Abstract
While postnatal psychological distress has been widely studied for many years, particularly with a focus on postpartum depression, symptoms of maternal depression, stress, and anxiety are not more common or severe after childbirth than during pregnancy. This paper reviews the newer body of research aimed at identifying the effects of women’s antenatal psychological distress on fetal behavior and child development, and the biological pathways for this influence. These studies are in line with the growing body of literature supporting the “fetal origins hypothesis” that prenatal environmental exposures — including maternal psychological state–based alterations in in utero physiology — can have sustained effects across the lifespan.
Keywords: fetal heart rate, fetal movement, HPA axis, neurobehavioral development, developmental psychopathology
Introduction
While postnatal psychological distress has been widely studied for many years, particularly with a focus on postpartum depression, symptoms of maternal depression, stress, and anxiety are not more common or severe after childbirth than during pregnancy [1]. In a recent meta-analysis of 28 articles regarding depression during pregnancy, Gavin et al. found that up to 13% of women experience depressive episodes at some point during pregnancy or within the first year postpartum [2]. Thus, a newer body of research has emerged aimed at identifying the effects of women’s antenatal psychological distress on fetal behavior and child development, and the biological pathways for this influence. These studies are in line with the growing body of literature supporting the “fetal origins hypothesis” that prenatal environmental exposures — including maternal psychological state–based alterations in in utero physiology — can have sustained effects across the lifespan.
The prenatal period is a critical time for neurodevelopment and is thus a period of vulnerability during which a range of exposures have been found to exert long-term changes on brain development and behavior with implications for physical and psychiatric health. For example, maternal consumption of essential fatty acids during pregnancy is linked to lower birth weight and decrements in cognitive and motor function, while fetal exposure to PCBs and methylmercury, via seafood in women’s diet, is linked to neurocognitive deficits [3].
While toxins have direct effects on the processes of neurogenesis, neuronal migration, cellular differentiation and synaptic refinement that are occurring during the prenatal period, there also is evidence for the interaction between these types of prenatal exposures and maternal psychosocial health. Risk of developmental delay in children exposed prenatally to tobacco smoke has been found to be much greater among those infants whose mothers also experienced material hardship during pregnancy[3]. This finding is consistent with extensive epidemiological research on birth cohorts from the Dutch Hunger Winter of 1944 based on offspring of women who were pregnant at the time when food intake was reduced to 500 – 1500 kcal per day during WWII. In a series of studies, Brown and colleagues have shown that fetal development during this period is associated with a two–fold increased risk for schizophrenia, schizoid/schizoptypal personality disorder, as well as comparable risk for major affective disorders in adulthood [4]. Two interpretations regarding the causal mechanisms of these effects have been suggested: 1) deficiency in many micro– and macronutrients, such as folate and/or overall calorie nutrition could directly alter brain development or 2) maternal stress, secondary to famine, could have neurotoxic effects on brain regions relevant to mental illness. Though these mechanisms are not mutually exclusive and could both contribute to the increased risk of psychopathology observed in these studies, there are extensive studies on prenatal stress in animals that provide support for the latter interpretation and largely rule out genetic factors for the prenatal stress effects [5].
Clinical studies link pregnant women’s exposure to a range of traumatic, as well as chronic and common life stressors (i.e., bereavement, daily hassles, and earthquake), to significant alterations in children’s neurodevelopment, including increased risk for mixed handedness, autism, affective disorders, and reduced cognitive ability[6]. More recently, maternal antenatal anxiety and/or depression have been shown to predict increased risk for neurodevelopmental disorders in children, and to confer risk for future mental illness. Reports show that elevated levels of antenatal depression and anxiety are associated with poor emotional adjustment in young children [7]. The impact of women’s anxiety (and/or depression) during pregnancy has been found to extend into childhood and adolescence, as well as to affect the hypothalamic-pituitary-adrenal (HPA) axis, predicting attention deficit hyperactivity disorder (ADHD) symptoms in 8–9 year old children [8]as well as alterations in HPA axis activation in 4 month olds in our laboratory [9] and in 10 [10], and 14–15 year olds [11]. The majority of these studies have controlled for women’s postnatal mood, as well as other demographic factors, yet the possibility that the women’s’ antenatal mood is a marker for qualities in the postnatal environment that affect child development cannot be ruled out. What these data suggest is that, in addition to the known pathways for the familial transmission of risk for mental illness, genetics, environment, and gene X environment interactions, there is another possibility: that some of the risk is conferred prenatally via changes in women’s mood–based physiology affecting fetal neurobehavioral development.
If pregnant women’s distress, similar to their nutrition, is influencing children’s long–term development, i.e., if fetal exposure to the physiological alterations associated with women’s psychological distress affects child outcomes, evidence of this maternal influence should be detectable during the prenatal period. This review will cover the recent studies showing associations between prenatal maternal psychological states and alterations in fetal behavior and physiology, as well as the two possible pathways for the ‘transmission’ of maternal mood to the fetus: (1) maternal-fetal HPA axis dysregulation and (2) intrauterine environment disruption due to variations in uterine artery flow. Implications for clinical intervention will be discussed.
Women’s Antenatal Psychological State: Influences on Fetal Physiology and Behavior
Fetal Heart Rate
Characteristics of fetal heart rate (FHR) activity are associated with a range of dysphoric psychological states in pregnant women, including perceived stress, lab-induced stress, self-reported depression, clinically appraised depression, anxiety disorders, state anxiety, and anxiodepressive comorbidities (Table 1). Importantly, the indices of FHR used in these studies are distinct from those of cardiac activity used by obstetricians and others in prenatal pediatrics to investigate physical disease and anomalies. Instead, common markers such as FHR reactivity to a stimulus, or heart rate variability, reflect emerging individual differences in the development of the autonomic and central nervous systems related to styles of future emotion regulation and risk for psychopathology. For example, in both child and adult psychiatry research, lower levels of high frequency heart rate variability are associated with less adaptive transitions in responding to emotion–eliciting cues [12] and (in adults) greater hostility [13]. In other studies, greater heart rate increases to novelty in infancy predicts increased fearful behavior and an increased risk for anxiety disorders in school age children[14]. That there is continuity in fetal to infant neurobehavior [15–18] further supports the relevance of these autonomic nervous system (ANS) indices for characterizing the influence of women’s antental distress on fetal development.
Table 1.
Women’s Antenatal Psychological State: Influences on Fetal Physiology and Behavior
| Authors | Title | Design | Results |
|---|---|---|---|
| DiPietro et al., 1996 | Fetal Neurobehavioral Development | N=31; Fetal activity and fetal heart rate digitized using fetal actocardiograph over 50 min. periods at 20w, 24w, 28w, 32w, 36w, and 38w–39w gestation while maternal abdomen underwent vibroacoustic stimulation; HSUP |
|
| Allister et al., 2001 | The effects of maternal depression on fetal heart rate response to vibroacoustic stimulation | N=20; FHR monitored using actocardiograph while maternal abdomen underwent vibroacoustic stimulation between 32w–36w gestation; BDI |
|
| Dieter et al., 2008a | Maternal depression and anxiety effects on the human fetus: preliminary findings and clinical implications | N=90: Fetal activity observed via ultrasound for 5 continuous minutes between 18w and 36w gestation and movements were categorized; CES-D, STAI |
|
| DiPietro et al., 2002 | Maternal stress and affect influence fetal neurobehavioral development | N=52; Fetal activity monitored via fetal actocardiograph at 24w, 30w, and 36w gestation; AIM, DSI, PES – utilized to form a composite score |
|
| Monk et al., 2004 | Fetal heart rate reactivity differs by women’s psychiatric status: an early marker for developmental risk? | N=57; Fetal activity monitored via actocardiograph as well as maternal EKG, BP, respiration, and salivary cortisol between 36w–38w gestation during a lab- induced stressor; SCID and STAI during 2nd trimester |
|
| Monk et al., 2000 | Maternal stress responses and anxiety during pregnancy: effects on fetal heart rate | N=17; Fetal activity monitored via actocardiograph as well as maternal EKG, BP, and respiration between 35w–38w gestation during a lab- induced stressor; STPI |
|
| Monk et al., 2003 | Effects of women’s stress- elicited physiological activity and chronic anxiety on fetal heart rate | N=32; Fetal activity monitored via actocardiograph as well as maternal EKG, BP, and respiration between 35w–38w gestation during a lab- induced stressor; STAI |
|
| Monk et al., in preparation | Prenatal origins of self- regulation: fetal sensory responses differ by women’s psychiatric status | N=113; Fetal activity monitored via actocardiograph as well as maternal EKG, BP, respiration, and salivary cortisol between 36w–38w gestation during a lab- induced stressor; SCID during 2nd trimester |
|
| DiPietro et al., 2003 | Fetal response to induced maternal stress | N=137; Fetal activity monitored via actocardiograph as well as maternal EKG and SCL at 24w and 36w gestation during a lab-induced stressor |
|
| Groome et al., 1995 | Maternal anxiety during pregnancy: effect on fetal behavior at 38 to 40 weeks of gestation | N=18; Fetal activity monitored for 60 consecutive minutes using fetal actocardiograph at 38w–40w to define in utero “sleep states;” STAI |
|
| Dieter et al., 2008b | Maternal depression and anxiety effects on the human fetus: preliminary findings and clinical implications | N=32; Fetal activity monitored with fetal actocardiograph while maternal abdomen underwent vibroacoustic stimulation at 33w gestation; BDI-II, BAI |
|
| DiPietro et al., 1996 | Development of fetal movement – fetal heart rate coupling from 20 weeks through term | N=31; Fetal activity and fetal heart rate digitized using fetal actocardiograph over 50 min. periods at 20w, 24w, 28w, 32w, 36w, and 38w–39w gestation while maternal abdomen underwent vibroacoustic stimulation; HSUP |
|
| DiPietro et al., 1998 | Fetal neurobehavioral development: associations with socioeconomic class and fetal sex | N=103; Fetal activity and fetal heart rate digitized using fetal actocardiograph over 50 min. periods at 24w, 30w, and 36w gestation; group stratification by maternal SES |
|
| Sandman et al., 1999 | Maternal corticotropin- releasing hormone and habituation in the human fetus | N=33; Fetal activity monitored via fetal actocardiograph while maternal abdomen underwent vibroacoustic stimulation and maternal plasma CRH through blood draw between 31w–32w gestation. |
|
| Field et al., 2004 | Prenatal maternal cortisol, fetal activity and growth | N=131; Fetal activity and estimated fetal weight coded from ultrasound, cortisol collected through urinalysis between 20w–28w gestation; CES-D, STAI |
|
| DiPietro et al., in press | Fetal motor activity is associated with maternal cortisol | N=92; Fetal activity monitoring via fetal actocardiograph and salivary cortisol collection at 32w and 36w gestation; PSS, PNAS, STAI, PES |
|
| Sandman et al., 2003 | Maternal hypothalamic- pituitary-adrenal disregulation during the third trimester influences human fetal responses | N=135; Fetal activity monitored via fetal actocardiograph and maternal plasma ACTH and β-endorphin through blood draw at 32w gestation |
|
| Teixeira et al., 1999 | Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study | N=100; Uterine artery flow assessed by color Doppler ultrasound at 32w gestation; STAI |
|
| Kent et al., 2002 | Uterine artery resistance and anxiety in the second trimester of pregnancy | N=96; Uterine artery flow assessed by color Doppler ultrasound at 20w gestation; HAD |
|
| DiPietro et al., 2008 | Fetal responses to induced maternal relaxation during pregnancy | N=99; Fetal activity monitored via actocardiograph as well as maternal EKG, SCL, respiration, and uterine artery flow as assessed by color Doppler ultrasound at 32w gestation during a lab- induced maternal relaxation session; salivary cortisol collected at 6 points during session |
|
In a longitudinal study of fetal ontogeny, DiPietro et al. assessed fetal variables in relation to pregnant women’s reports of daily stress at 6 testing sessions beginning at 20 weeks and ending at 36–68 weeks gestation [19]. Fetuses of mothers who reported greater stress showed significantly lower FHR variability than the low stress group, which suggests that exposure to maternal psychological distress may contribute to diminished parasympathetic control of the fetal heart [19]. Similarly, fetuses of women suffering from maternal depression have displayed higher baseline FHR and a delayed FHR response to stimulus. In a fetal reactivity study, Allister et al. monitored fetal behavior in women with untreated depression and controls at 32–36 weeks gestation during a baseline period, a period of fetal stimulation via a vibroacoustic stimulus administered to the mother’s abdomen, and during a recovery period [20]. Fetuses of depressed mothers displayed a higher baseline FHR, a slower FHR reaction to the external stimulus, and a longer period to return to FHR baseline levels following the stimulus compared to fetuses in a control group [20].
In a series of studies from our group, we have shown that fetuses of mothers experiencing psychological distress also show significantly different reactivity to acute maternal stress. Fetuses of depressed mothers showed a significantly higher increase in FHR when the mother was introduced to a lab-induced stressor [21]. Similar increases in FHR were found in fetuses of anxious and anxiodepressive comorbid women [22–24]. In these studies, there were no group differences in baseline FHR, or in women’s cardiorespiratory reactivity to the laboratory challenge, which demonstrated significant increases. We interpret these results as indicating that fetuses have group differences in their acute reactivity to changes in the intrauterine environment, that is, to changes in maternal heart rate, respiration, and blood pressure, which may function as auditory and kinesthetic stimuli to the fetus and thereby reveal variation in fetuses’ central nervous system development. In a similar study of pregnant women exposed to a laboratory–based stressor, DiPietro et al, found increased FHR variability (which usually is associated with lowered FHR) and reduced movement during the challenge period compared to baseline [25]. The contrasting results between our studies and this one potentially underscore the role of chronic maternal mood in shaping fetal responses to changes in the intrauterine environment associated with maternal experience. That is, when fetal responses are examined in relation to women’s psychiatric symptoms and/or chronic mood, divergent responses emerge such that the subsample of those whose mothers have symptoms have a different response from those of ‘controls.’ Consequently, when all fetuses are examined together, the FHR result of the larger ‘control’ group is the average response, and contrasts with the finding from a clinical sample.
Another set of studies examining the effects of prenatal depression and anxiety found that fetuses of depressed and anxious mothers habituated more quickly and more fully to a vibroacoustic stimulus placed on the maternal abdomen than fetuses unexposed to maternal depression and anxiety [26]. One possible explanation for these results, which are in direct opposition to the findings of Allister et al. [20] and to the greater FHR reactivity to a different stimulus in our studies [21, 22], is that, due to methodological differences, the fetuses in the Dieter research are not experiencing “true” habituation to the vibroacoustic stimulus; rather, they are experiencing “receptor adaptation” or “effector fatigue” [27] such that fetuses of depressed and anxious women experience a diminished ability to react to repeated stimulation and sustain a robust response, as opposed to habituation. A better understanding of the effects of maternal psychological state on fetal reactivity and habituation requires more research and utilizing protocols other than vibroacoustic stimulation and laboratory stressors [26].
Fetal Activity
In addition to FHR, fetal activity, sleep pattern and movement have been shown to be influenced by maternal psychological states (Table 1), suggesting that maternal mood may also affect central nervous system development. When observed with an ultrasound monitor for 5 continuous minutes between 18 and 36 weeks gestation in a study of the effects maternal anxiety and depression on fetal development, fetuses of depressed mothers spent a greater percent of time active than fetuses of nondepressed mothers. This effect of maternal mood on fetal activity was strengthened when measurements of anxiety were included concurrently with depression, explaining 35% of the variance in fetal activity [26]. Maternal stress has also been shown to have a significant association with increased fetal motor activity at 24, 30, and 36 weeks gestation [28]. In contrast, fetuses of mothers with high anxiety have also been found to spend more time in “quiet sleep” and to be less active in “active sleep” than fetuses of mothers without high anxiety, with a linear relationship emerging between maternal anxiety and percent quiet sleep over a 4 hour monitoring period [29]. The long duration of this monitoring period may serve to explain the converse findings, as most other studies have only assessed a brief snapshot of fetal behavior. This could suggest that short observation periods are capturing acute changes in fetal behavior as a result of maternal testing conditions. Further observation of prolonged fetal monitoring will help to clarify the effects of maternal psychological distress on fetal behavior.
Finally, studies by DiPietro and colleagues have used as indices of fetal neurobehavioral the coupling of FHR and movement. They have shown that over the course of gestation, there is more often a coincidence between changes in heart rate and body movement [30]. They interpret this finding as indicating that the coupling index is reflecting the level of integration of the central nervous system, which increases with gestational age. In several studies, they have found associations between maternal health characteristics and the coupling of FHR and movement. Fetuses of women in lower socio–economic groups, and those of women reporting greater daily stress, and who had a higher resting heart rate, had less FHR and movement coupling compared to higher socio-economic status (SES) women and those with low daily stress scores [31].
Taken together, data from these studies, even with some contrary results, support the hypothesis that maternal psychological distress can affect the fetal autonomic and central nervous systems. In particular, depression and multiple assessments of daily stress are chronic mood states supporting the idea that, over the course of pregnancy, repeated exposure to mood–based alterations in women’s physiology shapes fetal neurobehavioral development.
Physiological Pathways for Transmission of Maternal Distress to the Fetus
Though maternal psychological distress has been shown to elicit both maternal cardiorespiratory and FHR and movement variations in baseline and lab-induced stress reactivity in many of these studies, as indicated, these physiologic changes have been found to exist largely independent of one another. Few significant relationships between concurrent maternal autonomic measures and FHR and movement are found, with the exception of a small association between increases in maternal skin conductance reactivity and increases in fetal movement [32], as well as an association between maternal blood pressure and FHR [21]. Researchers have looked to the HPA axis and uterine functioning as possible pathways by which maternal psychological state is transmitted to the fetus.
Maternal-Fetal HPA Axis Dysregulation
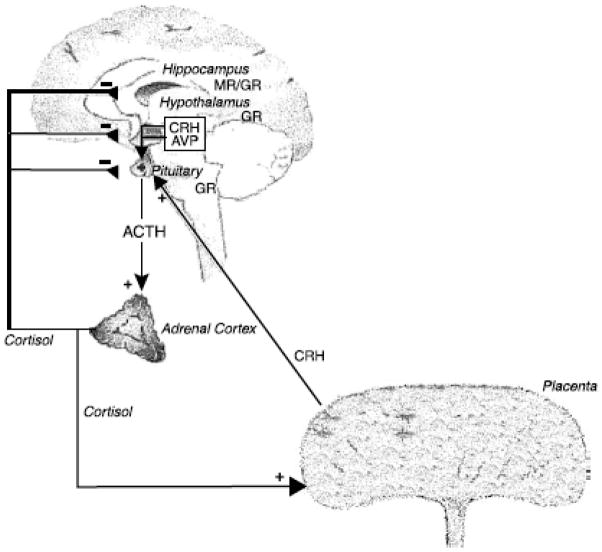
The HPA axis is often regarded as the central regulatory system for psychological distress[33]. The principal modulator of the HPA axis for psychological distress is corticotropin-releasing hormone (CRH), which is primarily released by the hypothalamus into the hypothalamo-hypophyseal portal system. This portal leads CRH into the anterior of the pituitary gland, stimulating corticotropes and secreting adrenocorticotropic horomone (ACTH) [34, 35]. ACTH stimulates the ACTH receptors of the adrenal gland, causing the synthesis and secretion of glucocorticoids into the blood stream, primarily cortisol. Cortisol elicits physiologic responses, such as increased blood pressure and heart rate, as well as down-regulating the hypothalamic release of CRH [34]. During pregnancy, in addition to hypothalamic CRH, the placenta also generates and releases CRH into the blood stream, causing hyperactivation of the HPA axis, and a considerable rise in the ratio of free/bound cortisol, reaching values comparable to those found in Cushing’s disease [36, 37]. The production of placental CRH and the presence of excess cortisol begin during the second trimester and increases linearly to term, with a spike in the last 6–8 weeks of pregnancy. This prenatal HPA axis regulation is displayed in Fig. 1. It has been demonstrated that as pregnant women progress through gestation, cortisol responses to acute stress decline, suggesting a blunting of the HPA axis due to high levels of placental CRH [36, 38, 39]. In humans, by the 16th gestational week, the placental enzyme 11β–HSD–2, which converts cortisol to inactive cortisone, forms a barrier to maternal glucocorticoids. However, 10–20% of maternal cortisol passes through to the fetus [40, 41], which, under conditions of stress–induced elevated maternal HPA activity, may be sufficient to exert long-term effects on the developing fetal brain.
Figure 1.
(if use, NEEDS TO BE REDRAWN for copy right purposes)
In psychiatric studies, elevated reactivity of the HPA axis is commonly found in the neurobiology of depression and other psychiatric illnesses [42]. During pregnancy, the link has been less consistent. Sarkar and colleagues recently found that pregnant women’s anxiety did not predict amniotic fluid cortisol and that the modest association between maternal anxiety and plasma cortisol is no longer detectable after 17 weeks gestation, likely due to the hypercortilsolemia of the HPA axis in later stages of pregnancy [43]. However, another report from the same laboratory found that maternal plasma and amniotic fluid cortisol are correlated after 18 weeks gestation, and that state anxiety following amniocentesis collected across a range of trimesters moderated the association between maternal plasma and amniotic fluid cortisol such that there was a strong positive relationship (r =.59) for highly anxious women, and a non-significant correlation in the least anxious group [41]. These findings suggest that antenatal anxiety may affect placental function, which in turn, regulates fetal exposure to maternal cortisol. In another study, higher ratings of self–reported stress were associated with elevated levels of ACTH and cortisol at 28 weeks[44]. We have found that 3rd trimester women who are co–morbid for anxiety and depression have higher cortisol compared to healthy controls, as well as those with only one psychiatric disorder [45].
In a 2004 study of fetal activity and maternal cortisol between 20 and 28 weeks gestation, Field et al. found that maternal cortisol levels were significantly related to increased fetal activity and inversely related to estimated fetal weight [46]. Similarly, DiPietro et al. found maternal cortisol levels were associated with fetal motor vigor and total time spent moving [47]. In our work, maternal cortisol is positively associated with greater FHR during women’s exposure to laboratory stress [24]. Furthermore, maternal CRH concentration has been evidenced to have a linear relationship with baseline FHR among women in 31 to 32 weeks gestation, while the inverse is demonstrated in FHR time to habituate to an abdominal vibroacoustic stimulus [48]. Interestingly, ACTH does not exhibit an association with FHR. However, dysregulated and uncoupled levels of ACTH and β-endorphin in maternal plasma displayed an effect on FHR increases [49].
Uterine Artery Resistance
Another mechanism by which maternal psychological state might affect the fetus is through the alteration of blood flow to the fetus via the uterine arteries. Uterine blood flow can be assessed by using a color Doppler ultrasound to measure uterine artery resistance and to detect the presence of notches in the ultrasound waveform pattern. The presence of a notch denotes a very high resistance to blood flow. Measures of high uterine artery resistance have been previously associated with underweight for gestational age babies and pre-eclampsia [50, 51].
This potential maternal-fetal psychological pathway remains largely unresearched, with only two conflicting studies. Teixeira et al. investigated uterine artery resistance among women experiencing anxiety versus controls at 32 weeks gestation. A significant positive association was discovered between both maximum artery resistance scores and mean artery resistance and anxiety levels [52]. There also was a significant dichotomous relationship between women with high anxiety and the presence of waveform notching. In a similarly designed study, though with women in the 20th week of gestation, Kent et al. could not repeat these findings, discovering no associative relationships between anxiety and uterine blood flow[53].
While the HPA axis may be involved with increasing uterine artery resistance, it has been suggested that noradrenaline is a likely mediator via sympathetic-adrenal activation [52]. Previous studies have shown a linear relationship between plasma noradrenaline concentration and anxiety [54]. Animal research has linked noradrenaline levels and decreased uterine blood flow [55, 56]. More research on this association utilizing a wider body of psychological, physiological, and neuroendocrine measurements is necessary.
Environmental imprinting?
Recently, DiPietro et al. utilized the notion of transmitting maternal state to the fetus in a lab-induced relaxation study [32]. During the 32nd week of gestation, maternal-fetal pairs were monitored for maternal EKG, skin conductance, and respiration as well as fetal heart rate and movement for 18 minutes of baseline, 18 minutes of guided relaxation, and 18 minutes of recovery. Salivary cortisol was collected at 6 different times during the study period including immediately following: subject arrival, ultrasound, baseline, relaxation, post-relaxation, and just before the subject departs.
All maternal physiological measures, except for respiration, declined significantly from baseline to relaxation, as well as their salivary cortisol levels. FHR and movement also declined significantly from baseline to relaxation, while FHR variability increased. Although maternal skin conductance level and respiration were significantly associated with FHR, the associations, as previously described, were small (rs. = .22 and –.21, respectively). Moreover, induced relaxation elicited a decline in fetal movement and an augmentation in FHR variability similar to the inducement of maternal stress [32]. Taken together, DiPietro et al. consider these unexpected results as suggesting that the fetal responses to both situations may, in part, reflect fetal perception of changes in the intrauterine environment resulting from the laboratory and relaxation manipulations. Because FHR decreases, they consider the possibility that the fetuses are showing an orienting response. This interpretation is similar to the previously discussed interpretation by Monk et al., in which group differences in FHR reactivity reflect group differences in fetal autonomic and central nervous system regulation in response to cardiorespiratory reactivity in women. It is possible that over the course of gestation, fetuses are conditioned by the stimuli in their prenatal environment to be better prepared for what they will encounter postnatally. Thus, some of the transmission of women’s psychological distress–based changes in physiology may shape fetal behavior as a result of in utero perception and learning.
Clinical Implications
Studies discussed here, all of which are ongoing, indicate that pregnant women’s psychological health may have consequences for fetal neurobehavioral development, and consequently, child outcomes. These findings underscore the importance of considering the effects of women’s mental health on child development during the prenatal, as well the postnatal, periods. While gestational diabetes is far less common than depression during pregnancy, women are routinely screened for this disorder, but not for depression, any psychiatric illness, nor even experiences of life stress. This is a short coming of public health policy, as it is an optimal time to perform mental health screenings since women most often are under regular care and thus available for referrals and follow up interventions, and because there is, potentially, more than one patient. Some forms of psychotherapy, as well as psychopharmacologic medications have been shown to be effective for depression and anxiety during pregnancy [57]. Further research likely will lead to targeted interventions for this population. For now, the first aim should be improved surveillance of women’s mood and mental health during pregnancy.
References
- 1.Evans J, et al. Cohort study of depressed mood during pregnancy and after childbirth. BMJ. 2001;323(7307):257–260. doi: 10.1136/bmj.323.7307.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavin NI, et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–83. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 3.Perera F, et al. Children’s environmental health research--highlights from the Columbia Center for Children’s Environmental Health. Ann N Y Acad Sci. 2006;1076:15–28. doi: 10.1196/annals.1371.018. [DOI] [PubMed] [Google Scholar]
- 4.Brown AS, et al. Further evidence of relation between prenatal famine and major affective disorder. American Journal of Psychiatry. 2000;157(2):190–195. doi: 10.1176/appi.ajp.157.2.190. [DOI] [PubMed] [Google Scholar]
- 5.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19(4):296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48(3–4):245–61. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor TG, et al. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry. 2003;44(7):1025–36. doi: 10.1111/1469-7610.00187. [DOI] [PubMed] [Google Scholar]
- 8.Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems and anxiety in 8–9 year olds. Child Dev. 2004;75(4):1085–97. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan LA, Evans L, Monk C. Effects of mothers’ prenatal psychiatric status and postnatal caregiving on infant biobehavioral regulation: Can prenatal programming be modified? Early Hum Dev. 2007;84(4):249–56. doi: 10.1016/j.earlhumdev.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor TG, et al. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58(3):211–7. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Van den Bergh BR, et al. Antenatal Maternal Anxiety is Related to HPA-Axis Dysregulation and Self-Reported Depressive Symptoms in Adolescence: A Prospective Study on the Fetal Origins of Depressed Mood. Neuropsychopharmacology. 2008;33(3):536–45. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- 12.Porges SW, et al. Infant regulation of the vagal “brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 13.Sloan RP, et al. Hostility, gender, and cardiac autonomic control. Psychosom Med. 2001;63(3):434–40. doi: 10.1097/00006842-200105000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Kagan J. Temperament and the reactions to unfamiliarity. Child Development. 1997;68:139–143. [PubMed] [Google Scholar]
- 15.DiPietro JA, et al. Antenatal origins of individual differences in heart rate. Developmental Psychobiology. 2000;37(4):221–228. doi: 10.1002/1098-2302(2000)37:4<221::aid-dev2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 16.DiPietro JA, Ghera MM, Costigan KA. Prenatal origins of temperamental reactivity in early infancy. Early Hum Dev. 2008;84(9):569–75. doi: 10.1016/j.earlhumdev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiPietro JA, et al. Fetal antecedents of infant temperament. Child Development. 1996;67:2568–2583. [PubMed] [Google Scholar]
- 18.Werner A, et al. Prenatal predictors of infant temperament. Developmental Psychobiology. 2007;49:474–484. doi: 10.1002/dev.20232. [DOI] [PubMed] [Google Scholar]
- 19.DiPietro JA, et al. Fetal neurobehavioral development. Child Development. 1996;67:2553–2567. [PubMed] [Google Scholar]
- 20.Allister L, et al. The effects of maternal depression on fetal heart rate responce to vibroacoustic stimulation. Developmental Neuropsychology. 2001;20(3):639–651. doi: 10.1207/S15326942DN2003_6. [DOI] [PubMed] [Google Scholar]
- 21.Monk C, et al. Fetal heart rate reactivity differs by women’s psychiatric status: an early marker for developmental risk? Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43(3):283–290. doi: 10.1097/00004583-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Monk C, et al. Maternal stress responses and anxiety during pregnancy: Effects on fetal heart rate. Developmental Psychobiology. 2000;36:67–77. [PubMed] [Google Scholar]
- 23.Monk C, et al. The effects of women’s stress–elicited physiological activity and chronic anxiety on fetal heart rate. Developmental and Behavioral Pediatrics. 2003;24(1):32–38. doi: 10.1097/00004703-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Monk C, et al. Prenatal Origins of Self–Regulation: Fetal Heart Rate Reactivity is Associated with Women’s Psychiatric Status & Cortisol. (in submission) [Google Scholar]
- 25.DiPietro J, Costigan KA, Gurewitsch ED. Fetal response to induced maternal stress. Early Hum Dev. 2003;74(2):125–138. doi: 10.1016/j.earlhumdev.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Dieter J, et al. Maternal depression and anxiety effects on the human fetus: Preliminary findings and clinical implications. Infant Mental Health Journal. 2008;29(5):420–441. doi: 10.1002/imhj.20192. [DOI] [PubMed] [Google Scholar]
- 27.Thompson RFSW. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73(1):16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- 28.DiPietro JA, et al. Maternal stress and affect influence fetal neurobehavioral development. Dev Psychol. 2002;38(5):659–668. [PubMed] [Google Scholar]
- 29.Groome LJ, et al. Maternal anxiety during pregnancy: Effect on fetal behavior at 38 to 40 weeks of gestation. Developmental and Behavioral Pediatrics. 1995;16(6):391–396. [PubMed] [Google Scholar]
- 30.DiPietro JA, et al. Developmental of fetal movement – fetal heart rate coupling from 20 weeks through term. Early Human Development. 1996;44:139–151. doi: 10.1016/0378-3782(95)01704-6. [DOI] [PubMed] [Google Scholar]
- 31.Pressman EK, et al. Fetal neurobehavioral development: Associations with socioeconomic class and fetal sex. Developmental Psychobiology. 1998;33:79–91. [PubMed] [Google Scholar]
- 32.DiPietro JA, et al. Fetal responses to induced maternal relaxation during pregnancy. Biol Psychol. 2008;77(1):11–9. doi: 10.1016/j.biopsycho.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsigos C, Chrousos G. Physiology of the hypothalamic-pituitary-adrenal axis in health and dysregulation in psychiatric and autoimmune disorders. Endocrinology and metabolism clinics of North America. 1994;23(3):451–466. [PubMed] [Google Scholar]
- 34.Gold P, Goodwin F, Chrousos G. Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress. N Engl J Med. 1988;319(7):413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- 35.Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003 Nov;:136–149. doi: 10.1196/annals.1290.016. 1997. [DOI] [PubMed] [Google Scholar]
- 36.Kammerer M, Taylor A, Glover V. The HPA axis and perinatal depression: a hypothesis. Arch Womens Ment Health. 2006;9(4):187–196. doi: 10.1007/s00737-006-0131-2. [DOI] [PubMed] [Google Scholar]
- 37.Tsigos C, Chrousos G. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 38.Kammerer M, et al. Pregnant women become insensitive to cold stress. BMC Pregnancy Childbirth. 2002;2(1):8. doi: 10.1186/1471-2393-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petraglia F, et al. Lack of effect of psychosocial stress on maternal corticotropin-releasing factor and catecholamine levels at 28 weeks’ gestation. J Soc Gynecol Investig. 2001;8(2):83–88. [PubMed] [Google Scholar]
- 40.Gitau R, et al. Fetal exposure to maternal cortisol. The Lancet. 1998;352:707–708. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- 41.Glover V, et al. Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology. 2008 doi: 10.1016/j.psyneuen.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 42.Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology. 2004;29(4):641–8. doi: 10.1038/sj.npp.1300397. [DOI] [PubMed] [Google Scholar]
- 43.Sarkar P, et al. Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: possible implications for foetal programming. J Neuroendocrinol. 2008;20(4):489–96. doi: 10.1111/j.1365-2826.2008.01659.x. [DOI] [PubMed] [Google Scholar]
- 44.Wadhwa PD, Garite TJ, Sandman CA. The neurobiology of stress in human pregnancy: Implications for prematurity and development of the fetal central nervous system. Progress in Brain Research. 2001;133:131–142. doi: 10.1016/s0079-6123(01)33010-8. [DOI] [PubMed] [Google Scholar]
- 45.Evans LM, Myers MM, Monk C. Pregnant women’s cortisol is elevated with anxiety and depression - but only when comorbid. Arch Womens Ment Health. 2008 doi: 10.1007/s00737-008-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Field T, et al. Prenatal depression effects on the fetus and the newborn. Infant Behavior & Development. 2004;27(2):216–229. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 47.DiPietro Jea. Fetal motor acivity is associated with maternal cortisol. in press. [Google Scholar]
- 48.Sandman CA, et al. Maternal corticotropin-releasing hormone and habituation in the human fetus. Developmental Psychobiology. 1999;34:163–173. doi: 10.1002/(sici)1098-2302(199904)34:3<163::aid-dev1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 49.Sandman CA, et al. Maternal hypothalamic-pituitary-adrenal disregulation during the third trimester influences human fetal responses. Dev Neurosci. 2003;25(1):41–49. doi: 10.1159/000071467. [DOI] [PubMed] [Google Scholar]
- 50.Harrington K, et al. Doppler ultralsound of the uterine arteries: the importance of bilateral notching in the prediction of pre-eclampsia, placental abruption or delivery of a small-for-gestational-age baby. Ultrasound in Obstetrics & Gynecology. 1996;7(3):182–188. doi: 10.1046/j.1469-0705.1996.07030182.x. [DOI] [PubMed] [Google Scholar]
- 51.Bower S, Schuchter K, Campbell S. Doppler ultrasound screening as part of routine antenatal scanning: prediction of pre-eclampsia and intrauterine growth retardation. BJOG. 1993;100(11):989–994. doi: 10.1111/j.1471-0528.1993.tb15139.x. [DOI] [PubMed] [Google Scholar]
- 52.Teixeira JMA, Fisk NM, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: Cohort based study. British Medical Journal. 1999;318:153–157. doi: 10.1136/bmj.318.7177.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kent A, et al. Uterine artery resistance and anxiety in the second trimester of pregnancy. Ultrasound in Obstetrics & Gynecology. 2002;19(2):177–179. doi: 10.1046/j.0960-7692.2001.00546.x. [DOI] [PubMed] [Google Scholar]
- 54.Starkman MN, et al. Peripheral catecholamine levels and the symptoms of anxiety: Studies in patients with and without pheochromocytoma. Psychosomatic Medicine. 1990;52:129–142. doi: 10.1097/00006842-199003000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Fried G, Thoresen M. Effects of neuropeptide Y and noradrenaline on uterine artery blood pressure and blood flow velocity in the pregnant guinea-pig. Regul Pept. 1990;28(1):1–9. doi: 10.1016/0167-0115(90)90059-6. [DOI] [PubMed] [Google Scholar]
- 56.Rosenfield C, West J. Circulatory response to systemic infusion of norepinephrine in the pregnant ewe. Am J Obstet Gynecol. 1976;127:376–383. doi: 10.1016/0002-9378(77)90493-8. [DOI] [PubMed] [Google Scholar]
- 57.Pearlstein T. Perinatal depression: treatment options and dilemmas. J Psychiatry Neurosci. 2008;33(4):302–18. [PMC free article] [PubMed] [Google Scholar]