SCN neurons appears critical to the robustness of the SCN network oscillatory system.
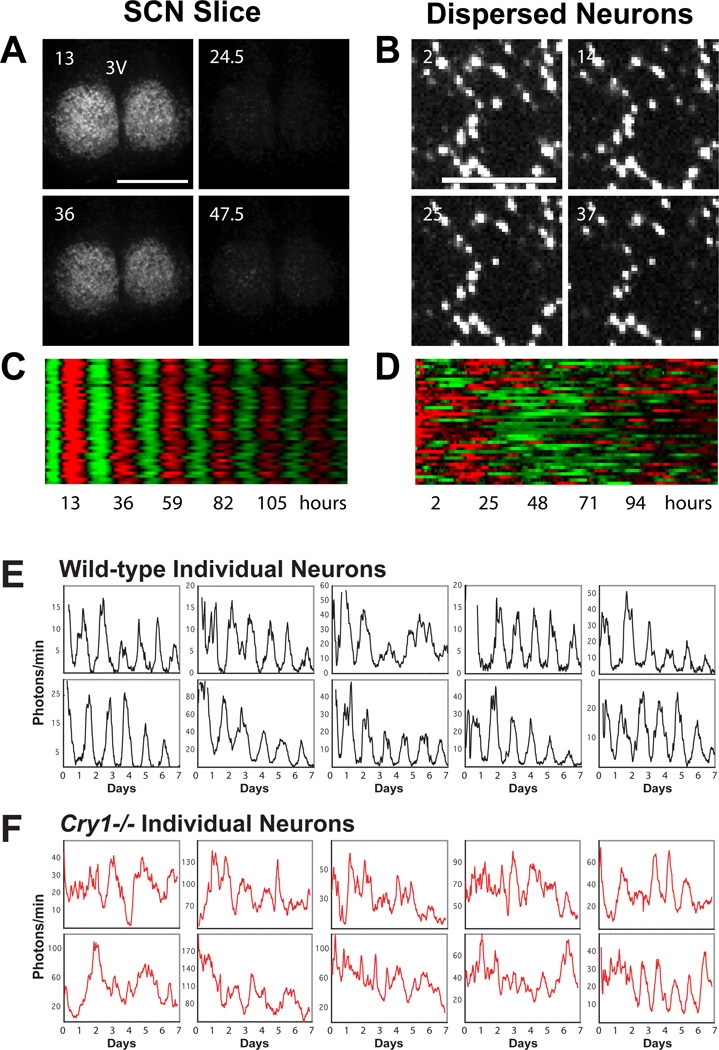
With the discovery of peripheral oscillators (Balsalobre et al 1998; Yamazaki et al 2000; Yoo et al 2004) and the apparent ubiquity of clock mechanisms (Yagita et al 2001), a critical question concerns the similarity and differences in the SCN pacemaker as compared to peripheral oscillators. To address this question, Liu et al. (Liu et al 2007a) examined whether canonical clock mutations previously assessed in vivo affected the SCN and peripheral oscillators in a similar manner. Using Per2::luciferase reporter mice, they found that the effects of the Period and Cryptochrome loss-of-function mutations were the same in SCN explants as that seen previously at the behavioral level. By contrast, in peripheral tissues, single loss-of-function mutations that are subtle at the behavioral level, such as Per1 or Cry1 knockouts, produced very strong loss-of-rhythm phenotypes. Interestingly, the effects of these mutations are cell autonomous in both fibroblasts and in isolated SCN neurons supporting the idea that the cell autonomous clock is similar in these two cell types. However, when the SCN population is coupled, the effects of these mutations are non-cell autonomous. This occurs as a consequence of the intercellular coupling in the SCN network, which is capable of rescuing a cell autonomous defect in the individual cells (Fig. 2). This transformation of the oscillatory capability of SCN neurons from damped to self-sustained is an important illustration of the robustness of the SCN network. Indeed, Ko et al. (Ko et al 2010) have found that the SCN network is capable of generating oscillations in the circadian domain in the complete absence of cell autonomous oscillatory potential. In Bmal1 knockout mice, which are arrhythmic at the behavioral level, SCN explants unexpectedly express stochastic oscillations in the circadian range that are highly variable. When the individual cells are no longer rhythmic, the coupling pathways within the SCN network can propagate stochastic rhythms that are a reflection of both feed-forward coupling mechanisms and intracellular noise. Thus, in a manner analogous to central pattern generators in neural circuits, rhythmicity can arise as an emergent property of the network in the absence of component pacemaker or oscillator cells.
Figure 2.
Network and autonomous properties of SCN neurons. Network properties of the SCN can compensate for genetic defects affecting rhythmicity at the cell autonomous level. A) Bioluminescence images of a Cry1−/− SCN in organotypic slice culture. Note the stable, synchronized oscillations. Numbers indicate hours after start of imaging; 3V indicates the 3rd ventricle. B) Bioluminescence images of dissociated individual Cry1−/− SCN neurons showing cell-autonomous, largely arrhythmic patterns of high bioluminescence intensity. C and D) Heatmap representations of bioluminescence intensity of individual Cry1−/− neurons in SCN slice (A) and dispersed culture (B). Values above and below the mean are shown in red and green, respectively, for 40 SCN neurons in each condition. E and F) Ten single SCN neuron rhythms from wild-type (E) and Cry1−/− (F) mice. Imaging began immediately following a media change at day 0. Dissociated Cry1−/− SCN neurons are largely arrhythmic, whereas dissociated wild-type cells are rhythmic. By contrast, in organotypic slice cultures, both wild-type and Cry1−/− SCN cells are robustly rhythmic and tightly synchronized. Figure and legend adapted and reprinted from (Liu et al 2007a), with permission from Elsevier.
In addition to the generation of sustained oscillations by the SCN network, the SCN is also robust to perturbations from environmental inputs. In wild type mice, the phase resetting curve to light pulses is characteristic of Type 1 or weak resetting (low amplitude) (Vitaterna et al 2006). This is a reflection of the robustness of the SCN pacemaker because inputs such as light can only perturb the phase of the oscillation to a limited extent. In contrast, genetic mutations that lower the amplitude of the molecular oscillation in the SCN lead to increases in the sensitivity to light-induced phase shifts (Type 0 resetting) without changing the strength of the light signals impinging on the SCN (Vitaterna et al 2006). Similar effects are seen with temperature cycles. Peripheral oscillators are exquisitely sensitive to the phase shifting effects of temperature and can be entrained strongly by low amplitude temperature cycles that are equivalent to the circadian fluctuation in core body temperature (~2.5°C oscillation in mice) (Brown et al 2002; Buhr et al 2010). Interestingly, the SCN is resistant to entrainment by low amplitude temperature cycles and this resistance depends on the intercellular coupling of SCN neurons (Abraham et al 2010; Buhr et al 2010). As was the case for genetic mutations on the generation of rhythms, the effects of temperature perturbations on the SCN are cell autonomous when intercellular coupling is eliminated. Thus, both SCN and peripheral oscillators are sensitive to temperature cycles at the cell autonomous level; however, coupling within the SCN network confers robustness and makes the SCN network resistant to temperature perturbations. As discussed below, the temperature resistance of the SCN makes functional sense since the SCN drives the body temperature rhythm and this temperature signal, which can serve as an entraining signal for peripheral clocks, might then feedback and interfere with the SCN (Buhr et al 2010).
PERIPHERAL CLOCKS
Rhythms of clock gene and/or protein expression have been observed in cells and tissues throughout the body in mammals and these rhythms persist in culture, demonstrating that non-SCN cells also contain endogenous circadian oscillators (Balsalobre et al 1998; Yamazaki et al 2000; Yoo et al 2004). Although the core clock machinery is conserved in these different cellular clocks, there are significant differences in the relative contributions of the individual clock components, as well as in the manner in which these peripheral clocks are reset and in the output pathways that are under their control. These endogenous cellular clocks drive extensive rhythms of gene transcription, with 3–10% of all mRNAs in a given tissue showing circadian rhythms in steady state levels (Akhtar et al 2002; Duffield et al 2002; Hughes et al 2009; Miller et al 2007; Panda et al 2002; Storch et al 2002). However, the genes that are under circadian control are largely non-overlapping in each tissue, reflecting the need to temporally control cellular physiology relevant to each unique cell type. As a result, the circadian clock exerts broad-ranging control over many biological processes, including many aspects of metabolism such as xenobiotic detoxification (Gachon et al 2006), glucose homeostasis (Lamia et al 2008; Marcheva et al 2010; So et al 2009; Turek et al 2005), and lipogenesis (Gachon et al 2011; Le Martelot et al 2009).
ORGANIZATION OF THE CIRCADIAN SYSTEM
For biological clocks to be effective, they must accurately keep time and adjust to environmental signals. In an organized circadian system, this requires SCN control of peripheral oscillators, and loss of the SCN results in peripheral circadian clocks that become desynchronized (Yoo et al 2004). However, tissue-specific gene expression patterns are likely to be regulated by both ‘local’ as well as central mechanisms. This concept was elegantly demonstrated through genetic disruption of the circadian clock mechanism specifically in the hepatocytes of mice, while leaving the circadian clock intact in the SCN and other cell types throughout the body (Kornmann et al 2007). Microarray analysis of mRNAs in the livers from these mice demonstrated that the disruption of the circadian molecular feedback loop specifically within the liver results in arrhythmicity of most hepatic transcripts. Thus, most circadian oscillations of hepatic function rely upon an intact liver clock. However, a subset of transcripts continued to cycle robustly even in the absence of a functional liver clock. Among these was the core clock component Per2. In livers maintained in explant culture, rhythms in Per2 transcription were observed in livers with intact clocks but were absent in livers with inactivated clocks. Thus, rhythmic gene expression can be driven by both local intracellular clocks and by extracellular systemic cues.
What are these systemic cues? The photically entrained SCN is thought to convey signals to light insensitive peripheral clocks to synchronize these systems, and SCN transplant studies (Ralph et al 1990; Silver et al 1996) and parabiosis experiments in mice (Guo et al 2005) have demonstrated that both humoral and non-humoral pathways are important for SCN coordination of circadian output rhythms. In addition, complex feedback loops link the circadian clock with rhythmic metabolic networks, integrating these systems in a light-independent manner. Circadian control of metabolism occurs at the central (SCN), as well as local levels, and involves clocks within a number of peripheral tissues including the liver, pancreas, skeletal muscle, intestine, and adipose tissue [for review see (Bass & Takahashi 2010; Green et al 2008)]. This intimate relationship between clocks and metabolism is an example of how the circadian “system” is integrated with, and influenced by, the physiology that is under its control. Therefore, organization of the circadian system requires a combination of 1) autonomic innervation of peripheral tissues, 2) endocrine signaling, 3) temperature, and 4) local signals (Fig. 3).
Figure 3.
Pathways of peripheral clock entrainment. The master circadian pacemaker within the SCN relays temporal information to peripheral oscillators through autonomic innervation, body temperature, humoral signals (such as glucocorticoids), and feeding-related cues. Local signaling pathways can also affect peripheral oscillators independently from the SCN.
Neural Control of Peripheral Oscillators: The Autonomic Nervous System
The SCN controls peripheral oscillators through both sympathetic and parasympathetic pathways (Kalsbeek et al 2010; Ueyama et al 1999). SCN projections through the paraventricular nucleus-superior cervical ganglia (PVN-SCG) pathway provide the dominant entraining signal for the submandibular salivary glands (Ueyama et al 1999; Vujovic et al 2008). Sympathetic innervation from the SCN to the PVN to the liver results in daily rhythms of plasma glucose, presumably by directly influencing the rhythm of hepatic gluconeogenesis (Cailotto et al 2005; Kalsbeek et al 2004).
Autonomic pathways from the SCN have been demonstrated to relay photic information to oscillators in the adrenal gland and liver (Buijs et al 1999; Cailotto et al 2009; Ishida et al 2005). Sympathetic innervation also modulates the sensitivity of the adrenal to adrenocorticotropic hormone (ACTH) and directly influences glucocorticoid release (Buijs et al 1999; Kalsbeek et al 2010; Kaneko et al 1981). Oscillators in both the adrenal cortex and medulla respond to neural input emanating from the SCN (Buijs et al 1999; Mahoney et al 2010). The adrenal clock is of particular interest given the strong case for glucocorticoids as a humoral entraining signal for peripheral clocks.
Hormonal Control of Peripheral Oscillators
While a number of hormones may have roles in mammalian circadian organization, glucocorticoids have received the most attention. Rhythmic glucocorticoids result from both the sympathetic inputs discussed above as well as an underlying rhythm of corticotropin releasing hormone (CRH) and ACTH function (Kaneko et al 1980; Kaneko et al 1981). The adrenal clock itself also provides temporal control of sensitivity to ACTH-induced glucocorticoid release (Oster et al 2006).
The demonstration that dexamethasone (a glucocorticoid analog) could shift the phase of peripheral tissues in vivo (Balsalobre et al 2000) provided the first definitive evidence that glucocorticoids were entraining signals for peripheral oscillators. Dexamethasone was initially shown to shift the phase of clock gene expression in liver, kidney, and heart, as well as cultured fibroblasts. What provides glucocorticoid input into the clock at the local level? Glucocorticoid-response elements (GREs) exist in the regulatory regions of the core clock genes, Bmal1, Cry1, Per1 (Reddy et al 2007; Yamamoto et al 2005b) and Per2 (So et al 2009). These GREs may lead to the transcriptional activation of a number of clock genes and clock-controlled genes by glucocorticoids.
Glucocorticoids can synchronize circadian expression of much of the oscillatory component of the liver transcriptome in SCN-lesioned mice (Reddy et al 2007). This is accomplished in part through activation of the nuclear receptor (and hepatic transcription factor) HNF4α. HNF4α is responsive to glucocorticoids (Reddy et al 2007), contains E-boxes, which may allow for transcriptional control by CLOCK:BMAL1 (Reddy et al 2007), and can interact with PER2 (Schmutz et al 2010). Other metabolically-relevant nuclear receptors, including PPARα, also respond to glucocorticoids (Lemberger et al 1994). Nuclear receptor activation by clock components and glucocorticoids provides another point of circadian input to metabolic pathways as described below.
Temperature
In most organisms, temperature is a powerful entraining agent for circadian rhythms. However, in mammals external temperature cycles are very weak entraining agents (Refinetti 2010), and this has been attributed to the fact that homeotherms regulate their body temperature and can defend their body temperature against environmental fluctuations. It has long been known that body temperature is circadian and the rhythm is driven by the SCN. Peripheral oscillators, including fibroblasts, liver, kidney, and lung, are exquisitely sensitive to temperature changes (Abraham et al 2010; Brown et al 2002; Buhr et al 2010; Kornmann et al 2007). These oscillators can be strongly reset by low amplitude temperature pulses that mimic the range of circadian variation; and temperature profiles that match circadian body temperature rhythms strongly entrain peripheral clocks (Brown et al 2002; Buhr et al 2010). However, as described above, the SCN is resistant to temperature cycles in the circadian range. Because of this system design, the SCN is ideally situated to utilize circadian temperature cycles as a universal entraining signal for peripheral oscillators. The influence of temperature on peripheral oscillators likely occurs through the transcription factor heat shock factor 1 (HSF1). HSF1 transcriptional activity oscillates with a circadian rhythm in the liver and can be driven by temperature cycles (Reinke et al 2008). HSF1 inhibitors block temperature-induced resetting in extra-SCN oscillators (Buhr et al 2010). Because HSF1 is influenced by a wide range of signaling pathways in the cell (Akerfelt et al 2010), temperature and HSF1 may form a final common pathway for the integration of resetting signals in peripheral clocks.
Behavioral and Homeostatic Regulation: Local cues feed back into the clock
In addition to controlling hormone secretion and body temperature directly, the SCN coordinates rhythms in behavioral processes, such as locomotor activity and feeding, which can influence endocrine function and body temperature. These behaviors, feeding in particular, can regulate peripheral clocks at the local level, modulating local signaling pathways and metabolic processes. Homeostatic signaling pathways also affect peripheral clocks and their function, allowing for extra-SCN control of circadian processes. Studies in which mealtime has been experimentally manipulated to occur antiphase to the normal SCN-driven feeding rhythm have attempted to elucidate local mechanisms for controlling clocks in peripheral tissues.
The liver clock, unlike the SCN, is particularly sensitive to resetting by feeding. Hepatic rhythms of clock gene and protein expression rapidly shift their phase to follow the timing of a scheduled meal (Damiola et al 2000; Stokkan et al 2001). Similarly, livers of Cry1/Cry2 null mice display rhythms in many transcripts (including a number of transcripts involved in metabolic processes) when fed in regular 24h intervals (Vollmers et al 2009). Feeding appears to result in cues that bypass the core circadian feedback loop to drive these rhythms. These cues may include feeding-induced changes in temperature and HSF1 activity (Kornmann et al 2007) or activation of other metabolically-sensitive pathways.
A number of local mediators of both core clock components and clock-controlled rhythmic transcripts have been identified, which can respond to SCN-driven inputs as well as local signals related to homeostasis and metabolic state. These include members of the nuclear receptor family of transcription factors, many of which exhibit circadian rhythms of transcription within the liver and other metabolically-relevant tissues (Yang et al 2006). These rhythmic nuclear receptors regulate transcription of downstream metabolic pathways. Among the rhythmic nuclear receptors are PPARs (peroxisome proliferator-activated receptors) and members of the REV-ERB and ROR families. As described above, RORα and REV-ERBα participate directly in the clock mechanism by regulating Bmal1 transcription (Preitner et al 2002; Sato et al 2004), but are also important for many aspects of metabolic regulation. Like REV-ERBs and RORs, many of the other rhythmic nuclear receptors are also regulators of clock function, providing a mechanism by which signals of metabolic status can influence rhythmicity. Glucocorticoid receptors, as discussed above, induce transcription of Per and potentially a number of other clock and clock controlled genes (Reddy et al 2007; So et al 2009; Yamamoto et al 2005a). PPARα, which responds to lipid status and glucocorticoids, may also regulate Bmal1 transcription (Canaple et al 2006).
PPARγ coactivator-1α (PGC-1α, a transcriptional coactivator) provides a link between changes in metabolic status and the clock. PGC-1α is critical for adaptive responses to nutritional and metabolic state, particularly following fasting [reviewed in (Lin et al 2005)]. PGC-1α is itself rhythmic, and activates expression of Bmal1 and Rev-erbα through coactivation of RORs (Liu et al 2007b). PGC-1α null mice display disruptions in a number of circadian outputs including locomotor activity, oxygen consumption rate, and expression of both clock and metabolic genes (Liu et al 2007b). PGC-1α also interacts with SIRTUIN 1 (SIRT1), a nicotinamide adenine dinucleotide (NAD)-dependent histone deacetylase (Rodgers et al 2005).
Another mechanism by which metabolic signals can feed into the clock is through the adenosine monophosphate-activated protein kinase (AMPK) (Bass & Takahashi 2010). This kinase is a central mediator of metabolic signals and its activity is robustly rhythmic in mouse liver and regulated by nutrient status, as reflected in the ratio of AMP to ATP. Active AMPK directly regulates the central clock mechanism by phosphorylating and destabilizing the clock component CRY1 (Lamia et al 2009).
Cellular redox state can also serve as a mechanism by which the metabolic status of the cell can impact the circadian system. NAD levels exhibit circadian oscillations in the liver, likely due to transcriptional regulation of nicotinamide phosphoribosyltransferase (Nampt, encoding the rate-limiting enzyme in the NAD+ salvage pathway) by CLOCK:BMAL1 (Nakahata et al 2009; Ramsey et al 2009). NAD levels also vary with cellular redox state as a consequence of metabolic changes and this, in turn, can directly impact clock function. The ratio of NAD+ to NADH influences binding of the NPAS2:BMAL1 and CLOCK:BMAL1 to DNA in vitro suggesting one way in which NAD could interact with clock components (Rutter et al 2001).
NAD+-dependent SIRT1 also displays daily oscillations and feeds back onto the circadian clock. SIRT1 forms a complex with CLOCK:BMAL1, leading to the deacetylation of PER2 (Asher et al 2008) and BMAL1 (Nakahata et al 2008). SIRT1 also suppresses CLOCK:BMAL1-mediated transcription, resulting in decreased expression of Per2 (Nakahata et al 2008; Ramsey et al 2009) and the clock-controlled gene Dbp (Nakahata et al 2008).
Another molecule with NAD+-sensitive activity, poly(ADP-ribose) polymerase 1 (PARP-1), was recently shown to interact with the circadian clock (Asher et al 2010). PARP-1 activity is rhythmic in the liver, and this rhythm persists even in the absence of a functional hepatic circadian clock. The rhythm of PARP-1 activity can be entrained by scheduled meals, however, suggesting that the circadian activity of PARP-1 is driven by feeding-related cues. PARP-1 interacts with CLOCK:BMAL1 in a rhythmic fashion and inhibits DNA binding by the CLOCK:BMAL1 complex. PARP-1 also polyADP-ribosylates CLOCK, and appears to temporally-regulate interaction of CLOCK:BMAL1 with PER2 and CRYs. The circadian regulation of PARP-1 by feeding, and subsequent consequences for the circadian clock, are likely not entirely mediated through NAD+. Regardless of the mechanism, PARP-1 provides another way for metabolic signals to influence timekeeping by the core molecular clock.
OTHER OSCILLATORS: FOOD AND DRUGS
The circadian system consists of a web of interconnected oscillators and feedback loops. The core molecular clock within cells keeps time and responds to cues from the SCN (through neural, hormonal, and activity-driven pathways), as well as signals from the local cellular environment. These cellular and tissue-level clocks result in rhythms of physiologically-relevant outputs, including glucose production, fat storage, and hormone production. These outputs, in turn, become circadian time-keeping cues relayed to other clocks throughout the body, likely ultimately feeding back to the central nervous system and even the SCN. Under normal circumstances, the SCN maintains temporal organization of body temperature, activity, feeding, and neural output rhythms. This keeps local and systemic circadian signals aligned. In the absence of the SCN, however, the system becomes disorganized. Activity is nonrhythmic and peripheral tissues and cells drift out of phase with one another. There are two striking exceptions to this phenomenon. Scheduled, restricted feeding and chronic administration of methamphetamine, a psychostimulant drug of abuse, are both capable of organizing the circadian outputs in the absence of the SCN.
The Food Entrainable Oscillator
It is not surprising that food and food-related cues would be salient for many biological processes, including circadian rhythms. The ability of animals to anticipate food availability is well established, and persists even when food is provided at a time that is out of phase with the animal’s normal feeding time (Richter 1922; Stephan et al 1979). When food is temporally restricted to the daytime (the normal rest period), nocturnal rodents will anticipate the arrival of the meal with an increase in activity; if the timing of that meal is shifted, rats will display transients, gradually shifting their food-anticipatory activity bout each day until it again precedes the start of food availability.
In animals with lesions of the SCN, temporal food restriction will induce circadian rhythmicity of locomotor behavior (Stephan et al 1979) and an accompanying temperature rhythm (Krieger et al 1977). Food anticipatory activity persists on days of total food deprivation, demonstrating that these cycles are not merely an hourglass phenomenon, but driven by an underlying oscillator (Stephan 2002). This food-entrainable oscillator (FEO) can take on pacemaking functions—organizing rhythms of activity, body temperature, and peripheral tissues in SCN-lesioned animals. Peripheral tissues from both SCN-intact and SCN-ablated mice are sensitive to temporally restricted feeding. In SCN-intact animals, phase desynchrony among peripheral oscillators can occur, with some remaining in phase with the (food-unaffected) SCN, and some following the phase of food availability (Damiola et al 2000; Pezuk et al 2010). Cues related to the meal itself must be the dominant entraining signals in this latter group of tissues. In SCN-lesioned mice, food entrainment organizes rhythms throughout the periphery, with stable phase relationships observed among tissues (Hara et al 2001; Pezuk et al 2010).
Interestingly, the FEO does not appear to require a functional molecular clock since Bmal1−/− and Per1/Per2−/− mice can entrain to restricted feeding (Pendergast et al 2009; Storch & Weitz 2009). The mechanism by which food drives oscillatory behavior throughout the organism is unknown. It is possible that the FEO exploits some of the same pathways used by the SCN, such as hormone and temperature-dependent cues, to organize peripheral tissues. The locus (or loci) of the FEO is also unknown. A number of structures, including the olfactory bulbs (Davidson et al 2001), the ventromedial hypothalamus (Mistlberger & Rechtschaffen 1984), the paraventricular thalamic nucleus (Landry et al 2007), and a large portion of the digestive system (Davidson et al 2003), have been ruled out.
The dorsomedial hypothalamus (DMH) has received considerable attention for its role in food entrainment. Data supporting a role for the DMH in the generation of food-anticipatory circadian activity are controversial (Gooley et al 2006; Landry et al 2006; Moriya et al 2009), and the DMH may not be essential for the expression of the FEO (Landry et al 2006; Moriya et al 2009). The DMH does, however, interact with the SCN under conditions of food restriction and may influence the strength of the FEO output, particularly in SCN-intact animals (Acosta-Galvan et al 2011).
The Methamphetamine-Sensitive Circadian Oscillator (MASCO)
Chronic or scheduled methamphetamine treatment affects circadian outputs in a manner similar to food restriction (Honma & Honma 2009). Methamphetamine, provided in the drinking water of rats and mice, is capable of driving circadian rhythms of locomotor behavior in the absence of the SCN (Honma et al 1987; Tataroglu et al 2006). In SCN-intact animals, this appears as a lengthening of the free-running period of locomotor activity and in some cases with two activity components that are relatively coordinated with each other. Much like the FEO, these rhythms persist when the stimulus (in this case, methamphetamine) is withdrawn (Tataroglu et al 2006), as well as in the absence of a functional molecular clock (Honma et al 2008; Mohawk et al 2009). The MASCO is capable of functioning as a pacemaker driving rhythms in locomotor activity, body temperature, endocrine function, and the oscillators of peripheral tissues (Honma et al 1988; Pezuk et al 2010). In the presence of the SCN, methamphetamine results in desynchrony among internal oscillators, as some follow the SCN and some follow the presumed phase of the MASCO (Pezuk et al 2010). When the SCN is ablated, however, the MASCO organizes oscillators in tissues throughout the organism, resulting in a coordinated system (Pezuk et al 2010).
The site of the MASCO is also unknown. It is possible that the FEO and MASCO share an anatomical and mechanistic basis, or, indeed, that they represent a single oscillator. Research focused on understanding how the MASCO and FEO relay circadian information to peripheral tissues will likely uncover novel (or underappreciated) mechanisms of control of rhythms. The role of these oscillators in the absence of food restriction and methamphetamine must also be determined. It is unlikely that these oscillators are dormant under normal conditions; instead, it seems probable that the FEO, MASCO, and SCN cooperate in a hierarchically organized, perhaps necessarily redundant, timing network.
SUMMARY
It is now clear that there is feedback at nearly every level of the circadian system. “Outputs” such as body temperature and feeding become inputs to other oscillators, and are capable of influencing the core molecular clockwork, generating complex interconnectivity between the circadian system and the biological outputs it controls. Reciprocity between the circadian and metabolic systems makes it likely that perturbations in one system will affect the other. This idea is supported both genetically in which circadian mutants have metabolic phenotypes and environmentally in which nutrient intake can modulate circadian rhythms (Bass & Takahashi 2010). In recent years, there has been considerable progress made in unraveling the connections between the circadian clock and metabolism. The role of circadian clocks in governing many other physiological systems has been established, but far less well characterized.
We still know very little about how oscillators and timing cues are integrated at the local and organismal level to coordinate the circadian architecture of the animal. Peripheral clocks must balance (sometimes conflicting) inputs arising from the SCN with those signaling local cellular and metabolic state. Moreover, recent work has revealed that the cell autonomous oscillator, which normally lies at the foundation of the circadian clockwork, is not absolutely crucial for the expression of rhythms by other components of the system. Within the SCN, coupling among individual neurons gives rise to a heterogeneous, yet elegantly organized, robust oscillatory network, which can overcome impaired rhythmicity at the cellular level (Ko et al 2010; Liu et al 2007a). Food- and drug-sensitive oscillators (FEO, MASCO) exist which can influence circadian rhythms and drive rhythmic outputs in the absence of the core molecular clock mechanism (Honma et al 2008; Mohawk et al 2009; Pendergast et al 2009; Storch & Weitz 2009). The ability of rhythmic circadian outputs to persist in the absence of the SCN necessitates any model of the circadian network to include alternative mechanisms for controlling circadian rhythms at the level of the cell, the tissue, and the organism.
Acronyms
- DMH
dorsomedial hypothalamus
- FEO
food entrainable oscillator
- HSF1
heat shock factor 1
- MASCO
methamphetamine sensitive circadian oscillator
- NAD
nicotinamide adenine dinucleotide
- PPAR
peroxisome proliferator-activated receptor
- SCN
suprachiasmatic nucleus
Glossary
- Circadian rhythm
an endogenously generated oscillation with a period of ~24h. Circadian rhythms can entrain to external cues and are temperature compensated.
- Entrainment
synchronization and phase control of a rhythm to a regularly occurring environmental cycle (usually ~24h). Light is a strong entraining signal for circadian clocks.
- Free-running period
the period of an oscillation in the absence of entraining signals. The free-running period reflects the intrinsic period of the oscillator, uninfluenced by environmental timing cues.
- Suprachiasmatic Nucleus
the site of the master circadian pacemaker in mammals. The suprachiasmatic nucleus (SCN) consists of two, bilateral nuclei positioned above the optic chiasm and on either side of the 3rd ventricle. The SCN is composed of ~20,000 individual neurons, which couple to form a complex, robust oscillatory network.
Footnotes
Related Articles and Reviews
Lowrey PL, Takahashi JS. 2011. Genetics of circadian rhythms in mammalian model organisms. Adv. Genet. 74: 175–230
Welsh DK, Takahashi JS, Kay SA. 2010. Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol. 72: 551-77
Dibner C, Schibler U, Albrecht U. 2010. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 72: 517-49
Bass J, Takahashi JS. 2010. Circadian integration of metabolism and energetics. Science 330: 1349-54
Do MT, Yau KW. 2010. Intrinsically photosensitive retinal ganglion cells. Physiol. Rev. 90: 1547-81
Hogenesch JB, Ueda HR. 2011. Understanding systems-level properties: timely stories from the study of clocks. Nat. Rev. Genet. 12: 407-16
Contributor Information
Jennifer A. Mohawk, Email: Jennifer.Mohawk@UTSouthwestern.edu.
Carla B. Green, Email: Carla.Green@UTSouthwestern.edu.
Joseph S. Takahashi, Email: Joseph.Takahashi@UTSouthwestern.edu.
LITERATURE CITED
- Abraham U, Granada AE, Westermark PO, Heine M, Kramer A, Herzel H. Coupling governs entrainment range of circadian clocks. Mol. Syst. Biol. 2010;6:438. doi: 10.1038/msb.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Galvan G, Yi CX, van der Vliet J, Jhamandas JH, Panula P, et al. Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proc. Natl. Acad. Sci. USA. 2011;108:5813–5818. doi: 10.1073/pnas.1015551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, et al. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–550. doi: 10.1016/s0960-9822(02)00759-5. [DOI] [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell. 2010;142:943–953. doi: 10.1016/j.cell.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N, Schibler U. Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr. Biol. 2002;12:1574–1583. doi: 10.1016/s0960-9822(02)01145-4. [DOI] [PubMed] [Google Scholar]
- Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, et al. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur. J. Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Cailotto C, La Fleur SE, Van Heijningen C, Wortel J, Kalsbeek A, et al. The suprachiasmatic nucleus controls the daily variation of plasma glucose via the autonomic output to the liver: are the clock genes involved? Eur. J. Neurosci. 2005;22:2531–2540. doi: 10.1111/j.1460-9568.2005.04439.x. [DOI] [PubMed] [Google Scholar]
- Cailotto C, Lei J, van der Vliet J, van Heijningen C, van Eden CG, et al. Effects of nocturnal light on (clock) gene expression in peripheral organs: a role for the autonomic innervation of the liver. PLoS One. 2009;4:e5650. doi: 10.1371/journal.pone.0005650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaple L, Rambaud J, Dkhissi-Benyahya O, Rayet B, Tan NS, et al. Reciprocal regulation of brain and muscle Arnt-like protein 1 and peroxisome proliferator-activated receptor alpha defines a novel positive feedback loop in the rodent liver circadian clock. Mol. Endocrinol. 2006;20:1715–1727. doi: 10.1210/me.2006-0052. [DOI] [PubMed] [Google Scholar]
- Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–95. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ, Aragona BJ, Werner RM, Schroeder E, Smith JC, Stephan FK. Food-anticipatory activity persists after olfactory bulb ablation in the rat. Physiol. Behav. 2001;72:231–235. doi: 10.1016/s0031-9384(00)00417-0. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Poole AS, Yamazaki S, Menaker M. Is the food-entrainable circadian oscillator in the digestive system? Genes Brain Behav. 2003;2:32–39. doi: 10.1034/j.1601-183x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol. Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC. Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol. 2002;12:551–557. doi: 10.1016/s0960-9822(02)00765-0. [DOI] [PubMed] [Google Scholar]
- Evans JA, Leise TL, Castanon-Cervantes O, Davidson AJ. Intrinsic regulation of spatiotemporal organization within the suprachiasmatic nucleus. In PLoS One. 2011:e15869. doi: 10.1371/journal.pone.0015869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley NC, Tong TY, Foley D, Lesauter J, Welsh DK, Silver R. Characterization of orderly spatiotemporal patterns of clock gene activation in mammalian suprachiasmatic nucleus. In Eur J Neurosci. 2011 doi: 10.1111/j.1460-9568.2011.07682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachon F. Physiological function of PARbZip circadian clock-controlled transcription factors. Ann. Med. 2007;39:562–571. doi: 10.1080/07853890701491034. [DOI] [PubMed] [Google Scholar]
- Gachon F, Leuenberger N, Claudel T, Gos P, Jouffe C, et al. Proline- and acidic amino acid-rich basic leucine zipper proteins modulate peroxisome proliferator-activated receptor alpha (PPARalpha) activity. Proc Natl Acad Sci U S A. 2011;108:4794–4799. doi: 10.1073/pnas.1002862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachon F, Olela FF, Schaad O, Descombes P, Schibler U. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab. 2006;4:25–36. doi: 10.1016/j.cmet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat. Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, et al. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Brewer JM, Champhekar A, Harris RB, Bittman EL. Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc. Natl. Acad. Sci. USA. 2005;102:3111–3116. doi: 10.1073/pnas.0409734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, et al. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 2001;6:269–278. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- Herzog ED. Neurons and networks in daily rhythms. Nat. Rev. Neurosci. 2007;8:790–802. doi: 10.1038/nrn2215. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: a reliable clock from less reliable neurons. J. Biol. Rhythms. 2004;19:35–46. doi: 10.1177/0748730403260776. [DOI] [PubMed] [Google Scholar]
- Hogenesch JB, Ueda HR. Understanding systems-level properties: timely stories from the study of clocks. Nat. Rev. Genet. 2011;12:407–416. doi: 10.1038/nrg2972. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S. The SCN-independent clocks, methamphetamine and food restriction. Eur. J. Neurosci. 2009;30:1707–1717. doi: 10.1111/j.1460-9568.2009.06976.x. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S, Hiroshige T. Activity rhythms in the circadian domain appear in suprachiasmatic nuclei lesioned rats given methamphetamine. Physiol. Behav. 1987;40:767–774. doi: 10.1016/0031-9384(87)90281-2. [DOI] [PubMed] [Google Scholar]
- Honma S, Honma K, Shirakawa T, Hiroshige T. Rhythms in behaviors, body temperature and plasma corticosterone in SCN lesioned rats given methamphetamine. Physiol. Behav. 1988;44:247–255. doi: 10.1016/0031-9384(88)90146-1. [DOI] [PubMed] [Google Scholar]
- Honma S, Yasuda T, Yasui A, van der Horst GT, Honma K. Circadian behavioral rhythms in Cry1/Cry2 double-deficient mice induced by methamphetamine. J. Biol. Rhythms. 2008;23:91–94. doi: 10.1177/0748730407311124. [DOI] [PubMed] [Google Scholar]
- Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, et al. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5:e1000442. doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Honma S, Ono D, Tanahashi Y, Honma K. Separate oscillating cell groups in mouse suprachiasmatic nucleus couple photoperiodically to the onset and end of daily activity. Proc. Natl. Acad. Sci. USA. 2007;104:7664–7669. doi: 10.1073/pnas.0607713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, et al. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Bruinstroop E, Yi CX, Klieverik LP, La Fleur SE, Fliers E. Hypothalamic control of energy metabolism via the autonomic nervous system. Ann. N. Y. Acad. Sci. 2010;1212:114–129. doi: 10.1111/j.1749-6632.2010.05800.x. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J. Neurosci. 2004;24:7604–7613. doi: 10.1523/JNEUROSCI.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Hiroshige T, Shinsako J, Dallman MF. Diurnal changes in amplification of hormone rhythms in the adrenocortical system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1980;239:R309–R316. doi: 10.1152/ajpregu.1980.239.3.R309. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Kaneko K, Shinsako J, Dallman MF. Adrenal sensitivity to adrenocorticotropin varies diurnally. Endocrinology. 1981;109:70–75. doi: 10.1210/endo-109-1-70. [DOI] [PubMed] [Google Scholar]
- Ko CH, Yamada YR, Welsh DK, Buhr ED, Liu AC, et al. Emergence of noise-induced oscillations in the central circadian pacemaker. In PLoS Biol. 2010:e1000513. doi: 10.1371/journal.pbio.1000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5:e34. doi: 10.1371/journal.pbio.0050034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger DT, Hauser H, Krey LC. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science. 1977;197:398–399. doi: 10.1126/science.877566. [DOI] [PubMed] [Google Scholar]
- Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;290:R1527–R1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Yamakawa GR, Mistlberger RE. Robust food anticipatory circadian rhythms in rats with complete ablation of the thalamic paraventricular nucleus. Brain Res. 2007;1141:108–118. doi: 10.1016/j.brainres.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Le Martelot G, Claudel T, Gatfield D, Schaad O, Kornmann B, et al. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7:e1000181. doi: 10.1371/journal.pbio.1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberger T, Staels B, Saladin R, Desvergne B, Auwerx J, Wahli W. Regulation of the peroxisome proliferator-activated receptor alpha gene by glucocorticoids. J. Biol. Chem. 1994;269:24527–24530. [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007a;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007b;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nuclei. Cell. 1997;91:855–860. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Mammalian circadian biology: Elucidating genome-wide levels of temporal organization. Annu. Rev. Genom. Hum. Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS. Genetics of circadian rhythms in Mammalian model organisms. Adv. Genet. 2011;74:175–230. doi: 10.1016/B978-0-12-387690-4.00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney CE, Brewer D, Costello MK, Brewer JM, Bittman EL. Lateralization of the central circadian pacemaker output: a test of neural control of peripheral oscillator phase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:R751–R761. doi: 10.1152/ajpregu.00746.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BH, McDearmon EL, Panda S, Hayes KR, Zhang J, et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci U S A. 2007;104:3342–3347. doi: 10.1073/pnas.0611724104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Rechtschaffen A. Recovery of anticipatory activity to restricted feeding in rats with ventromedial hypothalamic lesions. Physiol. Behav. 1984;33:227–235. doi: 10.1016/0031-9384(84)90104-5. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Baer ML, Menaker M. The methamphetamine-sensitive circadian oscillator does not employ canonical clock genes. Proc. Natl. Acad. Sci. USA. 2009;106:3519–3524. doi: 10.1073/pnas.0813366106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya T, Aida R, Kudo T, Akiyama M, Doi M, et al. The dorsomedial hypothalamic nucleus is not necessary for food-anticipatory circadian rhythms of behavior, temperature or clock gene expression in mice. Eur. J. Neurosci. 2009;29:1447–1460. doi: 10.1111/j.1460-9568.2009.06697.x. [DOI] [PubMed] [Google Scholar]
- Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Pendergast JS, Nakamura W, Friday RC, Hatanaka F, Takumi T, Yamazaki S. Robust food anticipatory activity in BMAL1-deficient mice. PLoS One. 2009;4:e4860. doi: 10.1371/journal.pone.0004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezuk P, Mohawk JA, Yoshikawa T, Sellix MT, Menaker M. Circadian organization is governed by extra-SCN pacemakers. J. Biol. Rhythms. 2010;25:432–441. doi: 10.1177/0748730410385204. [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AB, Maywood ES, Karp NA, King VM, Inoue Y, et al. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology. 2007;45:1478–1488. doi: 10.1002/hep.21571. [DOI] [PubMed] [Google Scholar]
- Refinetti R. Entrainment of circadian rhythm by ambient temperature cycles in mice. J. Biol. Rhythms. 2010;25:247–256. doi: 10.1177/0748730410372074. [DOI] [PubMed] [Google Scholar]
- Reinke H, Saini C, Fleury-Olela F, Dibner C, Benjamin IJ, Schibler U. Differential display of DNA-binding proteins reveals heat-shock factor 1 as a circadian transcription factor. Genes Dev. 2008;22:331–345. doi: 10.1101/gad.453808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C. A behavioristic study of the activity of the rat. Comp Psychol Monographs. 1922;1:1–55. [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rutter J, Reick M, McKnight SL. Metabolism and the control of circadian rhythms. Annu. Rev. Biochem. 2002;71:307–331. doi: 10.1146/annurev.biochem.71.090501.142857. [DOI] [PubMed] [Google Scholar]
- Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293:510–514. doi: 10.1126/science.1060698. [DOI] [PubMed] [Google Scholar]
- Sato TK, Panda S, Miraglia LJ, Reyes TM, Rudic RD, et al. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43:527–537. doi: 10.1016/j.neuron.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: many subtypes, diverse functions. Trends Neurosci. 2011 doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz I, Ripperger JA, Baeriswyl-Aebischer S, Albrecht U. The mammalian clock component PERIOD2 coordinates circadian output by interaction with nuclear receptors. Genes Dev. 2010;24:345–357. doi: 10.1101/gad.564110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- So AY, Bernal TU, Pillsbury ML, Yamamoto KR, Feldman BJ. Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci U S A. 2009;106:17582–17587. doi: 10.1073/pnas.0909733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK. The “other” circadian system: food as a Zeitgeber. J. Biol. Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Swann JM, Sisk CL. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol. 1979;25:545–554. doi: 10.1016/s0163-1047(79)90332-7. [DOI] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc. Natl. Acad. Sci. USA. 2009;106:6808–6813. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat. Rev. Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataroglu O, Davidson AJ, Benvenuto LJ, Menaker M. The methamphetamine-sensitive circadian oscillator (MASCO) in mice. J. Biol. Rhythms. 2006;21:185–194. doi: 10.1177/0748730406287529. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama T, Krout KE, Nguyen XV, Karpitskiy V, Kollert A, et al. Suprachiasmatic nucleus: a central autonomic clock. Nat. Neurosci. 1999;2:1051–1053. doi: 10.1038/15973. [DOI] [PubMed] [Google Scholar]
- VanderLeest HT, Houben T, Michel S, Deboer T, Albus H, et al. Seasonal encoding by the circadian pacemaker of the SCN. Curr. Biol. 2007;17:468–473. doi: 10.1016/j.cub.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, Ko CH, Chang AM, Buhr ED, Fruechte EM, et al. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc. Natl. Acad. Sci. USA. 2006;103:9327–9332. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl. Acad. Sci. USA. 2009;106:21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujovic N, Davidson AJ, Menaker M. Sympathetic input modulates, but does not determine, phase of peripheral circadian oscillators. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R355–R360. doi: 10.1152/ajpregu.00498.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc. Natl. Acad. Sci. USA. 2009;106:16493–16498. doi: 10.1073/pnas.0902768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu. Rev. Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr. Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita K, Tamanini F, van Der Horst GT, Okamura H. Molecular mechanisms of the biological clock in cultured fibroblasts. Science. 2001;292:278–281. doi: 10.1126/science.1059542. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, et al. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, et al. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem. 2005a;280:42036–42043. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, et al. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J. Biol. Chem. 2005b;280:42036–42043. doi: 10.1074/jbc.M509600200. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Yang X, Downes M, Yu RT, Bookout AL, He W, et al. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126:801–810. doi: 10.1016/j.cell.2006.06.050. [DOI] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc. Natl. Acad. Sci. USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]